Abstract
Metabolites produced by gut microbiota could be pathogenic or beneficial to the host. Gut microbiota varies among countries, age and sex, there is a possibility that the microbe‐associated metabolites might vary among countries, age and sex. Further studies are required to clarify the relationship among diet, gut microbiota, gut microbiota‐related metabolites and development of type 2 diabetes.
![]()
Patients with type 2 diabetes are increasing worldwide, especially in Asian countries. Diabetes mellitus is a multifactorial disease that develops and progresses due to the interaction of genetic and environmental factors. In addition to these factors, dietary factors have an effect on type 2 diabetes. In fact, the Westernization of the Asian diet is known to be responsible for the increase in type 2 diabetes in Asian countries 1 .
It has been clarified that gut microbiota plays a pivotal role in human health and disease, including type 2 diabetes. The microbiota is involved in the digestion of food, modulation of immune responses and the generation of different metabolites. This complex community of trillions of bacteria, fungi and viruses is highly metabolically active, and has co‐evolved with the human host.
Several studies have investigated the role of the human gut microbiota in type 2 diabetes patients. In fact, we recently showed that gut microbiota of Japanese patients with type 2 diabetes is different from that of healthy individuals, and this difference in gut microbiota is associated with sucrose intake and medium‐chain fatty acid intake, which are associated with characteristics of Western diet 2 . The gut microbiota has been shown to interact with host metabolism leading to insulin resistance and type 2 diabetes through several mechanisms including induction of low‐grade inflammation, and alterations of energy homoeostasis and glucose metabolism. Recently, it has emerged that metabolites produced by these gut microbiota could be pathogenic or beneficial to the host. Metabolites, which are end‐products or intermediates of metabolisms, usually refer to small molecules. Primary metabolites are substances directly involved in growth and reproduction, whereas secondary metabolites are substances that are not directly involved in those processes, but have important ecological functions, such as antibiotics and pigments. These metabolites might play crucial roles in host biosynthetic and metabolic networks, as well as various immunological and neurobiological processes. Metabolites are the products and intermediates of cellular metabolism. Metabolites can have a multitude of functions, including energy conversion, signaling, epigenetic influence and cofactor activity. Metabolites have been described as proximal reporters of disease, because their abundances in biological specimens are often directly related to pathogenic mechanisms, and this concept is routinely shown in clinical chemistry laboratory results. One of the gut microbiota‐related metabolites is trimethylamine N‐oxide. Trimethylamine N‐oxide is produced by gut bacteria by metabolizing choline, lecithin and carnitine, which are contained in red meat, liver and other animal products, and are known to be associated with the development of cardiovascular diseases 3 . Thus, trimethylamine N‐oxide has now become not only a marker, but also a treatment target for the development of cardiovascular diseases.
In practice, metabolomics presents a significant analytical challenge, because, unlike genomic and proteomic methods, it aims to measure molecules that have disparate physical properties (e.g., ranging in polarity from very water‐soluble organic acids to very non‐polar lipids). Because of the wide range of endogenous metabolites, gas chromatography–mass spectrometry (GC/MS), liquid chromatography–mass spectrometry (LC/MS) and capillary electrophoresis–mass spectrometry (CE/MS) analytical techniques are required for a truly comprehensive metabolomic study. Samples suitable for GC/MS analysis include plant terpenes and essential oils. Fatty acids can also be measured by GC/MS by derivatization. Liquid chromatography can separate non‐volatile and non‐derivatized metabolites. As a result, LC/MS can analyze a wider range of samples than GC/MS. The advantages of CE/MS are that it can measure most ionic substances, and is not affected by ion suppression and is highly quantitative. As LC, in which the separation is based on the hydrophilic/hydrophobic distribution of compounds, is complementary, it is expected that a comprehensive analysis of the majority of (non‐volatile) metabolites can be achieved by integrating the analytical data of CE/MS and LC/MS. Furthermore, nuclear magnetic resonance has an advantage for measurement, as it allows non‐invasive measurement of samples, and thus enables live tissue and cells to be measured, although nuclear magnetic resonance has a lower sensitivity than other MS.
Diabetes mellitus is a multifactorial disease, and the interaction of genetic and environmental factors are associated with the development of diabetes and its complications. To develop preventive and therapeutic methods, and realize a healthy and long‐lived society, it is necessary to understand the association among diet, gut microbiota, gut microbiota‐related metabolites and diabetes condition (Figure 1). Recently, several studies have begun to shed light on gut microbiota dysbiosis; however, our understanding of the overall metabolic interactions of microbial communities with their host with respect to disease development is still limited. A recent study from Finland showed that microbial‐associated metabolites are associated with the development of type 2 diabetes 4 .
Figure 1.
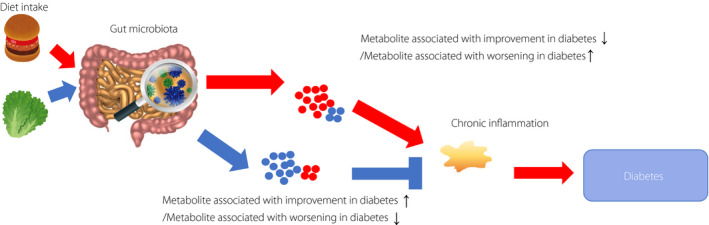
Scheme of association among diet, gut microbiota, gut microbiota‐related metabolites and development of diabetes. There is an association among diet, gut microbiota, gut microbiota‐related metabolites and development of diabetes. Habitual diet intake alters gut microbiota and at the same time changes the gut microbiota‐related metabolite. Thereafter, these metabolites are involved in the development of diabetes through several pathways, including chronic inflammation.
In the present study, using the LC/MS, a total of 857 unique metabolites were found and 86 metabolites related to gut microbiota were included in statistical analysis. Bile acids; choline metabolism; aromatic amino acid metabolism; non‐aromatic amino acid metabolism; xenobiotic metabolism; energy metabolism; lipid metabolism, including short chain fatty acid and other metabolites; lysophosphatidylcholines and lysophosphatidylethanolamines were chosen. According to the study, creatine, 1‐palmitoleoylglycerol (16:1), urate, 2‐hydroxybutyrate/2‐hydroxyisobutyrate, xanthine, xanthurenate, kynurenate, 3‐(4‐hydroxyphenyl)lactate, 1‐oleoylglycerol (18:1), 1‐myristoylglycerol (14:0), dimethylglycine and 2‐hydroxyhippurate (salicylurate) were significantly associated with the risk of type 2 diabetes.
Monoacylglycerols, including 1‐oleoylglycerol (18:1), 1‐myristoylglycerol (14:0) and 1‐palmitoleoylglycerol (16:1) increased the risk of type 2 diabetes. Furthermore, products of the metabolism of amino acid tryptophan, including kynurenate and xanthurenate, were found to be associated with an increased risk of type 2 diabetes. In addition, some metabolites, including dimethylglycine and 2‐hydroxyhippurate, increased the risk of incident diabetes without having a significant effect on insulin secretion or insulin sensitivity. In contrast, 1‐lignoceroyl‐glycerophosphocholine (24:0) was associated with a decreased risk of type 2 diabetes. Lysophospholipids stimulate glucose‐dependent insulin release through lipid signaling. Unfortunately, the present study did not show the association between microbe‐associated metabolites and gut microbiota or habitual dietary intakes. However, this study showed the association between microbe‐associated metabolites and incident diabetes, insulin sensitivity, and insulin resistance.
As the gut microbiota varies among countries, age and sex 5 , there is a possibility that the microbe‐associated metabolites might vary among countries, age and sex, and that many unknown microbe‐associated metabolites might be present. To propose personalized medicine, deep understanding of the association among diet, gut microbiota, gut microbiota‐related metabolites and diabetic condition is necessary. Therefore, further studies are required to clarify the relationship among diet, gut microbiota, gut microbiota‐related metabolites and the development of type 2 diabetes.
Disclosure
Dr Hashimoto reports grants from Asahi Kasei Pharma and personal fees from Mitsubishi Tanabe Pharma Corp., Novo Nordisk Pharma Ltd., Sanofi K.K. and Daiichi Sankyo Co. Ltd. outside the submitted work. Dr Hamaguchi reports grants from Takeda Pharma Co. Ltd, Sanofi K.K., Mitsubishi Tanabe Pharma Corp., Asahi Kasei Pharma, Sumitomo Dainippon Pharma Co. Ltd., Kyowa Kirin Co. Ltd., Daiichi Sankyo Co. Ltd., Astellas Pharma Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co. Ltd. and Eli Lilly Japan K.K. outside the submitted work. Professor Fukui received grants from Takeda Pharma Co. Ltd., Sanofi K.K., Kissei Phama Co. Ltd., Mitsubishi Tanabe Pharma Corp, Astellas Pharma Inc., Nippon Boehringer Ingelheim Co. Ltd., Daiichi Sankyo Co. Ltd., MSD K.K., Sanwa Kagagu Kenkyusho CO., LtD., Kowa Pharma Co. Ltd., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharma Co. Ltd., Eli Lilly Japan K.K., Taisho Pharma Co., Ltd., Tejin Pharma Ltd., Nippon Chemiphar Co., Ltd., Johnson & Johnson k.k. Medical Co., Abbott Japan Co. Ltd. and Terumo Corp., and received honoraria from Teijin Pharma Ltd., Arkray Inc., Kissei Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Mitsubishi Tanabe Pharma Corp., Sanofi K.K., Takeda Pharma Co. Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Kirin Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Daiichi Sankyo Co. Ltd., Kowa Pharma Co. Ltd., Ono Pharma Co. Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., Nippon Boehringer Ingelheim Co., Ltd., Taisho Pharma Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca K.K., Mochida Pharma Co. Ltd., Abbott japan Co. Ltd., Eli Lilly Japan K.K., Medtronic Japan Co. Ltd. and Nipro Corp. outside the submitted work.
Acknowledgment
We thank Editage (www.editage.com) for English language editing. No funding was received for this study.
J Diabetes Investig.2021.
References
- 1. Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011; 34: 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hashimoto Y, Hamaguchi M, Kaji A, et al. Intake of sucrose affects gut dysbiosis in patients with type 2 diabetes. J Diabetes Investig 2020; 11: 1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Z, Bergeron N, Levison BS, et al. Impact of chronic dietary red meat, white meat, or non‐meat protein on trimethylamine N‐oxide metabolism and renal excretion in healthy men and women. Eur Heart J 2019; 40: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vangipurapu J, Fernandes Silva L, Kuulasmaa T, et al. Microbiota‐related metabolites and the risk of type 2 diabetes. Diabetes Care 2020; 43: 1319–1325. [DOI] [PubMed] [Google Scholar]
- 5. Nishijima S, Suda W, Oshima K, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res 2016; 23: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]


