Abstract
Aims/Introduction
The Thai Type 1 Diabetes and Diabetes Diagnosed Before Age 30 Years Registry, Care and Network was established in 2014 and involved 31 hospitals. The objective of the registry was to evaluate glycemic control and complications of patients with type 1 diabetes.
Materials and Methods
Patients’ demographics, clinical data, frequencies of daily self‐monitoring of blood glucose (SMBG), glycemic control and complications were collected.
Results
Among the 1,907 type 1 diabetes patients, the mean age was 21.2 ± 11.3 years. The mean glycated hemoglobin level was 9.35 ± 2.41%, with significant variations among age groups (P < 0.001). Conventional insulin treatment and intensive insulin treatment were used in 43 and 57% of patients, respectively. Mean glycated hemoglobin levels were significantly higher in patients treated with conventional insulin treatment compared to those treated with intensive insulin treatment (9.63 ± 2.34 vs 9.17 ± 2.46%, P = 0.002). Compared to the conventional insulin treatment group, significantly more patients in the intensive insulin treatment group achieved good glycemic control (P < 0.001), and fewer had diabetic retinopathy (P = 0.031). The prevalence of microvascular complications increased significantly with age (P < 0.001). Multivariate analysis showed good glycemic control to be associated with age 25 to <45 years, intensive insulin treatment with SMBG three or more times daily and diabetes duration of 1 to <5 years.
Conclusions
Most Thai type 1 diabetes patients were not meeting the recommended glycemic target. As a result of this study, the national program to improve the quality of diabetes treatment and education has been implemented, and the results are ongoing.
Keywords: Intensive insulin treatment, Multiple daily insulin injection, Type 1 diabetes mellitus
This study reflects a nationwide snapshot of the management and outcomes of 1,907 patients with type 1 diabetes in Thailand. A total of 43% of patients were found to be receiving conventional insulin treatment, and 57% were receiving intensive insulin treatment. Most of the Thai type 1 diabetes patients that we enrolled in this study were not meeting their recommended glycemic target. Good glycemic control was achieved by just 13% of patients receiving conventional insulin treatment, and by just 19% of patients receiving intensive insulin treatment. Multivariate analysis showed good glycemic control to be significantly associated with the 25 to <45 years age group, intensive insulin therapy with self‐monitoring of blood glucose three or more times daily and duration of disease of 1 to <5 years.
Introduction
Thailand is an upper‐middle income developing country that has provided universal health coverage for its entire population since 2002 through the implementation of three health insurance programs, including: (i) the Civil Servant Medical Benefit Scheme for government officials and their dependents; (ii) the Social Security Scheme for private sector employees; and (iii) the Universal Health Coverage Scheme for the remaining population not covered by the Civil Servant Medical Benefit Scheme or Social Security Scheme 1 . In Thailand, universal health coverage benefits include the majority of medicines, investigations and medical equipment, but it excludes high‐cost investigations and treatments. For diabetes treatment, self‐monitoring of blood glucose (SMBG), continuous subcutaneous insulin infusion (CSII) and continuous glucose monitoring are not covered, so the costs of these important evaluation and treatment modalities place a significant financial burden on individuals living with type 1 diabetes. Difficult access to insulin analogs, and the shortage of knowledgeable healthcare professionals to provide diabetes self‐management education and support (DSMES) are also important obstacles to intensive insulin treatment (IIT) for type 1 diabetes in Thailand. The inability of parents and schools to provide support are also important barriers to IIT among children with type 1 diabetes in Thailand. As a consequence of these barriers to patient care and education, conventional insulin treatment (CIT) is a common treatment among Thai type 1 diabetes patients that are indicated for IIT. These obstacles, both individually and collectively, prevent the optimal management of type 1 diabetes patients in Thailand.
The incidence of type 1 diabetes is increasing globally 2 . This has also been observed in Thailand 3 , 4 , 5 , 6 , 7 . The incidence of type 1 diabetes among children aged 0–15 years in Thailand was increased from 0.2 per 100,000/year in 1984–1985 to 1.65 per 100,000/year in 1991–1995 5 . In 2003, the Thailand Diabetes Registry Project reported the prevalence of type 1 diabetes diagnosed before the age of 18 years to be 2.07% from 11 tertiary centers 8 . Data on type 1 diabetes in Thailand have been lacking since 2003. Established in 2014, the Thai Type 1 Diabetes and Diabetes Diagnosed Before Age 30 Years Registry, Care and Network (T1DDAR CN) is a collaboration among the Thai Society for Pediatric Endocrinology, the Endocrine Society of Thailand and the Diabetes Association of Thailand; government entities, such as the Siriraj Diabetes Center, Faculty of Medicine Siriraj Hospital, Mahidol University; the Northern Diabetes Center, Faculty of Medicine, Chiang Mai University; and the National Health Security Office (NHSO) of Thailand. The current network covers 31 hospitals around Thailand. Due to limited resource setting for type 1 diabetes and young‐onset diabetes patients in Thailand, T1DDAR CN had the following objectives: (i) to strengthen the clinical knowledge of medical professionals; (ii) to develop a referral system and network for IIT and specific DSMES program; (iii) to create DSMES teaching modules and education materials that focus on both survival skills and continuing education in simple and easy‐to‐use Thai language; and, (iv) to initiate cohort data of type 1 diabetes patients of all ages, and of patients who were diagnosed with diabetes before the age of <30 years.
The objectives of the present study were to assess glycemic control and diabetes complications in patients enrolled in the database of T1DDAR CN, and to determine factors associated with good glycemic control among patients with type 1 diabetes in Thailand.
Methods
Data of type 1 diabetes patients from 31 T1DDAR CN network hospitals (see Appendix 1) diagnosed during January 2005–2016 were retrospectively reviewed. The T1DDAR CN network hospitals are tertiary care level, with healthcare professionals who are interested in strengthening the education/support team for their type 1 diabetes patients. These hospitals function as referral centers for local hospitals located within their referral area. An electronic case record form was developed using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, TN, USA), which is a web‐based program. REDCap is hosted by the Research Institute for Health Sciences of Chiang Mai University, Chiang Mai, Thailand. Siriraj Diabetes Center together with Research Institute for Health Sciences operates as the administrative and data coordination center of the T1DDAR CN study. The researchers and research coordinators from each participating center received training in an effort to standardize data collection. Written instruction in how to complete the electronic case record form was also provided. The researchers and research coordinators had secure sign‐in authorization, and they could only access their own data. Data were collected at each site during September 2015 to March 2016. Data entry activities were closely monitored and reviewed monthly by the data coordinators and the principal investigators.
Data, including characteristics, treatment, glycemic control, daily SMBG, acute diabetic complications (including diabetic ketoacidosis [DKA] and severe hypoglycemia) and chronic diabetic complications (including diabetic retinopathy, diabetic nephropathy and diabetic neuropathy), were retrospectively reviewed. Obesity was considered if the patients had a body mass index of ≥25 kg/m2 for those aged >18 years or weight for height of ≥140% for those aged <18 years. Dyslipidemia was diagnosed if low density lipoprotein cholesterol was >100 mg/dL or the patients were receiving hyperlipidemia treatment. Hypertension was defined if the patients had elevated blood pressure or were treated with antihypertensive medication. Diabetic retinopathy was defined if the patients had macular edema, proliferative or non‐proliferative retinopathy, vitreous hemorrhage, or tractional retinal detachment. Diabetic nephropathy was considered if persistent albuminuria (>30 mg/g creatinine) was identified. Diabetic neuropathy was diagnosed by monofilament examination, loss of reflex or loss of vibratory sensation.
To assess glycemic control, treatment regimen and the prevalence of complications among different age groups, patients were stratified into seven age groups, similarly to the previous studies: the Australasian Diabetes Data Network 9 and the study of Type 1 Diabetes Exchange clinic registry 10 . Insulin regimen was categorized as follows: CIT (premixed or self‐mixed insulin 1–3 injections per day) and IIT (multiple daily injections ≥4 injections per day or CSII).
Glycemic control was classified as: (i) good glycemic control: glycated hemoglobin (HbA1c) <7.5% in the <18 years age group, and <7.0% in the ≥18 years age group; (ii) fair glycemic control: HbA1c within the range of 7.5–9.0% in the <18 years age group, and within the range of 7.0–9.0% in the ≥18 years age group; and (iii) poor glycemic control: HbA1c >9% in all age groups.
The study protocol was approved by the Central Research Ethics Committee of Thailand (approval number CREC 009/2559‐ Bm), and each participating site obtained local institutional board approval.
Statistical analysis
Data analysis was carried out using Stata/IC version 14.0 for Windows (StataCorp LP, College Station, TX, USA). Patients with missing data were omitted from the analyses involving that variable, but were included in the rest of the study. For normally distributed variables, data are presented as the number and percentage for categorical data, and as the mean ± standard deviation for continuous data. For non‐normally distributed continuous variables, data are presented as the median and interquartile range. For comparison between groups, Student’s t‐test, Mann–Whitney U‐test, weighted analysis of variance (anova), F‐test and Kruskal–Wallis test were used for continuous variables, as appropriate. The χ2‐test was used to compare categorical variables, and Scheffé’s method was used for multiple comparisons. Logistic regression analysis was carried out to identify independent predictors of optimal glycemic control. Only patients with a duration of type 1 diabetes of >1 year were included. Achievement of HbA1c targets (HbA1c <7.5% in the <18 years age group and <7.0% in the ≥18 years age group) was entered as the dependent variable. Potential factors associated with glycemic target achievement, including age, duration of disease, sex, health insurance schemes, educational level, insulin regimen and frequency of SMBG, were analyzed in univariate analysis. Factors identified as significant in univariate analysis were entered into multivariate logistic regression analysis. The household income per month was not included in the logistic regression analysis because of a high percentage of missing values (32.8%). A P‐value of <0.05 was considered statistically significant.
Results
Patient characteristics
A cohort of 1,907 type 1 diabetes patients (778 males/1,129 females), with a mean age at diagnosis of 13.5 ± 9.2 years, a current mean age of 21.2 ± 11.3 years and a mean duration of disease of 7.7 ± 6.4 years, were included and analyzed (Table 1). Notably, just 3.17 and 3.54% of the patients were in the age groups of <6 years and ≥45 years, respectively. Just 13 patients were aged ≥65 years; thus, they were categorized into the ≥45 years age group (Table 2). Regarding health insurance schemes, the majority of patients (67.4%) had Universal Health Coverage Scheme, 13.1% had Civil Servant Medical Benefit Scheme and 10.1% were covered by Social Security Scheme. More than half of patient families (57.4%) had a monthly household income <$600, and 30.9% of families earned $600–<1,500 per month. Just 11.7% had a monthly household income ≥$1,500. Obesity, dyslipidemia and hypertension were identified in 6.9, 27.6 and 9.1% of patients, respectively (Table 1). The prevalence of autoimmune thyroid disease was 5.2%.
Table 1.
Characteristics of the 1,907 type 1 diabetes patients included in this study
| Characteristics | n | Value |
|---|---|---|
| Current age (years) | 1,907 | 21.2 ± 11.3 |
| Age at diagnosis (years) | 1,892 | 13.5 ± 9.2 |
| Duration of type 1 diabetes (years) | 1,868 | 7.7 ± 6.4 |
| Sex (female) | 1,907 | 1129 (59.2%) |
| Health insurance schemes | 1,900 | |
| Civil servant medical benefit scheme | 249 (13.1%) | |
| Social security scheme | 192 (10.1%) | |
| Universal health coverage scheme | 1280 (67.4%) | |
| Others | 179 (9.4%) | |
| Household income per month | 1,191 | |
| <$300 | 272 (22.8%) | |
| $300 to <600 | 412 (34.6%) | |
| $600 to <900 | 216 (18.1%) | |
| $900 to <1,500 | 152 (12.8%) | |
| ≥$1,500 | 139 (11.7%) | |
| Mean HbA1c (%) | 1,820 | 9.35 ± 2.41 |
| Good glycemic control | 293 (16.1%) | |
| Fair glycemic control | 668 (36.7%) | |
| Poor glycemic control | 859 (47.2%) | |
| Frequency of SMBG (mean ± SD) | 1,687 | 2.06 ± 1.41 |
| ≤1/day | 659 (39.1%) | |
| 2/day | 350 (20.8%) | |
| 3/day | 337 (20.0%) | |
| ≥4/day | 341 (20.2%) | |
| Comorbidity | 1,897 | |
| Obesity | 131 (6.9%) | |
| Dyslipidemia | 523 (27.6%) | |
| Hypertension | 173 (9.1%) | |
| Prevalence of DKA per year | 1,815 | 186 (10.2%) |
| Prevalence of severe hypoglycemia per year | 1,638 | 140 (8.5%) |
| Diabetic retinopathy | 1,339 | 142 (10.6%) |
| Diabetic nephropathy | 1,330 | 305 (22.9%) |
| Diabetic neuropathy | 577 | 30 (5.2 %) |
Data presented as number and percentage or mean ± standard deviation. Income is shown in US dollars. Good glycemic control: glycated hemoglobin (HbA1c) <7.5% in the <18 years age group, and <7.0% in the ≥18 years age group; fair glycemic control: HbA1c within the range of 7.5–9.0% in the <18 years age group, and within the range of 7.0–9.0% in the ≥18 years age group; and, poor glycemic control: HbA1c >9% in all age groups. DKA, diabetic ketoacidosis; SBMG, self‐monitoring of blood glucose.
Table 2.
Participant characteristics stratified by age group
| Total | Age group (years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| <6 | 6 to <10 | 10 to <14 | 14 to <18 | 18 to <25 | 25 to <45 | ≥45 | P‐value | ||
| (n = 1,892) | (n = 60) | (n = 146) | (n = 314) | (n = 353) | (n = 462) | (n = 490) | (n = 67) | ||
| Sex (female) | 1,121 (59.2%) | 30 (50.0%) | 80 (54.8%) | 192 (61.2%) | 208 (58.9%) | 270 (58.4%) | 301 (61.4%) | 40 (59.7%) | 0.556 |
| Duration of disease (years) | 1,861 | 1 (0–2) | 0 (1–4) | 3 (1–5) | 5 (3–8) | 8 (4–11) | 10 (5–16) | 19 (10–27) | <0.001* |
| HbA1c (%) | 1,820 | 8.98 ± 1.90 | 9.20 ± 1.88 | 9.54 ± 2.21 | 9.89 ± 2.60 | 9.63 ± 2.68 | 8.85 ± 2.29 | 8.03 ± 1.26 | <0.001* |
| Good glycemic control | 13 (22.0%) | 19 (13.5%) | 47 (15.4%) | 62 (18.1%) | 57 (12.9%) | 85 (18.4%) | 10 (14.9%) | <0.001* | |
| Fair glycemic control | 21 (35.6%) | 58 (41.1%) | 89 (29.1%) | 84 (24.6%) | 162 (36.6%) | 205 (44.3%) | 49 (73.1%) | ||
| Poor glycemic control | 25 (42.4%) | 64 (45.4%) | 170 (55.6%) | 196 (57.3%) | 223 (50.4%) | 173 (37.4%) | 8 (11.9%) | ||
| Insulin regimen | 1,862 | ||||||||
| Conventional treatment | 30 (50.0%) | 88 (60.3%) | 149 (48.4%) | 149 (42.8%) | 180 (39.6%) | 208 (43.3%) | 18 (27.3%) | 0.001* | |
| Intensive treatment | 30 (50.0%) | 58 (39.7%) | 159 (51.6%) | 199 (57.2%) | 274 (60.4%) | 272 (56.7%) | 48 (72.7%) | ||
| Basal–bolus regimen | 29 (48.3%) | 58 (39.7%) | 155 (50.3%) | 197 (56.6%) | 267 (58.8%) | 266 (55.4%) | 48 (72.7%) | ||
| Continuous subcutaneous insulin infusion | 1 (1.7%) | 0 (0.0%) | 4 (1.3%) | 2 (0.6%) | 7 (1.5%) | 6 (1.2%) | 0 (0.0%) | ||
| SMBG | 1,687 | 3 (2–4) | 3 (2–4) | 3 (2–4) | 2 (1–3) | 2 (1–3) | 1 (0–2) | 2 (1–3) | |
| <1/day | 9 (17.0%) | 25 (18.1%) | 67 (22.7%) | 111 (33.6%) | 201 (49.1%) | 226 (55.7%) | 20 (35.7%) | <0.001* | |
| 2/day | 11 (20.8%) | 23 (16.7%) | 50 (17.0%) | 82 (24.8%) | 87 (21.3%) | 83 (20.4%) | 14 (25.0%) | ||
| 3/day | 12 (22.6%) | 39 (28.3%) | 88 (29.8%) | 71 (21.5%) | 62 (15.2%) | 53 (13.0%) | 12 (21.4%) | ||
| >4/day | 21 (39.6%) | 51 (37.0%) | 90 (30.5%) | 66 (20.0%) | 59 (14.4%) | 44 (10.8%) | 10 (17.9%) | ||
| Prevalence of DKA per year | 1,815 | 3 (5.1%) | 8 (5.6%) | 45 (14.6%) | 38 (11.0%) | 58 (13.2%) | 31 (6.8%) | 3 (4.6%) | 0.001* |
| Prevalence of severe hypoglycemia per year | 1,638 | 3 (5.3%) | 10 (7.0%) | 20 (6.6%) | 19 (5.5%) | 35 (8.1%) | 44 (10.1%) | 9 (14.3%) | 0.105 |
| Diabetic retinopathy | 1,339 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 20 (6.1%) | 98 (25.4%) | 24 (40.0%) | <0.001* |
| Diabetic nephropathy | 1,330 | 0 (0.0%) | 5 (9.4%) | 15 (7.8%) | 47 (17.5%) | 90 (25.6%) | 123 (31.6%) | 25 (41.0%) | <0.001* |
| Diabetic neuropathy | 577 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (2.2%) | 16 (6.1%) | 10 (33.3%) | <0.001* |
Data presented as number and percentage, median and interquartile range or mean ± standard deviation. *A P‐value <0.05 shows statistical significance. Good glycemic control: glycated hemoglobin (HbA1c) <7.5% in the <18 years age group, and <7.0% in the ≥18 years age group; fair glycemic control: HbA1c within the range of 7.5–9.0% in the <18 years age group, and within the range of 7.0–9.0% in the ≥18 years age group; and poor glycemic control: HbA1c >9% in all age groups. DKA, diabetic ketoacidosis; SBMG, self‐monitoring of blood glucose.
Glycemic control
The mean HbA1c level was 9.35 ± 2.41%, and mean HbA1c levels varied significantly according to age (P < 0.001; Figure 1a). HbA1c levels gradually increased from 8.98 ± 1.90% in the <6 years age group to the highest level of 9.89 ± 2.60% in the 14 to <18 years age group. The lowest level of 8.03 ± 1.26% was observed in the ≥45 years age group (Table 2; Figure 1a). Good glycemic control was achieved in just 16.1% of patients (Table 1). The percentage of patients achieving HbA1c targets was lowest (12.9%) in the 18 to <25 years age group, and highest (22.0%) in the <6 years age group. The percentage of poor glycemic control was highest in the 14 to <18 years age group (57.3%), and lowest in the ≥45 years age group (11.9%; Figure 1b).
Figure 1.
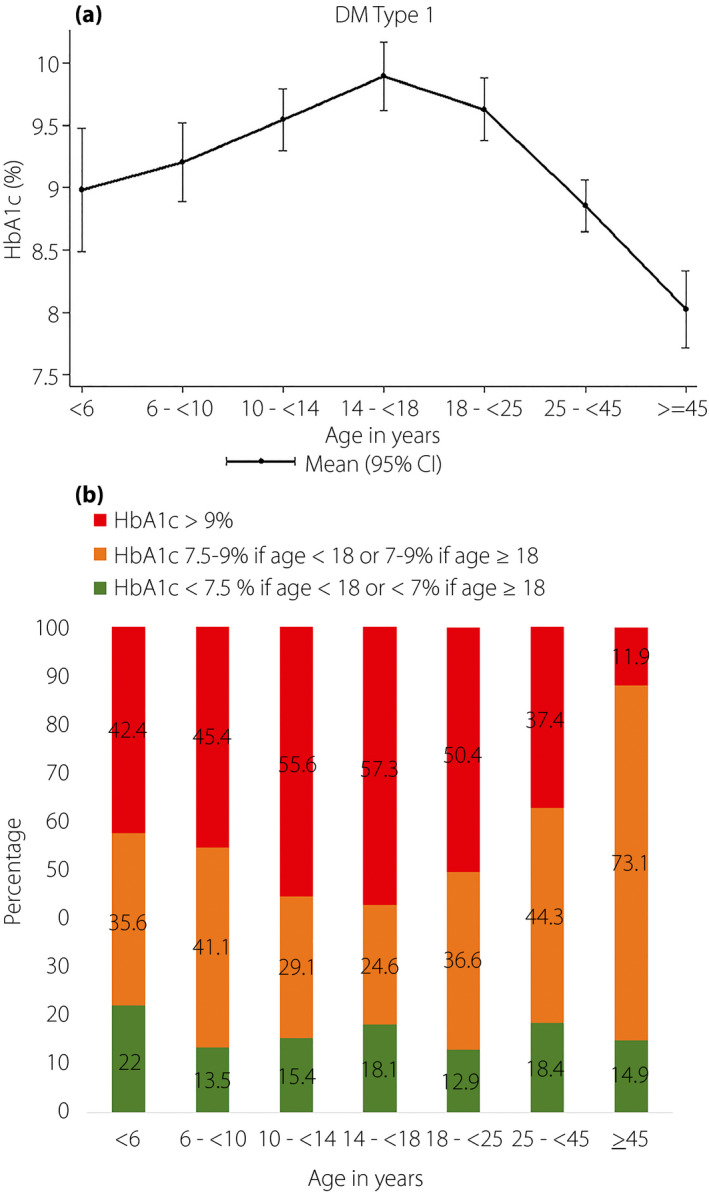
(a) Mean glycated hemoglobin (HbA1c) of 1,820 patients with type 1 diabetes stratified by age. (b) Percentage of patients achieving HbA1c targets stratified by age group. The HbA1c target for those aged <18 years was <7.5%, and the HbA1c target for those aged ≥18 years was <7.0%. CI, confidence interval; DM, diabetes mellitus.
SMBG
The mean frequency of SMBG was 2.06 ± 1.41 times daily. A higher frequency of SMBG was found to be significantly associated with lower HbA1c levels (P < 0.001; Figure 2). Multiple comparisons (Scheffé’s method) showed that SMBG carried out four times daily was significantly associated with lower HbA1c compared with SMBG one or fewer to three times daily (SMBG four times daily vs SMBG three times daily [P = 0.032], SMBG four times daily vs SMBG two times daily (P = 0.001), SMBG four times daily vs SMBG ≤1 time daily [P < 0.001]). However, individuals carrying out SMBG three times daily had similar levels of HbA1c to those who carried out SMBG two times daily (P = 0.684).
Figure 2.
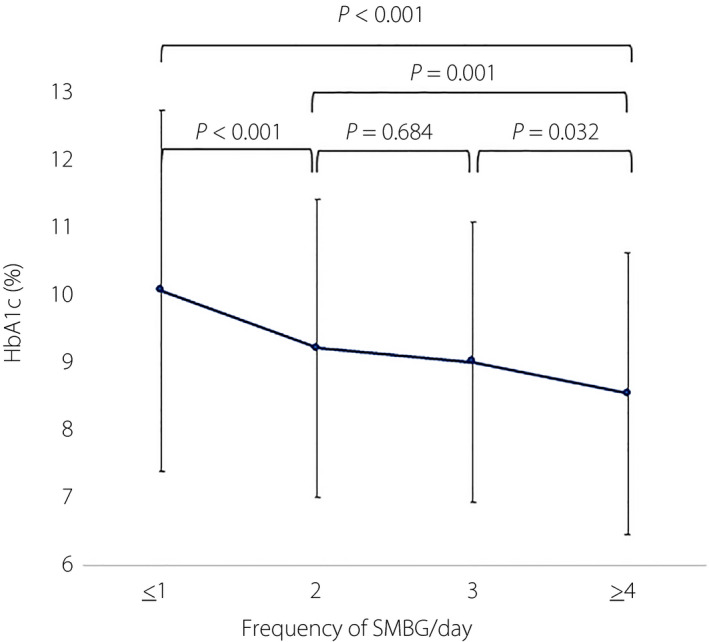
Frequency of self‐monitoring of blood glucose (SMBG) relative to glycated hemoglobin (HbA1c) among patients with type 1 diabetes.
Insulin regimens and treatment outcomes
A total of 43% of patients were on CIT, and 57% were on IIT (Table 3). CSII was used in only 1.1% of patients. Mean HbA1c levels were significantly higher in patients treated with CIT than in those treated with IIT (9.63 ± 2.34% vs 9.17 ± 2.46%, P = 0.002). Just around 19% of patients with IIT achieved optimal glycemic control, and just 13% of patients with CIT reached the HbA1c goal (P < 0.001).
Table 3.
Clinical and biochemical characteristics of patients with type 1 diabetes stratified by insulin regimen
| Characteristics | n (1,748) | Conventional insulin treatment (n = 755) | Intensive insulin treatment (n = 993) | P‐value |
|---|---|---|---|---|
| Health insurance scheme | 1,741 | |||
| Civil servant medical benefit scheme | 69 (9.2%) | 160 (16.2%) | <0.001* | |
| Social security scheme | 65 (8.6%) | 100 (10.1%) | ||
| Universal health coverage scheme | 558 (74.0%) | 632 (64.0%) | ||
| Others | 62 (8.2%) | 95 (9.6%) | ||
| Gender, female | 1,748 | 421 (55.8%) | 616 (62.0%) | <0.001* |
| Age at diagnosis (years) | 1,717 | 12.3 ± 7.8 | 13.3 ± 9.4 | 0.023* |
| Current age (years) | 1,733 | 19.1 ± 10.0 | 21.5 ± 11.4 | <0.001* |
| Duration of type 1 diabetes (years) | 1,714 | 5.6 (2.5–9.5) | 6.2 (3.1–11.9) | 0.003* |
| HbA1c (%) | 1,676 | 9.63 ± 2.34 | 9.17 ± 2.46 | 0.002* |
| Good glycemic control | 94 (13.4%) | 181 (18.6%) | <0.001* | |
| Fair glycemic control | 233 (33.2%) | 372 (38.2%) | ||
| Poor glycemic control | 375 (53.4%) | 421 (43.2%) | ||
| Frequency of SMBG | 1,563 | 2 (1–3) | 3 (1–4) | <0.001* |
| ≤1/day | 318 (49.3%) | 254 (27.7%) | <0.001* | |
| 2/day | 161 (25.0%) | 169 (18.4%) | ||
| 3/day | 110 (17.0%) | 211 (23.0%) | ||
| ≥4/day | 56 (8.7%) | 284 (30.9%) | ||
| Prevalence of DKA | 1,677 | 70 (10.0%) | 104 (10.7%) | 0.657 |
| Prevalence of severe hypoglycemia | 1,646 | 48 (7.0%) | 81 (8.5%) | 0.271 |
| Diabetic retinopathy | 1,205 | 59 (12.3%) | 62 (8.5%) | 0.031* |
| Diabetic nephropathy | 1,204 | 112 (23.8%) | 155 (21.2%) | 0.238 |
| Diabetic neuropathy | 505 | 9 (5.4%) | 19 (5.6%) | 0.951 |
Data presented as number and percentage, mean ± standard deviation, or median and interquartile range. *A P‐value <0.05 shows statistical significance. Good glycemic control: glycated hemoglobin (HbA1c) <7.5% in the <18 years age group, and <7.0% in the ≥18 years age group; fair glycemic control: HbA1c within the range of 7.5–9.0% in the <18 years age group, and within the range of 7.0–9.0% in the ≥18 years age group; and poor glycemic control: HbA1c >9% in all age groups. DKA, diabetic ketoacidosis; SBMG, self‐monitoring of blood glucose.
The annual incidence of the acute diabetic complications, DKA and severe hypoglycemia, was 10.2 and 8.5%, respectively (Table 1). The prevalence of DKA was highest in the 10 to <14 years age group (14.6%). The prevalence of hypoglycemia was highest in participants aged ≥45 years (14.3%; Table 2).
Diabetic retinopathy, diabetic nephropathy and diabetic neuropathy in this cohort was identified in 10.6, 22.9 and 5.2% of patients, respectively (Table 1). The prevalence of both diabetic retinopathy and diabetic nephropathy increased in our older patients. The prevalence of diabetic retinopathy was 6.1% in the 18 to <25 years age group, it increased to 25.4% in the 25 to <45 years age group, and it further increased to 40% in the ≥45 years age group. The prevalence of diabetic nephropathy was 25.6% in the 18 to <25 years age group, and the prevalence increased with age (41% in the ≥45 years age group). The prevalence of diabetic neuropathy was 2.2% in the 18 to <25 years age group, and it increased to 33.3% in the ≥45 years age group (Table 2). The prevalence of DKA, severe hypoglycemia, diabetic nephropathy and diabetic neuropathy were not significantly different between the two insulin regimens. However, the prevalence of diabetic retinopathy was significantly lower in the IIT group (8.5 vs 12.3%, P = 0.031; Table 3).
Factors associated with achievement of HbA1c targets
Associations between patients’ characteristics and HbA1c target achievement were explored by univariate and multivariate logistic regression analyses. In univariate analysis, good glycemic control was associated with a current age of 25 to <45 years, other type of health insurance scheme (e.g., self‐payment, unknown etc.), finishing a bachelor’s or master’s degree, IIT with frequency of SMBG three or more times daily and duration of disease 1 to <5 years. Multivariate analysis showed good glycemic control to be associated with current age of 25 to <45 years (P = 0.030), IIT and frequency of SMBG three or more times daily (P < 0.001), and duration of disease 1 to <5 years (P = 0.014; Table 4).
Table 4.
Analysis for predictors of metabolic control achievement in patients with type 1 diabetes
| Factors | n | Achieved metabolic control | Univariate analysis | Multivariate analysis † | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | AOR | 95% CI | P‐value | |||
| Age (years) | ||||||||
| <6 | 35 | 6 (17.1%) | 1.39 | 0.45–3.62 | 0.487 | 0.65 | 0.20–2.10 | 0.471 |
| 6 to <10 | 106 | 12 (11.3%) | 0.86 | 0.40–1.71 | 0.649 | 0.60 | 0.27–1.34 | 0.213 |
| 10 to <14 | 265 | 37 (14.0%) | 1.09 | 0.67–1.76 | 0.716 | 0.86 | 0.48–1.54 | 0.603 |
| 14 to <18 | 318 | 55 (17.3%) | 1.40 | 0.91–2.17 | 0.108 | 1.31 | 0.79–2.19 | 0.296 |
| 18 to <25 | 393 | 51 (13.0%) | Ref. | Ref. | ||||
| 25 to <45 | 375 | 74 (19.7%) | 1.65 | 1.10–2.48 | 0.011* | 2.00 | 1.07–3.76 | 0.030* |
| ≥45 | 50 | 8 (16.0%) | 1.28 | 0.49–2.96 | 0.554 | 1.16 | 0.39–3.48 | 0.792 |
| Duration of disease (years) | ||||||||
| 1 to <5 | 607 | 106 (17.5%) | 1.45 | 1.02–2.08 | 0.031* | 1.65 | 1.11–2.45 | 0.014* |
| 5 to <10 | 480 | 61 (12.7%) | Ref. | Ref. | ||||
| 10 to <20 | 364 | 61 (16.8%) | 1.38 | 0.92–2.07 | 0.098 | 1.18 | 0.74–1.88 | 0.478 |
| 20 to <30 | 74 | 12 (16.2%) | 1.33 | 0.62–2.67 | 0.406 | 0.94 | 0.39–2.26 | 0.893 |
| ≥30 | 18 | 3 (16.7%) | 1.37 | 0.25–5.06 | 0.622 | 0.86 | 0.17–4.49 | 0.863 |
| Sex | ||||||||
| Male | 635 | 97 (15.3%) | Ref. | |||||
| Female | 908 | 146 (16.1%) | 1.06 | 0.80–1.42 | 0.670 | |||
| Health insurance schemes | ||||||||
| Civil servant medical benefit scheme | 205 | 37 (18.0%) | 1.28 | 0.83–1.92 | 0.225 | |||
| Social security scheme | 151 | 21 (13.9%) | 0.94 | 0.54–1.55 | 0.794 | |||
| Universal health coverage scheme | 1040 | 153 (14.7%) | Ref. | |||||
| Others | 140 | 31 (22.1%) | 1.65 | 1.03–2.58 | 0.023* | |||
| Education level | ||||||||
| Currently studying | 887 | 132 (14.9%) | Ref. | Ref. | ||||
| Less than bachelor’s degree | 233 | 27 (11.6%) | 0.75 | 0.46–1.18 | 0.200 | 0.63 | 0.33–1.20 | 0.161 |
| Bachelor’s degree, master’s degree or higher | 213 | 48 (22.5%) | 1.66 | 1.12–2.44 | 0.007* | 1.27 | 0.63–2.55 | 0.499 |
| Insulin regimen and frequency of SMBG | ||||||||
| Conventional and ≤1 time/day | 274 | 32 (11.7%) | Ref. | Ref. | ||||
| Conventional and 2 times/day | 139 | 23 (16.6%) | 1.50 | 0.80–2.78 | 0.169 | 1.89 | 0.99–3.60 | 0.053 |
| Conventional and ≥3 times/day | 148 | 18 (12.2%) | 1.05 | 0.53–2.01 | 0.883 | 1.39 | 0.69–2.80 | 0.352 |
| Intensive and ≤1 time/day | 232 | 29 (12.5%) | 1.08 | 0.61–1.91 | 0.777 | 1.05 | 0.57–1.96 | 0.867 |
| Intensive and 2 times/day | 161 | 18 (11.2%) | 0.95 | 0.48–1.82 | 0.875 | 0.99 | 0.50–1.93 | 0.972 |
| Intensive and ≥3 times/day | 430 | 101 (23.5%) | 2.32 | 1.49–3.69 | <0.001* | 2.80 | 1.71–4.58 | <0.001* |
Categorical data are presented as number and percentage. *A P‐value <0.05 shows statistical significance.
Input variables were age, duration of disease, health insurance schemes, educational level, insulin regimen and self‐monitoring of blood glucose (SMBG). AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio; Ref., reference
Discussion
The present nationwide study improves our understanding of the current status and influencing factors of glycemic control (e.g., types of insulin regimen, frequency of SMBG, age and duration of diabetes) among individuals with type 1 diabetes in Thailand. Consistent with the reports from Western countries 11 , 12 , 13 , we found poor glycemic control to be common during adolescence and early adulthood, with subsequent gradual improvement in individuals aged >25 years. We also found a higher frequency of SMBG to be significantly associated with better glycemic control, as established by other studies 14 , 15 , 16 . Interestingly, just 0.69% of the present patients were aged ≥65 years. Although studies have shown that type 1 diabetes patients had a shorter life expectancy 17 , 18 , it is unclear if this is responsible for the low detection rate in this age group in the current study. Lower awareness of type 1 diabetes in older adults among healthcare providers could partially explain this finding.
Importantly, the results of the present study clearly show that most patients with type 1 diabetes in Thailand have not achieved optimal glycemic control. Alarmingly, the mean HbA1c and the percentage of patients that met the glycemic target in the current study (9.35 ± 2.41% and 16%, respectively) are not different from those reported from the Thailand Diabetes Registry Project, which was carried out in 2003 (a mean HbA1c of 9.3 ± 2.5% in 195 children and adolescents, and 17% met glycemic target) 8 . Similarly, a previous study from Asia and the Western Pacific region that included 159 Thai children and adolescents during 2001–2002 found a mean HbA1c of 9.0 ± 2.3%, 89.3% of them were receiving one or two injections daily, 10.7% were receiving three injections daily and none were receiving four injections daily or CSII 19 . Although the proportion of patients using IIT in this cohort (57%) was significantly higher than a decade ago, their HbA1c was still high at 9.17%, and just 18.6% achieved glycemic target. The present T1DDAR CN project clearly shows the worrisome fact that there has been no improvement in glycemic control in our type 1 diabetes patients during the past decade.
When comparing glycemic control between the present cohort and individuals with type 1 diabetes from developed countries 9 , 11 , 13 , 20 , 21 , the present patients had a higher HbA1c and a lower proportion of individuals achieving targeted glycemic control. Among these countries, Sweden had the lowest HbA1c (7.6%) and the highest percentage of patients who achieved optimal glycemic control (49%) 20 . Sweden has a well‐established national program that helps participating centers to improve their care for children with diabetes, along with a national quality registry (SWEDIABKIDS) that provides open online data that each center can access their performance and compare with other centers’ as well as nationally 22 . Germany, and Austria had the next lowest mean HbA1c (7.7–7.8%) 20 . In contrast, Australia, the UK, the USA and Wales showed higher HbA1c levels (8.3–8.8%), and just 17–27% of patients had optimal glycemic control 9 , 20 . Similar to the findings from the Type 1 Diabetes Exchange clinic registry 11 , the present patients aged >25 years had better glycemic control than our younger patients. However, just 15–18% of the patients met the targeted glycemic control, and their average HbA1c was 8.03–8.85%, which is higher than the reported 7.6–7.7% from the Type 1 Diabetes Exchange 11 .
The present cohort also showed patterns of insulin delivery in Thailand. Similar to findings from the Australasian Diabetes Data Network registry 9 , CIT was commonly used in younger children, with 50–60% of Thai children aged <10 years using CIT. In Thailand, multiple daily injections are mainly prescribed for children who are aged >10 years who are deemed able to carry out insulin injections, or for those who have caregivers that can give insulin injection(s) during school hours. The fact that CIT is commonly prescribed for young school‐aged children in Thailand might suggest that parents or school personnel have difficulty giving insulin injections or providing support for IIT. Another possible explanation might be the lack of a type 1 diabetes‐specialized healthcare team to provide support for school personnel. Only 1% of our patients were on CSII. The high cost of CSII and the low number of experienced medical teams can explain the very low percentage of CSII use in Thailand.
The results from the T1DDAR CN study have illuminated the current status of type 1 diabetes care in Thailand. Despite more than half of patients being on IIT, the majority did not achieve optimal glycemic control. This can be explained by several factors. First, just 20% of the present patients carried out SMBG four or more times per day. The cost of the glucose test strips, currently not covered by any insurance, might partly explain the infrequency of SMBG. A study from Korea emphasized the necessity of a national reimbursement policy for blood glucose test strips 23 . The Korea National Health Insurance Service has reimbursed the cost of blood glucose test strips for up to four times a day for patients with type 1 diabetes since 2011. A study among 466 Korean patients with type 1 diabetes showed an increased proportion of patients who carried out SMBG four or more times per day after registering for a national reimbursement program: 28.4% at baseline and 44.1% at 12 month follow up 23 . Furthermore, an increase in SMBG frequency was associated with >5% reduction of HbA1c at 12‐month follow up 23 . Socioeconomic status has been reported to be associated with glycemic control 12 . More than half of our cohort had a monthly household income <$600, which is below the $840 average national income 24 . In addition to adversely affecting the adequacy of basic diabetes needs (e.g., glucose test strips, types and quality of meals etc.), low socioeconomic status might also affect the state of care and supervision in the home. The IDREAM study in India found parental involvement in insulin administration to be associated with better glycemic control 25 . Suboptimal glycemic control in our cohort might also be due to the omission of pre‐main meal insulin injection or an extra injection for snack, especially in patients receiving multiple daily injections. Other possible factors include lack of the routine use of flexible insulin dosing coupled with carbohydrate counting. We then arrive at the important question – what is the availability and effectiveness of the DSMES provided among hospitals in Thailand. A recent nationwide survey showed that 30% of diabetes educators in Thailand reported that the diabetes education in their hospitals was successful, whereas 37–43% reported uncertainty regarding the program’s effectiveness, and 24–32% said that the program’s effectiveness was not evaluated 26 . One of the obstacles reported was lack of time for diabetes educators to provide education due to their need to attend to other duties 26 . The uncertainty of the effectiveness and lack of formal evaluation of diabetes education are weak points in the process of diabetes care in Thailand 26 . Finally, psychological factors might influence glycemic control. The burden and the demands of having type 1 diabetes in managing daily diabetes‐related tasks can lead to negative emotions or “diabetes distress” 27 and depressive symptoms 28 , 29 . Depressive symptoms are common in adolescents with type 1 diabetes, and have been shown to be related to decreased self‐care and poor glycemic control 28 , 29 .
The Thailand Diabetes Registry Project in 2003 reported a prevalence of diabetic retinopathy and diabetic nephropathy of 21.6 and 44.4%, respectively in type 1 diabetes patients 30 , 31 . Although the overall prevalence of diabetic retinopathy and nephropathy was lower in the current study than the previous study, the prevalence of both complications increased dramatically in older patients. It is also of great concern that diabetic nephropathy had already developed in our young cohort. We found that patients who were treated with IIT had a lower prevalence of diabetic retinopathy, which supports the reported benefit of IIT on microvascular complications 32 . The overall prevalence of diabetic neuropathy in our cohort was 5.2%, which is quite similar to the prevalence of 7% in the SEARCH for Diabetes in Youth study 33 . However, only one‐third of our patients had the neuropathy assessment, suggesting that improvement in care process is required.
Patterns of acute complications (hypoglycemia and DKA) in the current study are also worrisome. Previously, data from Asia and the Western Pacific Region in 2001–2002 showed an incidence of hypoglycemia events in Thailand of 75.9 events per 100 patient‐years, and the incidence of DKA events was 11.4 per 100 patient‐years 19 . The incidence of hypoglycemia among Thai children was similar to that of the region (74 events per 100 patient‐years); however, the incidence of DKA was modestly higher (region – 9.9 events per 100 patient‐years) 19 . The prevalence of severe hypoglycemia in the T1DDAR CN project was 8.5% per year, and the prevalence was highest in participants aged ≥45 years. The present finding is similar to that reported from the Type 1 Diabetes Exchange registry, which found that 6% of participants had severe hypoglycemia within the previous 3 months, and that the rate increased in older patients 11 . However, the present cohort had a considerably higher prevalence of DKA (10.2% per year) than that of the Type 1 Diabetes Exchange registry (3% of participants reported having a DKA event within the previous 3 months) 11 . This observed high rate of DKA can partly be explained by poor glycemic control and the fact that most participating hospitals did not have a system in place to assist patients with impending DKA (e.g., no 24‐h telephone consultation service). The present results showed no difference in the prevalence of DKA and severe hypoglycemia between CIT and IIT groups.
The present study had some limitations. First, this study recruited only patients from tertiary centers, so these results might not be generalizable for the entire country. Second, this study did not evaluate other factors that might influence glycemic control, such as psychological status and self‐care behaviors. Finally, HbA1c was assayed at each tertiary hospital and not at one centralized laboratory.
In conclusion, the T1DDAR CN study comprises the largest national cohort of type 1 diabetes patients to date in Thailand. The results of this important study showed that most patients do not meet the recommended glycemic target, facing a high risk of complications. Further study is required to prioritize the factors that will influence improvement in glycemic control. Changes to Thailand’s national health policy in type 1 diabetes care, including the provision of optimal glucose test strips and glucometer, establishing a referral system to experienced diabetologists, and the development and implementation of a standardized DSMES system at the national level, are urgently required. As the results of the present study, a national program to improve the quality of diabetes treatment and DSMES has been implemented since October 2018, and the result is ongoing.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the Siriraj Diabetes Center, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, and the Research Institute for Health Sciences of Chiang Mai University, Chiang Mai, Thailand, for managing the centralized administrative and data coordination responsibilities for the T1DDAR CN study. The authors also thank the staff and nurses at each center for their contributions to this study, Jiraporn Nilsu for her support as a project manager, Suthathip Wongsrithep and Jarun Chuayen for their valuable technical support on data curation, and Antika Wongthanee for her kind assistance with statistical analysis. The present study was supported by the Thai Society for Pediatric Endocrinology, the Endocrine Society of Thailand, the Diabetes Association of Thailand and the National Health Security Office of Thailand.
Appendix 1.
The following persons participated in the T1DDAR CN:
Central region
University Hospitals: King Chulalongkorn Memorial Hospital, Chulalongkorn University, Bangkok (Taninee Sahakitrungruang, Suphab Aroonparkmongkol, Vichit Supornsilchai); Ramathibodi Hospital, Mahidol University, Bangkok (Chardpraorn Ngarmukos, Hataikarn Nimitphong, Manassawee Korwutthikulrangsri, Patcharin Khlairit, Pat Mahachoklertwattana, Preamrudee Poomthavorn, Ratanaporn Jerawatana, Sarunyu Pongratanakul, Sirimon Reutrakul); Siriraj Hospital, Mahidol University, Bangkok (Apiradee Sriwijitkamol, Jeerunda Santiprabhob, Lukana Preechasuk, Ornsuda Lertbannaphong, Raweewan Lertwattanarak, Sriwan Thongpaeng, Supawadee Likitmaskul, Supitcha Patjamontri); Thammasat University Hospital, Pathum Thani (Nattamon Tanathornkirati, Pitvara Panpitpat, Pontipa Engkakul, Thipaporn Tharavanij); Vajira Hospital, Navamindradhiraj University, Bangkok (Natphassorn Dermkhuntod, Petch Rawdaree, Thanyaros Sinsophonphap, Warunee Sunpakaew).
Hospitals in the Ministry of Public Health: Charoenkrung Pracharak Hospital, Bangkok (Phatharaporn Kiatpanabhikul, Supawut Suksantilirs); Queen Sirikit National Institute of Child Health, Bangkok (Chawkaew Kongkanka, Nutlita Boonkong, Sirinya Somsaen); Rajavithi Hospital, College of Medicine, Rangsit University, Bangkok (Apatsara Vansaksri, Chaicharn Deerochanawong); Sawanpracharak Hospital, Nakhon Sawan (Chattama Chairat, Kamonwan Chanchalam, Sanguansak Siang‐ruangsang); Taksin Hospital, Bangkok (Worraporn Tantichattanon).
Hospitals in the Ministry of Defense: Bhumibol Adulyadej Hospital, Bangkok (Chulalak Nganlasome, Karnsuda Pichetsin, Kesinee Boonpakdee)
HRH Princess Maha Chakri Sirindhorn Medical Center‐MSMC Hospital, Nakhon Nayok (Nattakarn Wongjitrat); Phramongkutklao Hospital, Bangkok (Jiraporn Nuphonthong, Nattapol Sathavarodom, Nawaporn Numbenjapon); Somdejprapinklao Hospital, Bangkok (Chantraporn Keamseng).
North region
University Hospitals: Chiang Mai University Hospital, Chiang Mai (Danil Wongsa, Laddawan Limpijankit, Mattabhorn Phimphilai, Prapai Dejkhamron).
Hospitals in the Ministry of Public Health: Buddhachinaraj Hospital, Phitsanulok (Meijinee Densriwiwat); Chiangrai Prachanukroh Hospital, Chiang Rai City (Kiran Sony, Orathai Mahawongsanan, Pataree Maneerat); Nakornping Hospital, Chiang Mai (Hataitip Tangngam, Tattiwa Nirach).
Northeast region
University Hospitals: Srinagarind Hospital, Khon Kaen University, Khon Kaen (Chatlert Pongchaiyakul, Ouyporn Panamonta, Pattara Wiromrat).
Hospitals in the Ministry of Public Health: Khon Kaen Hospital, Khon Kaen (Chatchai Suesirisawad); Maharat Nakhon Ratchasima Hospital, Nakhon Ratchasima (Priya Sanguanwongwichit, Puntip Tantiwong, Sirilak Setthalak); Mukdahan Hospital, Mukdahan (Akanit Jindamaneemas, Nattakarn Suwansaksri); Sunpasitthiprasong Hospital, Ubon Ratchathani (Jaturat Petchkul).
East region
University Hospitals: Burapha University Hospital, Chonburi (Krittha Jeerawongpanich).
Hospitals in the Ministry of Public Health: Chonburi Hospital (Somlak Tongmeesee); Prapokklao Hospital, Chanthaburi (Thapana Roonghiranwat); Rayong Hospital, Rayong (Chotima Sornsiriwong, Naruewan Piriyabanjong, Tippawan Kongvitayanon).
South region
University Hospitals: Songklanagarind Hospital, Prince of Songkla University, Songkhla (Rattana Leelawattana, Somchit Jaruratanasirikul).
Hospitals in the Ministry of Public Health: Hat Yai Hospital, Songkhla (Pathikan Dissaneevate); Maharaj Nakhon Si Thammarat Hospital, Nakhon Si Thammarat (Saowanee Nakkaew); Surat Thani Hospital, Surat Thani (Palinee Nantarakchaikul).
J Diabetes Investig. 2021
References
- 1. Tangcharoensathien V, Witthayapipopsakul W, Panichkriangkrai W, et al. Health systems development in Thailand: a solid platform for successful implementation of universal health coverage. Lancet 2018; 391: 1205–1223. [DOI] [PubMed] [Google Scholar]
- 2. Patterson C, Guariguata L, Dahlquist G, et al. Diabetes in the young ‐ a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract 2014; 103: 161–175. [DOI] [PubMed] [Google Scholar]
- 3. Deerochanawong C, Ferrario A. Diabetes management in Thailand: a literature review of the burden, costs, and outcomes. Global Health 2013; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Likitmaskul S, Angsusingha K, Morris S, et al. Type 1 diabetes in Thai children aged 0–14 years. J Med Assoc Thai 1999; 82: 826–832. [PubMed] [Google Scholar]
- 5. Tuchinda C, Likitmaskul S, Unachak K, et al. The epidemiology of type 1 diabetes in Thai children. J Med Assoc Thai 2002; 85: 648–652. [PubMed] [Google Scholar]
- 6. Jaruratanasirikul S, Thammaratchuchai S, Sriplung H. Trends of childhood diabetes in Southern Thailand: 20‐year experience in a tertiary medical center. World J Pediatr 2017; 13: 566–570. [DOI] [PubMed] [Google Scholar]
- 7. Panamonta O, Thamjaroen J, Panamonta M, et al. The rising incidence of type 1 diabetes in the northeastern part of Thailand. J Med Assoc Thai 2011; 94: 1447–1450. [PubMed] [Google Scholar]
- 8. Likitmaskul S, Wacharasindhu S, Rawdaree P, et al. Thailand diabetes registry project: type of diabetes, glycemic control and prevalence of microvascular complications in children and adolescents with diabetes. J Med Assoc Thai 2006; 89(Suppl 1): S10–S16. [PubMed] [Google Scholar]
- 9. Phelan H, Clapin H, Bruns L, et al. The Australasian Diabetes Data Network: first national audit of children and adolescents with type 1 diabetes. Med J Aust 2017; 206: 121–125. [DOI] [PubMed] [Google Scholar]
- 10. McCarthy MM, Grey M. Type 1 diabetes self‐management from emerging adulthood through older adulthood. Diabetes Care 2018; 41: 1608–1614. [DOI] [PubMed] [Google Scholar]
- 11. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015; 38: 971–978. [DOI] [PubMed] [Google Scholar]
- 12. Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes 2016; 17: 327–336. [DOI] [PubMed] [Google Scholar]
- 13. Carlsen S, Skrivarhaug T, Thue G, et al. Glycemic control and complications in patients with type 1 diabetes ‐ a registry‐based longitudinal study of adolescents and young adults. Pediatr Diabetes 2017; 18: 188–195. [DOI] [PubMed] [Google Scholar]
- 14. Ziegler R, Heidtmann B, Hilgard D, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes 2011; 12: 11–17. [DOI] [PubMed] [Google Scholar]
- 15. Redondo MJ, Connor CG, Ruedy KJ, et al. Pediatric Diabetes Consortium Type 1 Diabetes New Onset (NeOn) Study: factors associated with HbA1c levels one year after diagnosis. Pediatr Diabetes 2014; 15: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self‐monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 2013; 36: 2009–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petrie D, Lung TW, Rawshani A, et al. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia 2016; 59: 1167–1176. [DOI] [PubMed] [Google Scholar]
- 18. Huo L, Harding JL, Peeters A, et al. Life expectancy of type 1 diabetic patients during 1997–2010: a national Australian registry‐based cohort study. Diabetologia 2016; 59: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 19. Craig ME, Jones TW, Silink M, et al. Diabetes care, glycemic control, and complications in children with type 1 diabetes from Asia and the Western Pacific Region. J Diabetes Complications 2007; 21: 280–287. [DOI] [PubMed] [Google Scholar]
- 20. Charalampopoulos D, Hermann JM, Svensson J, et al. Exploring variation in glycemic control across and within eight high‐income countries: a cross‐sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care 2018; 41: 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rica I, Mingorance A, Gomez‐Gila AL, et al. Achievement of metabolic control among children and adolescents with type 1 diabetes in Spain. Acta Diabetol 2017; 54: 677–683. [DOI] [PubMed] [Google Scholar]
- 22. Samuelsson U, Akesson K, Peterson A, et al. Continued improvement of metabolic control in Swedish pediatric diabetes care. Pediatr Diabetes 2018; 19: 150–157. [DOI] [PubMed] [Google Scholar]
- 23. Jin SM, Baek JH, Suh S, et al. Factors associated with greater benefit of a national reimbursement policy for blood glucose test strips in adult patients with type 1 diabetes: a prospective cohort study. J Diabetes Investig 2018; 9: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Statistical Office, Ministry of Information and Communication Technology . The 2015 Household Socio‐Economic Survey Whole Kingdom [Internet], 2016. Available from: http://web.nso.go.th/en/survey/house_seco/data/Full_Report2015.pdf Accessed August 2, 2019
- 25. Friedemann‐Sanchez G, Capistrant BD, Ron J, et al. Caregiving for children with type 1 diabetes and clinical outcomes in central India: the IDREAM study. Pediatr Diabetes 2018; 19: 527–533. [DOI] [PubMed] [Google Scholar]
- 26. Preechasuk L, Sriussadaporn P, Likitmaskul S. The obstacles to diabetes self‐management education and support from healthcare professionals’ perspectives: a nationwide survey. Diabetes Metab Syndr Obes 2019; 12: 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hagger V, Hendrieckx C, Sturt J, et al. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr Diab Rep 2016; 16: 9. [DOI] [PubMed] [Google Scholar]
- 28. Buchberger B, Huppertz H, Krabbe L, et al. Symptoms of depression and anxiety in youth with type 1 diabetes: a systematic review and meta‐analysis. Psychoneuroendocrinology 2016; 70: 70–84. [DOI] [PubMed] [Google Scholar]
- 29. Hood KK, Huestis S, Maher A, et al. Depressive symptoms in children and adolescents with type 1 diabetes: association with diabetes‐specific characteristics. Diabetes Care 2006; 29: 1389–1391. [DOI] [PubMed] [Google Scholar]
- 30. Chetthakul T, Likitmaskul S, Plengvidhya N, et al. Thailand diabetes registry project: prevalence of diabetic retinopathy and associated factors in type 1 diabetes mellitus. J Med Assoc Thai 2006; 89(Suppl 1): S17–S26. [PubMed] [Google Scholar]
- 31. Rawdaree P, Ngarmukos C, Deerochanawong C, et al. Thailand diabetes registry (TDR) project: clinical status and long term vascular complications in diabetic patients. J Med Assoc Thai 2006; 89(Suppl 1): S1–S9. [PubMed] [Google Scholar]
- 32. Diabetes Control Complications Trial Research Group , Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 33. Jaiswal M, Divers J, Dabelea D, et al. Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for diabetes in youth study. Diabetes Care 2017; 40: 1226–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]


