Abstract
Aims/Introduction
Vaspin is linked to obesity and its metabolic abnormalities. However, the role of vaspin serum levels in diabetic retinopathy (DR) is unknown. In the present study, we investigated the association between serum levels of vaspin and both DR and vision‐threatening DR.
Materials and Methods
This was a cross‐sectional single‐center observational study from December 2018 to September 2019. We evaluated circulating serum levels of vaspin in 372 participants with type 2 diabetes. DR was screened through detailed ocular examination. DR patients were also divided two groups: vision‐threatening DR and non‐vision‐threatening DR. The relationship between vaspin and DR was investigated by univariate and multivariate logistic regression analyses, and the results are shown as odds ratios with 95% confidence intervals.
Results
The vaspin serum levels of 372 patients were obtained, with a median value of 1.50 ng/mL (interquartile range 0.94–2.18 ng/mL). The median age of those patients was 53 years (interquartile range 44–62 years), and 44.4% were women. Patients with DR and VDTR had significantly increased vaspin serum levels (P < 0.001 andP < 0.001). A multivariable regression model found that patients with high levels of vaspin were approximately 1.85‐fold (odds ratio for per unit increase 1.85, 95% confidence interval 1.43–2.55; P < 0.001) more likely to experience DR, and 3.76‐fold (odds ratio for per unit increase 3.76, 95% confidence interval 2.05–6.55; P < 0.001) more likely to experience VTDR. The predictive value of vaspin was stronger in women than in men.
Conclusion
Higher vaspin serum levels were associated with an increased risk of DR and VDTR in patients with type 2 diabetes, which showed that vaspin is an important indicator factor for DR.
Keywords: Diabetes, Diabetic retinopathy, Vaspin
The regulation of vaspin serum concentrations in diabetic retinopathy is unknown. This study showed that higher vaspin serum levels were associated with increased risk of diabetic retinopathy and vision‐threatening diabetic retinopathy in patients with type 2 diabetes, which showed that vaspin is an important indicator factor for diabetic retinopathy.

Introduction
The World Health Organization report states that >422 million adults globally were suffering from diabetes in 2014, and a continuous rise in prevalence is expected 1 . A cross‐sectional national survey showed that the estimated prevalence of diabetes was 10.9% (prediabetes 35.7%) in the Chinese population 2 . Diabetic retinopathy (DR) affects >30% of patients with diabetes, and remains one of the main causes of blindness in working people 3 .
Adipocytokines play a role in the pathogenesis of diabetes and its complications 4 . A meta‐analysis confirmed that leptin and adiponectin levels are higher in type 2 diabetes mellitus patients with microvascular complications 5 . Yilmaz et al. 6 suggested that adiponectin might take part in the pathogenesis of DR. Uckaya et al. 7 declared that the more advanced the DR, the higher the plasma leptin levels, suggesting leptin might play a role in the progression of human DR to a proliferative phase. In addition, circulating visfatin was increased with progressive β‐cell deterioration 8 , and serum omentin‐1 concentration correlated negatively with DR presence and severity in type 2 diabetes mellitus patients 9 . Jung et al. 10 found that higher serum adiponectin was related to increased odds for diabetic nephropathy in type 2 diabetes mellitus.
Vaspin is a novel adipocytokine produced by visceral and subcutaneous adipose tissues 11 . Vaspin messenger ribonucleic acid expression and elevated serum levels were associated with obesity, type 2 diabetes, metabolic syndrome and atherosclerosis 12 , 13 , 14 , 15 . A meta‐analysis showed that significantly higher levels of serum vaspin were observed in obese individuals and type 2 diabetes mellitus patients 16 ; however, another study showed that low serum concentration of vaspin is a risk factor for the progression type 2 diabetes mellitus 17 .
Furthermore, previous studies assessed the association between circulating vaspin and insulin sensitivity, yielding conflicting results 18 , 19 , and the mechanisms of how vaspin might play a role in glucose metabolism and insulin sensitivity are still unknown 20 . Interestingly, vaspin can affect vascular systems to prevent or exacerbate obesity‐related vascular complications, such as diabetes‐related vascular dysfunction 21 , hypertension 22 and atherosclerosis 23 . To our knowledge, no previous studies have clarified the relationship of serum vaspin with DR prevalence in patients with type 2 diabetes mellitus. The aim of the present study was to investigate the association between serum vaspin levels and both DR and vision‐threatening DR (VTDR) in type 2 diabetes mellitus patients.
Methods
Patients
This was a cross‐sectional, single‐center, observational study. A total of 506 patients with type 2 diabetes mellitus were screened from the Department of Endocrinology of Shengjing Hospital of China Medical University (Shenyang, China). The study period was from December 2018 to September 2019. The diagnosis of diabetes was based on the 1999 World Health Organization diagnostic criteria 24 . Patients with malignant tumor, liver and/or renal insufficiency, infectious and inflammatory conditions, cardiovascular disease, psychological and/or neurological disorders, and eye infections were excluded. The research protocol of this study was reviewed and approved by the Human Studies Ethics Committee of the Shengjing Hospital of China Medical University (No. 2018‐SJEC‐003). No patients were included unless their written informed consent was obtained.
Data collection
Demographic information (age, sex and body mass index [BMI]), diabetes duration, hypertension, hyperlipoproteinemia, smoking habits, alcohol abuse and treatments information (insulin, lipid‐lowering and blood pressure‐lowering) were recorded. Intensive glucose treatment was defined as treatment with sulfonylurea or insulin or, if >120% of ideal bodyweight, metformin. DR was screened through detailed ocular examination using dilated ophthalmoscopy and slit lamp biomicroscopy. The severity of DR was defined by the Early Treatment Diabetic Retinopathy Study grading standards, which was divided into two groups: non‐proliferative DR and proliferative DR 25 . In addition, DR patients were also divided two groups: vision‐threatening DR (VTDR; included severe non‐proliferative DR and proliferative DR patients) and non‐VTDR (included mild and moderate non‐proliferative DR) 26 . We used the eye with the more severe symptoms to group the patients.
Laboratory testing
A fasting blood sample was collected from the cubital vein. Serum samples were separated and stored at −80°C. Serum glucose, lipids (total cholesterol, triglycerides, low density lipoprotein cholesterol, high‐density lipoprotein cholesterol) and high‐sensitivity C‐reactive protein (Hs‐CRP) were assessed using BS800M (MINDRAY, Shenzhen, China). Serum levels of vaspin and insulin were tested using commercial enzyme‐linked immunosorbent assay kits, as per the manufacturers’ instructions (BioVision, Inc., Milpitas, CA, USA), with intra‐ and interassay coefficients of variation of 4.5–8.5%/6.0–9.5% and 4.0–7.0%/5.0–8.5%, respectively. Insulin resistance was assessed by the homeostasis model assessment of insulin resistance (HOMA‐IR) index (fasting serum insulin [µU/mL] × fasting blood glucose [mmol/L] / 22.5) 27 .
Statistical analysis
The categorical data are presented as the number and percentage (%), whereas distributed data are presented as the median and interquartile range (IQR). The difference between groups was assessed by the χ2‐test (categorical data) or Mann–Whitney U‐test (distributed data). The correlation between different factors was assessed by Spearman’s rank correlation test. In addition, those included patients were divided into two groups based on the vaspin levels (Q1–3 vs Q4), and the relationship between serum vaspin and other factors was analyzed.
The relationship between vaspin and DR (and VTDR) was also investigated by the univariate and multivariate logistic regression analyses (adjusted for age, sex, BMI, duration of diabetes, conventional risk factors, treatments and serum levels of glucose, lipids, insulin, and Hs‐CRP), and results are shown as odds ratios (ORs) with 95% confidence intervals (CIs). The receiver operating characteristic curve was used to elaborate the role of vaspin in diagnosing DR (and VTDR), and results are shown as the area under the curve with 95% CI. All statistical analyses were tested by IBM SPSS software (version 22.0; IBM Corporation, Armonk, NY, USA), and a P < 0.05 (two‐sided) was considered significant.
Results
Patient characteristics
We recorded 375 patients with type 2 diabetes mellitus (excluded: 8 with malignant tumor; 6 with liver and/or renal insufficiency; 12 with infectious and inflammatory conditions; 58 with cardiovascular disease; 31 with psychological and/or neurological disorders; 5 with infection in one and/or both eyes; and 11 without informed consent). The vaspin serum level was obtained for 372 patients (99.2%) with a median value of 1.50 ng/mL (IQR 0.94–2.18 ng/mL).
The characteristics of patients according to vaspin levels (Q1–3 vs Q4) are shown in Table 1. Patients with a vaspin level in the highest quartile (Q4) were more likely women and with hypertension. BMI, disease duration, HOMA‐IR, serum levels of Hs‐CRP, fasting serum glucose and fasting insulin were significantly greater in this group. Furthermore, patients were more likely to have DR (54.3% vs 18.6%; P < 0.001) and VTDR (33.3% vs 3.5%) in this group (Table 1).
Table 1.
Basal characteristic of diabetes patients
| Characteristics | All | Vaspin (ng/mL) | P † | |
|---|---|---|---|---|
| Q1–3 (<2.18) | Q4 (≥2.18) | |||
| n | 372 | 280 | 92 | – |
| Age (years) | 53 (44–62) | 54 (43–63) | 53 (45–59) | 0.61 |
| Female | 165 (44.4) | 110 (39.3) | 55 (59.8) | 0.001 |
| BMI (kg/m2) | 25.5 (23.2–27.5) | 24.9 (22.6–27.0) | 26.5 (25.0–28.4) | <0.001 |
| Disease duration | 8 (4–11) | 7 (3–10) | 10 (6–12) | <0.001 |
| Hypertension | 151 (40.6) | 103 (36.8) | 48 (52.2) | 0.009 |
| Hyperlipidemia | 127 (34.1) | 92 (32.9) | 35 (38.0) | 0.36 |
| Smoking status | 85 (22.8) | 58 (20.7) | 27 (29.3) | 0.09 |
| Alcohol intake | 68 (18.3) | 44 (15.7) | 24 (26.1) | 0.03 |
| Intensive glucose treatment ‡ | 148 (39.8) | 120 (42.9) | 28 (30.4) | 0.03 |
| Use of lipid‐lowering medication | 87 (23.4) | 67 (23.9) | 27 (29.3) | 0.30 |
| Antihypertensive treatment | 135 (36.3) | 108 (38.6) | 27 (29.3) | 0.11 |
| Laboratory testing | ||||
| HbA1c (%) | 7.4 (6.2–8.5) | 6.9 (6.0–8.2) | 8.6 (7.4–9.5) | <0.001 |
| Hs‐CRP (mg/dL) | 0.41 (0.15–0.84) | 0.32 (0.14–0.71) | 0.75 (0.32–1.00) | <0.001 |
| FSG (mmol/L) | 6.12 (5.15–7.01) | 5.94 (5.05–6.73) | 7.11 (5.15–9.11) | <0.001 |
| Fasting insulin (µU/mL) | 7.12 (5.93–8.94) | 6.85 (5.76–8.32) | 7.75 (6.53–9.84) | <0.001 |
| HOMA‐IR | 4.33 (2.46–5.44) | 3.90 (2.15–5.14) | 5.32 (4.76–5.87) | <0.001 |
| Vaspin (ng/mL) | 1.50 (0.94–2.18) | 1.14 (0.84–1.64) | 2.72 (2.52–2.85) | <0.001 |
| Diagnosis of DR | 102 (27.4) | 52 (18.6) | 50 (54.3) | <0.001 |
| PDR | 25 (6.7) | 6 (2.1) | 19 (20.7) | <0.001 |
| VTDR | 40 (10.8) | 10 (3.5) | 30 (33.3) | <0.001 |
BMI, body mass index; DR, diabetic retinopathy; FBG, fasting blood glucose; FSG, fasting serum glucose; HbA1c, hemoglobin A1c; HOMA‐IR, homeostasis model assessment of insulin resistance; Hs‐CRP, high‐sensitivity‐C‐reactive protein; PDR, proliferative diabetic retinopathy; VTDR; vision‐threatening diabetic retinopathy.
Results are expressed as percentages or as medians (interquartile range).
P‐values were compared by Mann–Whitney U‐test or χ2‐test as appropriate.
Sulfonylurea or insulin or, if >120% of ideal bodyweight, metformin.
Serum vaspin and other factors
A positive correlation between vaspin and BMI (r [Spearman] = 0.258, P < 0.001) was found (Figure 1a). Serum levels of vaspin were associated with HOMA‐IR (r = 0.518, P < 0.001; Figure 1b). Furthermore, positive correlations between vaspin and Hs‐CRP (r = 0.343, P < 0.001), fasting serum glucose (r = 0.158, P = 0.002), fasting insulin (r = 0.305, P < 0.001), disease duration (r = 0.340, P < 0.001) and sex (r = 0.129, P = 0.013) were shown.
Figure 1.
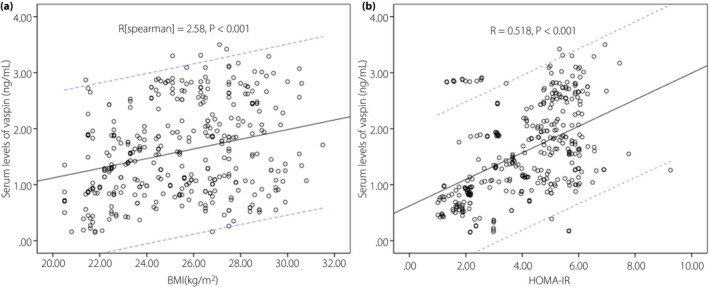
The correlation between vaspin and other factors. (a) The correlation between vaspin and body mass index (BMI). (b) The correlation between vaspin and homeostasis model assessment of insulin resistance (HOMA‐IR). The solid line represents the trend line and the dashed line represents the 95% confidence interval.
Serum vaspin and DR
In the present study, 102 patients (27.4%) experienced DR, and vaspin serum levels in those patients were higher than in those patients without DR (median 2.07 ng/mL, IQR 1.23–2.73 ng/mL vs median 1.29 ng/mL, IQR 0.90–1.88 ng/mL; P < 0.001; Figure 2). A multivariable regression model showed that patients with high levels of vaspin were approximately 1.85‐fold (OR for per unit increase 1.85, 95% CI 1.43–2.55; P < 0.001) more likely to experience DR after adjustment for sex, BMI, disease duration, intensive glucose treatment, HbA1c, Hs‐CRP and HOMA‐IR (Table 2).
Figure 2.
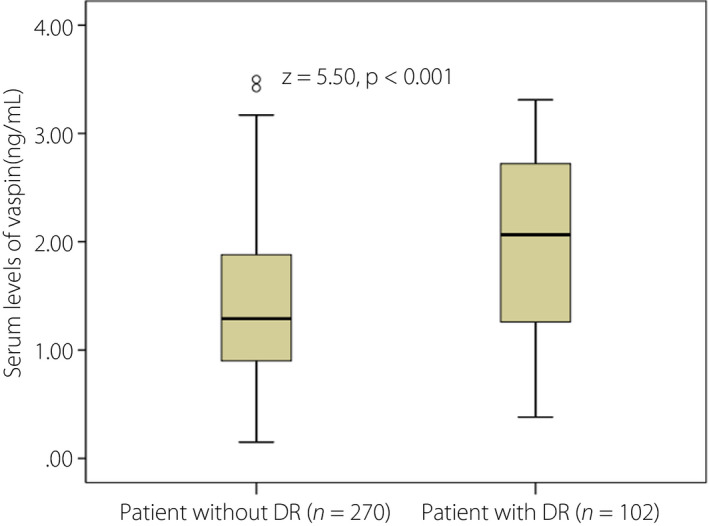
Box plots of serum levels of vaspin in type 2 diabetes mellitus patients with diabetic retinopathy (DR) and without DR. The Mann–Whitney U‐test was used. All data are medians and interquartile ranges.
Table 2.
Logistic regression model for vaspin and other predictors using diabetic retinopathy and vision‐threatening diabetic retinopathy as the dependent variables
| Univariate analysis | Multivariate analysis † | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95%CI) | P | |
| DR | ||||
| Vaspin (increase per unit) | 2.48 (1.82–3.37) | <0.001 | 1.85 (1.43–2.55) | <0.001 |
| Age (increase per unit) | 0.99 (0.73–1.03) | 0.38 | – | |
| Sex (female vs male) | 3.17 (1.98–5.05) | <0.001 | 1.85 (1.43–3.02) | 0.003 |
| BMI (increase per unit) | 1.15 (1.05–1.26) | 0.003 | 1.07 (1.02–1.15) | 0.009 |
| Disease duration (increase per unit) | 1.08 (1.02–1.13) | 0.007 | 1.05 (0.95–1.21) | 0.09 |
| Hypertension (yes vs no) | 1.56 (1.02–2.51) | 0.075 | – | |
| Hyperlipidemia (yes vs no) | 1.22 (0.95–1.98) | 0.32 | – | |
| Smoking status (yes vs no) | 1.68 (0.98–2.97) | 0.58 | – | |
| Alcohol intake (yes vs no) | 2.03 (1.32–3.28) | 0.13 | – | |
| Intensive glucose treatment (yes vs no) ‡ | 0.85 (0.77–0.95) | 0.012 | 0.95 (0.88–1.06) | 0.08 |
| HbA1c (increase per unit) | 1.48 (1.26–1.74) | 0.001 | 1.32 (1.21–1.55) | 0.008 |
| Hs‐CRP (increase per unit) | 1.67 (1.22–2.15) | 0.012 | 1.43 (1.15–1.94) | 0.039 |
| HOMA‐IR (increase per unit) | 1.14 (1.08–1.19) | <0.001 | 1.08 (1.02–1.15) | 0.002 |
| VTDR | ||||
| Vaspin (increase per unit) | 7.65 (3.16–11.15) | <0.001 | 3.76 (2.05–6.55) | <0.001 |
| Age (increase per unit) | 0.98 (0.95–1.02) | 0.11 | – | |
| Sex (female vs male) | 2.55 (2.01–3.16) | 0.003 | 2.03 (1.33–2.93) | 0.009 |
| BMI (increase per unit) | 1.22 (1.06–1.39) | 0.004 | 1.15 (1.03–1.33) | 0.011 |
| Disease duration (increase per unit) | 1.09 (1.03–1.18) | 0.009 | 1.04 (1.01–1.14) | 0.021 |
| Hypertension (yes vs no) | 1.72 (0.89–3.32) | 0.11 | – | |
| Hyperlipidemia (yes vs no) | 1.05 (0.52–2.08) | 0.82 | – | |
| Smoking status (yes vs no) | 1.32 (0.63–2.77) | 0.43 | – | |
| Alcohol intake (yes vs no) | 1.87 (0.88–3.96) | 0.10 | – | |
| Intensive glucose treatment (yes vs no) ‡ | 0.77 (0.63–0.92) | 0.015 | 0.89 (0.75–1.03) | 0.08 |
| HbA1c (increase per unit) | 1.60 (1.29–2.00) | <0.001 | 1.40 (1.14–1.92) | 0.003 |
| Hs‐CRP (increase per unit) | 1.44 (1.20–1.63) | 0.013 | 1.31 (1.09–1.62) | 0.04 |
| HOMA‐IR (increase per unit) | 1.15 (1.09–1.22) | <0.001 | 1.09 (1.02–1.20) | <0.001 |
BMI, body mass index; CI, confidence interval; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HOMA‐IR, homeostasis model assessment of insulin resistance; Hs‐CRP, high‐sensitivity C‐reactive protein; OR, odds ratio; VTDR; vision‐threatening diabetic retinopathy.
Factors included in the multivariate analysis were confirmed in the univariate analysis
Sulfonylurea or insulin or, if >120% of ideal bodyweight, metformin
A receiver operating characteristic model showed that the optimal value of vaspin for diagnosing DR was 2.22 ng/mL (Youden’s index), yielding a best value of specificity (49.0%) and sensitivity (84.8%), with the area under the curve of vaspin of 0.69 (95% CI 0.62–0.75, P < 0.001; Figure 3).
Figure 3.
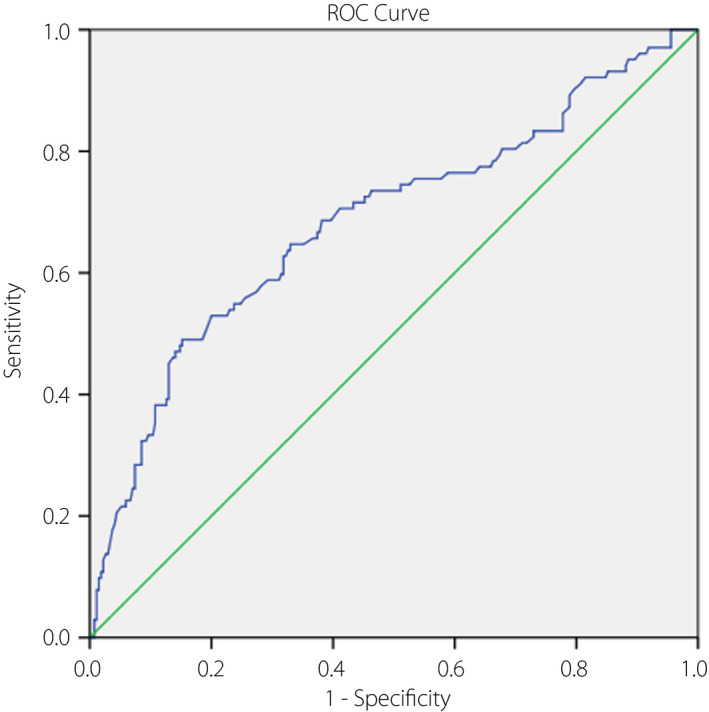
The receiver operating characteristic (ROC) curve was utilized to assess the accuracy of serum vaspin levels to diagnose diabetic retinopathy.
Serum vaspin and VTDR
Finally, 40 patients (10.8%) experienced VTDR, and vaspin serum levels in those patients were higher than in those patients without VTDR (median 2.67, IQR 2.39–2.86 vs median 1.31, IQR 0.88–1.94 ng/mL; P < 0.001; Fig. 4). A multivariable regression model showed that patients with high levels of vaspin were approximately 3.76‐fold (OR for per unit increase 3.76, 95% CI 2.05–6.55; P < 0.001) more likely to experience VTDR after adjustment for sex, BMI, disease duration, intensive glucose treatment, HbA1c, Hs‐CRP and HOMA‐IR (Table 2).
Figure 4.
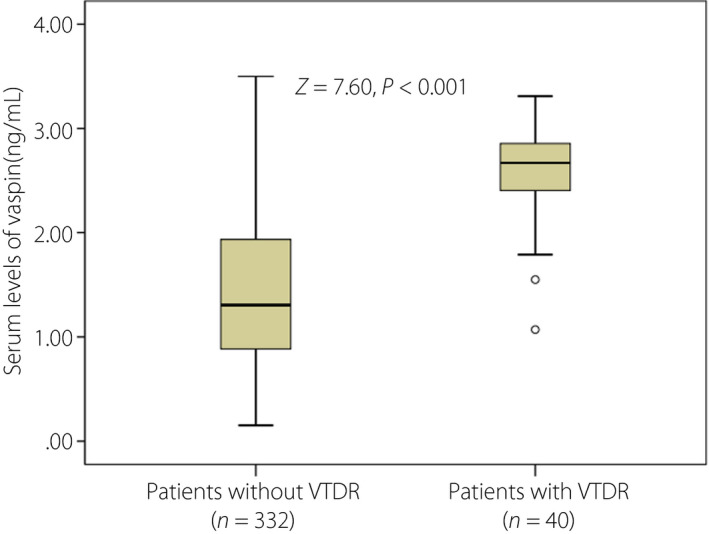
Box plots of serum levels of vaspin in type 2 diabetes mellitus patients with vision‐threatening diabetic retinopathy (VTDR) and without VTDR. Mann–Whitney U‐test. All data are medians and interquartile ranges.
A receiver operating characteristic model showed that the critical value of vaspin for diagnosing VTDR was 2.03ng/mL (Youden’s index), yielding a best value of specificity (85.0%) and sensitivity (77.7%), with the area under the curve of vaspin of 0.87 (95% CI 0.82–0.91, P < 0.001; Figure 5).
Figure 5.
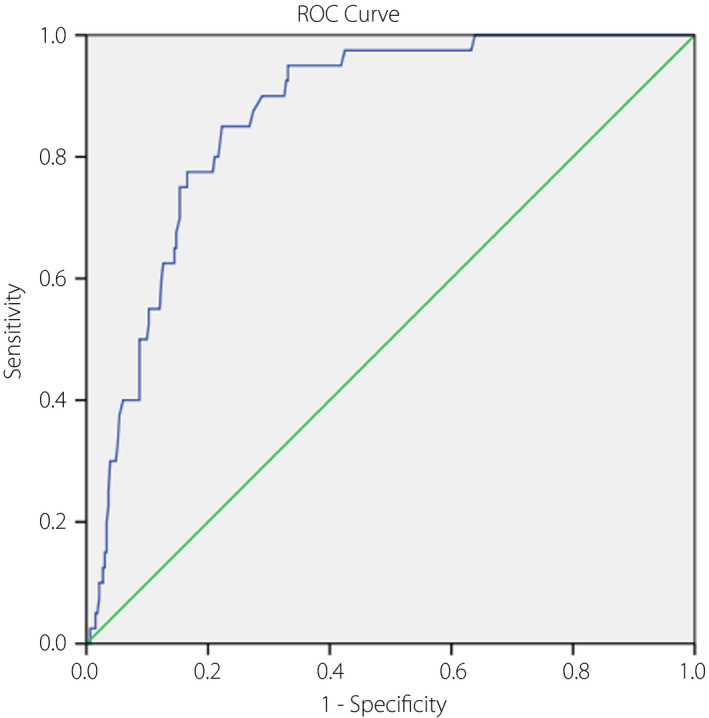
The receiver operating characteristic (ROC) curve was utilized to assess the accuracy of serum vaspin levels to diagnose vision‐threatening diabetic retinopathy.
Subgroup analysis
We carried out analyses separately among men and women. Vaspin levels in women were higher than in men (median 1.60 ng/mL, IQR 0.95–2.53 ng/mL vs median 1.40 ng/mL, IQR 0.91–1.96 ng/mL; P < 0.001). Interestingly, the predictive value of vaspin to predict DR was stronger in women than in men (OR 2.01, 95% CI 1.59–2.78 vs OR 1.77, 95% CI 1.40–2.49). Similarly, the predictive value of vaspin to predict VTDR was also stronger in women than in men (OR 3.98, 95% CI 2.21–7.02 vs OR 3.31, 95% CI 1.98–6.03).
Discussion
Adipokines influence vessel wall homeostasis by influencing endothelial cell function and modulating inflammation 28 . However, the role of vaspin in DR is still unknown. In the present study, we evaluated serum levels of vaspin in Chinese patients with type 2 diabetes mellitus and investigated the role of vaspin in DR. The results showed that: (i) higher vaspin serum levels were associated with an increased risk of DR and VTDR in type 2 diabetes mellitus patients, and the specificity was relatively low to detect DR (OR for per unit increase 1.85, 95% CI 1.43–2.55), but fair specificity/sensitivity to detect VTDR (OR 3.76, 95% CI 2.05–6.55); (ii) the predictive value of vaspin to predict DR/VTDR was stronger in women than in men; and (iii) vaspin levels were positively related to BMI and HOMA‐IR. The present study suggested that more frequent retinal examination should be highlighted for type 2 diabetes patients with the highest quartile range of vaspin.
Insulin resistance might influence the correlation between serum vaspin concentration and visceral adipose tissue 29 . In the present study, vaspin was positively associated with insulin resistance. One study found that serum vaspin concentration was significantly higher in diabetes patients than that in control participants (P = 0.020) among women 30 , and another study showed that vaspin might play an important role in the pathogenesis of type 2 diabetes mellitus 31 . However, Stepan et al. 32 did not find different circulating vaspin levels between gestational diabetes patients and control participants. In addition, circulating vaspin was not related to insulin sensitivity 19 , 33 . Thus, the association between vaspin, insulin sensitivity and diabetes is more complex, and more work should be carried out to explore it further.
The role of vaspin in vascular health had been proposed. Rashad et al. 34 showed that among Egyptian type 2 diabetes patients, serum vaspin and vaspin expression levels were significantly higher in the stroke group compared with the non‐stroke group, whereas another study 35 showed that the arterial ischemic stroke group had significantly lower vaspin levels compared with controls. One study found that serum vaspin levels correlated positively with carotid intima‐media thickness (c‐IMT) and atherosclerosis in humans 36 . Hao et al. 37 suggested that vaspin correlated positively with coronary artery disease in type 2 diabetes mellitus patients. Yang et al. 38 showed that serum vaspin levels were higher in type 2 diabetes mellitus patients without macrovascular complications than in type 2 diabetes mellitus patients with macrovascular complications (P < 0.001), whereas another study by Gulcelik et al. 39 also found that type 2 diabetes mellitus patients with macrovascular complications had low vaspin levels. It should be noted that Gulcelik et al. only evaluated 37 female type 2 diabetes patients 39 . A small sample without male patients means the data validity is worth further study. In the present study, the data showed that the serum vaspin level was positively associated with DR and VTDR. Similarly, one study reported that vaspin levels were significantly higher in patients with cardiovascular disease than in in patients without cardiovascular disease, and elevated vaspin levels were associated with a 1.7‐fold increased risk of cardiovascular disease in type 2 diabetes patients (P = 0.001) 40 . However, one study found that low vaspin levels were a risk factor for diabetic nephropathy in type 2 diabetes mellitus patients 41 , whereas another study showed that vaspin levels did not change within the early stages in patients with diabetic nephropathy 42 .
Vaspin serum concentrations were significantly higher in women compared with men 15 , which was supported by the present findings and a previous study showing sex differences in serum vaspin levels 19 . The sex differences in serum vaspin levels might be caused by the sex hormone levels in men and women. However, there was no significant difference of serum vaspin levels between men and women in another study 30 . The effect of sex on vaspin levels requires further exploration. In addition, previous studies showed that treatment with metformin could reduce vaspin serum concentrations 18 , 39 . Similarly, the present results showed that low serum levels vaspin were more likely from patients receiving metformin treatment.
Vaspin, a novel cytokine, plays role in endothelial dysfunction and inflammation 43 , 44 . Vaspin had been used for drug development for the treatment of obesity‐related complications 45 , 46 . In fact, endothelial dysfunction 47 , 48 and chronic low‐grade inflammation 48 , 49 are the core elements in the pathophysiology of diabetes and its complications, such as DR. Thus, the role of vaspin in DR might be mediated by vascular endothelial dysfunction and inflammation. We found a positive relationship between vaspin and Hs‐CRP. However, we could not test other inflammation biomarkers and endothelial dysfunction status in our participants. The direct influence of vaspin on DR through the endothelial dysfunction and inflammation in type 2 diabetes mellitus patients cannot be confirmed. Future research needs to prove this hypothesis.
Some study limitations could not be ignored. First, the present study was limited by its cross‐sectional design. The relationship of causality between vaspin and DR could not be confirmed. Second, estrogen might influence the sex differences in vaspin concentrations 18 . However, we did not test the sex hormone levels. Third, the association between vaspin gene variants (rs2236242) with obesity and type 2 diabetes mellitus has been proposed 50 . However, we did not obtain vaspin gene variants, and the association between vaspin gene variants with vaspin serum levels and DR could not be assessed. Finally, some potential confounding factors, such as dietary intake and outdoor physical activity, might influence vaspin serum levels 20 . However, that information was not collected in the present study.
In summary, higher vaspin serum levels were associated with an increased risk of DR and VTDR in type 2 diabetes mellitus patients, which showed that vaspin is an important indicator factor for DR, especially for VTDR. Further investigations are warranted to clarify this preliminary result.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We express our gratitude to all the patients who participated in this study, and thereby made this work possible. This work was supported by National Natural Science Foundation of China (81200718).
J Diabetes Investig. 2021
References
- 1. Lovic D, Piperidou A, Zografou I, et al. The growing epidemic of diabetes mellitus. Current vascular 2020; 18: 104–109. [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017; 317: 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong TY, Sabanayagam C. Strategies to tackle the global burden of diabetic retinopathy: from epidemiology to artificial intelligence. Ophthalmologica 2020; 243: 9–20. [DOI] [PubMed] [Google Scholar]
- 4. Gulcelik NE, Usman A, Gürlek A. Role of adipocytokines in predicting the development of diabetes and its late complications. Endocrine 2009; 36: 397–403. [DOI] [PubMed] [Google Scholar]
- 5. Rodríguez AJ, dos Santos Nunes V, Mastronardi CA, et al. Association between circulating adipocytokine concentrations and microvascular complications in patients with type 2 diabetes mellitus: A systematic review and meta‐analysis of controlled cross‐sectional studies. J Diabetes Complications 2016; 30: 357–367. [DOI] [PubMed] [Google Scholar]
- 6. Yilmaz MI, Sonmez A, Acikel C, et al. Adiponectin may play a part in the pathogenesis of diabetic retinopathy. Eur J Endocrinol 2004; 151: 135–140. [DOI] [PubMed] [Google Scholar]
- 7. Uckaya G, Ozata M, Bayraktar Z, et al. Is leptin associated with diabetic retinopathy? Diabetes Care 2000; 23: 371–376. [DOI] [PubMed] [Google Scholar]
- 8. López‐Bermejo A, Chico‐Julià B, Fernàndez‐Balsells M, et al. Serum visfatin increases with progressive β‐cell deterioration. Diabetes 2006; 55: 2871–2875. [DOI] [PubMed] [Google Scholar]
- 9. Yasir M, Senthilkumar GP, Jayashree K, et al. Association of serum omentin‐1, apelin and chemerin concentrations with the presence and severity of diabetic retinopathy in type 2 diabetes mellitus patients. Arch Physiol Biochem 2019; 1–8. [DOI] [PubMed] [Google Scholar]
- 10. Jung CH, Kim BY, Mok JO, et al. Association between serum adipocytokine levels and microangiopathies in patients with type 2 diabetes mellitus[J]. J Diabet Invest 2014; 5: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Escoté X, Gómez‐Zorita S, López‐Yoldi M, et al. Role of omentin, vaspin, cardiotrophin‐1, TWEAK and NOV/CCN3 in obesity and diabetes development. Int J Mol Sci 2017; 18: 1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dimova R, Tankova T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. Biomed Res Int 2015; 2015: 823481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kloting N, Berndt J, Kralisch S, et al. Vaspin gene expression in human adipose tissue: association with obesity and type 2 diabetes. Biochem Biophys Res Commun 2006; 339: 430–436. [DOI] [PubMed] [Google Scholar]
- 14. Teshigawara S, Wada J, Hida K, et al. Serum vaspin concentrations are closely related to insulin resistance, and rs77060950 at SERPINA12 genetically defines distinct group with higher serum levels in Japanese population. J Clin Endocrinol Metab 2012; 97: E1202–E1207. [DOI] [PubMed] [Google Scholar]
- 15. Youn BS, Kloting N, Kratzsch J, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008; 57: 372–377. [DOI] [PubMed] [Google Scholar]
- 16. Feng R, Li Y, Wang C, et al. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: a meta‐analysis. Diabetes Res Clin Pract 2014; 106: 88–94. [DOI] [PubMed] [Google Scholar]
- 17. Jian W, Peng W, Xiao S, et al. Role of serum vaspin in progression of type 2 diabetes: a 2‐year cohort study. PLoS One 2014; 9: e94763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan BK, Heutling D, Chen J, et al. Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes 2008; 57: 1501–1507. [DOI] [PubMed] [Google Scholar]
- 19. Seeger J, Ziegelmeier M, Bachmann A, et al. Serum levels of the adipokine vaspin in relation to metabolic and renal parameters. J Clin Endocrinol Metab 2008; 93: 247–251. [DOI] [PubMed] [Google Scholar]
- 20. Blüher M. Vaspin in obesity and diabetes: pathophysiological and clinical significance. Endocrine 2012; 41: 176–182. [DOI] [PubMed] [Google Scholar]
- 21. Yamawaki H. Vascular effects of novel adipocytokines: focus on vascular contractility and inflammatory responses. Biol Pharm Bulletin 2011; 34: 307–310. [DOI] [PubMed] [Google Scholar]
- 22. Kameshima S, Sakamoto Y, Okada M, et al. Vaspin prevents elevation of blood pressure through inhibition of peripheral vascular remodelling in spontaneously hypertensive rats. Acta Physiol 2016; 217: 120–129. [DOI] [PubMed] [Google Scholar]
- 23. Choi SH, Kwak SH, Lee Y, et al. Plasma vaspin concentrations are elevated in metabolic syndrome in men and are correlated with coronary atherosclerosis in women. Clin Endocrinol 2011; 75: 628–635. [DOI] [PubMed] [Google Scholar]
- 24. Department of Noncommunicable Disease Surveillance . Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1. Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization, 1999. (Accessed February 26, 2010, at http://www.staff.ncl.ac.uk/philip.home/who_dmg.pdf. opens in new tab.). [Google Scholar]
- 25. Early Treatment Diabetic Retinopathy Study Research Group . Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10: Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98(suppl): 786–806. [PubMed] [Google Scholar]
- 26. Zhao Q, Wu XX, Zhou J, et al. Elevated plasma levels of copeptin linked to diabetic retinopathy in type 2 diabetes. Mol Cell Endocrinol 2017; 442: 106–112. [DOI] [PubMed] [Google Scholar]
- 27. Hanley AJ, Williams K, Stern MP, et al. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 2002; 25: 1177–1184. [DOI] [PubMed] [Google Scholar]
- 28. Aust G, Richter O, Rohm S, et al. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis 2009; 204: 262–266. [DOI] [PubMed] [Google Scholar]
- 29. Chang HM, Park HS, Park CY, et al. Association between serum vaspin concentrations and visceral adipose tissue in Korean subjects. Metabolism 2010; 59: 1276–1281. [DOI] [PubMed] [Google Scholar]
- 30. Yin YE, Hou XH, Pan XP, et al. Serum vaspin level in relation to postprandial plasma glucose concentration in subjects with diabetes. Chin Med J 2009; 122: 2530–2533. [PubMed] [Google Scholar]
- 31. El‐Mesallamy HO, Kassem DH, El‐Demerdash E, et al. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism 2011; 60: 63–70. [DOI] [PubMed] [Google Scholar]
- 32. Stepan H, Kralisch S, Klostermann K, et al. Preliminary report: circulating levels of the adipokine vaspin in gestational diabetes mellitus and preeclampsia. Metabolism 2010; 59: 1054–1056. [DOI] [PubMed] [Google Scholar]
- 33. Akbarzadeh S, Nabipour I, Jafari SM, et al. Serum visfatin and vaspin levels in normoglycemic first‐degree relatives of Iranian patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2012; 95(1): 132–138. [DOI] [PubMed] [Google Scholar]
- 34. Rashad NM, Ahmed HS, Ashour WMR, et al. Association of vaspin gene expression and its serum level on the risk of ischemic stroke in type 2 diabetic Egyptian patients: Prospective case‐control study. Biotechnol Appl Biochem 2019. 10.1002/bab.1850 [DOI] [PubMed] [Google Scholar]
- 35. Kadoglou NP, Fotiadis G, Lambadiari V, et al. Serum levels of novel adipokines in patients with acute ischemic stroke: potential contribution to diagnosis and prognosis. Peptides 2014; 57: 12–16. [DOI] [PubMed] [Google Scholar]
- 36. Esaki E, Adachi H, Hirai Y, et al. Serum vaspin levels are positively associated with carotid atherosclerosis in a general population. Atherosclerosis 2014; 233: 248–252. [DOI] [PubMed] [Google Scholar]
- 37. Hao F, Zhang H, Zhu J, et al. Association between vaspin level and coronary artery disease in patients with type 2 diabetes. Diabetes Res Clin Pract 2016; 113: 26–32. [DOI] [PubMed] [Google Scholar]
- 38. Yang W, Li Y, Tian T, et al. Serum vaspin concentration in elderly patients with type 2 diabetes mellitus and macrovascular complications. BMC End Dis 2017; 17: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gulcelik NE, Karakaya J, Gedik A, et al. Serum vaspin levels in type 2 diabetic women in relation to microvascular complications. Eur J Endocrinol 2009; 160: 65–70. [DOI] [PubMed] [Google Scholar]
- 40. El‐Lebedy DH, Ibrahim AA, Ashmawy IO. Novel adipokines vaspin and irisin as risk biomarkers for cardiovascular diseases in type 2 diabetes mellitus. Diabetes Metab Syndr 2018; 12: 643–648. [DOI] [PubMed] [Google Scholar]
- 41. Mihanfar A, Rahmati‐Yamchi M, Mota A, et al. Serum levels of vaspin and its correlation with nitric oxide in type 2 diabetic patients with nephropathy. Curr Diabetes Rev 2018; 14: 162–167. [DOI] [PubMed] [Google Scholar]
- 42. Karadag S, Sakci E, Uzun S, et al. The correlation of inflammatory markers and plasma vaspin levels in patients with diabetic nephropathy. Ren Fail 2016; 38: 1044–1049. [DOI] [PubMed] [Google Scholar]
- 43. Wang HH, Wang QF. Low vaspin levels are related to endothelial dysfunction in patients with ankylosing spondylitis[J]. Braz J Med Biol Res 2016; 49: e5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun N, Wang H, Wang L. Vaspin alleviates dysfunction of endothelial progenitor cells induced by high glucose via PI3K/Akt/eNOS pathway. Int J Clin Exp Pathol 2015; 8: 482–489. [PMC free article] [PubMed] [Google Scholar]
- 45. Yin C, Hu W, Wang M, et al. The role of the adipocytokines vaspin and visfatin in vascular endothelial function and insulin resistance in obese children. BMC Endo Dis 2019; 19: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heiker JT. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation. J Pept Sci 2014; 20: 299–306. [DOI] [PubMed] [Google Scholar]
- 47. Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 2001; 88: e14–e22. [DOI] [PubMed] [Google Scholar]
- 48. Stehouwer CD, Gall MA, Twisk JW, et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low‐grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 2002; 51: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 49. Kolb H, Mandrup‐Poulsen T. The global diabetes epidemic as a consequence of lifestyle‐induced low‐grade inflammation. Diabetologia 2010; 53: 10–20. [DOI] [PubMed] [Google Scholar]
- 50. Li J, Li Q, Zhu YC, et al. Association of vaspin rs2236242 gene variants with type 2 diabetes and obesity in a Chinese population: A prospective, single‐center study. J Cell Physiol 2019; 234: 16097–16101. [DOI] [PubMed] [Google Scholar]


