Abstract
Aims/Introduction
This study determined the prevalence and risk factors for diabetic peripheral neuropathy (DPN) and painful DPN (pDPN) in patients with type 2 diabetes in primary healthcare (PHC) and secondary healthcare (SHC) in Qatar.
Materials and Methods
This was a cross‐sectional multicenter study. Adults with type 2 diabetes were randomly enrolled from four PHC centers and two diabetes centers in SHC in Qatar. Participants underwent assessment of clinical and metabolic parameters, DPN and pDPN.
Results
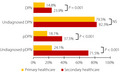
A total of 1,386 individuals with type 2 diabetes (297 from PHC and 1,089 from SHC) were recruited. The prevalence of DPN (14.8% vs 23.9%, P = 0.001) and pDPN (18.1% vs 37.5%, P < 0.0001) was significantly lower in PHC compared with SHC, whereas those with DPN at high risk for diabetic foot ulceration (31.8% vs 40.0%, P = 0.3) was comparable. The prevalence of undiagnosed DPN (79.5% vs 82.3%, P = 0.66) was comparably high, but undiagnosed pDPN (24.1% vs 71.5%, P < 0.0001) was lower in PHC compared with SHC. The odds of DPN and pDPN increased with age and diabetes duration, and DPN increased with poor glycemic control, hyperlipidemia and hypertension, whereas pDPN increased with obesity and reduced physical activity.
Conclusions
The prevalence of DPN and pDPN in type 2 diabetes is lower in PHC compared with SHC, and is attributed to overall better control of risk factors and referral bias due to patients with poorly managed complications being referred to SHC. However, approximately 80% of patients had not been previously diagnosed with DPN in PHC and SHC. Furthermore, we identified a number of modifiable risk factors for PDN and pDPN.
Keywords: Diabetes neuropathy, Diagnosis, Painful neuropathy
The prevalence of diabetic neuropathy and painful diabetic neuropathy is lower in primary healthcare, and is attributed to better control of risk factors for diabetic peripheral neuropathy. However, approximately 80% of patients with diabetic neuropathy were undiagnosed in both primary and secondary healthcare. Diabetic neuropathy is associated with poor glycemic control, hyperlipidemia and hypertension, whereas painful neuropathy is associated with obesity and reduced physical activity.
Introduction
Diabetic peripheral neuropathy (DPN) is the most common complication of diabetes and yet often remains undiagnosed 1 . Late diagnosis can lead to significant morbidity in the form of painful DPN (pDPN) 2 , erectile dysfunction 3 , diabetic foot ulceration (DFU) 4 and amputation 5 , as well as increased mortality 6 .
Early diagnosis and management of DPN might limit or reduce disease progression 1 . However, screening for DPN and pDPN is inadequate 7 , 8 , 9 . The prevalence of DPN and pDPN have been shown to range from 2.4 to 24.1% 10 , 11 and 16 to 19% 12 , 13 in primary care, and 32.1 14 and 21.0% 15 in secondary care in patients with type 2 diabetes, respectively. This wide range has been attributed to differing populations and methods used to identify DPN and pDPN. We have recently reported that approximately 80% of patients with DPN 9 and pDPN 2 have not previously been diagnosed in hospital clinics in secondary health care (SHC) in Qatar, which might lead to late presentation with DFU. Indeed, in Qatar it has been reported that 25% of patients with diabetes in SHC have foot problems 16 . This has serious consequences given that one in four patients with DFU is at risk of amputation 5 , and the 5‐year mortality of people with a DFU is higher than many common cancers 17 . Currently, the American Diabetes Association recommends annual screening of DPN at diagnosis of type 2 diabetes and 5 years after the diagnosis of type 1 diabetes by neurological examination or monofilament testing, but there is no specific recommendation for pDPN 1 .
There are currently no US Food and Drug Administration (FDA) approved therapies for DPN 6 . Lifestyle interventions, including physical activity 18 and avoidance of smoking 19 , 20 are advised, and optimization of glycemic control 19 , 21 , treatment of hypertension 22 , 23 and hyperlipidemia 20 , 24 might improve DPN. FDA approved medications for treating painful symptoms include duloxetine, pregabalin and tapentadol 25 .
According to the International Diabetes Federation, the prevalence of diabetes in adults aged 20–79 years in Qatar was 15.5% in 2020 26 , which is almost twofold greater than the 2019 reported prevalence of 8.3% in the rest of the world 27 . Given the high prevalence of diabetes, in 2015 Qatar launched the National Diabetes Strategy to improve the management of people with diabetes and its complications by establishing common clinical care pathways within and between primary healthcare (PHC) and SHC. We have, therefore, applied the same methods and diagnostic criteria in patients with type 2 diabetes to establish the prevalence and risk factors for DPN and pDPN in PHC and SHC. We believe the findings of the present study will be key to planning strategies to enable earlier diagnosis and optimal management of the often forgotten complication of diabetic neuropathy in Qatar and the region.
Methods
This was a cross‐sectional, multicenter study. Individuals aged 18–85 years with type 2 diabetes mellitus were enrolled from four PHC centers (Umm Ghuwailina, Al Khor, Al Daayen and Al Rayyan) and the only two National Diabetes centers in Qatar (Hamad General Hospital and Al‐Wakra Hospital). Participants were randomly enrolled and screened for eligibility on the day they attended the clinic for their diabetes review between June 2017 and February 2019. Exclusion criteria included type 1 diabetes and other causes of neuropathy, including severe vitamin B12 deficiency, chronic hypothyroidism and chemotherapy.
The present study was approved by the institutional review board of Weill Cornell Medicine‐Qatar and Hamad Medical Corporation. All participants gave informed consent to take part in the study. The research adhered to the tenets of the declaration of Helsinki.
Demographic and metabolic measures
Sex, ethnicity, age, duration of diabetes and body mass index were recorded. The average systolic and diastolic blood pressure of two readings were obtained from the participant’s left arm while seated with the arm at heart level, using a standard zero mercury sphygmomanometer after 10–15 min of rest. A non‐fasting blood sample was collected through venepuncture from each participant into ethylenediaminetetraacetic acid tubes and transported within 2 h to a central certified laboratory at Hamad General Hospital. Glycated hemoglobin, total cholesterol and triglyceride were measured by an autoanalyzer (Hitachi 747 autoanalyzer; Tokyo, Japan). Poor glycemic control was defined as glycated hemoglobin ≥9%. Hypertension was defined according to either an average systolic blood pressure ≥140 mmHg and/or the use of antihypertensive medication, as described in the World Health Organization/International Society of Hypertension guidelines 28 . Hyperlipidemia was defined according to a total cholesterol level ≥6.2 mmol/L and/or triglyceride level of ≥2.3 mmol/L or if the patient was treated with a statin. Obesity was classified according to World Health Organization criteria with a body mass index ≥30 kg/m2 29 . Current cigarette smoking was defined as having smoked at least one cigarette every day for ≥1 year preceding the study visit. Physical activity was defined as doing some physical activity, including walking for ≥30 min/day, at least three times a week over the past year.
Assessment of diabetic neuropathy and painful neuropathy
The diagnosis of DPN was based on the presence of one or more neuropathic symptoms and impaired vibration perception threshold (VPT) in the feet. Subjective neurological symptoms, such as burning pain, painful cold, electric shocks, tingling, pins and needles, and numbness, were acquired through a face‐to‐face interview with the investigators. VPT was measured by a Horwell neurothesiometer (Scientific Laboratory Supplies, Nottingham, UK) on the pulp of the large toe on both feet, and the average value of three measurements was recorded in volts (V) ranging from 0 to 50 V. A VPT ≥15 V was defined as impaired vibration perception consistent with the presence of DPN 30 and a VPT ≥25 V as high risk for DFU 31 .
Painful DPN was assessed using the Douleur Neuropathique en 4 (DN4) questionnaire in Arabic and English, as previously described 32 . The DN4 questionnaire has been validated for its ability to distinguish neuropathic pain from non‐neuropathic pain 33 and in the Arabic version 34 , and for pDPN 32 . It consists of 10 questions: seven questions relating to the pain description (burning, painful cold, electric shocks) and associated abnormal sensations (tingling, pins and needles, numbness, itching), and the other three neurological examination outcomes in the painful area for hypoesthesia to touch and pin prick using disposable examination pins and allodynia to brushing. The scoring is based on a yes (1 point) or no (0 point) answer, and each question is equally weighted. A score ≥4 has a high sensitivity (80%) and specificity (92%) for pDPN 32 . The questionnaire was administered by the investigator in either English or Arabic. Previously diagnosed pDPN was self‐reported. Medications for pDPN were recorded.
All investigators underwent a formal training session on the use and interpretation of the neurothesiometer and DN4 questionnaire.
Statistical analysis
The prevalence of DPN and pDPN across different demographics and risk factors as categorical variables were summarized using frequency distributions. Variables in patients with DPN or pDPN were compared between PHC and SHC using the χ2‐test.
Binary logistic regression analysis was carried out with age, duration of diabetes, sex, poor glycemic control, obesity, hyperlipidemia, hypertension, physical activity, smoking, ethnicity and healthcare as independent variables, and DPN or pDPN as the dependent variable. The multiple logistic regression model included all variables with a P‐value of ≤0.05 at the bivariate level. Adjusted odds ratios, their corresponding 95% confidence intervals and P‐value are presented.
All analyses were carried out using IBM SPSS (version 26; SPSS Inc., Armonk, NY, USA). A two‐tailed P‐value of ≤0.05 was considered significant.
Results
Prevalence of DPN and pDPN in PHC compared with SHC
A total of 1,386 individuals with type 2 diabetes were recruited from PHC (n = 297) and SHC (n = 1,089; Figure 1; Table 1). The prevalence of DPN (14.8% vs 23.9%, P = 0.001) was significantly lower in PHC compared with SHC. The percentage of patients undiagnosed with DPN was comparable (79.5% vs 82.3%, P = 0.66) between PHC and SHC. The prevalence of pDPN (18.1% vs 37.5%, P < 0.0001) and percentage of patients undiagnosed with pDPN (24.1% vs 71.5%, P < 0.0001) was significantly lower in PHC compared with SHC. The mean VPT (10.4 ± 7.2 V vs 12.5 ± 9.4 V, P < 0.0001), DN4 score (1.0 ± 1.6 vs 2.5 ± 2.6, P < 0.0001) and percentage of patients with all neuropathic symptoms including burning, painful cold, electric shocks, tingling, pins and needles, numbness, and itching were significantly lower in PHC compared with SHC (Table 2). Although no patients in PHC had DFU, 6.2% had DFU in SHC. However, the prevalence of those at high risk for DFU was comparable (31.8% vs 40.0%, P = 0.3) between PHC and SHC.
Figure 1.

Prevalence of diabetic peripheral neuropathy (DPN), painful diabetic neuropathy (pDPN), undiagnosed DPN and pDPN in type 2 diabetes in primary and secondary healthcare. NS, not significant.
Table 1.
Prevalence of diabetic peripheral neuropathy, painful diabetic neuropathy, undiagnosed diabetic peripheral neuropathy and painful diabetic neuropathy, and those at high risk of diabetic foot ulceration and their risk factors in type 2 diabetes in primary and secondary healthcare
| Primary health care | Secondary health care |
P‐value PHC vs SHC |
|||
|---|---|---|---|---|---|
| Diabetic peripheral neuropathy | 44/297 | 14.8% | 260/1,089 | 23.9% | 0.001 |
| High risk for diabetic foot ulceration | 14/44 | 31.8% | 104/260 | 40.0% | 0.30 |
| Diabetic foot ulcer | 0/44 | 0.0% | 16/260 | 6.2% | 0.13 |
| Painful diabetic neuropathy | 54/298 | 18.1% | 410/1,092 | 37.5% | <0.0001 |
| Undiagnosed cases | |||||
| Undiagnosed diabetic peripheral neuropathy | 35/44 | 79.5% | 214/260 | 82.3% | 0.66 |
| Undiagnosed painful diabetic neuropathy | 13/54 | 24.1% | 293/410 | 71.5% | <0.0001 |
| Risk factors | |||||
| Age | |||||
| 20–50 years | 88/295 | 29.8%† | 445/1,073 | 41.5%‡ | 0.001 |
| 51–60 years | 117/295 | 39.7%† | 379/1,073 | 35.3%† | |
| >60 years | 90/295 | 30.5%† | 249/1,073 | 23.2%‡ | |
| Duration of diabetes | |||||
| ≤10 years | 204/296 | 68.9%† | 690/1,080 | 63.9%† | 0.26 |
| 11–20 years | 73/296 | 24.7%† | 303/1,080 | 28.1%† | |
| >20 years | 19/296 | 6.4%† | 87/1,080 | 8.1%† | |
| Lifestyle modifiable risk factors | |||||
| Physical activity | 158/275 | 57.5%† | 326/854 | 38.2%‡ | <0.0001 |
| Smoking | 27/274 | 9.9% | 157/909 | 17.3% | 0.003 |
| Cardiovascular modifiable risk factors | |||||
| Poor glycemic control | 98/266 | 36.8%† | 436/991 | 44.0%‡ | 0.04 |
| Hyperlipidemia | 208/259 | 80.3%† | 738/1,008 | 73.2%‡ | 0.02 |
| Hypertension | 176/274 | 64.2%† | 669/1,040 | 64.3%† | 0.98 |
| Obesity | 87/213 | 40.8%† | 510/957 | 53.3%‡ | 0.001 |
| Systolic blood pressure (mmHg) | 131.7 ± 15.5 | 132.5 ± 18.0 | 0.42 | ||
| Diastolic blood pressure (mmHg) | 78.8 ± 8.0 | 78.2 ± 10.2 | 0.32 | ||
| BMI (kg/m2) | 29.8 ± 5.4 | 31.5 ± 7.4 | 0.0003 | ||
| HbA1c (mmol/mol) | 63.1 ± 19.7 | 65.5 ± 21.9 | 0.08 | ||
| HbA1c (%) | 7.9 ± 1.8 | 8.1 ± 2.0 | 0.08 | ||
| Cholesterol (mmol/L) | 3.9 ± 1.0 | 4.4 ± 1.2 | <0.0001 | ||
| Triglyceride (mmol/L) | 1.8 ± 1.0 | 1.8 ± 1.2 | 0.96 | ||
Variables were summarized using means and standard deviations for numeric variables, and frequency distribution for categorical variables. Continues and categorical variables were compared using unpaired t‐test and χ2‐test, respectively.†,‡Rows with the same symbols are not statistically significant, and different symbols are significantly different. BMI, body mass index; HbA1c, glycated hemoglobin; PHC, primary healthcare; SHC, secondary healthcare.
Table 2.
Mean vibration perception threshold, Douleur Neuropathique en 4 questionnaire score, and percentage of neuropathic symptoms in type 2 diabetes in primary and secondary healthcare
| PHC | SHC |
P‐value PHC vs SHC |
|
|---|---|---|---|
| Vibration perception threshold (V) | 10.4 ± 7.2 | 12.5 ± 9.4 | <0.0001 |
| DN4 score | 1.0 ± 1.6 | 2.5 ± 2.6 | <0.0001 |
| Burning pain (%) | 22.6 | 46.7 | <0.0001 |
| Painful cold (%) | 7.7 | 26.5 | <0.0001 |
| Electric shocks (%) | 5.1 | 22.3 | <0.0001 |
| Tingling (%) | 20.2 | 32.0 | <0.0001 |
| Pins and needles (%) | 16.2 | 35.1 | <0.0001 |
| Numbness (%) | 12.5 | 42.0 | <0.0001 |
| Itching (%) | 7.8 | 16.7 | <0.0001 |
Variables were summarized using means and standard deviations for numeric variables and frequency distribution for categorical variables. Continues and categorical variables were compared using unpaired t‐test and x 2, respectively. DN4, Douleur Neuropathique en 4 questionnaire; PHC, primary healthcare; SHC, secondary healthcare.
Risk factor management in PHC compared with SHC
More patients with type 2 diabetes aged >60 years (30.5% vs 23.2%, P = 0.001) and fewer patients aged 20–50 years (29.8% vs 41.5%, P = 0.001) were under the care of PHC compared with SHC. The body mass index (29.8 kg/m2 vs 31.5 kg/m2, P = 0.0003) and percentage of patients with obesity (40.8% vs 53.3%, P = 0.001) was significantly lower in PHC compared with SHC. Glycated hemoglobin was comparable, but the percentage of patients with poor glycemic control (36.8% vs 44.0%, P = 0.04) was lower in PHC compared with SHC. In PHC, the total cholesterol (3.9 mmol/L vs 4.4 mmol/L, P < 0.0001) was lower and triglycerides were comparable compared with SHC. However, hyperlipidemia was present in a significantly lower percentage of patients in SHC compared with PHC (73.2% vs 80.3%, P = 0.02). The systolic and diastolic blood pressure, and percentage of patients with hypertension was comparable between PHC and SHC. More patients undertook physical activity (57.5% vs 38.2%, P < 0.0001), and fewer patients smoked cigarettes (9.9% vs 17.3%, P = 0.003) in PHC compared with SHC.
Association of risk factors for DPN in PHC and SHC
The odds of developing DPN increased by 2.4‐fold in patients aged 51–60 years (P < 0.0001), and 2.9‐fold in those aged >60 years compared with patients aged 20–50 years (P < 0.0001; Table 3). The odds increased 2.2‐fold with 11–20 years of diabetes (P < 0.0001), to 3.9‐fold with >20 years of diabetes (P < 0.0001) compared with ≤10 years of diabetes. The odds for DPN was 1.4‐fold greater in men (P = 0.02). The odds increased 1.5‐fold in those with poor glycemic control (P = 0.02), and 1.6‐fold in patients treated with insulin and other antidiabetic therapy compared with patients treated with metformin and other antidiabetic therapy (P = 0.006). The odds increased 1.8‐fold in those with hyperlipidemia (P = 0.006), and 1.5‐fold in those with hypertension (P = 0.05). The association with obesity and ethnicity for DPN was lost after controlling for risk factors. However, even after adjusting for all risk factors, the odds of developing DPN in SHC remained 2.1‐fold higher than in PHC (P = 0.001).
Table 3.
Predictors for diabetic peripheral neuropathy in primary and secondary healthcare
| Diabetic peripheral neuropathy | AOR | 95% CI | P‐value |
|---|---|---|---|
| Sex | |||
| Male | 1 | ||
| Female | 0.7 | 0.5–0.9 | 0.02 |
| Ethnic groups | |||
| Arabs | 1 | ||
| South Asians | 0.8 | 0.5–1.1 | 0.19 |
| Age | |||
| 20–50 years | 1 | ||
| 51–60 years | 2.4 | 1.6–3.5 | <0.0001 |
| >60 years | 2.9 | 1.9–4.5 | <0.0001 |
| Duration of diabetes | |||
| ≤10 years | 1 | ||
| 11–20 years | 2.2 | 1.6–3.0 | <0.0001 |
| >20 years | 3.9 | 2.4–6.4 | <0.0001 |
| Poor glycemic control | 1.5 | 1.1–2.0 | 0.02 |
| Hyperlipidemia | 1.8 | 1.2–2.8 | 0.006 |
| Hypertension | 1.5 | 1.0–2.2 | 0.05 |
| Obesity | 1.3 | 0.9–1.8 | 0.20 |
| Antidiabetic therapy | |||
| Metformin/plus | 1 | ||
| Insulin/plus | 1.6 | 1.2–2.3 | 0.006 |
| Primary healthcare | 1 | ||
| Secondary healthcare | 2.1 | 1.4–3.2 | 0.001 |
The multiple logistic regression model included all variables with P‐values of ≤0.05 at the bivariate level. Adjusted odds ratios (AOR), their corresponding 95% confidence intervals (CI) and P‐value are presented.
Association of risk factors for pDPN in PHC and SHC
The odds of developing pDPN was 1.5‐fold greater in patients aged >50 years (P = 0.02) compared with those aged 20–50 years (P < 0.0001; Table 4). The odds increased from 2.2‐fold with 11–20 years of diabetes (P < 0.0001) to 4.4‐fold with >20 years of diabetes (P < 0.0001) compared with ≤10 years of diabetes. The odds also increased by 1.7‐fold in participants treated with insulin/plus other antidiabetic therapy compared with those treated with metformin/plus other antidiabetic therapy (P < 0.0001). The odds increased 1.6‐fold with obesity (P = 0.002), and 1.4‐fold in Arab participants compared with South Asian participants (P = 0.03). However, the odds decreased by 1.7‐fold with physical activity (P = 0.01). The association of poor glycemic control, hyperlipidemia, hypertension and sex with pDPN was lost after controlling for these risk factors. However, even after adjusting for all risk factors, the odds of developing pDPN in SHC was 2.4‐fold higher than in PHC (P < 0.0001).
Table 4.
Predictors for diabetic painful neuropathy in primary and secondary healthcare
| Painful neuropathy | AOR | 95% CI | P‐value |
|---|---|---|---|
| Sex | |||
| Male | 1 | ||
| Female | 1.2 | 0.9–1.6 | 0.32 |
| Ethnic groups | |||
| Arabs | 1 | ||
| South Asians | 0.7 | 0.5–1.0 | 0.03 |
| Age | |||
| 20–50 years | 1 | ||
| 51–60 years | 1.5 | 1.1–2.0 | 0.02 |
| >60 years | 1.5 | 1.1–2.2 | 0.02 |
| Duration of diabetes | |||
| ≤10 years | 1 | ||
| 11–20 years | 2.2 | 1.6–3.0 | <0.0001 |
| >20 years | 4.4 | 2.7–7.1 | <0.0001 |
| Poor glycemic control | 1.2 | 0.9–1.6 | 0.2 |
| Hyperlipidemia | 1.1 | 0.8–1.5 | 0.58 |
| Hypertension | 1.3 | 0.9–1.8 | 0.13 |
| Obesity | 1.6 | 1.2–2.2 | 0.002 |
| Physical activity | 0.6 | 0.4–0.9 | 0.01 |
| Antidiabetic therapy | |||
| Metformin/plus other therapy | 1 | ||
| Insulin/plus | 1.7 | 1.3–2.4 | <0.0001 |
| Primary healthcare | 1 | ||
| Secondary healthcare | 2.4 | 1.6–3.5 | <0.0001 |
The multiple logistic regression model included all variables with P‐value of ≤0.05 at the bivariate level. Adjusted odds ratios (AOR), their corresponding 95% confidence intervals (CI) and P‐value are presented.
Discussion
The present study shows that the prevalence of DPN is 1.6‐fold lower and pDPN is twofold lower in PHC compared with SHC. Furthermore, the percentage of patients with undiagnosed DPN (~80%) and those at risk of DFU (32–40%) was extremely high, and comparable between PHC and SHC, despite the introduction of national diabetes care pathways. DPN was associated with poor glycemic control, hyperlipidemia and hypertension, whereas pDPN was associated with obesity, and was lower in patients undertaking physical activity at least 3 days per week. The higher prevalence of DPN and pDPN in SHC remained significant, even after controlling for risk factors. This might partly be attributed to referral bias, with more patients with poorer control of risk factors and diabetic complication being referred to SHC.
The DN4 questionnaire was chosen to define pDPN for three reasons: (i) its diagnostic ability to distinguish neuropathic pain from non‐neuropathic pain, including osteoarthritis, inflammation and mechanical low back pain (common differentials, especially in PHC) for which it has been validated with 86% sensitivity and 83% specificity 33 ; (ii) its diagnostic ability specifically for pDPN with 80% sensitivity and 92% specificity 32 , and (iii) validation using the Arabic version showing 88% sensitivity and 75% specificity 34 . The higher prevalence of pDPN compared with DPN might be attributed to the criteria used to define these conditions. pDPN was defined according to DN4, whereas DPN was based on symptoms and an elevated VPT (>15 V).
There are currently no FDA approved therapies for DPN 6 . However, screening annually for symptoms and signs of DPN starting at diagnosis of type 2 diabetes is advocated on the basis that early management of risk factors for DPN might reduce the rate of disease progression, and treatment to relieve neuropathic symptoms might improve the patient’s quality of life 1 . We have recently assessed the prevalence of DPN and pDPN in SHC in Qatar 2 , 9 . This is the first study to compare the prevalence of DPN and pDPN in PHC and SHC using the same criteria and in the same population. The prevalence of DPN in both PHC and SHC in Qatar is lower compared with the prevalence in SHC in other countries; for example, 37% in Bahrain 19 , 60% in Turkey 21 , 49% in Iran 35 , 45% in the USA 36 , 32% in the UK 14 , 31% in Italy 37 and 62% in China 38 . The prevalence of pDPN is also lower in both SHC and PHC in Qatar compared with studies from SHC, with a reported prevalence of 65% in Saudi Arabia 39 , 61% in Egypt 40 , 58% in Jordan and 54% in Lebanon.
Despite the introduction of national diabetes pathways in Qatar, the present study confirms an alarmingly high prevalence of undiagnosed DPN in PHC and SHC 2 , 9 . It highlights the considerable need to educate both patients and physicians on DPN and pDPN 41 . This might explain why up to 25% of patients with diabetes in SHC in Qatar have foot problems 16 . Currently, DPN is not assessed systematically, even using the 10‐g monofilament, which in itself identifies only those with advanced neuropathy 1 . Given that one in four patients with DFU are at risk of amputation 5 , the present study highlights the need for the National Diabetes Strategy to implement annual DPN screening in PHC and SHC. This should be carried out using evidence‐based screening tests to detect incipient small fiber damage to detect sudomotor dysfunction using Sudoscan 42 or Neuropad 43 , or vibration perception using a neurothesiometer 44 , or cold or warm perception thresholds using NerveCheck 45 ; as opposed to monofilament testing, which is convenient, but only detects advanced large fiber neuropathy. A common reason for the underdiagnosis of pDPN is that patients with symptoms are often unaware that the pain is related to DPN and do not report them to their physician 13 , 46 . Questionnaire, such as the DN4 32 , and Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale 47 , can be used to screen for neuropathic pain, and the Neuropathic Pain Scale (NPS) 48 , Neuropathic Pain Symptom Inventory (NPSI) 49 and Diabetic Peripheral Neuropathic Pain Impact measure (DPNPI) 50 can characterize subgroups of neuropathic pain patients who might benefit from specific therapies, as well as monitor the impact of treatment 51 . Reassuringly, we show a much lower prevalence of patients with undiagnosed pDPN in PHC, which might reflect a more systematic approach to identify neuropathic symptoms as part of a general screen for complications, as opposed to SHC, where there is no formal screening unless the physician refers for further assessment.
The lack of an FDA approved therapy for DPN often creates a negative attitude toward the need to diagnose early DPN 52 . However, the present study has identified a range of modifiable risk factors for DPN, including poor glycemic control 19 , 21 , hypertension 22 , 23 and hyperlipidemia 20 , 24 , and for pDPN, including obesity 40 , 53 , 54 , 55 and reduced physical activity 56 , 57 . Although, intensive glycemic control is advocated, the data for an impact on DPN in type 2 diabetes patients are limited 58 , 59 , 60 , and other cardiovascular risk factors, such as hypertension, hyperlipidemia and obesity, might play a more important role. Indeed, treatment with angiotensin converting enzyme inhibitors 22 , 23 , statins 61 , 62 or glucagon‐like peptide‐1 receptor agonists 63 , 64 , 65 might have a beneficial effect on DPN.
A limitation of the present study was the relatively small number of participants from PHC, thus limiting the generalizability of the findings. A further limitation was the cross‐sectional design of this study, which limited the predictive validity of the observed associations between the various risk factors with DPN and pDPN.
In conclusion, the present study identified a lower prevalence of DPN and pDPN in PHC compared with SHC, which might be attributed to better overall risk factor control in PHC and referral bias due to patients who are poorly managed with complications being referred to SHC. Alarmingly, an equally high proportion (~80%) of patients with DPN were undiagnosed in both PHC and SHC, highlighting the need for the National Diabetes Strategy to implement annual DPN screening. The identification of hyperglycemia, hyperlipidemia and hypertension as modifiable risk factors for DPN, and obesity and physical activity as modifiable risk factors of pDPN provide a robust argument to establish protocols for the early diagnosis and management of DPN and pDPN.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank all the participants for their efforts, will and commitment to be involved in the study. Qatar National Research Fund, Grant BMRP‐5726113101; Pfizer Gulf FZ LLC, W1230787
J Diabetes Investig. 2021
References
- 1. Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and risk factors for painful diabetic neuropathy in secondary healthcare in Qatar. J Diabetes Investig 2019; 10: 1558–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kouidrat Y, Pizzol D, Cosco T, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta‐analysis of 145 studies. Diabet Med 2017; 34: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 4. Raghav A, Khan ZA, Labala RK, et al. Financial burden of diabetic foot ulcers to world: a progressive topic to discuss always. Ther Adv Endocrinol Metab 2018; 9: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apelqvist J, Agardh CD. The association between clinical risk factors and outcome of diabetic foot ulcers. Diabetes Res Clin Pract 1992; 18: 43–53. [DOI] [PubMed] [Google Scholar]
- 6. Azmi S, Ferdousi M, Kalteniece A, et al. Diagnosing and managing diabetic somatic and autonomic neuropathy. Ther Adv Endocrinol Metab 2019; 10: 2042018819826890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care 2005; 28: 1480–1481. [DOI] [PubMed] [Google Scholar]
- 8. Wang W, Balamurugan A, Biddle J, et al. Diabetic neuropathy status and the concerns in underserved rural communities: challenges and opportunities for diabetes educators. Diabetes Educ 2011; 37: 536–548. [DOI] [PubMed] [Google Scholar]
- 9. Ponirakis G, Elhadd T, Chinnaiyan S, et al. Prevalence and management of diabetic neuropathy in secondary care in Qatar. Diabetes Metab Res Rev 2020; 36: e3286. [DOI] [PubMed] [Google Scholar]
- 10. Cabezas‐Cerrato J. The prevalence of clinical diabetic polyneuropathy in Spain: a study in primary care and hospital clinic groups. Neuropathy Spanish Study Group of the Spanish Diabetes Society (SDS). Diabetologia 1998; 41: 1263–1269. [DOI] [PubMed] [Google Scholar]
- 11. Kostev K, Jockwig A, Hallwachs A, et al. Prevalence and risk factors of neuropathy in newly diagnosed type 2 diabetes in primary care practices: a retrospective database analysis in Germany and U.K. Prim Care Diabetes 2014; 8: 250–255. [DOI] [PubMed] [Google Scholar]
- 12. Davies M, Brophy S, Williams R, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006; 29: 1518–1522. [DOI] [PubMed] [Google Scholar]
- 13. Daousi C, MacFarlane IA, Woodward A, et al. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med 2004; 21: 976–982. [DOI] [PubMed] [Google Scholar]
- 14. Young MJ, Boulton AJ, MacLeod AF, et al. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993; 36: 150–154. [DOI] [PubMed] [Google Scholar]
- 15. Abbott CA, Malik RA, van Ross ER, et al. Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabetes Care 2011; 34: 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al‐Thani AA, Farghaly A, Akram H, et al. Knowledge and perception of diabetes and available services among diabetic patients in the state of Qatar. Cent Asian J Glob Health 2019; 8: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes‐related wounds and amputations worse than cancer? Int Wound J 2007; 4: 286–287. [DOI] [PubMed] [Google Scholar]
- 18. Al‐Kaabi JM, Al‐Maskari F, Zoubeid T, et al. Prevalence and determinants of peripheral neuropathy in patients with type 2 diabetes attending a tertiary care center in the United Arab Emirates. J Diabetes Metab 2014; 5: 1–7. [Google Scholar]
- 19. Al‐Mahroos F, Al‐Roomi K. Diabetic neuropathy, foot ulceration, peripheral vascular disease and potential risk factors among patients with diabetes in Bahrain: a nationwide primary care diabetes clinic‐based study. Ann Saudi Med 2007; 27: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 341–350. [DOI] [PubMed] [Google Scholar]
- 21. Boru UT, Alp R, Sargin H, et al. Prevalence of peripheral neuropathy in type 2 diabetic patients attending a diabetes center in Turkey. Endocr J 2004; 51: 563–567. [DOI] [PubMed] [Google Scholar]
- 22. Malik RA, Williamson S, Abbott C, et al. Effect of angiotensin‐converting‐enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: randomised double‐blind controlled trial. Lancet 1998; 352: 1978–1981. [DOI] [PubMed] [Google Scholar]
- 23. Reja A, Tesfaye S, Harris ND, et al. Is ACE inhibition with lisinopril helpful in diabetic neuropathy? Diabet Med 1995; 12: 307–309. [DOI] [PubMed] [Google Scholar]
- 24. Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complications 2013; 27: 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Javed S, Petropoulos IN, Alam U, et al. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis 2015; 6: 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. IDF Middle East and North Africa Region . Prevalence of diabetes in adults in Qatar 2020. Available from: https://idf.org/our‐network/regions‐members/middle‐east‐and‐north‐africa/members/45‐qatar.html Accessed March 2020.
- 27. International Diabetes Federation . IDF DIABETES ATLAS 9th edition 2019. Available from: https://www.diabetesatlas.org/en/sections/demographic‐and‐geographic‐outline.html Accessed March 2020.
- 28. Moser M. World Health Organization‐International Society of Hypertension Guidelines for the Management of Hypertension‐Do These Differ From the U.S. Recommendations? Which guidelines should the practicing physician follow? J Clin Hypertens (Greenwich) 1999; 1: 48–54. [PubMed] [Google Scholar]
- 29. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: i–xii, 1–253. [PubMed] [Google Scholar]
- 30. Wiles PG, Pearce SM, Rice PJ, et al. Vibration perception threshold: influence of age, height, sex, and smoking, and calculation of accurate centile values. Diabet Med 1991; 8: 157–161. [DOI] [PubMed] [Google Scholar]
- 31. Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds – a prospective‐study. Diabetes Care 1994; 17: 557–560. [DOI] [PubMed] [Google Scholar]
- 32. Spallone V, Morganti R, D'Amato C, et al. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med 2012; 29: 578–585. [DOI] [PubMed] [Google Scholar]
- 33. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005; 114: 29–36. [DOI] [PubMed] [Google Scholar]
- 34. Terkawi AS, Abolkhair A, Didier B, et al. Development and validation of Arabic version of the douleur neuropathique 4 questionnaire. Saudi J Anaesth 2017; 11(Suppl 1): S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kiani J, Moghimbeigi A, Azizkhani H, et al. The prevalence and associated risk factors of peripheral diabetic neuropathy in Hamedan, Iran. Arch Iran Med 2013; 16: 17–19. [PubMed] [Google Scholar]
- 36. Mold JW, Vesely SK, Keyl BA, et al. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract 2004; 17: 309–318. [DOI] [PubMed] [Google Scholar]
- 37. Salvotelli L, Stoico V, Perrone F, et al. Prevalence of neuropathy in type 2 diabetic patients and its association with other diabetes complications: The Verona Diabetic Foot Screening Program. J Diabetes Complications 2015; 29: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 38. Lu B, Yang Z, Wang M, et al. High prevalence of diabetic neuropathy in population‐based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract 2010; 88: 289–294. [DOI] [PubMed] [Google Scholar]
- 39. Halawa MR, Karawagh A, Zeidan A, et al. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin 2010; 26: 337–343. [DOI] [PubMed] [Google Scholar]
- 40. Jambart S, Ammache Z, Haddad F, et al. Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res 2011; 39: 366–377. [DOI] [PubMed] [Google Scholar]
- 41. Malik RA, Andag‐Silva A, Dejthevaporn C, et al. Diagnosing peripheral neuropathy in South‐East Asia: a focus on diabetic neuropathy. J Diabetes Investig 2020; 11: 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Selvarajah D, Cash T, Davies J, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One 2015; 10: e0138224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ponirakis G, Petropoulos IN, Fadavi H, et al. The diagnostic accuracy of Neuropad for assessing large and small fibre diabetic neuropathy. Diabet Med 2014; 31: 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bril V, Perkins BA. Comparison of vibration perception thresholds obtained with the Neurothesiometer and the CASE IV and relationship to nerve conduction studies. Diabet Med 2002; 19: 661–666. [DOI] [PubMed] [Google Scholar]
- 45. Ponirakis G, Odriozola MN, Odriozola S, et al. NerveCheck: an inexpensive quantitative sensory testing device for patients with diabetic neuropathy. Diabetes Res Clin Pract 2016; 113: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eichholz M, Alexander AH, Cappelleri JC, et al. Perspectives on the impact of painful diabetic peripheral neuropathy in a multicultural population. Clin Diabetes Endocrinol 2017; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001; 92: 147–157. [DOI] [PubMed] [Google Scholar]
- 48. Jensen MP, Friedman M, Bonzo D, et al. The validity of the neuropathic pain scale for assessing diabetic neuropathic pain in a clinical trial. Clin J Pain 2006; 22: 97–103. [DOI] [PubMed] [Google Scholar]
- 49. Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004; 108: 248–257. [DOI] [PubMed] [Google Scholar]
- 50. Brod M, Blum SI, Bushnell DM, et al. Development and validation of the Diabetic Peripheral Neuropathic Pain Impact (DPNPI) measure, a patient‐reported outcome measure. Qual Life Res 2015; 24: 3001–3014. [DOI] [PubMed] [Google Scholar]
- 51. Attal N, Bouhassira D, Baron R. Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol 2018; 17: 456–466. [DOI] [PubMed] [Google Scholar]
- 52. Malik RA, Aldinc E, Chan SP, et al. Perceptions of painful diabetic peripheral neuropathy in South‐East Asia: results from patient and physician surveys. Adv Ther 2017; 34: 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009; 35: 206–213. [DOI] [PubMed] [Google Scholar]
- 54. Ziegler D, Landgraf R, Lobmann R, et al. Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res Clin Pract 2018; 139: 147–154. [DOI] [PubMed] [Google Scholar]
- 55. Aslam A, Singh J, Rajbhandari S. Prevalence of painful diabetic neuropathy using the self‐completed leeds assessment of neuropathic symptoms and signs questionnaire in a population with diabetes. Can J Diabetes 2015; 39: 285–295. [DOI] [PubMed] [Google Scholar]
- 56. Ziegler D, Rathmann W, Meisinger C, et al. Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre‐diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain 2009; 13: 582–587. [DOI] [PubMed] [Google Scholar]
- 57. Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre‐diabetic neuropathy. Diabetes Care 2006; 29: 1294–1299. [DOI] [PubMed] [Google Scholar]
- 58. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 59. Ismail‐Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pop‐Busui R, Lu J, Brooks MM, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care 2013; 36: 3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davis TM, Yeap BB, Davis WA, et al. Lipid‐lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia 2008; 51: 562–566. [DOI] [PubMed] [Google Scholar]
- 62. Villegas‐Rivera G, Roman‐Pintos LM, Cardona‐Munoz EG, et al. Effects of Ezetimibe/Simvastatin and Rosuvastatin on oxidative stress in diabetic neuropathy: a randomized, double‐blind, placebo‐controlled clinical trial. Oxid Med Cell Longev 2015; 2015: 756294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kan M, Guo G, Singh B, et al. Glucagon‐like peptide 1, insulin, sensory neurons, and diabetic neuropathy. J Neuropathol Exp Neurol 2012; 71: 494–510. [DOI] [PubMed] [Google Scholar]
- 64. Himeno T, Kamiya H, Naruse K, et al. Beneficial effects of exendin‐4 on experimental polyneuropathy in diabetic mice. Diabetes 2011; 60: 2397–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ponirakis G, Abdul‐Ghani MA, Jayyousi A, et al. Effect of treatment with exenatide and pioglitazone or basal‐bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res Care 2020; 8: e001420. [DOI] [PMC free article] [PubMed] [Google Scholar]


