Abstract
Aims/Introduction
The triglyceride–glucose (TyG) index has been proposed as a reliable and simple marker of insulin resistance. We investigated the association between TyG index and diabetic nephropathy (DN) in patients with type 2 diabetes.
Materials and Methods
A consecutive case series of 682 adult patients with type 2 diabetes hospitalized in the Department of Endocrinology at the Tongji Hospital (Wuhan, Hubei, China) from January 2007 to December 2009 was included in this cross‐sectional analysis. Receiver operating characteristics curve analysis, correlation analysis and multiple logistic regression analysis were carried out.
Results
A total of 232 (34.0%) participants were identified with DN. Compared with the non‐DN group, the DN group had longer disease duration, and higher bodyweight, systolic blood pressure, diastolic blood pressure, glycated hemoglobin, triglycerides, total cholesterol, serum uric acid, 24 h‐urinary albumin, TyG index and homeostasis model assessment 2 estimates for insulin resistance (HOMA2‐IR; P < 0.05 for each). The TyG index with an optimal cut‐off point >9.66 showed a higher area under the receiver operating characteristic curve of 0.67 (P = 0.002) than HOMA2‐IR (area under the curve 0.61, P = 0.029) on receiver operating characteristic curve analysis for DN identification. Additionally, the TyG index positively correlated with the levels of metabolic indicators (bodyweight, glycated hemoglobin, triglycerides, total cholesterol, serum uric acid, fasting glucose and HOMA2‐IR) and natural logarithmic 24 h‐urinary albumin (P < 0.05 for each), but not natural logarithm of estimated glomerular filtration rate. On multiple regression analysis, an increased TyG index was shown to be an independent risk factor (odds ratio 1.91, P = 0.001) for DN.
Conclusions
The TyG index was independently associated with DN in patients with type 2 diabetes, and was a better marker than HOMA2‐IR for identification of DN in type 2 diabetes patients.
Keywords: Diabetic nephropathy, Insulin resistance, Triglyceride–glucose index
The triglyceride–glucose index provides an affordable and easily interpreted biomarker for monitoring the degree of insulin resistance, and was independently associated with diabetic nephropathy in patients with type 2 diabetes. It was a better marker than homeostasis model assessment for insulin resistance for identification of diabetic nephropathy in type 2 diabetes patients.
![]()
Introduction
Diabetic nephropathy (DN), as assessed by the development of albuminuria or a reduction in the glomerular filtration rate, increases cardiovascular morbidity and mortality in patients with type 2 diabetes, and remains the most important cause of end‐stage renal disease 1 . There is a high prevalence of DN among Asian patients with type 2 diabetes (China 26–41%, Japan 22–32%, Singapore 53%) 2 , 3 , 4 , 5 . Owing to the large population of patients with diabetes in the Asia–Pacific region, the number of patients with DN is a tremendous burden on the healthcare system. Clinical trials have established that the development and progression of DN are closely associated with glycemic control 6 . However, the timing and severity of DN vary in type 2 diabetes patients with poor glycemic control, and DN might also occur in patients with well‐controlled blood glucose levels. Therefore, hyperglycemia might not be the only risk factor for renal damage, suggesting that other factors are also involved in the clinical manifestation of DN.
In the context of renal disease, patients with diabetes or microalbuminuria are often more insulin resistant (IR), suggesting insulin resistance might lead to accelerated progression of DN 7 , 8 , 9 , 10 . Increased albuminuria is a strong predictor for the development of overt DN. Although the role of insulin resistance in the pathogenesis of increased albuminuria is well illustrated in type 1 diabetes 11 , its contribution in type 2 diabetes is controversial. Both positive 10 , 12 , 13 , 14 and negative associations 15 , 16 between insulin resistance and albuminuria have been reported in different studies. These conflicting findings might be attributable to the small number of patients included in the aforementioned studies, and in some circumstances, by studying different markers of insulin resistance 17 , 18 . Exploring the relationship between insulin resistance and DN will help us further understand the pathogenesis of DN, and potentially identify new intervention points to improve the outcomes in type 2 diabetes.
Most methods of evaluating insulin resistance, such as the hyperinsulinemic euglycemic glucose clamp (HEGC) 19 , are costly and difficult to operate. Alternatively, the homeostasis model assessment for insulin resistance (HOMA‐IR) index is widely used in clinical practice to evaluate insulin resistance using fasting state measurements 20 . However, the plasma insulin or C‐peptide assay is expensive, or it is not easily available in all laboratories and has poor reproducibility. Thus, there is a need for new biomarkers that are easier to detect and more affordable. The triglyceride–glucose (TyG) index is the product of fasting plasma glucose and triglycerides levels, and has shown an excellent predictive performance to determine the insulin resistance when compared with HOMA‐IR 21 , 22 and HEGC 23 .
However, few studies have investigated the association between the TyG index and DN. Thus, the aim of the present study was to investigate the association between the TyG index, as a simple surrogate measure of insulin resistance, and DN in patients with type 2 diabetes.
Methods
Participants
This was a retrospective collection of a consecutive case series including 682 patients with type 2 diabetes hospitalized in the Department of Endocrinology at the Tongji Hospital of Huazhong University of Science and Technology (Wuhan, Hubei, China) from January 2007 to December 2009. Inclusion criteria for analysis included: (i) diagnosis of type 2 diabetes according to the World Health Organization 24 and Chinese Diabetes Society criteria 25 ; (ii) age ≥18 years; (iii) patients diagnosed with diabetes for at least 1 year; and (iv) no documented ketosis or ketoacidosis in the 3 months before enrolment. Individuals with any febrile or infectious illness, obstructive uropathy, severe heart failure, stroke, liver disease, cancer, autoimmune disease, changed lifestyle and pharmacological treatment during the past 3 months, and pregnant woman were excluded. DN was defined as an albumin excretion rate (AER) of ≥30 mg/day or an AER of ≥20 µg/min in at least two of three consecutive overnight urine collections. For patients with DN, any evidence of albuminuria in non‐diabetic renal disease was an additional exclusion criterion. The study was approved by the ethics committee of Tongji Hospital of Huazhong University of Science and Technology, and was carried out in accordance with the Declaration of Helsinki. Appropriate consent and assent were obtained from all participants.
Clinical and biochemical measurements
Anthropometric measurements included bodyweight and blood pressure assessments. Blood pressure was measured using a mercury sphygmomanometer after the participants sat at rest for 5 min. Fasting blood specimens were obtained from the participants in the early morning after refraining from eating, drinking and smoking for at least 8 h. All blood and urine specimens were tested immediately after collection. Glycated hemoglobin (HbA1C) was measured using high performance liquid chromatography (D‐10™; Bio‐Rad Laboratories, Hercules, CA, USA). Fasting glucose, triglycerides, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, uric acid and creatinine were measured using an automatic analyzer (Cobas8000; Roche Diagnostics Ltd., Basel, Switzerland). Insulin and C‐peptide levels were measured with a chemiluminescent immunometric assay (Cobas e601; Roche Diagnostics Ltd.). Urinary albumin was measured using the immunoturbidimetric method (Cobas8000; Roche Diagnostics Ltd.). Evidence of fatty liver and vessel plaque were analyzed with a medical ultrasonic apparatus.
Definition
The estimated glomerular filtration rate (eGFR) was calculated from serum creatinine and cystatin C using the CRIC Study equation 26 . The homoeostasis model assessment 2 estimates of insulin resistance (HOMA2‐IR) and β‐cell function (HOMA2‐B) based on fasting C‐peptide concentrations (which performs better than insulin in patients with diabetes) were calculated using the HOMA calculator (University of Oxford, Oxford, UK, available online from http://www.dtu.ox.ac.uk) 27 . The TyG index was calculated as follows: TyG = ln(fasting triglycerides [mg/dL] × fasting glucose [mg/dL] / 2) 21 . Diabetic retinopathy was diagnosed by an ophthalmologist based on fundus photographs 28 . Diabetic peripheral neuropathy was assessed by neurologists according to the Toronto criteria 29 . Macrovascular complications were identified by clinical evaluation of coronary, cerebral and peripheral artery diseases, and aortic aneurysms 30 . Smokers were defined as those with a daily or occasional smoking habit at the time of recruitment. Drinkers were also defined as those with a daily or occasional drinking habit at the time of recruitment. Normo‐, micro‐ and macroalbuminuria were defined as AER <30, 30–300 and >300 mg/24 h 31 , respectively.
Statistical analysis
Data were analyzed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard error or median (interquartile range) for continuous variables, and as percentages for categorical variables. Comparisons between the two groups (DN vs non‐DN) were carried out using the Student’s t‐test, Mann–Whitney U‐test or χ2‐test. One‐way anova was used to compare the difference in TyG levels between the three groups of macroalbuminuria, microalbuminuria and normoalbuminuria. The general linear model was used to calculate and compare the corrected means. Pearson’s correlation and partial correlation analyses were carried out for correlation analysis of TyG with other variables. HbA1C, HOMA2‐IR, AER and eGFR were natural logarithmic (ln) transformed in the correlation analysis. Receiver operating characteristic curve (ROC) analysis was constructed to evaluate the discriminatory performance for DN presence according to the value of the area under the ROC curve (AUC). A binary logistic regression multivariable analysis with DN categorized as a binary variable (presence or absence of DN) was used to evaluate the associations between the measured risk factors and DN. Statistical significance was defined by a P‐value <0.05.
Results
Characteristics of type 2 diabetes patients with and without DN
A total of 375 men and 307 women, aged 58.0 ± 0.5 years, with a median disease duration of 7 years (interquartile range 4–11 years) were included. A total of 34% (232/682) of patients with type 2 diabetes were identified with DN. Table 1 shows the participant characteristics stratified by groups (non‐DN and DN). Compared with the patients without DN, patients with DN had a longer disease duration, and were more likely to have hypertension, fatty liver disease, diabetic retinopathy and diabetic peripheral neuropathy (P < 0.05 for each). Of note, the DN group had greater bodyweight, systolic blood pressure, diastolic blood pressure, HbA1C, triglycerides, total cholesterol, serum uric acid and 24h‐AER (P < 0.05 for each), showing a greater burden of metabolic syndrome. Similarly, there was more severe insulin resistance in patients with DN, as shown by the TyG index and HOMA2‐IR (P = 0.000 and P = 0.011, respectively); however, there was no difference in the fasting insulin levels between groups. In addition, patients in the DN group were more likely to use insulin, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, calcium channel blockers, β‐blockers and diuretics, and less likely to use biguanides, sulfonylureas and α‐glucosidase inhibitors than patients in the non‐DN group (P < 0.05 for each).
Table 1.
Characteristics of type 2 diabetes patients with and without diabetic nephropathy
| Variable | Non‐DN (n = 450) | DN (n = 232) | P‐value |
|---|---|---|---|
| Age (years) | 57.4 ± 0.6 | 59.0 ± 0.7 | 0.094 |
| Women (%) | 46.0 | 43.1 | 0.471 |
| Disease duration (years) | 6 (3–10) | 10 (5–13) | 0.000 |
| Hypertension (%) | 38.2 | 65.5 | 0.000 |
| Fatty liver disease (%) | 4.7 | 9.1 | 0.024 |
| Retinopathy (%) | 20.7 | 41.8 | 0.000 |
| Macrovascular complications (%) | 17.6 | 22.4 | 0.127 |
| Peripheral neuropathy (%) | 47.8 | 58.2 | 0.010 |
| Smoking (%) | 31.3 | 34.9 | 0.344 |
| Drinking (%) | 23.8 | 26.7 | 0.398 |
| Bodyweight (kg) | 63.6 ± 0.5 | 66.8 ± 6.6 | 0.001 |
| SBP (mmHg) | 133.4 ± 1.0 | 146.4 ± 1.8 | 0.000 |
| DBP (mmHg) | 79.5 ± 0.6 | 84.0 ± 1.0 | 0.000 |
| HbA1C (%) | 7.5 (6.6–9.0) | 8.1 (6.6–10.35) | 0.002 |
| Triglycerides (mmol/L) | 1.40 ± 0.04 | 2.05 ± 0.15 | 0.000 |
| Total cholesterol (mmol/L) | 4.42 ± 0.05 | 4.94 ± 0.11 | 0.000 |
| HDL‐C (mmol/L) | 1.29 ± 0.15 | 1.14 ± 0.03 | 0.448 |
| LDL‐C (mmol/L) | 3.45 ± 0.83 | 2.96 ± 0.19 | 0.663 |
| Serum uric acid (µmol/L) | 318.8 ± 5.7 | 361.7 ± 9.2 | 0.000 |
| Serum creatinine (µmol/L) | 58.7 (48.5–71.3) | 82.0 (61.5–159.4) | 0.000 |
| eGFR (mL/min/1.73 m2) | 128.9 (100.7–157.7) | 76.1 (38.0–121.8) | 0.000 |
| AER (mg/24 h) | 15 (6–25) | 245 (55–829) | 0.000 |
| Fasting glucose (mmol/L) | 6.8 ± 0.1 | 7.3 ± 0.3 | 0.203 |
| Fasting insulin (mU/L) | 5.1 (3.5–8.8) | 6.1 (3.0–11.2) | 0.365 |
| Fasting C‐peptide (µg/L) | 1.9 (1.5–2.6) | 2.2 (1.7–3.3) | 0.012 |
| HOMA2‐B | 72.4 (48.3–112.0) | 70.9 (47.4–123.0) | 0.829 |
| HOMA2‐IR | 1.53 (1.14–2.09) | 1.79 (1.28–2.69) | 0.011 |
| TyG index | 9.10 ± 0.38 | 9.42 ± 0.74 | 0.000 |
| Medication | |||
| Insulin (%) | 65.8 | 83.2 | 0.000 |
| Biguanides (%) | 34.7 | 23.3 | 0.000 |
| Sulfonylureas (%) | 8.4 | 2.6 | 0.003 |
| Meglitinides (%) | 12.9 | 10.8 | 0.424 |
| α‐Glucosidase inhibitors (%) | 32.2 | 22.4 | 0.007 |
| Thiazolidinediones (%) | 10.7 | 11.2 | 0.830 |
| ACEI/ARB (%) | 20.7 | 44.0 | 0.000 |
| CCB (%) | 19.1 | 41.8 | 0.000 |
| β‐Blockers (%) | 4.2 | 10.3 | 0.002 |
| Diuretics (%) | 3.8 | 15.9 | 0.000 |
| Lipid lowering (%) | 31.9 | 38.4 | 0.093 |
Data are presented as the mean ± standard error or median (interquartile range) for continuous variables, and the percentage for categorical variables. Missing data: 237 participants without triglyceride–glucose (TyG) index data.
ACEI/ARB, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers; AER, albumin excretion rate; CCB, calcium channel blockers; DBP, diastolic blood pressure; DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; HbA1C, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA2‐B, homoeostasis model assessment 2 estimates of β‐cell function; HOMA2‐IR, homoeostasis model assessment 2 estimates of insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure.
ROC analysis for identification of patients with risk of DN
ROC analysis was carried out to evaluate the performance of the TyG index for identifying patients with the risk of DN. The AUC value of the TyG index was 0.67 (95% confidence interval [CI] 0.57–0.78, P = 0.002), which was higher than that of HOMA2‐IR (AUC 0.61, 95% CI 0.55–0.77, P = 0.029). When the Youden Index reached the maximum, the optimal cut‐off point of the TyG index was defined as >9.66. The corresponding sensitivity and specificity were 61.7% and 76.0%, respectively.
Association of TyG with metabolic indicators
Then, patients were separated into two groups as a low‐TyG group (TyG ≤9.66) and a high‐TyG group (TyG >9.66), according to the cut‐off value determined in the ROC analysis. Compared with the low‐TyG group, patients with a high level of TyG index had higher levels of risk factors of metabolic syndrome and higher proportion of DN, as indicated by older age, fatty liver disease, smoking, and a higher bodyweight, HbA1C, triglycerides, total cholesterol, serum uric acid, fasting glucose, HOMA2‐IR and 24h‐AER (P < 0.05 for each; Table 2). Accordingly, the TyG index positively correlated with the levels of these metabolic indicators (bodyweight, HbA1C, triglycerides, total cholesterol, serum uric acid, fasting glucose and HOMA2‐IR) and negatively correlated with high‐density lipoprotein cholesterol (P < 0.05 for each; Table S1).
Table 2.
Clinical and metabolic characteristics in patients with different levels of the triglyceride–glucose index
| Variables | TyG index ≤9.66 (n = 310) | TyG index >9.66 (n = 108) | P‐value |
|---|---|---|---|
| DN (%) | 25 | 50 | 0.000 |
| Age (years) | 54.7 ± 1.7 | 58.7 ± 0.7 | 0.003 |
| Women (%) | 44.2 | 37.0 | 0.195 |
| Disease duration (years) | 7 (3–11) | 7 (3–10) | 0.420 |
| Hypertension (%) | 43.9 | 51.9 | 0.152 |
| Fatty liver disease (%) | 3.9 | 13.0 | 0.001 |
| Smoking (%) | 31.0 | 41.7 | 0.043 |
| Bodyweight (kg) | 63.1 ± 0.6 | 70.1 ± 1.3 | 0.000 |
| SBP (mmHg) | 136 ± 1 | 139 ± 2 | 0.152 |
| DBP (mmHg) | 80 ± 1 | 83 ± 1 | 0.063 |
| HbA1C (%) | 7.4 (6.4–8.9) | 8.5 (7.3–10.5) | 0.000 |
| Triglycerides (mmol/L) | 1.21 ± 0.03 | 2.73 ± 0.21 | 0.000 |
| Total cholesterol (mmol/L) | 4.51 ± 0.06 | 5.00 ± 0.14 | 0.000 |
| HDL‐C (mmol/L) | 1.20 ± 0.03 | 1.00 ± 0.02 | 0.000 |
| LDL‐C (mmol/L) | 2.67 ± 0.05 | 2.85 ± 0.12 | 0.113 |
| Serum uric acid (µmol/L) | 315.5 ± 5.8 | 355.0 ± 11.5 | 0.001 |
| Serum creatinine (µmol/L) | 61.7 (49.6–76.1) | 65.1 (53.3–91.1) | 0.038 |
| eGFR (mL/min/1.73 m2) | 122.9 (94.5–153.3) | 110.9 (70.3–152.2) | 0.163 |
| AER (mg/24 h) | 40 (15–74) | 55 (30–240) | 0.002 |
| Fasting glucose (mmol/L) | 6.9 ± 1.3 | 10.0 ± 0.4 | 0.000 |
| Fasting insulin (mU/L) | 5.3 (3.4–9.0) | 7.5 (3.8–14.1) | 0.050 |
| HOMA2‐IR | 1.55 (1.19–2.13) | 1.98 (1.38–2.65) | 0.006 |
| TyG index | 8.88 ± 0.03 | 10.11 ± 0.04 | 0.000 |
Data are presented as the mean ± standard error or median (interquartile range) for continuous variables, and the percentage for categorical variables.
AER, albumin excretion rate; DBP, diastolic blood pressure; DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; HbA1C, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA2‐IR, homoeostasis model assessment 2 estimates of insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TyG, triglyceride–glucose.
Correlation of TyG index with albuminuria and eGFR
We also investigated the relationship between the TyG index and albuminuria and eGFR. In the correlation analysis, the TyG index positively correlated with lnAER (r = 0.190, P = 0.003). After adjustment for age, sex, disease duration, bodyweight, presence of hypertension, HbA1C and serum uric acid, the positive relationship between TyG index and lnAER remained significant (r = 0.173, P = 0.006). Consistently, the TyG index was higher in patients with macro‐ and microalbuminuria than those with normoalbuminuria (P < 0.05; Figure 1). The difference remained significant even after adjusting for the aforementioned confounding factors (P < 0.05). However, there was no significant correlation between the TyG index and lneGFR with (r = −0.095, P = 0.138) or without (r = −0.016, P = 0.805) adjustment for confounding factors. There was also no difference in the TyG index among DN patients with eGFR <30, 30–59, 60–89 and ≥90 mL/min/1.73 m2 (P = 0.786).
Figure 1.
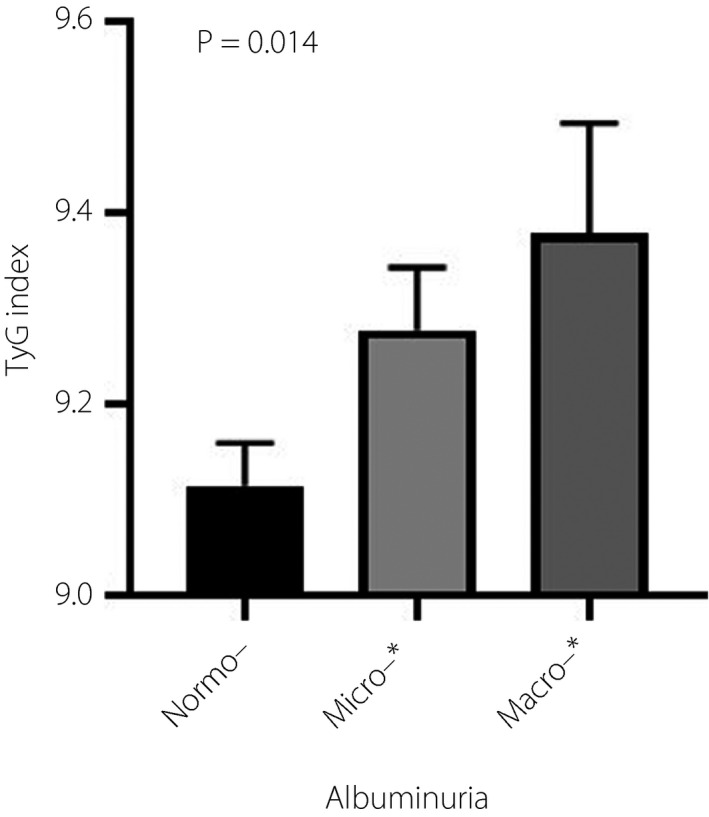
Levels of the triglyceride–glucose (TyG) index in patients with type 2 diabetes stratified by normoalbuminuria, microalbuminuria and macroalbuminuria. The difference remained significant even after adjusting for age, sex, disease duration, bodyweight, presence of hypertension, glycated hemoglobin and serum uric acid (P = 0.04). *P < 0.05 compared with normoalbuminuria.
Association of TyG index with diabetic nephropathy on multivariate analysis
On multivariate logistic stepwise regression analysis (Table 3), the TyG index was independently associated with DN in adult patients with type 2 diabetes after adjustment for age, sex, disease duration, bodyweight, presence of hypertension, HbA1C and serum uric acid. It is noteworthy that the odds ratio (OR) of TyG index (OR 1.91, P = 0.001) was higher than that of HbA1C (OR 1.35, P = 0.000).
Table 3.
Odds ratio of diabetic nephropathy in patients with type 2 diabetes
| Variable | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| TyG index (per 1 unit increase) | 1.91 | 1.29–2.85 | 0.001 |
| ≤9.66 | 1.00 | – | – |
| >9.66 | 2.99 | 1.61–5.06 | 0.000 |
| Hypertension | 2.46 | 1.40–4.30 | 0.002 |
| HbA1C | 1.35 | 1.19–1.53 | 0.000 |
| Disease duration | 1.07 | 1.02–1.12 | 0.009 |
The triglyceride–glucose (TyG) index was adjusted for age, sex, disease duration, bodyweight, presence of hypertension, glycated hemoglobin (HbA1C) and serum uric acid.
CI, confidence interval.
We further assessed the effect of the TyG index in the subgroups of patients (Table 4). An increased TyG index remained significantly associated with DN in the subgroups of age ≥60 years (OR 2.24, P = 0.032), age <60 years (OR 2.14, P = 0.004), HbA1C ≥7% (OR 2.28, P = 0.002) and eGFR ≥90 mL/min/1.73 m2 (OR 2.60, P = 0.001), and patients with or without hypertension (OR 2.04, P = 0.018; OR 1.89, P = 0.034) after multivariable adjustment. However, this association was not significant in patients with HbA1C <7% and eGFR <90 mL/min/1.73 m2.
Table 4.
Subgroups analysis of odds ratios of the triglyceride–glucose index with diabetic nephropathy in patients with type 2 diabetes
| Subgroups | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Age | ||||||
| ≥60 years | 1.74 (1.05–2.89) | 0.031 | 2.30 (1.21–4.37) | 0.011 | 2.24 (1.07–4.68) | 0.032 |
| <60 years | 2.32 (1.53–3.53) | 0.000 | 2.25 (1.40–3.61) | 0.001 | 2.14 (1.27–3.62) | 0.004 |
| HbA1C | ||||||
| ≥7.0% | 2.47 (162–3.77) | 0.000 | 2.50 (1.55–4.02) | 0.000 | 2.28 (1.37–3.80) | 0.002 |
| <7.0% | 1.40 (0.75–2.60) | 0.295 | 1.39 (0.67–2.88) | 0.373 | 1.52 (0.72–3.23) | 0.275 |
| eGFR | ||||||
| <90 mL/min/1.73 m2 | 0.85 (0.50–1.47) | 0.565 | 1.01 (0.50–2.01) | 0.983 | 1.34 (0.57–3.15) | 0.509 |
| ≥90 mL/min/1.73 m2 | 2.99 (1.89–4.73) | 0.000 | 3.00 (1.81–4.97) | 0.000 | 2.60 (1.49–4.54) | 0.001 |
| Hypertension | ||||||
| Yes | 1.53 (0.98–2.39) | 0.063 | 1.64 (0.98–2.72) | 0.058 | 2.04 (1.13–3.67) | 0.018 |
| No | 2.23 (1.41–3.52) | 0.001 | 2.42 (1.43–4.09) | 0.001 | 1.89 (1.05–3.40) | 0.034 |
Model 1: adjusted for age and sex; model 2: model 1 + bodyweight, disease duration, presence of hypertension and serum uric acid; model 3: model 2 + glycated hemoglobin (HbA1C).
CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio.
Discussion
In the present study, patients with type 2 diabetes and DN manifested a greater burden of clinical parameters associated with metabolic syndrome, including greater bodyweight, blood pressure, HbA1C, triglycerides, total cholesterol, serum uric acid and 24h‐AER. Importantly, patients with type 2 diabetes and DN showed more severe insulin resistance, as indicated by a higher TyG index and HOMA2‐IR scores compared with patients without DN (P < 0.05 for each). The TyG index showed a greater ROC AUC score (AUC 0.67, P = 0.002) for identification of DN in type 2 diabetes patients in comparison with HOMA2‐IR (AUC 0.61, P = 0.029), and the optimal cut‐off point for the TyG index was defined as >9.66, with a corresponding sensitivity and specificity of 61.7% and 76.0%, respectively. The TyG index was positively correlated with the levels of metabolic indicators (bodyweight, HbA1C, triglycerides, total cholesterol, serum uric acid, fasting glucose and HOMA2‐IR) and lnAER (P < 0.05 for each), but not lneGFR. Multiple regression analysis showed that an increased TyG index, as a surrogate marker of insulin resistance, was independently associated with DN in patients with type 2 diabetes (OR 1.91, P = 0.001).
The TyG index has been proposed as a simple and reliable surrogate marker for metabolic syndrome and insulin resistance 32 , 33 , 34 . Accumulating evidence has also confirmed the important role of TyG index in predicting macrovascular disease 35 , 36 , 37 . However, data on the association between TyG index and DN in patients with type 2 diabetes are limited. In the present study, we observed a significant positive correlation between the TyG index and DN in type 2 diabetes patients. Indeed, several studies have shown that insulin resistance is implicated in the development of DN. Although the mechanism underlying the relationship has not been fully elucidated, insulin resistance is associated with an elevation in the glomerular hydrostatic pressure, leading to increased renal vascular permeability and ultimately glomerular hyperfiltration 9 . Other possible mechanistic pathways linking insulin resistance to DN are inflammation 38 , oxidative stress 39 , metabolic acidosis 40 and increased lipotoxicity 41 , leading to the development of microangiopathy. Several reports have suggested that dyslipidemia has an important role in the progression of renal disease in both type 2 diabetes 42 and type 1 diabetes 43 .
Previous studies have reported the association of DN with HOMA‐IR, another surrogate marker of insulin resistance. De Cosmo et al. 44 showed that adult male patients with type 2 diabetes in the highest quartile of HOMA‐IR were more likely to have albuminuria than those in the lowest quartile. Others have shown a longitudinal relationship between insulin resistance, as assessed by baseline HOMA‐IR, and development of microalbuminuria in a 5‐year prospective cohort study 12 . HOMA2‐IR is an improvement over its predecessor, as it integrates the estimation of peripheral resistance with the fasting C‐peptide level, and some authorities consider it a better metric for Asian populations 45 . The present results coincide with these previously reported findings that patients with DN typically have a higher HOMA2‐IR. Importantly, we indeed found that, compared with HOMA2‐IR, the TyG index showed a stronger association with DN in type 2 diabetes patients and a greater ROC AUC, indicating that TyG is a better marker for identification of DN in type 2 diabetes patients compared with HOMA2‐IR. In addition, the TyG index incorporates indicators of both glucose and lipid metabolisms, demonstrating the importance of serum triglycerides and glucose in the pathophysiology of insulin resistance in DN.
Additionally, the subgroup analysis revealed that patients with more frequent episodes of insufficient glycemic control (HbA1C ≥7%) showed greater OR values for DN, findings that were not seen in patients with HbA1C <7%. One explanation is that insulin resistance might be involved in the early phase of DN in type 2 diabetes patients, but not the late phase. Interestingly, patients in the subgroup of DN with HbA1C <7% showed higher blood pressure, worse renal function and a greater degree of albuminuria (Table S2), indicating that some patients with HbA1C <7% had progressed to a more serious stage of renal disease. Furthermore, the present results showed a significant association between TyG index and DN in patients with eGFR ≥90 mL/min/1.73 m2, but an insignificant association in patients with eGFR <90 mL/min/1.73 m2. The TyG index was associated with the development of albuminuria, but not grade of albuminuria. In addition, previous evidence also shows that reduced insulin sensitivity is independently associated with microalbuminuria in type 2 diabetes patients, but is not significantly associated with macroalbuminuria. Therefore, we speculate that the role of insulin resistance might be more obvious in the early phase of DN than in the late phase. In contrast, hypertension might also play a central role in this stage, as seen in Table S3, which shows that these patients were more likely to be treated with one or more antihypertensive drugs, including angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers and calcium channel blockers, all of which have been reported to improve insulin sensitivity. Hsu et al. 12 previously also reported that in the subgroup of type 2 diabetes patients with HbA1C <8% or blood pressure >130/80 mmHg, the role of insulin resistance in the incidence of DN was reduced. To summarize, the role of insulin resistance in the development of DN in these patients remains unclear, especially in the late phase, and requires further studies.
Several limitations to this study should be acknowledged. First, this was a cross‐sectional observational study. A causal relationship cannot be established directly based on the results of this study. Second, we continuously collected all participants at a particular location over a period of time; thus, our participants are well representative of hospitalized patients with type 2 diabetes, but not the general population with type 2 diabetes. Indeed, prospective cohort studies are required to evaluate the predictive potential of the TyG index for development of DN in patients with type 2 diabetes, especially in the general population. Third, we did not use the HEGC for measuring insulin resistance, as HEGC is time‐consuming and costly, and thus not suitable for the present large sample study. This limitation was compensated by the use of the TyG index, which is easy to detect and has been proposed as a reliable surrogate of insulin resistance with high sensitivity, when compared with HEGC 23 .
In conclusion, we showed a significant association between an increased TyG index and DN in patients with type 2 diabetes. We found that the TyG index was a better marker than HOMA2‐IR for the identification of DN in type 2 diabetes patients. Insulin resistance is an important and crucial player in the pathophysiology of DN, and might be an important target for its treatment and prevention. Future studies are required for a detailed understanding of the link between the insulin resistance parameters, especially the TyG index, and renal dysfunction in type 2 diabetes patients at different stages of DN.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Correlation analysis of the triglyceride–glucose index with metabolic indicators.
Table S2 | Characteristics of type 2 diabetes patients with and without diabetic nephropathy according to glycated hemoglobin.
Table S3 | Medication of patients with diabetic nephropathy in the subgroups of glycated hemoglobin.
Acknowledgments
We thank the National Natural Science Foundation of China (81770817 to GY and 81700727 to LL) and China Diabetes Young Scientific Talent Research Project of China International Medical Foundation (2018‐N‐01 to LL). We also thank all the doctors, nurses, technicians and participating patients for their dedication to this study. The study was funded by National Natural Science Foundation of China (81770817 to GY and 81700727 to LL) and China Diabetes Young Scientific Talent Research Project of China International Medical Foundation (2018‐N‐01 to LL).
J Diabetes Investig. 2021
Contributor Information
Shengzhong Li, Email: lishengzhong1010@163.com.
Gang Yuan, Email: gangyuan@tjh.tjmu.edu.cn.
References
- 1. Collins AJ, Foley RN, Herzog C, et al. US renal data system 2010 annual data report. Am J Kidney Dis 2011; 57: e1–526. [DOI] [PubMed] [Google Scholar]
- 2. Jia W, Gao X, Pang C, et al. Prevalence and risk factors of albuminuria and chronic kidney disease in Chinese population with type 2 diabetes and impaired glucose regulation: Shanghai diabetic complications study (SHDCS). Nephrol Dial Transplant 2009; 24: 3724–3731. [DOI] [PubMed] [Google Scholar]
- 3. Chan JC, So W, Ma RC, et al. The complexity of vascular and non‐vascular complications of diabetes: the Hong Kong Diabetes Registry. Curr Cardiovasc Risk Rep 2011; 5: 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yokoyama H, Araki SI, Kawai K, et al. Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46). BMJ Open Diabetes Res Care 2018; 6: e000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loh PT, Toh MP, Molina JA, et al. Ethnic disparity in prevalence of diabetic kidney disease in an Asian primary healthcare cluster. Nephrology 2015; 20: 216–223. [DOI] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, et al. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 7. Pham H, Robinson‐Cohen C, Biggs ML, et al. Chronic kidney disease, insulin resistance, and incident diabetes in older adults. Clin J Am Soc Nephrol 2012; 7: 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 2016; 311: F1087–F1108. [DOI] [PubMed] [Google Scholar]
- 9. Sasson AN, Cherney DZ. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 2012; 3: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross‐sectional, case‐control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes 2006; 55: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 11. Svensson M, Yu ZW, Eriksson JW. A small reduction in glomerular filtration is accompanied by insulin resistance in type I diabetes patients with diabetic nephropathy. Eur J Clin Invest 2002; 32: 100–109. [DOI] [PubMed] [Google Scholar]
- 12. Hsu CC, Chang HY, Huang MC, et al. Association between insulin resistance and development of microalbuminuria in type 2 diabetes: a prospective cohort study. Diabetes Care 2011; 34: 982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vedovato M, Lepore G, Coracina A, et al. Effect of sodium intake on blood pressure and albuminuria in Type 2 diabetic patients: the role of insulin resistance. Diabetologia 2004; 47: 300–303. [DOI] [PubMed] [Google Scholar]
- 14. Bjornstad P, Maahs DM, Cherney DZ, et al. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care 2014; 37: 3033–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jager A, Kostense PJ, Nijpels G, et al. Microalbuminuria is strongly associated with NIDDM and hypertension, but not with the insulin resistance syndrome: the Hoorn Study. Diabetologia 1998; 41: 694–700. [DOI] [PubMed] [Google Scholar]
- 16. Rizvi A, Varasteh B, Chen YD, et al. Lack of a relationship between urinary albumin excretion rate and insulin resistance in patients with non‐insulin‐dependent diabetes mellitus. Metabolism 1996; 45: 1062–1064. [DOI] [PubMed] [Google Scholar]
- 17. Jia T, Huang X, Qureshi AR, et al. Validation of insulin sensitivity surrogate indices and prediction of clinical outcomes in individuals with and without impaired renal function. Kidney Int 2014; 86: 383–391. [DOI] [PubMed] [Google Scholar]
- 18. Placzkowska S, Pawlik‐Sobecka L, Kokot I, et al. Indirect insulin resistance detection: Current clinical trends and laboratory limitations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2019; 163: 187–199. [DOI] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–E223. [DOI] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 21. Simental‐Mendia LE, Rodriguez‐Moran M, Guerrero‐Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008; 6: 299–304. [DOI] [PubMed] [Google Scholar]
- 22. Kang B, Yang Y, Lee EY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes (Lond) 2017; 41: 789–792. [DOI] [PubMed] [Google Scholar]
- 23. Guerrero‐Romero F, Simental‐Mendia LE, Gonzalez‐Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab 2010; 95: 3347–3351. [DOI] [PubMed] [Google Scholar]
- 24. Alberti K, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 25. Chinese Diabetes S, National Office for Primary Diabetes C . [National guidelines for the prevention and control of diabetes in primary care(2018)]. Zhonghua Nei Ke Za Zhi 2018; 57: 885–893. [DOI] [PubMed] [Google Scholar]
- 26. Lash JP, Go AS, Appel LJ, et al. Chronic renal insufficiency cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009; 4: 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21: 2191–2192. [DOI] [PubMed] [Google Scholar]
- 28. Martinell M, Dorkhan M, Stalhammar J, et al. Prevalence and risk factors for diabetic retinopathy at diagnosis (DRAD) in patients recently diagnosed with type 2 diabetes (T2D) or latent autoimmune diabetes in the adult (LADA). J Diabetes Complications 2016; 30: 1456–1461. [DOI] [PubMed] [Google Scholar]
- 29. Dyck PJ, Albers JW, Andersen H, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 2011; 27: 620–628. [DOI] [PubMed] [Google Scholar]
- 30. Dal Canto E, Ceriello A, Ryden L, et al. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol 2019; 26: 25–32. [DOI] [PubMed] [Google Scholar]
- 31. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150. [Google Scholar]
- 32. Mohd Nor NS, Lee S, Bacha F, et al. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic‐euglycemic clamp. Pediatr Diabetes 2016; 17: 458–465. [DOI] [PubMed] [Google Scholar]
- 33. Khan SH, Sobia F, Niazi NK, et al. Metabolic clustering of risk factors: evaluation of Triglyceride‐glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr 2018; 10: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li R, Li Q, Cui M, et al. Clinical surrogate markers for predicting metabolic syndrome in middle‐aged and elderly Chinese. J Diabetes Investig 2018; 9: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee EY, Yang HK, Lee J, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis 2016; 15: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park K, Ahn CW, Lee SB, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care 2019; 42: 1569–1573. [DOI] [PubMed] [Google Scholar]
- 37. Jin JL, Sun D, Cao YX, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new‐onset, stable coronary artery disease: a nested case‐control study. Ann Med 2018; 50: 576–586. [DOI] [PubMed] [Google Scholar]
- 38. Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 2012; 7: 1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gnudi L. Cellular and molecular mechanisms of diabetic glomerulopathy. Nephrol Dial Transplant 2012; 27: 2642–2649. [DOI] [PubMed] [Google Scholar]
- 40. Marunaka Y. Roles of interstitial fluid pH in diabetes mellitus: glycolysis and mitochondrial function. World J Diabetes 2015; 6: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006; 440: 944–948. [DOI] [PubMed] [Google Scholar]
- 42. Dominguez JH, Tang N, Xu W, et al. Studies of renal injury III: lipid‐induced nephropathy in type II diabetes. Kidney Int 2000; 57: 92–104. [DOI] [PubMed] [Google Scholar]
- 43. Jenkins AJ, Lyons TJ, Zheng D, et al. Lipoproteins in the DCCT/EDIC cohort: associations with diabetic nephropathy. Kidney Int 2003; 64: 817–828. [DOI] [PubMed] [Google Scholar]
- 44. De Cosmo S, Minenna A, Ludovico O, et al. Increased urinary albumin excretion, insulin resistance, and related cardiovascular risk factors in patients with type 2 diabetes: evidence of a sex‐specific association. Diabetes Care 2005; 28: 910–915. [DOI] [PubMed] [Google Scholar]
- 45. Song YS, Hwang YC, Ahn HY, et al. Comparison of the Usefulness of the Updated Homeostasis Model Assessment (HOMA2) with the Original HOMA1 in the Prediction of Type 2 Diabetes Mellitus in Koreans. Diabetes Metab J 2016; 40: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Correlation analysis of the triglyceride–glucose index with metabolic indicators.
Table S2 | Characteristics of type 2 diabetes patients with and without diabetic nephropathy according to glycated hemoglobin.
Table S3 | Medication of patients with diabetic nephropathy in the subgroups of glycated hemoglobin.


