Key Points
Question
What are performance and feasibility rates of prediction scales for large anterior vessel occlusion when validated externally and compared head to head in the emergency medical services setting?
Findings
In this cohort study of 2007 patients who received acute stroke codes, 7 prediction scales showed good accuracy scores, high specificity, and low sensitivity, statistically favoring the Los Angeles Motor Scale and the Rapid Arterial Occlusion Evaluation scale. Feasibility rates favored the Prehospital Acute Stroke Severity scale.
Meaning
The present results suggest that small but statistically significant differences in accuracies appear to be clinically meaningful in larger populations for reducing treatment delays, with subsequent improved clinical outcomes, and that feasibility should be considered before implementing a scale.
Abstract
Importance
The efficacy of endovascular thrombectomy (EVT) for symptomatic large anterior vessel occlusion (sLAVO) sharply decreases with time. Because EVT is restricted to comprehensive stroke centers, prehospital triage of patients with acute stroke codes for sLAVO is crucial, and although several prediction scales are already in use, external validation, head-to-head comparison, and feasibility data are lacking.
Objective
To conduct external validation and head-to-head comparisons of 7 sLAVO prediction scales in the emergency medical service (EMS) setting and to assess scale feasibility by EMS paramedics.
Design, Setting, and Participants
This prospective cohort study was conducted between July 2018 and October 2019 in a large urban center in the Netherlands with a population of approximately 2 million people and included 2 EMSs, 3 comprehensive stroke centers, and 4 primary stroke centers. Participants were consecutive patients aged 18 years or older for whom an EMS-initiated acute stroke code was activated. Of 2812 acute stroke codes, 805 (28.6%) were excluded, because no application was used or no clinical data were available, leaving 2007 patients included in the analyses.
Exposures
Applications with clinical observations filled in by EMS paramedics for each acute stroke code enabling reconstruction of the following 7 prediction scales: Los Angeles Motor Scale (LAMS); Rapid Arterial Occlusion Evaluation (RACE); Cincinnati Stroke Triage Assessment Tool; Prehospital Acute Stroke Severity (PASS); gaze-face-arm-speech-time; Field Assessment Stroke Triage for Emergency Destination; and gaze, facial asymmetry, level of consciousness, extinction/inattention.
Main Outcomes and Measures
Planned primary and secondary outcomes were sLAVO and feasibility rates (ie, the proportion of acute stroke codes for which the prehospital scale could be reconstructed). Predictive performance measures included accuracy, sensitivity, specificity, the Youden index, and predictive values.
Results
Of 2007 patients who received acute stroke codes (mean [SD] age, 71.1 [14.9] years; 1021 [50.9%] male), 158 (7.9%) had sLAVO. Accuracy of the scales ranged from 0.79 to 0.89, with LAMS and RACE scales yielding the highest scores. Sensitivity of the scales ranged from 38% to 62%, and specificity from 80% to 93%. Scale feasibility rates ranged from 78% to 88%, with the highest rate for the PASS scale.
Conclusions and Relevance
This study found that all 7 prediction scales had good accuracy, high specificity, and low sensitivity, with LAMS and RACE being the highest scoring scales. Feasibility rates ranged between 78% and 88% and should be taken into account before implementing a scale.
This cohort study performs external validations, head-to-head comparisons, and feasibility ratings of 7 scales used by emergency medical services in the field to predict symptomatic large anterior vessel occlusion among patients suspected of acute stroke.
Introduction
In acute ischemic stroke, clinical efficacy of intravenous thrombolysis (IVT) and endovascular thrombectomy (EVT) is highly time dependent.1,2,3 Endovascular thrombectomy can only be given to a subset of patients with a symptomatic large anterior vessel occlusion (sLAVO), constituting 4.9% to 14.5% of all patients with suspected stroke.4,5 Contrary to IVT, which is available in most primary stroke centers (PSCs), EVT is an elaborate treatment and, therefore, restricted to comprehensive stroke centers (CSCs) with EVT facilities. Because sLAVO cannot be reliably identified in the ambulance, patients suspected of acute stroke are often transferred to the nearest hospital (often a PSC) to start IVT as soon as possible. For patients with sLAVO, this routing leads to a median of 60 to 109 minutes’ delay due to interhospital transfers, with associated worse functional outcomes.6,7 Prehospital identification of patients with sLAVO enabling direct allocation to a CSC would greatly reduce delays to EVT treatment and improve clinical outcomes.
Several clinical prediction scales have been developed with this purpose; however, most scales were validated only in the hospital and not in the field (ie, prehospital by emergency medical service [EMS] paramedics), and external validation is often lacking.8,9 Moreover, to decide which scale is preferred, head-to-head comparison in the field is required, which is currently lacking. Finally, feasibility ratings of the scales have not been investigated systematically although this is an important feature to consider before adopting a scale in clinical practice.
The aims of the present study are therefore to (1) externally validate field performance, including head-to-head comparisons, of 7 prediction scales and (2) assess feasibility rates (ie, the proportion of acute stroke codes for which the prehospital scale could be reconstructed) of these scales in the EMS setting.
Methods
Study Design and Study Population
This is a prospective, multiregional, observational cohort study. All consecutive patients 18 years of age or older for whom an EMS-initiated acute stroke code was activated between July 2018 and October 2019 were included. Patients were recruited from the Leiden and The Hague regions, encompassing 2 EMSs, 3 CSCs, and 4 PSCs, serving a total population of approximately 2 million inhabitants. Results are reported according to the Standards for Reporting of Diagnostic Accuracy (STARD) reporting guideline for diagnostic accuracy studies.10 The institutional review boards of Leiden University Medical Center and of the participating hospitals approved this study and waived the need for obtaining informed consent because the extent of the effort required by the large number of health care providers to obtain permission from the participants was disproportionate compared with the relatively limited sensitivity of the collected encoded data and the related limited intrusion to the personal privacy. This study is registered with ClinicalTrials.gov.11
An acute stroke code was initiated by EMS if there was a prehospital suspicion of acute stroke with a positive face-arm-speech test (FAST) or other focal neurologic symptoms. When symptom onset or last seen well was 6 hours or less, it was routine policy to transport these patients to the nearest hospital, and when symptom onset was 6 to 24 hours, it was policy to transport patients to a CSC. The guidelines allow for protocol deviation based on a paramedic’s individual judgment.
Sample size calculation for external validation of the prediction models was estimated at a minimum of 100 patients with the primary outcome event (sLAVO).12,13 To increase power, we aimed to include more than 120 patients with sLAVO. On the basis of the literature, we estimated that 4.9% to 14.5% of patients receiving an acute stroke code have sLAVO.4,5 Considering the lower bound of the estimated sLAVO incidence, we therefore expected that a total of 2000 acute stroke codes had to be included.
Prediction Scales and Prehospital Applications
Seven prediction scales were simultaneously assessed and subsequently validated: the Los Angeles Motor Scale (LAMS),14 the Rapid Arterial Occlusion Evaluation (RACE),15 the Cincinnati Stroke Triage Assessment Tool (C-STAT; formerly CPSSS),16 the Prehospital Acute Stroke Severity (PASS) scale,17 the gaze-face-arm-speech-time (G-FAST) test,18 the Field Assessment Stroke Triage for Emergency Destination (FAST-ED),19 and the gaze, facial asymmetry, level of consciousness, extinction/inattention (GACE) scale.8 The scales were selected based on a previous systematic review.8 All are ordinal scales with a cut point to decide whether or not a patient has sLAVO, except for the GACE scale, which uses a 4-item decision tree. Some scales are already implemented in clinical practice (eg, FAST-ED and RACE).20,21
The EMS paramedics were instructed to fill in an application on site or during transport for each EMS-initiated acute stroke code. The application contained 10 to 13 items to structure neurologic observations, enabling reconstruction of all 7 prediction scales according to the authors’ scoring instructions and prespecified cut points. The application was designed and tested in close collaboration with Research and Development (R&D) EMS Hollands Midden and filled in online or directly in the electronic transport record. Because the transport record system is part of standard care and the application merely structures routine clinical observations, it largely fit within the regular workflow.
For 1 of the EMS regions, filling in the application was not mandatory. We anticipated that this would result in acute stroke codes in which the application would not be used at all. To investigate possible selection bias, we collected clinical data from patients with an acute stroke code in this region without a filled-in application for comparison.
Hospital Data Collection
Clinical data were retrieved from electronic patient records and included demographic characteristics, medical history, medication use, and stroke severity as assessed with the National Institutes of Health Stroke Scale (NIHSS). In cases for which an NIHSS score was not noted, the score was reconstructed from neurologic examination at admission by NIHSS-certificated research members with a validated algorithm as described previously.22 In-hospital performance metrics included symptom-onset-to-door time, door-to-needle time, and door-to-groin-puncture time (the door was defined as the door of the first hospital).23 Finally, data on neuroimaging and diagnoses at admission, discharge, and after 3 months were retrieved from electronic patient records. Clinical outcomes according to the modified Rankin Scale were also retrieved after 3 months (patients with stroke only), which is a mandatory outcome parameter in Dutch stroke centers.
Data Privacy
A trusted third party was installed to safeguard privacy, storage, and use of data. Application data and clinical data were coupled, encoded, and then transferred by the trusted third party to a data safe allowing access to investigators only.
Outcomes
The primary outcome was sLAVO clinically assessed by the treating stroke team taking the following radiologic criteria into account: occlusion of the intracranial carotid artery, tandem intracranial carotid artery, middle cerebral artery (M1 or M2 segment), or anterior cerebral artery (A1 or A2 segment).
For feasibility, we considered the cutoff reconstruction rate the most important parameter. The reconstruction rate was defined as the proportion of acute stroke codes for which the authors’ prespecified scale’s cut point could be determined with the available data. This was possible if (1) the cut point was reached with the points scored by EMS paramedics or (2) assigning maximal scores to missing or untestable items would still lead to a total number of points below the authors’ prespecified cut point.
We also calculated the full-scale feasibility: the proportion of acute stoke codes for which the full scale could be reconstructed. A scale could not be reconstructed and therefore was deemed not feasible when any required item to reconstruct a scale was missing or untestable. We excluded the GACE scale for feasibility analysis because this would always reach 100% owing to its decision tree construction.
Finally, for each scale, we assessed the item that was reported missing or untestable most frequently to provide insight to the most important limiting factor for a scale to be feasible. In addition, to estimate how the use of a prediction scale might have influenced patient allocation in our cohort, we provided a hypothetical example by applying the scale with the highest diagnostic accuracy.
Statistical Analysis
Categorical variables were compared with Pearson χ2 tests and presented as proportions. Continuous variables were compared using the t test or Mann-Whitney test and presented as mean (SD) values or median values and interquartile ranges (IQRs) as appropriate. A 2-sided P ≤ .05 was considered statistically significant.
Scale performance was assessed by calculating diagnostic accuracies (C statistic) of the authors’ prespecified cut point as well as sensitivity, specificity, the Youden index, positive predictive value, and negative predictive value, with corresponding 95% CIs.
Accuracy was considered excellent for values 0.9 to 1.0, good for 0.8 to 0.9, fair for 0.7 to 0.8, poor for 0.6 to 0.7, and failed for 0.5 to 0.6.24 Accuracies between the scales were compared with the McNemar test. The Youden index was used to evaluate the overall discriminative power of a diagnostic test and was calculated by deducting 1 from the sum of the test’s sensitivity and specificity. The Youden index equals 0 for poor accuracy and 1 for excellent accuracy.25
In addition, the accuracy for full-scale range was assessed by the area under the curve (AUC). Since hitherto the in-hospital NIHSS score holds the highest accuracy in predicting sLAVO, we also included this as a reference.26
Reconstruction rates are provided with 95% CIs for comparison. Data analyses were performed using SPSS, version 24.0, or R, version 3.5.1, CRAN (R-CRAN project).
Results
Patient Inclusion
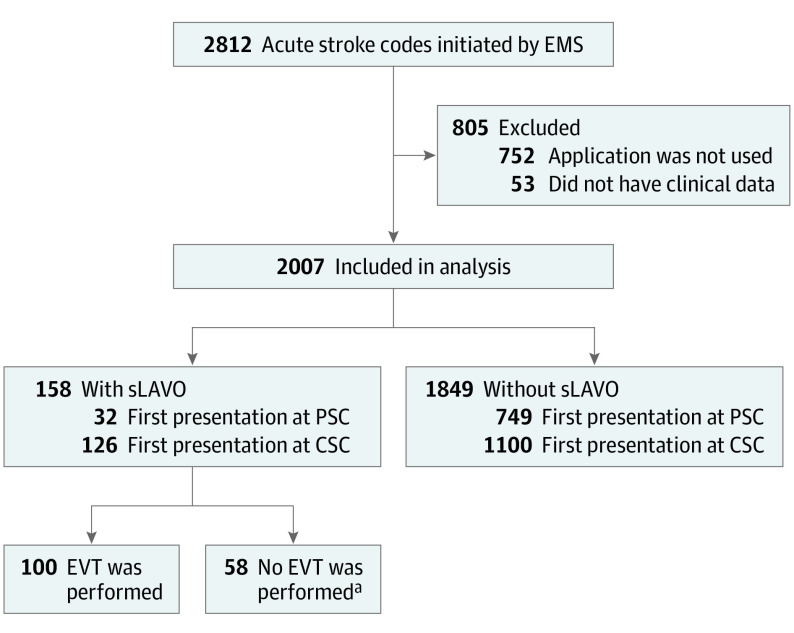
Between July 2018 and October 2019, 2812 acute stroke codes were activated (Figure 1). We excluded 805 acute stroke codes (28.6%), because no application was used (752 [26.7%]) or because no clinical data were available in the electronic patient record (53 [1.9%]).
Figure 1. Flowchart of Patient Recruitment.
CSC represents comprehensive stroke center; EMS, emergency medical service; EVT, endovascular thrombectomy; PSC; primary stroke center; and sLAVO, symptomatic large anterior vessel occlusion.
aImaging did not show an occlusion or a perfusion mismatch; the occlusion was technically not accessible. Patients showed clinical recovery or deteriorated neurologic status, or patients participated in the MR CLEAN LATE study randomized for no EVT.
We collected clinical data on 442 of 752 patients with acute stroke codes (58.8%) for whom the application was not used. These patients had similar baseline characteristics, incidence of sLAVO, and stroke severity (median [IQR] NIHSS score, 4 [2-8] vs 4 [2-10]) and more often had hemorrhagic stroke or a stroke mimic compared with patients with application data (eTable 1 in the Supplement).
Patient Characteristics
Of 2007 included patients with acute stroke codes, 1021 (50.9%) were men, the mean (SD) age was 71.1 (14.9) years, and the median (IQR) NIHSS score was 4 (2-8) (Table 1). Of 2007 patients with acute stroke codes, 781 (38.9%) first presented in a PSC, and 1226 (61.1%) first presented in a CSC. The final diagnosis after 3 months was ischemic stroke in 842 patients (41.9%), intracerebral hemorrhage in 148 patients (7.4%), transient ischemic attack in 264 patients (13.2%), and stroke mimic in 753 patients (37.5%). In addition, 158 patients (7.9%) with an acute stroke code received a diagnosis of sLAVO.
Table 1. Baseline Patient Characteristics.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Total (N = 2007) | sLAVO | ||
| Yes (n = 158) | No (n = 1849) | ||
| Age, mean (SD), y | 71.1 (14.9) | 72.4 (13.3) | 70.9 (14.9) |
| Male sex | 1021 (50.9) | 92 (58.2) | 929 (50.2) |
| Stroke logistics | |||
| Primary stroke center | 781 (38.9) | 32 (20.3)a | 749 (40.5) |
| Comprehensive stroke center | 1226 (61.1) | 126 (79.7) | 1100 (59.5) |
| Medical history | |||
| Ischemic stroke or transient ischemic attack | 671 (33.4) | 33 (20.9) | 638 (34.5) |
| Intracerebral hemorrhage | 83 (4.1) | 2 (1.3) | 81 (4.4) |
| Atrial fibrillation | 273 (13.6) | 30 (19.0) | 243 (13.2) |
| Diabetes | 421 (21.0) | 37 (23.4) | 384 (20.8) |
| Hyperlipidemia | 817 (40.7) | 48 (30.4) | 769 (41.6) |
| Hypertension | 1179 (58.7) | 88 (55.7) | 1091 (59.0) |
| Myocardial infarction | 223 (11.1) | 20 (12.7) | 203 (11.0) |
| Medication use | |||
| Oral anticoagulation | 337 (16.8) | 24 (15.2) | 313 (16.9) |
| Antiplatelets | 690 (34.4) | 50 (31.6) | 640 (34.6) |
| Hospital admission | |||
| NIHSS score, median (IQR)b | 4 (2-8) | 11 (5-17) | 3 (2-6) |
| ODT, median (IQR), minb | 140 (59-441) | 115 (45-340) | 142 (62-446) |
| Wake-up strokeb | 235 (23.7) | 46 (29.7) | 189 (22.6) |
| Blood pressure, median (IQR), mm Hg | |||
| Systolic | 160 (140-182) | 157 (139-182) | 160 (140-182) |
| Diastolic | 87 (77-100) | 87 (75-99) | 87 (77-100) |
| Glucose level, median (IQR), mg/dL | 6.6 (5.7-8.1) | 6.8 (5.9-8.6) | 6.6 (5.7-8.1) |
| Atrial fibrillation de novo | 79 (3.9) | 13 (8.2) | 66 (3.6) |
| Reperfusion therapy | |||
| IVTc | 314 (15.6) | 61 (38.6) | 253 (13.7) |
| EVTd | 100 (5.0) | 100 (63.3) | NA |
| IVT + EVT | 43 (2.1) | 43 (27.2) | NA |
| In-hospital performance metrics, median (IQR), min | |||
| DNT | 25 (19-34) | 24 (18-33) | 25 (19-34) |
| DGT | 72 (54-105) | 72 (54-105) | NA |
| Site of occlusion | |||
| ICA(-T) | 28 (1.4) | 24 (15.2) | NA |
| MCA M1 | 77 (3.8) | 75 (47.5) | NA |
| MCA M2 | 75 (3.7) | 73 (46.2) | NA |
| ACA 1/2 | 5 (0.2) | 5 (3.2) | NA |
| Othere | 29 (1.4) | 5 (3.2) | 24 (1.3) |
| Final diagnosis after 90 d | |||
| Ischemic stroke | 842 (41.9) | 155 (98.1) | 687 (37.2) |
| Intracerebral hemorrhage | 148 (7.4) | 0 | 148 (8.0) |
| Transient ischemic attack | 264 (13.2) | 3 (1.9) | 261 (14.1) |
| Stroke mimic | 753 (37.5) | 0 | 753 (40.7) |
| mRS after 90 d, median (IQR)b,f | 2 (1-4) | 3 (2-6) | 2 (1-4) |
Abbreviations: ACA, anterior cerebral artery; DGT, first-door-to-groin-puncture time for EVT; DNT, door-to-needle time for IVT; EVT, endovascular thrombectomy; ICA(-T), intracranial carotid artery or tandem ICA; IQR, interquartile range; IVT, intravenous thrombolysis; MCA M1 or M2, middle cerebral artery segment M1 or M2; mRS, modified Rankin scale; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; ODT, onset to hospital door; sLAVO, symptomatic large anterior vessel occlusion.
SI conversion factor: To convert glucose level to mmol/L, multiply by 0.0555.
Of 32 patients with sLAVO, 27 (84.4%) were transferred to a comprehensive stroke center for EVT or observation.
Only provided for patients with stroke (ie, ischemic stroke and intracerebral hemorrhage).
Missing data in 3.5%.
No missing data.
Large posterior vessel occlusion locations: basilar artery, vertebral artery, and posterior cerebral artery, segments P1 or P2.
Missing data in 36.2%.
Compared with patients without sLAVO, patients with sLAVO less often had a history of ischemic stroke or transient ischemic attack (33 of 158 [20.9%] vs 638 of 1849 [34.5%]; P = .001), had higher median (IQR) NIHSS scores (11 [5-17] vs 3 [2-6]; P < .001), and had higher incidence of atrial fibrillation de novo (13 of 158 [8.2%] vs 66 of 1849 [3.6%]; P = .01). Other baseline characteristics are given in Table 1.
Treatment and Logistic Metrics
Of 158 patients with sLAVO, 32 (20.3%) first presented in a PSC and 126 (79.7%) in a CSC (vs 1100 of 1849 patients without sLAVO [59.5%]) (Table 1). Median [IQR] symptom-onset-to-door time was shorter in patients with sLAVO compared with patients without sLAVO (115 [45-340] vs 142 [62-446] minutes; P = .02). More patients with than without sLAVO received IVT (61 of 158 [38.6%] vs 253 of 1849 [13.7%]; P < .001), and EVT was performed in 100 patients with sLAVO (63.3%), with a median (IQR) door-to-groin-puncture time of 72 (54-105) minutes. For patients who presented directly to a CSC, the median (IQR) door-to-groin-puncture time was shorter (61 [51-81] minutes) compared with patients who first presented in a PSC (114 [103-140] minutes; P < .001) (eTable 2 in the Supplement).
Scale Performance
Table 2 gives the accuracy for identifying sLAVO with sensitivity, specificity, and predictive values. Accuracies ranged from 0.79 to 0.89, with LAMS (0.89; 95% CI, 0.87-0.90) and RACE (0.88; 95% CI, 0.86-0.89) having the highest accuracies. Head-to-head comparisons showed that these scales significantly outperformed the other scales (eTable 3 in the Supplement). Specificity was high for all scales (range, 80%-93%), whereas sensitivity was low (range, 38%-62%). The Youden index ranged from 0.30 to 0.47, and RACE had the highest index score (0.47; 95% CI, 0.37-0.56). In addition, negative predictive value was high for all scales (range, 95%-96%), and positive predictive value was low (range, 21%-32%).
Table 2. Diagnostic Performance of the Prediction Scales According to Prespecified Cut Points.
| Prediction scalea | Accuracy (95% CI)b | Sensitivity (95% CI) | Specificity (95% CI) | Youden’s index (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| C-STAT ≥2 | 0.79 (0.77-0.81) | 0.62 (0.54-0.69) | 0.80 (0.78-0.82) | 0.42 (0.34-0.50) | 0.21 (0.18-0.24) | 0.96 (0.95-0.96) |
| PASS ≥2 | 0.81 (0.79-0.83) | 0.55 (0.47-0.64) | 0.83 (0.81-0.85) | 0.39 (0.30-0.48) | 0.21 (0.18-0.25) | 0.95 (0.95-0.96) |
| G-FAST ≥3 | 0.82 (0.81-0.84) | 0.61 (0.53-0.69) | 0.84 (0.82-0.86) | 0.46 (0.37-0.54) | 0.24 (0.21-0.27) | 0.96 (0.95-0.97) |
| FAST-ED ≥4 | 0.83 (0.81-0.85) | 0.60 (0.53-0.69) | 0.85 (0.83-0.87) | 0.46 (0.37-0.54) | 0.25 (0.22-0.29) | 0.96 (0.95-0.97) |
| RACE ≥5 | 0.88 (0.86-0.89) | 0.56 (0.46-0.65) | 0.90 (0.89-0.92) | 0.47 (0.37-0.56) | 0.32 (0.27-0.38) | 0.96 (0.95-0.97) |
| LAMS ≥4 | 0.89 (0.87-0.90) | 0.38 (0.29-0.46) | 0.93 (0.91-0.94) | 0.30 (0.22-0.40) | 0.28 (0.22-0.34) | 0.95 (0.94-0.96) |
Abbreviations: C-STAT, Cincinnati Stroke Triage Assessment Tool; FAST-ED, Field Assessment Stroke Triage for Emergency Destination; GACE, gaze, facial asymmetry, level of consciousness, extinction/inattention; G-FAST, gaze-face-arm-speech-time; LAMS, Los Angeles Motor Scale; NPV, negative predictive value; PASS, Prehospital Acute Stroke Severity; PPV, positive predictive value; RACE, Rapid Arterial Occlusion Evaluation.
GACE is not included in this analysis because GACE has no cut point.
Accuracy at cut point: ([true positives + true negatives]/total number of patients).
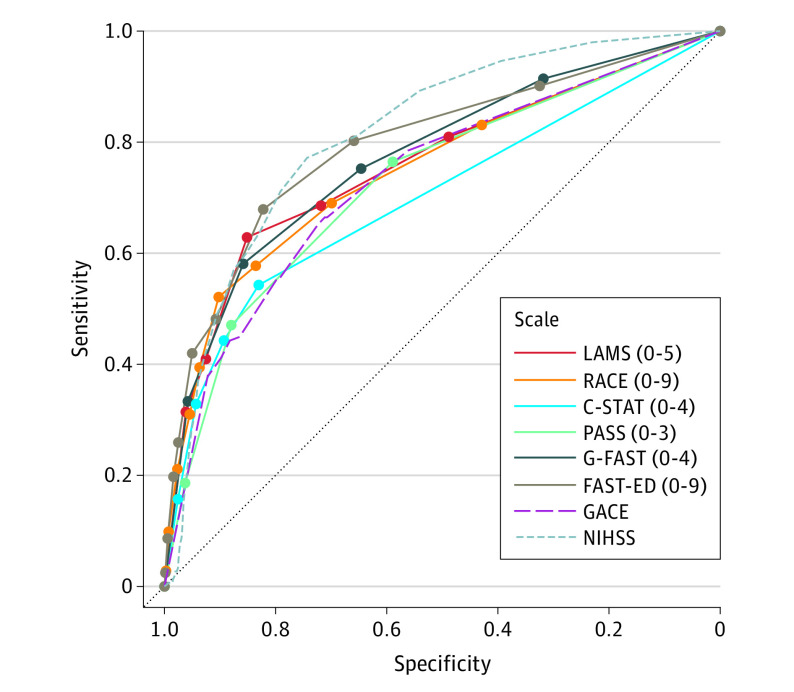
The AUC for the full-range accuracies for sLAVO prediction are shown in Figure 2. Scales showed fair to good performance, with accuracies ranging from 0.70 to 0.80 (eTable 4 in the Supplement). Although FAST-ED (AUC, 0.80; 95% CI, 0.74-0.85) had the highest accuracy, the accuracy was not statistically significantly different from G-FAST (AUC, 0.77; 95% CI, 0.72-0.82; P = .46), LAMS (AUC, 0.76; 95% CI, 0.71-0.81; P = .10), or RACE (AUC, 0.75; 95% CI, 0.69-0.82; P = .53).
Figure 2. Receiver Operating Characteristic Curves of Prediction Scale Diagnostic Performance.
C-STAT indicates Cincinnati Stroke Triage Assessment Tool; FAST-ED, Field Assessment Stroke Triage for Emergency Destination; GACE, gaze, facial asymmetry, level of consciousness, extinction/inattention; G-FAST, gaze-face-arm-speech-time; LAMS, Los Angeles Motor Scale; NIHSS, National Institutes of Health Stroke Scale; PASS, Prehospital Acute Stroke Severity; and RACE, Rapid Arterial Occlusion Evaluation. Numerals in parentheses indicate the full range of the scale.
Feasibility
The mean reconstruction rate of the scales’ cut point was 84.1% (range, 78.1%-87.9%) (Table 3). The PASS scale had the highest reconstruction rate (87.9%; 95% CI, 86.5-89.4). Compared with reconstruction rates of the full scale, calculating the rates for the cut point allowed scale reconstruction of 6.1% to 24.1% more acute stroke codes (eg, for RACE, 78.1% vs 57.2%). Missing or untestable items that mainly prevented the reconstruction of a scale’s cut point were neglect or motor function (Table 3).
Table 3. Scale Feasibility in the Field: Reconstruction Rate.
| Prediction scalea | No./total No. (%) [95% CI]b | Most frequent missing or untestable item, No. item/total No. (%)c | |
|---|---|---|---|
| Cutoff reconstruction rates | Full reconstruction rates | ||
| PASS | 1765/2007 (87.9) [86.5-89.4] | 1533/2007 (76.4) [74.5-78.2] | Motor deficit, arm 226/242 (93.4) |
| G-FAST | 1753/2007 (87.3) [85.9-88.8] | 1532/2007 (76.3) [74.5-78.2] | Motor deficit, arm 238/254 (93.7) |
| C-STAT | 1750/2007 (87.2) [85.7-88.7] | 1267/2004 (63.1) [61.0-65.2] | Motor deficit, arm 224/257 (87.2) |
| LAMS | 1662/2007 (82.8) [81.1-84.5] | 1540/2007 (76.7) [74.9-78.6] | Motor deficit, arm 322/345 (93.3) |
| FAST-ED | 1632/2007 (81.3) [79.6-83.0] | 1325/2007 (66.0) [63.9-68.1] | Motor deficit, arm; neglect 301/375 (80.3) |
| RACE | 1568/2007 (78.1) [76.3-79.9] | 1148/2007 (57.2) [55.0-59.4] | Motor deficit, legs 418/439 (95.2) |
Abbreviations: C-STAT, Cincinnati Stroke Triage Assessment Tool; FAST-ED, Field Assessment Stroke Triage for Emergency Destination; GACE, gaze, facial asymmetry, level of consciousness, extinction/inattention; G-FAST, gaze-face-arm-speech-time; LAMS, Los Angeles Motor Scale; PASS, Prehospital Acute Stroke Severity; RACE, Rapid Arterial Occlusion Evaluation.
GACE is not included in this analysis because the feasibility would always reach 100% owing to its decision tree construction.
Total number of reconstructed scales/all acute stroke codes.
Number of highest missing or untestable item/total missing or untestable items.
Hypothetical Example
Applying LAMS to our cohort, an urban region with relatively short distances between PSCs and CSCs and a low prevalence of sLAVO, indicated that 13 patients with sLAVO who first presented to a PSC would have benefited from direct allocation to a CSC, 17 patients with ischemic stroke treated with IVT allocated to a PSC would have unnecessarily bypassed a PSC, and 38 patients without sLAVO (including stroke mimics) allocated to a PSC would have been allocated to a CSC (including 6 patients with clinically severe intracerebral hemorrhage) (eFigure in the Supplement).
Discussion
In this prospective cohort study assessing more than 2000 patients with acute stroke codes, we found that several established sLAVO prediction scales had good accuracy when used in the EMS setting, with RACE and the LAMS showing the highest accuracies. Feasibility rates were relatively high for all scales, with the highest feasibility for PASS. We also found that feasibility rates could increase by using all available information to reconstruct the scale’s cut point, thereby enabling additional inclusion of acute stroke codes with incomplete or untestable items.
The prevalence of sLAVO in our cohort is in line with previous reports using a similar reference group of acute stroke codes (ie, also including hemorrhagic strokes and stroke mimics), indicating that our cohort was a good reflection of patients in general clinical practice.4,5 The accuracies in our study (0.79-0.89) were at the higher end of the spectrum of accuracies presented in earlier studies (0.51-0.91) and were comparable with a recent report investigating a novel clinical prediction scale in a similar cohort (including all acute stroke codes), although additional teleconsultation with a stroke neurologist was incorporated in that study.9,27,28,29 In practice, the preferred sLAVO prediction scale will depend on the local context, which will include such factors as prevalence of sLAVO, differences in transport times between hospitals, in-hospital performance metrics, and local policies.30,31
Although differences among the scales in accuracies were small, in larger populations, these small differences may result in clinically meaningful outcomes. Taking into account median delays associated with transferring patients with sLAVO between a PSC and a CSC (ie, 53 minutes in our cohort) against a background of relatively small distances between a PSC and CSC (approximately 10 minutes’ driving time), our hypothetical example showed a clear benefit for reducing delays to reperfusion treatment when using a sLAVO prediction scale in the ambulance setting. Despite local policy to always allocate a patient with an acute stroke code to the nearest hospital, in our cohort, a relatively high proportion of patients with sLAVO were allocated directly to a CSC (79.7% vs 59.5% of patients without sLAVO). In addition, the use of LAMS would have resulted in meaningful improvements in patient logistics.
Our study also adds important data on feasibility of sLAVO prediction scales. We show that by using all available information, additional acute stroke codes for patients with items that were missing or untestable could still be included, which resulted in higher feasibility rates for all scales.
The PASS scale had the highest reconstruction rate, probably as a result of fewer items that needed to be assessed compared with the other scales. The RACE scale had the lowest feasibility rates, although the cutoff reconstruction rate was much higher than the full reconstruction rate (78.1% vs 57.2%). The feasibility of RACE was shown to be low (40%) in the first study published using this scale,15 but increased to 78% in a later study.22 This suggests that feasibility may improve once EMS paramedics become more familiar with a scale. Focused training aimed at the items missing most frequently (ie, motor deficits in arm or legs) would improve this rate substantially.
Limitations
Our study has some limitations. First, the application was not used in 26.7% of acute stroke codes. However, acute stroke codes without application data differed only in final diagnosis, whereas stroke severity and percentage of sLAVO were comparable between both groups; therefore, we do not think that this lack of application data biased our results. Second, we selected the clinical sLAVO prediction scales based on a previous report,8 but other scales have been developed since our study onset. We do not believe that performance outcomes will differ greatly because clinical assessment of the scale items showed considerable overlap with the scales that we tested and reported accuracies are comparable to our findings.32,33,34,35 Third, we used the presence of sLAVO as assessed on computed tomography angiography performed by the radiologist in the local hospital although we did not have these images available for centralized review. Therefore, it is possible that we missed some patients with sLAVO. However, sLAVO detection is high across all levels of radiologist training according to a previous report,36 and during the present study, computed tomography angiography was part of the routine workup for patients with acute stroke codes in all hospitals. Fourth, to reconstruct all scale items for each separate scale according to our instructions, the applications we used contained more items than the original scales, which could have negatively influenced the scale’s feasibility.
Conclusions
Our study is the first to our knowledge to provide external validation in the field as well as to offer head-to-head comparisons of several established sLAVO prediction scales. Our results indicate that these scales had good diagnostic accuracies, with LAMS and RACE showing the highest accuracies. Scale feasibility rates ranged from 78% to 88%, and it is important to take feasibility into account before implementing a prediction scale in the field because focused training could substantially increase these rates.
eTable 1. Comparing Patients With and Without Application Data
eTable 2. Stroke Logistics and In-Hospital Performance Metrics in PSC vs CSC Presented Patients
eTable 3. Comparing Accuracies of the Prediction Scales According to Prespecified Cut Points
eTable 4. Comparison of Full Range Accuracy of the Prediction Scales
eFigure. Allocation of Acute Stroke Code Patients
References
- 1.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 3.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Coote S, Pesavento L, et al. Large vessel occlusion scales increase delivery to endovascular centers without excessive harm from misclassifications. Stroke. 2017;48(3):568-573. doi: 10.1161/STROKEAHA.116.016056 [DOI] [PubMed] [Google Scholar]
- 5.Dozois A, Hampton L, Kingston CW, et al. PLUMBER Study (Prevalence of Large Vessel Occlusion Strokes in Mecklenburg County Emergency Response). Stroke. 2017;48(12):3397-3399. doi: 10.1161/STROKEAHA.117.018925 [DOI] [PubMed] [Google Scholar]
- 6.Froehler MT, Saver JL, Zaidat OO, et al. ; STRATIS Investigators . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation. 2017;136(24):2311-2321. doi: 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venema E, Groot AE, Lingsma HF, et al. Effect of interhospital transfer on endovascular treatment for acute ischemic stroke. Stroke. 2019;50(4):923-930. doi: 10.1161/STROKEAHA.118.024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koster GT, Nguyen TTM, van Zwet EW, et al. Clinical prediction of thrombectomy eligibility: A systematic review and 4-item decision tree. Int J Stroke. 2019;14(5):530-539. doi: 10.1177/1747493018801225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith EE, Kent DM, Bulsara KR, et al. ; American Heart Association Stroke Council . Accuracy of prediction instruments for diagnosing large vessel occlusion in individuals with suspected stroke: a systematic review for the 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke. Stroke. 2018;49(3):e111-e122. doi: 10.1161/STR.0000000000000160 [DOI] [PubMed] [Google Scholar]
- 10.Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799. doi: 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prehospital prediction of large anterior vessel occlusion (PHPLAVO). ClinicalTrials.gov identifier: NCT04442659. Updated June 22, 2020. Accessed October 12, 2020. https://clinicaltrials.gov/ct2/show/NCT04442659
- 12.Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol. 2005;58(5):475-483. doi: 10.1016/j.jclinepi.2004.06.017 [DOI] [PubMed] [Google Scholar]
- 13.Collins GS, Ogundimu EO, Altman DG. Sample size considerations for the external validation of a multivariable prognostic model: a resampling study. Stat Med. 2016;35(2):214-226. doi: 10.1002/sim.6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazliel B, Starkman S, Liebeskind DS, et al. A brief prehospital stroke severity scale identifies ischemic stroke patients harboring persisting large arterial occlusions. Stroke. 2008;39(8):2264-2267. doi: 10.1161/STROKEAHA.107.508127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45(1):87-91. doi: 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 16.Katz BS, McMullan JT, Sucharew H, Adeoye O, Broderick JP. Design and validation of a prehospital scale to predict stroke severity: Cincinnati Prehospital Stroke Severity Scale. Stroke. 2015;46(6):1508-1512. doi: 10.1161/STROKEAHA.115.008804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastrup S, Damgaard D, Johnsen SP, Andersen G. Prehospital Acute Stroke Severity scale to predict large artery occlusion: design and comparison with other scales. Stroke. 2016;47(7):1772-1776. doi: 10.1161/STROKEAHA.115.012482 [DOI] [PubMed] [Google Scholar]
- 18.Scheitz JF, Abdul-Rahim AH, MacIsaac RL, et al. ; SITS Scientific Committee . Clinical selection strategies to identify ischemic stroke patients with large anterior vessel occlusion: results from SITS-ISTR (Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Registry). Stroke. 2017;48(2):290-297. doi: 10.1161/STROKEAHA.116.014431 [DOI] [PubMed] [Google Scholar]
- 19.Lima FO, Silva GS, Furie KL, et al. Field Assessment Stroke Triage for Emergency Destination: a simple and accurate prehospital scale to detect large vessel occlusion strokes. Stroke. 2016;47(8):1997-2002. doi: 10.1161/STROKEAHA.116.013301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrera D, Gorchs M, Querol M, et al. ; Catalan Stroke Code and Reperfusion Consortium (Cat-SCR) . Revalidation of the RACE scale after its regional implementation in Catalonia: a triage tool for large vessel occlusion. J Neurointerv Surg. 2019;11(8):751-756. doi: 10.1136/neurintsurg-2018-014519 [DOI] [PubMed] [Google Scholar]
- 21.Carr K, Yang Y, Roach A, Shivashankar R, Pasquale D, Serulle Y. Mechanical revascularization in the era of the Field Assessment Stroke Triage for Emergency Destination (FAST-ED): a retrospective cohort assessment in a community stroke practice. J Stroke Cerebrovasc Dis. 2020;29(1):104472. doi: 10.1016/j.jstrokecerebrovasdis.2019.104472 [DOI] [PubMed] [Google Scholar]
- 22.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862. doi: 10.1161/01.STR.31.4.858 [DOI] [PubMed] [Google Scholar]
- 23.Kruyt ND, Nederkoorn PJ, Dennis M, et al. Door-to-needle time and the proportion of patients receiving intravenous thrombolysis in acute ischemic stroke: uniform interpretation and reporting. Stroke. 2013;44(11):3249-3253. doi: 10.1161/STROKEAHA.113.001885 [DOI] [PubMed] [Google Scholar]
- 24.Li F, He H. Assessing the accuracy of diagnostic tests. Shanghai Arch Psychiatry. 2018;30(3):207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19(4):203-211. [PMC free article] [PubMed] [Google Scholar]
- 26.Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health Stroke Scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157. doi: 10.1161/STROKEAHA.111.000604 [DOI] [PubMed] [Google Scholar]
- 27.Vidale S, Agostoni E. Prehospital stroke scales and large vessel occlusion: a systematic review. Acta Neurol Scand. 2018;138(1):24-31. doi: 10.1111/ane.12908 [DOI] [PubMed] [Google Scholar]
- 28.Antipova D, Eadie L, Macaden A, Wilson P. Diagnostic accuracy of clinical tools for assessment of acute stroke: a systematic review. BMC Emerg Med. 2019;19(1):49. doi: 10.1186/s12873-019-0262-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazya MV, Berglund A, Ahmed N, et al. Implementation of a prehospital stroke triage system using symptom severity and teleconsultation in the Stockholm Stroke Triage Study. JAMA Neurol. 2020;77(6):691-699. doi: 10.1001/jamaneurol.2020.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 31.Ahmed N, Audebert H, Turc G, et al. Consensus statements and recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 11-13 November 2018. Eur Stroke J. 2019;4(4):307-317. doi: 10.1177/2396987319863606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H, Pesavento L, Coote S, et al. Ambulance clinical triage for acute stroke treatment: paramedic triage algorithm for large vessel occlusion. Stroke. 2018;49(4):945-951. doi: 10.1161/STROKEAHA.117.019307 [DOI] [PubMed] [Google Scholar]
- 33.Gong X, Chen Z, Shi F, et al. Conveniently-Grasped Field Assessment Stroke Triage (CG-FAST): a modified scale to detect large vessel occlusion stroke. Front Neurol. 2019;10:390. doi: 10.3389/fneur.2019.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Václavík D, Bar M, Klečka L, Holeš D, Čábal M, Mikulík R. Prehospital stroke scale (FAST PLUS Test) predicts patients with intracranial large vessel occlusion. Brain Behav. 2018;8(9):e01087. doi: 10.1002/brb3.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidale S, Arnaboldi M, Frangi L, Longoni M, Monza G, Agostoni E. The Large Artery Intracranial Occlusion Stroke Scale: a new tool with high accuracy in predicting large vessel occlusion. Front Neurol. 2019;10:130. doi: 10.3389/fneur.2019.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyd CA, Jayaraman MV, Baird GL, et al. Detection of emergent large vessel occlusion stroke with CT angiography is high across all levels of radiology training and grayscale viewing methods. Eur Radiol. 2020;30(8):4447-4453. doi: 10.1007/s00330-020-06814-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparing Patients With and Without Application Data
eTable 2. Stroke Logistics and In-Hospital Performance Metrics in PSC vs CSC Presented Patients
eTable 3. Comparing Accuracies of the Prediction Scales According to Prespecified Cut Points
eTable 4. Comparison of Full Range Accuracy of the Prediction Scales
eFigure. Allocation of Acute Stroke Code Patients