ABSTRACT
Background
High-quality diets reduce the risk of cardiometabolic and other chronic diseases. The dietary components that distinguish higher from lower quality diets, and their associations with health, have not been fully investigated.
Objectives
This study aimed to assess the component scores that underlie differences in total Healthy Eating Index (HEI)–2015 scores, quantify fatty acid (saturated, monounsaturated, polyunsaturated) intakes that comprise Fatty Acids component scores, and assess associations between component scores and cardiometabolic risk factors.
Methods
A cross-sectional analysis of data from the NHANES (2001–2016) was conducted. Total and component HEI-2015 scores were assessed in adult (≥19 y) participants who provided one 24-h dietary recall (n = 39,799). Survey-weighted mean component scores by quartile of total HEI-2015 score were determined. Regression analyses were conducted to assess fatty acid intakes across quartiles of Fatty Acids component scores. Separate regression analyses were conducted to assess associations between component scores and cardiometabolic risk factors, after adjusting for demographic characteristics and health behaviors.
Results
Scores for components related to dietary fat (Fatty Acids, Saturated Fats) and grain quality (Whole Grains, Refined Grains) accounted for the greatest differences in HEI-2015 scores. Higher Fatty Acids scores were primarily composed of lower saturated and greater polyunsaturated fat intakes. Whole Fruits, and Seafood and Plant Proteins, were most favorably associated with cardiometabolic risk factors including anthropometric measures (P < 0.001), systolic blood pressure (P < 0.01), glycemic markers (Whole Fruits only, P < 0.01), and HDL cholesterol and triglycerides (Seafood and Plant Proteins only, P < 0.001).
Conclusions
Average diet quality in US adults is suboptimal. Higher quality diets are primarily distinguished by the types of fats and grain-based foods that are consumed. Interventions targeting dietary components that are most favorably associated with cardiometabolic risk factors—whole fruits, seafood, and plant proteins—may have the greatest impact on disease risk.
Keywords: HEI-2015, diet quality, NHANES, cardiometabolic, cholesterol, dietary pattern
This study assessed component scores that underlie differences in total Healthy Eating Index (HEI)–2015 scores and assessed their associations with cardiometabolic risk factors in US adults.
Introduction
A high-quality dietary pattern provides essential nutrients to promote health within energy requirements by balancing intakes of a variety of nutrient-rich foods and limiting empty calorie sources. A commonly used tool to assess diet quality is the Healthy Eating Index (HEI), which scores dietary alignment with the Dietary Guidelines for Americans (DGA). Higher HEI scores—representing better adherence to the guidelines—have been prospectively associated with lower incident cardiovascular disease (CVD) (1), type 2 diabetes (2), and all-cause, CVD, and cancer mortality in US adults (1–4).
The most recent version, HEI-2015, is calculated as the sum of 13 individually scored components that assess alignment with specific recommendations in the 2015–2020 DGA to consume or limit certain foods and/or nutrients (5). Components are generally weighted equally at a maximum of 10 points each, with some components (vegetables, fruits, and proteins) divided into smaller 5-point subgroups, to represent importance of all dietary recommendations (5). Component scores sum to a maximum of 100 points for a diet that fully aligns with recommendations. Moderate HEI-2015 scores could indicate moderate alignment with guidelines on all components or full alignment with some and complete misalignment (0 score) with others. Examination of the component scores that comprise individuals’ total HEI scores is recommended to better understand the dietary pattern represented by the summative scores (6). However, associations between diet quality and disease risk are typically investigated using only the total HEI-2015 score, which obscures any differences in the strength of associations with particular dietary components(7). Given the diverse nutrient and bioactive profiles of the foods that make up each HEI-2015 component, associations with cardiometabolic disease risk likely differ by component, such that adherence to particular dietary recommendations more strongly explains the diet–disease relation. To the authors’ knowledge, the independent associations between individual HEI-2015 components and cardiometabolic risk factors have not yet been evaluated.
Furthermore, one of the components, Fatty Acids, is a composite score representing the ratio of unsaturated fatty acids to SFAs. The standard for a maximum score (10 points) is a ratio of ≥2.5, whereas a ratio of ≤1.2 receives zero points, regardless of the types of unsaturated fats (monounsaturated vs. polyunsaturated) consumed. The physiological effects of fatty acids vary by type, with the greatest atherogenic lipid-lowering and coronary heart disease risk reductions resulting from replacement of saturated fats with polyunsaturated fats (8–10). Yet, under the HEI-2015 scoring criteria, monounsaturated and polyunsaturated fats are valued equally. Thus, the differences in fat subtype intakes that underlie differences in Fatty Acids component scores in the population should be assessed to better understand associations between this HEI component and cardiometabolic health.
Understanding which foods and nutrients contribute most to higher diet quality scores, and how they are associated with risk factors for cardiometabolic diseases, can guide policies and interventions to improve Americans’ diets and help protect against such diseases. Therefore, the goals of this study were as follows: 1) to describe the differences in component scores that distinguish higher versus lower quality diets as scored by the HEI-2015, 2) quantify intakes of dietary fats (saturated, monounsaturated, and polyunsaturated) that comprise the Fatty Acids component scores, and 3) determine the associations between HEI-2015 component scores and cardiometabolic risk factors in US adults using data from the NHANES 2001–2016.
Methods
Study overview, study population, and analytic sample
Data from adults ≥19 y of age (n = 46,236) participating in NHANES 2001–2016 were used after the exclusion of individuals who did not provide a reliable in-person dietary recall (n = 5008) and pregnant or lactating females (n = 1547), for a final analytic sample of n = 39,799. A description of NHANES and analytical methods is available online (11, 12). Use of human subjects for NHANES has been approved by the National Center for Health Statistics Research Ethics Review Board and subjects consented to participate (13). Since the current study was a secondary data analysis lacking personal identifiers, additional institutional review board approval was not required.
Sample characteristics
Demographic and lifestyle characteristics were self-reported and included age, gender, race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other), family income-to-poverty guideline ratio [poverty-income ratio (PIR); <1.35, 1.35 to 1.85, and >1.85], physical activity (sedentary, moderate, and vigorous as developed from questionnaire responses), and smoking status (current, former, and never). Use of antihypertension, lipid-lowering, and hypoglycemic medications was self-reported (14).
Dietary intake data
Dietary data were based on a single 24-h dietary recall collected in person using the automated multiple-pass method (15). Detailed descriptions of the dietary recalls and data collection are available in the NHANES dietary data documentation (16). Energy and nutrient intakes from foods were determined using the Food and Nutrient Database for Dietary Studies (FNDDS) appropriate for each NHANES cycle (17), which are available from total nutrient intake files in the NHANES datasets. Food components (total vegetables, total fruit, etc.) and added sugars were determined using the USDA MyPyramid Patterns Equivalent Databases (for NHANES 2001–2004) and relevant Food Patterns Equivalent Databases for later NHANES cycles (18).
HEI-2015 scores were calculated for each person individually according to the simple scoring method, using an SAS program available on the website of the National Cancer Institute (19). Nine components score the adequacy of intakes for foods that are encouraged (Total Fruits, Whole Fruits, Total Vegetables, Greens and Beans, Whole Grains, Dairy, Total Protein Foods, Seafood and Plant Proteins, and Fatty Acids). The remaining 4 components (Refined Grains, Sodium, Added Sugars, and Saturated Fats) assess moderation, awarding maximum points for intakes that are at or below recommended limits. Detailed information on the development of the components, scoring standards, and density approach for the HEI-2015 has been described previously (4, 5, 20, 21). The construct validity, reliability, and criterion validity of the HEI-2015 have been established (4).
Body-weight and laboratory parameters
Height, weight, and waist circumference were obtained according to NHANES protocols (22). BMI was calculated as body weight (kilograms) divided by height (meters) squared. Systolic and diastolic blood pressures were determined using standard NHANES protocols (23). Laboratory methods for measuring total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, fasting blood glucose, and fasting insulin are detailed online (24). HOMA-IR was calculated as plasma insulin (mU/L) × plasma glucose (mmol/L)/22.5 (25).
Statistical analyses
Analyses were adjusted for the complex sampling design of NHANES and incorporated appropriate sample weights as advised by the NHANES analytical guidelines (12). All analyses were performed using SAS release 9.4 (SAS Institute) (26). Subjects were divided into quartiles based on total HEI scores and each component score (for 2 component scores, Greens and Beans and Total Protein Foods, only 3 groups could be created). Means/percentages and SEs of demographic data and total HEI and component scores by quartiles of total HEI score were determined. Regression analyses were used to assess whether total HEI scores and component scores were associated with body weight and laboratory parameters. Covariates in regression models included age; sex; race/ethnicity; family PIR; physical activity level; current smoking status; use of medications to lower blood pressure, lipids, or glucose; and BMI (excluding models for BMI and waist circumference). Further adjustment for total energy intake (kilocalories per day) did not substantively alter estimates and, thus, was not included in the final model. Least-squares means and SEs were output for quartiles of total HEI scores and component scores. Separate regression analyses were conducted to assess the intake of fat type (i.e., saturated, monounsaturated, and polyunsaturated) across quartiles of the HEI Fatty Acids component scores. A P value < 0.01 was considered significant.
Results
Sample characteristics
The mean total HEI-2015 scores in quartiles 1, 2, 3, and 4 were 33.82, 45.27, 54.42, and 68.37, respectively (Table 1). Adults with higher quality diets were, on average, older and reported higher family incomes relative to the poverty line; greater use of medications for lowering blood pressure, lipids, and glucose; greater physical activity; and lower smoking rates, compared with those with lower quality diets. The proportion of adults who identified as non-Hispanic Black or Mexican American declined across increasing total HEI-2015 score.
TABLE 1.
Demographic and lifestyle characteristics by quartile of HEI-2015 scores in nonpregnant, nonlactating US adults aged ≥19 y in NHANES 2001–20161
| HEI-2015 quartiles | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Unweighted n | 9945 | 10,018 | 10,071 | 9765 |
| Mean HEI | 33.82 | 45.27 | 54.42 | 68.37 |
| Sex, % | ||||
| Male | 52.5 | 50.9 | 49.6 | 44.2 |
| Female | 47.5 | 49.1 | 50.4 | 55.8 |
| Age, y | 43 | 46 | 48 | 52 |
| Race/ethnicity, % | ||||
| Non-Hispanic White | 68.8 | 67.9 | 68.3 | 70.9 |
| Non-Hispanic Black | 13.2 | 12.8 | 11.0 | 8.3 |
| Mexican American | 8.3 | 8.6 | 8.8 | 7.2 |
| Other Hispanic | 4.7 | 4.9 | 5.1 | 5.2 |
| Other race | 5.0 | 5.8 | 6.8 | 8.3 |
| Poverty-income ratio, % | ||||
| <1.35 | 29.2 | 24.4 | 22.5 | 16.4 |
| 1.35–1.85 | 10.3 | 10.0 | 10.0 | 8.7 |
| >1.85 | 60.5 | 65.6 | 67.5 | 74.9 |
| Antihypertensive medication, % | ||||
| Yes | 22.9 | 25.5 | 28.0 | 31.0 |
| No | 77.0 | 74.5 | 72.0 | 69.0 |
| Lipid-lowering medication, % | ||||
| Yes | 13.3 | 15.8 | 16.9 | 20.1 |
| No | 86.7 | 84.2 | 83.1 | 79.9 |
| Hypoglycemic medication, % | ||||
| Yes | 6.8 | 7.3 | 8.6 | 8.5 |
| No | 93.2 | 92.7 | 91.4 | 91.5 |
| Physical activity, % | ||||
| Sedentary | 29.0 | 28.3 | 26.0 | 20.9 |
| Moderate | 33.0 | 35.3 | 35.5 | 37.0 |
| Vigorous | 38.0 | 36.4 | 38.5 | 42.1 |
| Smoking, % | ||||
| Current | 30.5 | 24.4 | 18.6 | 9.9 |
| Former | 44.6 | 48.7 | 53.1 | 58.1 |
| Never | 24.7 | 26.7 | 28.2 | 32.0 |
Percentages are survey weighted. HEI, Healthy Eating Index; Q, quartile.
Diet quality: total and component scores
The mean HEI-2015 score was 50.47 ± 0.17 (median: 49.75 ± 0.20). All individual component scores increased significantly with total HEI-2015 score (all P < 0.001) (Figure 1A; Supplemental Table 1). Across the range of total HEI-2015 scores, components related to fat and grain quality exhibited the greatest differences. Each 1-point higher HEI-2015 score was linearly associated with a 0.12-point greater Fatty Acids component score, indicating greater consumption of unsaturated fats relative to saturated fats, and a 0.12-point greater Whole Grains component score, indicating greater consumption of whole grains. Each 1-point higher HEI-2015 score was also associated with a 0.11-point greater score for Saturated Fats and Refined Grains, indicating lower intakes of these foods/nutrients. In contrast, the Dairy and Total Protein Foods components changed least (0.02 point) per 1-point higher total HEI-2015 score. Expressed as percentages of maximum component scores, Total Fruits and Whole Fruits exhibited the greatest variation across the distribution of total HEI-2015 scores, while Dairy and Total Protein Foods varied least (Figure 1B).
FIGURE 1.
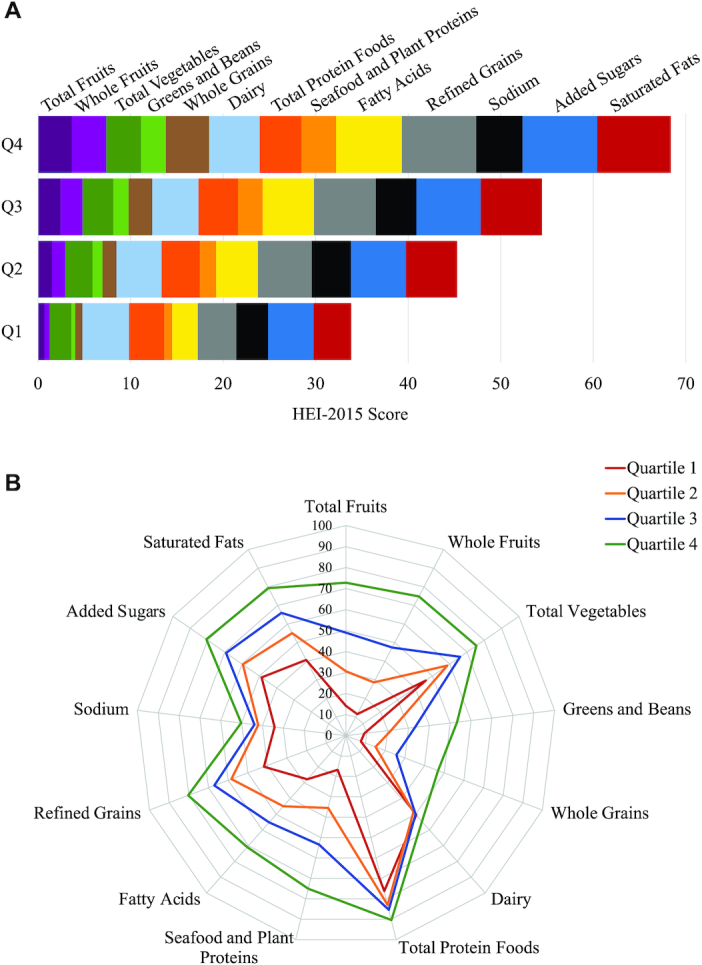
Mean component scores by quartile of total HEI-2015 scores in nonpregnant, nonlactating US adults aged ≥19 y in NHANES 2001–2016 (A) and expressed as percentages of maximum possible component scores (B). HEI, Healthy Eating Index; Q, quartile.
Fat-composition differences underlying the Fatty Acids component score
The mean Fatty Acids component score was 4.97 ± 0.03 (out of maximum of 10). Each 1-point greater Fatty Acids score was associated with 1.7-g lower saturated fat intake and 1.2-g greater intake of polyunsaturated fat (Table 2), which was mostly linoleic acid (18:2n–6; 1.1 g/point). Monounsaturated fat intake was 0.2-g greater per point. The leading food sources of these fats differed for individuals with the highest, versus lowest, quartile of Fatty Acids component scores (Supplemental Table 2). For the highest quartile, nuts and seeds were the primary sources of all fat types. Salad dressings and vegetable oils were also major sources of polyunsaturated fats in the highest quartile. In the lowest quartile of Fatty Acids component scores, pizza was the leading source of both polyunsaturated and monounsaturated fats, while cheese was the top source of saturated fat and closely followed pizza as a major source of monounsaturated fats.
TABLE 2.
Mean fat intakes by quartile of Fatty Acids component scores in nonpregnant, nonlactating US adults aged ≥19 y in NHANES 2001–20161
| Fatty Acids component score quartiles | Linear trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ß ± SE | P | |
| Fatty Acids score2 | 0.43 ± 0.01 | 3.12 ± 0.01 | 6.47 ± 0.02 | 9.87 ± 0.005 | ||
| Total fat, g | 81.7 ± 0.7 | 86.4 ± 0.7 | 84.3 ± 0.6 | 77.7 ± 0.8 | −0.5 ± 0.09 | <0.001 |
| Saturated fat, g | 34.3 ± 0.3 | 30.2 ± 0.2 | 25.4 ± 0.2 | 18.2 ± 0.2 | −1.7 ± 0.03 | <0.001 |
| Monounsaturated fat, g | 27.4 ± 0.3 | 31.3 ± 0.3 | 31.3 ± 0.2 | 29.4 ± 0.3 | 0.2 ± 0.04 | <0.001 |
| Polyunsaturated fat, g | 12.2 ± 0.1 | 17.0 ± 0.1 | 20.2 ± 0.2 | 23.6 ± 0.2 | 1.2 ± 0.02 | <0.001 |
| Linoleic acid, g | 10.6 ± 0.1 | 15.0 ± 0.1 | 17.9 ± 0.2 | 21.0 ± 0.2 | 1.1 ± 0.02 | <0.001 |
Values are means ± SEs. Q, quartile.
Scored out of a maximum of 10 points, based on the ratio of total unsaturated fats (monounsaturated and polyunsaturated) to saturated fats.
Associations between total diet quality and cardiometabolic risk factors
Diet quality was significantly associated with several cardiometabolic risk factors, after adjustment for demographic factors and health behaviors. Total HEI-2015 scores were inversely associated with BMI, waist circumference, systolic blood pressure (SBP), LDL cholesterol, triglycerides, glucose, and insulin and were associated with greater HDL cholesterol (all P < 0.01) (Table 3). These risk factors were carried forth to determine associations between individual HEI-2015 component scores and cardiometabolic risk.
TABLE 3.
Cardiometabolic risk factors by quartile of HEI-2015 in nonpregnant, nonlactating US adults aged ≥19 y in NHANES 2001–20161
| HEI-2015 quartiles | Linear trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ß ± SE | P | |
| BMI, kg/m2 | 29.51 ± 0.12 | 28.97 ± 0.10 | 28.55 ± 0.10 | 27.68 ± 0.08 | −0.05 ± 0.003 | <0.001 |
| Waist circumference, cm | 100.19 ± 0.27 | 98.97 ± 0.24 | 97.83 ± 0.25 | 95.66 ± 0.19 | 0.13 ± 0.01 | <0.001 |
| SBP, mm Hg | 123.14 ± 0.29 | 122.64 ± 0.25 | 122.25 ± 0.23 | 121.90 ± 0.24 | −0.04 ± 0.01 | <0.001 |
| DBP, mm Hg | 71.27 ± 0.22 | 71.25 ± 0.19 | 71.11 ± 0.21 | 70.84 ± 0.23 | −0.01 ± 0.01 | 0.18 |
| Total cholesterol, mg/dL | 196.59 ± 0.57 | 197.68 ± 0.60 | 196.72 ± 0.70 | 195.14 ± 0.66 | −0.06 ± 0.02 | 0.02 |
| LDL-C, mg/dL | 116.51 ± 0.77 | 116.45 ± 0.73 | 115.79 ± 0.78 | 113.90 ± 0.82 | −0.09 ± 0.03 | 0.005 |
| HDL-C, mg/dL | 51.47 ± 0.24 | 52.62 ± 0.23 | 53.33 ± 0.26 | 54.81 ± 0.24 | 0.10 ± 0.01 | <0.001 |
| Triglycerides, mg/dL | 137.84 ± 2.24 | 135.07 ± 2.37 | 136.58 ± 2.80 | 128.60 ± 1.88 | −0.20 ± 0.08 | 0.009 |
| Glucose, mg/dL | 105.97 ± 0.48 | 105.55 ± 0.49 | 104.24 ± 0.46 | 104.49 ± 0.58 | −0.05 ± 0.02 | 0.009 |
| Insulin, µU/mL | 13.76 ± 0.27 | 13.19 ± 0.27 | 12.93 ± 0.29 | 12.65 ± 0.20 | −0.03 ± 0.01 | 0.001 |
| HOMA-IR | 3.89 ± 0.10 | 3.70 ± 0.10 | 3.56 ± 0.09 | 3.61 ± 0.09 | −0.01 ± 0.004 | 0.02 |
Values are means ± SEs. Covariates include age, gender, race/ethnicity, poverty-income ratio, physical activity level, current smoking status, antihypertensive medication, lipid-lowering medication, hypoglycemic medication, and BMI (excluding models for BMI and waist circumference). DBP, diastolic blood pressure; HDL-C, HDL cholesterol; HEI, Healthy Eating Index; LDL-C, LDL cholesterol; Q, quartile; SBP, systolic blood pressure.
Associations between individual diet quality components and cardiometabolic risk factors
Adiposity measures
All components except for Dairy, Total Protein Foods, and Added Sugars were inversely associated with either BMI, waist circumference, or both measures of body composition (Tables 4 and 5). The greatest incremental differences in BMI and waist circumference were observed for the Total Fruits component score [BMI (in kg/m2): −0.27 per point, P < 0.001; waist circumference: −0.71 cm per point, P < 0.001], followed by the Whole Fruits component (BMI: −0.23 per point, P < 0.001; waist circumference: −0.59 cm per point, P < 0.001). In contrast, greater Total Protein Foods scores were associated with higher BMI (0.17 per point, P < 0.001) and waist circumference (0.28 cm per point, P = 0.005).
TABLE 4.
Mean cardiometabolic risk factors by quartiles of HEI-2015 adequacy component scores in nonpregnant, nonlactating US adults aged ≥19 y in NHANES 2001–20161
| Component score quartiles | Linear trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ß ± SE | P | |
| Total Fruit (52) | 0.01 ± 0.002 | 0.49 ± 0.01 | 2.93 ± 0.02 | 4.98 ± 0.002 | ||
| BMI, kg/m2 | 29.49 ± 0.09 | 28.84 ± 0.12 | 28.33 ± 0.11 | 27.92 ± 0.11 | −0.27 ± 0.02 | <0.001 |
| Waist circumference, cm | 100.22 ± 0.23 | 98.61 ± 0.28 | 97.36 ± 0.25 | 96.14 ± 0.23 | −0.71 ± 0.05 | <0.001 |
| Systolic blood pressure, mm Hg | 122.76 ± 0.25 | 122.33 ± 0.26 | 122.55 ± 0.26 | 122.22 ± 0.28 | −0.08 ± 0.06 | 0.18 |
| LDL-C, mg/dL | 117.27 ± 0.64 | 115.69 ± 0.90 | 114.20 ± 0.72 | 115.15 ± 0.83 | −0.36 ± 0.17 | 0.04 |
| HDL-C, mg/dL | 53.21 ± 0.22 | 53.14 ± 0.24 | 52.60 ± 0.21 | 53.25 ± 0.26 | −0.03 ± 0.06 | 0.58 |
| Triglycerides, mg/dL | 136.01 ± 2.31 | 132.21 ± 1.92 | 133.36 ± 2.32 | 136.01 ± 2.25 | 0.16 ± 0.58 | 0.78 |
| Glucose, mg/dL | 105.78 ± 0.53 | 105.18 ± 0.47 | 104.67 ± 0.49 | 104.49 ± 0.58 | −0.25 ± 0.14 | 0.08 |
| Insulin, µU/mL | 13.48 ± 0.30 | 13.55 ± 0.34 | 12.82 ± 0.22 | 12.64 ± 0.21 | −0.19 ± 0.07 | 0.006 |
| Whole Fruit (5) | 0.00 ± 0.002 | 0.26 ± 0.004 | 2.60 ± 0.02 | 4.99 ± 0.002 | ||
| BMI, kg/m2 | 29.25 ± 0.08 | 28.63 ± 0.17 | 28.48 ± 0.12 | 27.99 ± 0.11 | −0.23 ± 0.02 | <0.001 |
| Waist circumference, cm | 99.64 ± 0.21 | 97.97 ± 0.41 | 97.66 ± 0.27 | 96.40 ± 0.25 | −0.59 ± 0.05 | <0.001 |
| Systolic blood pressure, mm Hg | 122.92 ± 0.21 | 122.70 ± 0.42 | 122.28 ± 0.27 | 121.94 ± 0.26 | −0.15 ± 0.06 | 0.009 |
| LDL-C, mg/dL | 117.01 ± 0.59 | 112.75 ± 1.37 | 114.49 ± 0.88 | 115.24 ± 0.71 | −0.23 ± 0.15 | 0.13 |
| HDL-C, mg/dL | 53.04 ± 0.18 | 52.72 ± 0.34 | 53.05 ± 0.26 | 53.17 ± 0.26 | 0.01 ± 0.05 | 0.79 |
| Triglycerides, mg/dL | 135.53 ± 1.91 | 130.15 ± 3.59 | 135.12 ± 2.62 | 133.79 ± 2.03 | −0.10 ± 0.52 | 0.85 |
| Glucose, mg/dL | 105.78 ± 0.42 | 105.31 ± 1.06 | 104.71 ± 0.46 | 104.21 ± 0.42 | −0.28 ± 0.11 | 0.009 |
| Insulin, µU/mL | 13.48 ± 0.23 | 13.95 ± 0.75 | 12.91 ± 0.24 | 12.56 ± 0.19 | −0.19 ± 0.06 | 0.002 |
| Total Vegetables (5) | 0.80 ± 0.01 | 2.43 ± 0.01 | 4.03 ± 0.01 | 4.99 ± 0.001 | ||
| BMI, kg/m2 | 28.79 ± 0.10 | 28.82 ± 0.10 | 28.65 ± 0.11 | 28.46 ± 0.12 | −0.08 ± 0.03 | <0.01 |
| Waist circumference, cm | 98.52 ± 0.25 | 98.49 ± 0.23 | 98.19 ± 0.28 | 97.51 ± 0.27 | −0.21 ± 0.08 | 0.006 |
| Systolic blood pressure, mm Hg | 122.54 ± 0.22 | 122.79 ± 0.26 | 122.29 ± 0.26 | 122.32 ± 0.26 | −0.07 ± 0.07 | 0.31 |
| LDL-C, mg/dL | 116.38 ± 0.86 | 114.67 ± 0.79 | 115.56 ± 0.66 | 116.04 ± 0.81 | −0.16 ± 0.24 | 0.50 |
| HDL-C, mg/dL | 52.50 ± 0.21 | 53.00 ± 0.29 | 52.97 ± 0.22 | 53.71 ± 0.28 | 0.26 ± 0.07 | <0.001 |
| Triglycerides, mg/dL | 137.02 ± 3.19 | 133.76 ± 1.99 | 132.44 ± 2.49 | 134.90 ± 2.54 | −0.57 ± 0.80 | 0.48 |
| Glucose, mg/dL | 105.03 ± 0.50 | 105.71 ± 0.44 | 104.16 ± 0.44 | 105.33 ± 0.49 | −0.02 ± 0.14 | 0.90 |
| Insulin, µU/mL | 13.14 ± 0.23 | 13.19 ± 0.23 | 13.32 ± 0.33 | 12.91 ± 0.26 | −0.04 ± 0.08 | 0.57 |
| Greens and Beans (5) | 0.00 ± 0.0004 | —3 | 2.00 ± 0.03 | 4.93 ± 0.003 | ||
| BMI, kg/m2 | 28.85 ± 0.08 | —3 | 28.58 ± 0.16 | 28.28 ± 0.11 | −0.10 ± 0.02 | <0.001 |
| Waist circumference, cm | 98.61 ± 0.19 | —3 | 97.70 ± 0.35 | 97.28 ± 0.26 | −0.26 ± 0.06 | <0.001 |
| Systolic blood pressure, mm Hg | 122.71 ± 0.18 | —3 | 121.86 ± 0.34 | 122.19 ± 0.25 | −0.11 ± 0.05 | 0.03 |
| LDL-C, mg/dL | 115.88 ± 0.49 | —3 | 115.87 ± 1.19 | 115.05 ± 0.80 | −0.21 ± 0.18 | 0.23 |
| HDL-C, mg/dL | 52.52 ± 0.15 | —3 | 53.73 ± 0.38 | 54.09 ± 0.26 | 0.31 ± 0.06 | <0.001 |
| Triglycerides, mg/dL | 137.20 ± 1.71 | —3 | 130.15 ± 3.96 | 129.89 ± 2.01 | −1.55 ± 0.51 | 0.003 |
| Glucose, mg/dL | 105.46 ± 0.32 | —3 | 104.67 ± 0.54 | 104.29 ± 0.54 | −0.22 ± 0.12 | 0.08 |
| Insulin, µU/mL | 13.15 ± 0.16 | —3 | 13.15 ± 0.28 | 13.09 ± 0.26 | 0.00 ± 0.05 | 0.96 |
| Whole Grains (10) | 0.01 ± 0.002 | 0.32 ± 0.01 | 2.18 ± 0.01 | 7.29 ± 0.03 | ||
| BMI, kg/m2 | 28.94 ± 0.08 | 29.29 ± 0.32 | 28.52 ± 0.10 | 28.26 ± 0.10 | −0.10 ± 0.02 | <0.001 |
| Waist circumference, cm | 98.87 ± 0.21 | 99.41 ± 0.70 | 97.80 ± 0.24 | 97.07 ± 0.22 | −0.25 ± 0.03 | <0.001 |
| Systolic blood pressure, mm Hg | 122.80 ± 0.21 | 123.89 ± 0.73 | 122.40 ± 0.25 | 121.82 ± 0.25 | −0.14 ± 0.04 | <0.001 |
| LDL-C, mg/dL | 116.27 ± 0.62 | 113.58 ± 2.45 | 114.80 ± 0.74 | 115.62 ± 0.81 | −0.16 ± 0.13 | 0.19 |
| HDL-C, mg/dL | 52.95 ± 0.21 | 52.97 ± 0.79 | 53.35 ± 0.24 | 52.98 ± 0.25 | 0.01 ± 0.04 | 0.78 |
| Triglycerides, mg/dL | 136.29 ± 1.89 | 132.77 ± 7.13 | 135.06 ± 2.26 | 130.85 ± 1.96 | −0.71 ± 0.30 | 0.02 |
| Glucose, mg/dL | 105.74 ± 0.41 | 102.95 ± 1.08 | 104.65 ± 0.48 | 104.43 ± 0.52 | −0.15 ± 0.08 | 0.08 |
| Insulin, µU/mL | 13.24 ± 0.20 | 11.98 ± 0.70 | 13.22 ± 0.27 | 12.97 ± 0.23 | −0.01 ± 0.04 | 0.83 |
| Dairy (10) | 0.77 ± 0.01 | 3.45 ± 0.01 | 6.43 ± 0.02 | 9.70 ± 0.01 | ||
| BMI, kg/m2 | 28.81 ± 0.10 | 28.70 ± 0.09 | 28.63 ± 0.12 | 28.58 ± 0.10 | −0.03 ± 0.02 | 0.05 |
| Waist circumference, cm | 98.25 ± 0.25 | 98.22 ± 0.23 | 98.08 ± 0.26 | 98.14 ± 0.25 | −0.02 ± 0.03 | 0.50 |
| Systolic blood pressure, mm Hg | 122.73 ± 0.25 | 122.32 ± 0.24 | 122.67 ± 0.21 | 122.21 ± 0.26 | −0.04 ± 0.03 | 0.15 |
| LDL-C, mg/dL | 115.85 ± 0.76 | 115.54 ± 0.82 | 115.19 ± 0.81 | 116.10 ± 0.83 | 0.04 ± 0.12 | 0.74 |
| HDL-C, mg/dL | 53.24 ± 0.21 | 53.28 ± 0.23 | 52.90 ± 0.25 | 52.80 ± 0.26 | −0.06 ± 0.03 | 0.07 |
| Triglycerides, mg/dL | 137.95 ± 3.22 | 133.82 ± 2.16 | 132.31 ± 2.12 | 134.20 ± 1.86 | −0.29 ± 0.35 | 0.41 |
| Glucose, mg/dL | 105.67 ± 0.48 | 105.02 ± 0.50 | 104.86 ± 0.45 | 104.75 ± 0.48 | −0.10 ± 0.07 | 0.15 |
| Insulin, µU/mL | 13.05 ± 0.32 | 12.97 ± 0.25 | 13.26 ± 0.23 | 13.26 ± 0.22 | 0.02 ± 0.04 | 0.55 |
| Total Protein Foods (5) | 2.19 ± 0.01 | 4.33 ± 0.01 | —4 | 5.00 ± 0.001 | ||
| BMI, kg/m2 | 28.39 ± 0.11 | 28.48 ± 0.11 | —4 | 28.86 ± 0.09 | 0.17 ± 0.04 | <0.001 |
| Waist circumference, cm | 97.71 ± 0.27 | 98.07 ± 0.27 | —4 | 98.39 ± 0.20 | 0.28 ± 0.10 | 0.005 |
| Systolic blood pressure, mm Hg | 122.75 ± 0.27 | 122.49 ± 0.32 | —4 | 122.37 ± 0.16 | −0.11 ± 0.08 | 0.19 |
| LDL-C, mg/dL | 115.52 ± 0.87 | 115.36 ± 0.92 | —4 | 115.81 ± 0.50 | 0.26 ± 0.31 | 0.41 |
| HDL-C, mg/dL | 52.37 ± 0.27 | 52.94 ± 0.29 | —4 | 53.38 ± 0.18 | 0.44 ± 0.10 | <0.001 |
| Triglycerides, mg/dL | 134.55 ± 2.18 | 139.37 ± 2.99 | —4 | 133.22 ± 1.72 | −0.58 ± 0.86 | 0.50 |
| Glucose, mg/dL | 105.15 ± 0.49 | 104.90 ± 0.61 | —4 | 105.09 ± 0.33 | 0.11 ± 0.19 | 0.58 |
| Insulin, µU/mL | 13.01 ± 0.23 | 13.11 ± 0.28 | —4 | 13.19 ± 0.17 | 0.12 ± 0.08 | 0.12 |
| Seafood and Plant Proteins (5) | 0.00 ± 0.001 | 0.64 ± 0.01 | 3.03 ± 0.02 | 5.00 ± 0.001 | ||
| BMI, kg/m2 | 29.11 ± 0.10 | 28.76 ± 0.17 | 28.54 ± 0.10 | 28.22 ± 0.09 | −0.16 ± 0.02 | <0.001 |
| Waist circumference, cm | 99.34 ± 0.22 | 98.20 ± 0.40 | 97.68 ± 0.24 | 97.07 ± 0.21 | −0.40 ± 0.05 | <0.001 |
| Systolic blood pressure, mm Hg | 122.96 ± 0.21 | 122.70 ± 0.39 | 122.37 ± 0.31 | 121.93 ± 0.22 | −0.22 ± 0.05 | <0.001 |
| LDL-C, mg/dL | 116.16 ± 0.68 | 114.96 ± 1.08 | 116.12 ± 1.00 | 115.15 ± 0.61 | −0.23 ± 0.16 | 0.14 |
| HDL-C, mg/dL | 52.29 ± 0.18 | 52.51 ± 0.35 | 53.08 ± 0.26 | 54.08 ± 0.24 | 0.34 ± 0.05 | <0.001 |
| Triglycerides, mg/dL | 138.88 ± 1.91 | 138.92 ± 4.09 | 130.76 ± 2.63 | 130.23 ± 2.07 | −1.89 ± 0.51 | <0.001 |
| Glucose, mg/dL | 105.60 ± 0.33 | 104.84 ± 0.77 | 104.37 ± 0.51 | 104.92 ± 0.42 | −0.16 ± 0.09 | 0.08 |
| Insulin, µU/mL | 13.21 ± 0.23 | 13.28 ± 0.31 | 13.15 ± 0.39 | 13.00 ± 0.17 | −0.07 ± 0.05 | 0.11 |
| Fatty Acids (10) | 0.44 ± 0.01 | 3.12 ± 0.01 | 6.47 ± 0.02 | 9.87 ± 0.01 | ||
| BMI, kg/m2 | 28.74 ± 0.11 | 28.87 ± 0.11 | 28.65 ± 0.11 | 28.46 ± 0.10 | −0.03 ± 0.01 | 0.01 |
| Waist circumference, cm | 98.60 ± 0.26 | 98.59 ± 0.26 | 98.07 ± 0.25 | 97.41 ± 0.24 | −0.13 ± 0.03 | <0.001 |
| Systolic blood pressure, mm Hg | 122.86 ± 0.29 | 122.44 ± 0.27 | 122.28 ± 0.23 | 122.35 ± 0.24 | −0.06 ± 0.03 | 0.07 |
| LDL-C, mg/dL | 116.56 ± 0.78 | 116.26 ± 0.86 | 115.29 ± 0.82 | 114.56 ± 0.77 | −0.24 ± 0.11 | 0.02 |
| HDL-C, mg/dL | 52.54 ± 0.23 | 52.65 ± 0.23 | 53.11 ± 0.21 | 53.93 ± 0.25 | 0.14 ± 0.03 | <0.001 |
| Triglycerides, mg/dL | 137.22 ± 2.18 | 134.35 ± 2.29 | 136.64 ± 2.10 | 129.89 ± 2.75 | −0.58 ± 0.31 | 0.07 |
| Glucose, mg/dL | 105.35 ± 0.46 | 105.54 ± 0.46 | 104.80 ± 0.44 | 104.60 ± 0.44 | −0.10 ± 0.06 | 0.09 |
| Insulin, µU/mL | 13.22 ± 0.23 | 13.41 ± 0.27 | 12.94 ± 0.25 | 12.97 ± 0.27 | −0.03 ± 0.03 | 0.29 |
Values are means ± SEs. Covariates include age, gender, ethnicity, poverty-income ratio, physical activity level, current smoking status, antihypertensive medication, lipid-lowering medication, hypoglycemic medication, and BMI (excluding models for BMI and waist circumference). HDL-C, HDL cholesterol; HEI, Healthy Eating Index; LDL-C, LDL cholesterol; Q, quartile.
Maximum component score.
Quartiles 1 and 2 were merged because estimated scores were equivalent.
Quartiles 3 and 4 were merged because estimated scores were equivalent.
TABLE 5.
Mean cardiometabolic risk factors by quartiles of HEI-2015 moderation component scores in nonpregnant, nonlactating US adults aged ≥19 y in NHANES 2001–20161
| Component score quartiles | Linear trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ß ± SE | P | |
| Refined Grains (102) | 0.85 ± 0.02 | 5.17 ± 0.02 | 8.50 ± 0.01 | 9.99 ± 0.002 | ||
| BMI, kg/m2 | 28.94 ± 0.11 | 28.60 ± 0.10 | 28.66 ± 0.11 | 28.54 ± 0.10 | −0.04 ± 0.01 | 0.001 |
| Waist circumference, cm | 98.65 ± 0.27 | 97.95 ± 0.23 | 98.14 ± 0.26 | 97.96 ± 0.24 | −0.06 ± 0.03 | 0.04 |
| Systolic blood pressure, mm Hg | 122.76 ± 0.29 | 122.92 ± 0.22 | 122.46 ± 0.24 | 121.88 ± 0.24 | −0.09 ± 0.03 | 0.006 |
| LDL-C, mg/dL | 115.50 ± 0.67 | 115.60 ± 0.85 | 115.33 ± 0.82 | 116.14 ± 0.75 | 0.04 ± 0.08 | 0.68 |
| HDL-C, mg/dL | 52.11 ± 0.23 | 52.47 ± 0.22 | 53.34 ± 0.25 | 54.18 ± 0.24 | 0.22 ± 0.03 | <0.001 |
| Triglycerides, mg/dL | 135.35 ± 2.06 | 134.64 ± 2.14 | 134.42 ± 2.75 | 133.83 ± 2.24 | −0.18 ± 0.30 | 0.55 |
| Glucose, mg/dL | 104.73 ± 0.40 | 105.88 ± 0.46 | 104.89 ± 0.51 | 104.80 ± 0.44 | −0.01 ± 0.06 | 0.82 |
| Insulin, µU/mL | 13.25 ± 0.32 | 13.58 ± 0.25 | 12.96 ± 0.26 | 12.77 ± 0.20 | −0.06 ± 0.04 | 0.13 |
| Sodium (10) | 0.01 ± 0.002 | 2.30 ± 0.01 | 5.62 ± 0.01 | 9.09 ± 0.02 | ||
| BMI, kg/m2 | 29.10 ± 0.11 | 28.88 ± 0.10 | 28.60 ± 0.11 | 28.12 ± 0.10 | −0.10 ± 0.01 | <0.001 |
| Waist circumference, cm | 98.93 ± 0.25 | 98.49 ± 0.23 | 98.17 ± 0.25 | 97.08 ± 0.25 | −0.19 ± 0.03 | <0.001 |
| Systolic blood pressure, mm Hg | 122.47 ± 0.26 | 122.64 ± 0.22 | 122.42 ± 0.27 | 122.40 ± 0.23 | 0.00 ± 0.03 | 0.97 |
| LDL-C, mg/dL | 115.45 ± 0.75 | 115.70 ± 0.84 | 115.74 ± 0.67 | 115.79 ± 0.88 | 0.05 ± 0.12 | 0.68 |
| HDL-C, mg/dL | 53.10 ± 0.23 | 53.26 ± 0.24 | 52.71 ± 0.27 | 53.16 ± 0.24 | −0.01 ± 0.03 | 0.68 |
| Triglycerides, mg/dL | 136.80 ± 2.65 | 127.87 ± 1.85 | 134.22 ± 2.18 | 139.38 ± 2.87 | 0.50 ± 0.37 | 0.19 |
| Glucose, mg/dL | 105.43 ± 0.53 | 104.91 ± 0.47 | 105.01 ± 0.48 | 104.94 ± 0.47 | −0.03 ± 0.07 | 0.66 |
| Insulin, µU/mL | 13.06 ± 0.29 | 13.27 ± 0.26 | 13.25 ± 0.23 | 12.96 ± 0.23 | −0.01 ± 0.03 | 0.73 |
| Added Sugars (10) | 1.30 ± 0.02 | 5.76 ± 0.01 | 8.71 ± 0.01 | 9.98 ± 0.002 | ||
| BMI, kg/m2 | 28.77 ± 0.11 | 28.74 ± 0.12 | 28.43 ± 0.11 | 28.76 ± 0.10 | −0.02 ± 0.02 | 0.18 |
| Waist circumference, cm | 98.55 ± 0.28 | 98.20 ± 0.28 | 97.47 ± 0.25 | 98.38 ± 0.23 | −0.07 ± 0.04 | 0.05 |
| Systolic blood pressure, mm Hg | 122.34 ± 0.26 | 122.40 ± 0.26 | 122.26 ± 0.26 | 122.90 ± 0.27 | 0.05 ± 0.03 | 0.18 |
| LDL-C, mg/dL | 116.81 ± 0.86 | 115.65 ± 0.80 | 115.07 ± 0.73 | 115.15 ± 0.86 | −0.18 ± 0.13 | 0.18 |
| HDL-C, mg/dL | 49.94 ± 0.22 | 52.18 ± 0.19 | 54.16 ± 0.24 | 55.81 ± 0.28 | 0.66 ± 0.03 | <0.001 |
| Triglycerides, mg/dL | 139.83 ± 2.68 | 137.69 ± 2.71 | 130.64 ± 2.36 | 130.13 ± 2.09 | −1.19 ± 0.38 | 0.002 |
| Glucose, mg/dL | 104.88 ± 0.51 | 104.13 ± 0.38 | 104.08 ± 0.44 | 106.99 ± 0.57 | 0.14 ± 0.07 | 0.06 |
| Insulin, µU/mL | 13.74 ± 0.27 | 13.17 ± 0.21 | 12.83 ± 0.23 | 12.80 ± 0.29 | −0.10 ± 0.04 | 0.004 |
| Saturated Fats (10) | 1.06 ± 0.02 | 4.97 ± 0.01 | 8.09 ± 0.01 | 9.98 ± 0.002 | ||
| BMI, kg/m2 | 29.33 ± 0.12 | 28.92 ± 0.10 | 28.38 ± 0.10 | 28.07 ± 0.10 | −0.14 ± 0.02 | <0.001 |
| Waist circumference, cm | 99.84 ± 0.27 | 98.81 ± 0.23 | 97.48 ± 0.24 | 96.51 ± 0.22 | −0.37 ± 0.03 | <0.001 |
| Systolic blood pressure, mm Hg | 122.61 ± 0.24 | 122.06 ± 0.25 | 122.67 ± 0.21 | 122.60 ± 0.26 | 0.02 ± 0.03 | 0.42 |
| LDL-C, mg/dL | 117.15 ± 0.83 | 115.94 ± 0.75 | 114.76 ± 0.79 | 114.76 ± 0.85 | −0.33 ± 0.12 | 0.006 |
| HDL-C, mg/dL | 53.30 ± 0.23 | 52.60 ± 0.26 | 53.26 ± 0.21 | 53.07 ± 0.27 | −0.01 ± 0.03 | 0.69 |
| Triglycerides, mg/dL | 129.53 ± 2.41 | 134.27 ± 2.58 | 132.61 ± 2.11 | 142.05 ± 2.51 | 1.15 ± 0.33 | <0.001 |
| Glucose, mg/dL | 106.15 ± 0.51 | 105.09 ± 0.44 | 104.59 ± 0.48 | 104.42 ± 0.49 | −0.17 ± 0.07 | 0.01 |
| Insulin, µU/mL | 13.42 ± 0.26 | 13.08 ± 0.20 | 13.08 ± 0.29 | 12.96 ± 0.31 | −0.05 ± 0.03 | 0.16 |
Values are means ± SEs. Covariates include age, gender, ethnicity, poverty-income ratio, physical activity level, current smoking status, antihypertensive medication, lipid-lowering medication, hypoglycemic medication, and BMI (excluding models for BMI and waist circumference). HDL-C, HDL cholesterol; HEI, Healthy Eating Index; LDL-C, LDL cholesterol; Q, quartile.
Maximum component score.
Blood pressure
SBP differed most with increasing Seafood and Plant Proteins component scores (−0.22 mm Hg per point, P < 0.001). Whole Fruits (−0.15 mm Hg per point, P = 0.009), Whole Grains (−0.14 mm Hg per point, P < 0.001), and Refined Grains (−0.09 mm Hg per point, P = 0.006) components were also significantly associated with lower SBP.
Lipid concentrations
Higher scores for the Saturated Fats component (indicating lower percentages of calories consumed from saturated fat) were associated with lower LDL cholesterol. Several components were associated with HDL cholesterol. Higher scores for Added Sugars (indicating lower percentages of calories consumed from added sugars) were associated with the greatest differences in HDL cholesterol (0.66 mg/dL per point, P < 0.001; to convert cholesterol to mmol/L, multiply by 0.0259), followed by Total Protein Foods (0.44 mg/dL per point, P < 0.001). Lower triglycerides were observed with greater scores for Seafood and Plant Proteins (−1.89 mg/dL per point, P = 0.003; to convert triglycerides to mmol/L, multiply by 0.0113), Greens and Beans (−1.55 mg/dL per point, P = 0.003), and Added Sugars (−1.19 mg/dL per point, P = 0.002), whereas greater scores for Saturated Fats were associated with higher triglycerides (1.15 mg/dL per point, P < 0.001).
Glycemic measures
Higher scores for Whole Fruits were associated with lower fasting plasma glucose (−0.28 mg/dL per point, P = 0.009; to convert glucose to mmol/L, multiply by 0.0555). Higher scores for Added Sugars were associated with the greatest differences in fasting insulin (−0.31 µU/mL, P = 0.005; to convert insulin to pmol/L, multiply by 6). Higher Total Fruits (−0.19 µU/mL per point, P = 0.006) and Whole Fruits (−0.19 µU/mL per point, P = 0.002) component scores were also associated with lower fasting insulin.
Associations between unscored diet quality components and cardiometabolic risk factors
The mean score in the highest quartile of some components was at or near the maximum score. To ensure that truncation at upper score values did not prevent detection of associations, associations between the dietary factors represented by these components in unscored units of intake (actual cup-equivalents, ounce-equivalents, grams, or % of energy rather than component subscores) and risk factors were also assessed (Supplemental Table 3). Unlike HEI component scores, intakes were not expressed in relation to energy intake (i.e., per 1000 kcal or as a % of energy); thus, models were additionally adjusted for total energy intake. Most associations were confirmed, although in the opposite direction for moderation components (as would be expected), since these are reverse scored when calculating HEI scores. In addition, greater fatty acid ratios (ratio of unsaturated to saturated fat) were inversely associated with LDL cholesterol (ß = −1.43, P = 0.003).
Discussion
Among US adults in 2001–2016, higher versus lower quality diets scored by the HEI-2015 were primarily distinguished by higher scores for components that represent dietary fat and grain quality. Specifically, higher Fatty Acids component scores represented greater intakes of polyunsaturated fats and lower intakes of saturated fat, whereas monounsaturated fats varied minimally across the range of scores. Higher component scores were generally associated with better cardiometabolic health, although the Whole Fruits and Seafood and Plant Proteins components were most favorably associated with the assessed risk factors.
These findings partly align with a previous analysis that scored diet quality in adult participants in NHANES 2001–2008 using an earlier version of the HEI (27). Differences in total diet quality scored by HEI-2005 were primarily explained by a component that scored intakes of solid fats, alcohols, and added sugars (SoFAAS; scored out of 20 points; linear ß = 0.40, P = 0.0001), which is most comparable to the Added Sugars and Saturated Fats components in HEI-2015 (5). The next greatest differences were in scores for the Oils component (ß = 0.11, P = 0.0001), which is now encompassed by the Fatty Acids component, and Saturated Fats (ß = 0.10, P = 0.0001). Thus, differences in fat quality contributed substantially to variation in total diet quality previously scored by the HEI-2005 and by its modern version, HEI-2015, in the present study. In contrast, differences in grain components were minor contributors to variation in total HEI-2005 scores (Whole Grains, ß = 0.04; Total Grains, ß = 0.01), whereas the HEI-2015 counterparts, Whole Grains and Refined Grains, were among the top components that explained differences in total diet quality. The HEI-2005 only allocated a maximum of 5 points for the Whole Grains component (which was increased to 10 points in subsequent versions) and scored intake of Total Grains (out of 5 points) but not Refined Grains. Changes in component definitions and relative weights in the total HEI-2015 score may account for their greater contribution to total diet quality scores, compared with the HEI-2005.
While the Whole Grain component was among the leading contributors to higher HEI-2015 scores, it should be noted that the mean Whole Grains component score in the highest quartile of total diet quality (4.69) was still well below the maximum of 10. Whole Grains was also consistently the lowest scoring component (expressed as a percentage of maximum points) in all quartiles. In contrast, the mean Refined Grains score in the highest quartile was 80% of the maximum, indicating that adults with high diet quality are consuming fewer refined grains but still need to increase their intakes of whole grains.
The fat composition of most Americans adults’ diets is also suboptimal. The mean Fatty Acids component score for those with the highest diet quality (quartile 4) was just 7.09 out of 10. Higher Fatty Acids scores can reflect greater intakes of monounsaturated and/or polyunsaturated fats, lower intakes of saturated fats, or both. On average, adults with higher Fatty Acids component scores had lower intakes of saturated fat and greater intakes of polyunsaturated fats, primarily linoleic acid. The dietary sources of these fats differed as well. Polyunsaturated fats were largely from nuts/seeds and vegetable oils (e.g., corn, soy, canola, olive, etc.) in those with the highest scores, while the leading source of polyunsaturated fats for those with low scores was pizza. As pizza is not a particularly rich source of polyunsaturated fat, its ranking as a major source for individuals with low Fatty Acids scores reflects the large quantity consumed and the relative shortage of healthier polyunsaturated-rich foods in their diets. High-fat dairy products, namely cheese and ice cream, were major sources of saturated fat in the lowest quartile of scores, while nuts and seeds were leading sources for the highest quartile. Comparatively minimal differences in monounsaturated fat intakes were observed across the range of Fatty Acids component scores.
While both mono- and polyunsaturated fats are recommended to replace saturated fats (28), the strongest evidence supports the cardioprotective effect of replacing saturated fat with polyunsaturated fats (8, 29). Thus, the observed replacement of saturated fats with mostly polyunsaturated fats in US adults with higher Fatty Acids component scores is consistent with authoritative recommendations. Population scores could be improved by encouraging consumption of nuts, seeds, and nontropical oils (e.g., corn, soybean, canola) in place of saturated fat sources.
The HEI scoring metric distributes points among components according to a priori–defined standards, to quantify alignment with guidelines rather than their associations with health (5). The recommendations that correspond to these components are all intended to promote general health; however, particular components were more strongly associated with cardiometabolic risk factors than others. The Whole Fruits and Seafood and Plant Proteins components were most favorably associated with cardiometabolic risk factors, considering both the number and strength of risk factors with which they were associated. Prospective observational studies support an inverse association between fruit intake and cardiovascular disease incidence and mortality (30), while plant proteins (31) and fish consumption (32) have been associated with lower risk of all-cause and cardiovascular mortality and are recommended to decrease atherosclerotic CVD risk (28, 33). The favorable associations between these components and cardiometabolic risk factors are, therefore, consistent with previous research and current dietary recommendations. However, as this is a cross-sectional study, causation cannot be inferred from the observed associations. These dietary components may be markers of a healthy lifestyle and, thus, cluster with other unmeasured health-promoting behaviors or with other dietary components that explain the favorable associations with cardiometabolic risk. Whole Fruits and Seafood and Plant Proteins components have previously been shown to exhibit moderate correlations (0.46–0.56) with total HEI-2015 scores (4), although other components that were similarly correlated with total diet quality (e.g., Total Vegetables) were not associated with as many—or as strongly with—assessed cardiometabolic risk factors. Thus, the observed associations are likely not explained by the correlation of these components with total diet quality alone, but rather may be attributable to beneficial components in these foods (e.g., fruit polyphenols, seafood omega-3 fatty acids), their displacement of other less healthful foods, or their correlation with other health-promoting behaviors.
The 2 components that score dietary fat quality, Fatty Acids and Saturated Fats, were both associated with lower waist circumference but differed in their associations with other risk factors. Higher Fatty Acids component scores were associated with higher HDL cholesterol, while the Saturated Fats scores were inversely associated with LDL cholesterol and were unexpectedly associated with higher triglycerides. Replacement of saturated with polyunsaturated fat is associated with the greatest LDL-cholesterol reductions (8, 10). Given that higher Fatty Acids component scores were primarily composed of lower saturated and higher polyunsaturated fat, it is surprising that higher Fatty Acids component scores were not associated with LDL cholesterol. It is possible that truncation of scores at the maximum (10 points = ratio ≥2.5) attenuated the association to nonsignificance, as the unscored ratio was associated with LDL cholesterol in the expected direction. The heterogeneity in food sources underlying the calculation of the fatty acid ratio is another complicating factor, as certain foods contain bioactives that can affect LDL cholesterol independently of fatty acids. Oils, for example, vary widely in their effects on circulating lipid concentrations (34), which may be partly explained by variable phytosterol content (35, 36). Salad dressings and vegetable oils were among the leading contributors to unsaturated fat intakes. Thus, differences in specific types of oils within these broad categories (e.g., corn, soy, canola, olive) consumed by people with higher versus lower Fatty Acids component scores could explain why a significant association with LDL cholesterol was not observed.
The Saturated Fats component awards greater points for less saturated fat consumption as a percentage of total energy (maximum of 10 points = ≤8% kcal from saturated fat), regardless of the nutrient(s) replacing saturated fat. The physiological benefits of reducing saturated fat intake are dependent on the replacement nutrient, with the greatest atherogenic lipid-lowering achieved by replacement of saturated with polyunsaturated fats, while replacement of saturated fat with carbohydrates is associated with smaller LDL-cholesterol reductions and increased triglycerides (8). Failure to consider the replacement nutrient in Saturated Fats component scoring may explain why better scores for this component (i.e., lower intakes of saturated fat) were associated with higher triglycerides.
Dairy scores were not significantly associated with any cardiometabolic risk factors. This finding does not necessarily mean that dairy foods do not affect cardiometabolic risk, but rather that these risk factors did not substantially differ across the range of observed Dairy component scores in this population. This might be due to the heterogeneous nutrient profiles of dairy foods (e.g., cheese, milk, yogurt) encompassed by this component or the limited variation in Dairy scores (on average, 0.14-point greater per quartile increase) across the total HEI-2015 score distribution.
Over the past 18 y, the quality of Americans’ diets has not substantially improved, with mean total HEI-2015 scores increasing by just 2 points (37). This study may inform interventions to improve diet quality, as components that differ most across the distribution of total HEI-2015 scores may be more amenable to improvement. Closer study of the barriers and facilitators to adherence in individuals with the lowest and highest scores for these components, respectively, could potentially guide strategies to improve adherence in those with the poorest diet quality. In contrast, Dairy scores were consistently low (∼50% of maximum), regardless of overall diet quality, and Sodium scores also differed little between the highest and lowest quartiles (difference of 1.6 points out of 10). These dietary components may be challenging to modify, perhaps due to physiological barriers to consumption (i.e., lactose intolerance) (38, 39), consumers’ attitudes and beliefs (40, 41), and the food environment (42, 43), and require more complex solutions.
Strengths of this study include analysis of a large US sample with the application of appropriate survey procedures to obtain nationally representative estimates. Our examination of individual component scores yields valuable insights into how Americans are achieving better diet quality, as scored by the HEI-2015, and how the components individually relate to cardiometabolic health, which has recently been recommended to enhance understanding of the relation between diet quality and cardiovascular health (7). Further disaggregation of US adults’ Fatty Acids scores into fat subtype intakes, and assessment of their food sources, uniquely provides insight into how Americans are achieving better or worse fat quality scores, which may inform guidance to shift intakes toward more healthful types and sources. However, these analyses are limited by use of self-reported dietary data (a single 24-h recall), which may be subject to measurement error. A single recall per person is regarded as sufficient to estimate population mean dietary intakes, under the assumption that a 24-h recall is an unbiased measure of true intake but does not accurately represent the distribution of usual intakes (44). However, an objective of this study was to relate HEI total and component scores to individual cardiometabolic risk factors. The preferred statistical methods for such analyses—the multivariate Markov chain Monte Carlo method followed by regression—are under development and, thus, the available methodology cited by the National Cancer Institute was applied (45). Data were aggregated from 8 cycles spanning 16 y, a period throughout which the DGA have been updated 3 times. Although much of the advice remains unchanged, temporal trends may have contributed to some of the observed variation in total and component scores (37). The cross-sectional design prohibits causal inferences regarding the associations between diet and risk factors, as the temporality and direction of associations cannot be established. Finally, analyses were adjusted for known demographic and lifestyle covariates, but residual or unmeasured confounding may have influenced the observed associations. Adherence to dietary guidelines likely clusters with other unmeasured behaviors and dietary components that may explain the associations with cardiometabolic risk factors.
In conclusion, higher quality diets, as assessed by the HEI-2015, in US adults are primarily distinguished by consumption of healthier fats (less saturated and more polyunsaturated) and grain-based foods (more whole and fewer refined grains), and adherence to particular dietary recommendations is favorably associated with cardiometabolic risk factors. However, the average diet quality is suboptimal, and thus future investigation of interventions at the individual, community, and policy levels is needed to improve diet quality of the population and decrease the risk of many chronic diseases. Interventions targeting specific dietary components that are most favorably associated with cardiometabolic risk—namely, whole fruits, seafood, and plant proteins—may have the greatest impact on US adults’ health.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—VLF: performed statistical analyses; VKS, KSP, and PMK-E: wrote the first draft with contributions from VLF; and all authors: contributed to the study design, reviewed and commented on subsequent drafts of the manuscript, and read and approved the final manuscript.
Notes
Funding was provided by ACH Food Companies, Inc.
Author disclosures: KSP, PMK-E, and VLF received a grant from ACH Food Companies, Inc., to conduct this research. VLF, as senior vice president of Nutrition Impact LLC, performs consulting and database analyses for various food and beverage companies and related entities. FE, MEC, and MTB are employed by ACH Food Companies, Inc. VKS reports no conflicts of interest.
Scientists employed by the funder (FE, MEC, MTB) helped design the study and interpret the data; the funder had no role in data analysis.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: CVD, cardiovascular disease; DGA, Dietary Guidelines for Americans; HEI, Healthy Eating Index; PIR, poverty-income ratio; SBP, systolic blood pressure.
Contributor Information
Valerie K Sullivan, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA.
Kristina S Petersen, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA.
Victor L Fulgoni, III, Nutrition Impact, LLC, Battle Creek, MI, USA.
Fulya Eren, ACH Food Companies, Inc., Oakbrook Terrace, IL, USA.
Martha E Cassens, ACH Food Companies, Inc., Oakbrook Terrace, IL, USA.
Michael T Bunczek, ACH Food Companies, Inc., Oakbrook Terrace, IL, USA.
Penny M Kris-Etherton, Email: pmk3@psu.edu, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA.
References
- 1. Hu EA, Steffen LM, Coresh J, Appel LJ, Rebholz CM. Adherence to the Healthy Eating Index-2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J Nutr. 2020;150(2):312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118(1):74–100, e11. [DOI] [PubMed] [Google Scholar]
- 3. Panizza CE, Shvetsov YB, Harmon BE, Wilkens LR, Le Marchand L, Haiman C, Reedy J, Boushey CJ. Testing the predictive validity of the Healthy Eating Index-2015 in the Multiethnic Cohort: is the score associated with a reduced risk of all-cause and cause-specific mortality?. Nutrients. 2018;10(4):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reedy J, Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TRE, Wilson MM, Subar AF, Kahle LL, Tooze JA. Evaluation of the Healthy Eating Index-2015. J Acad Nutr Diet. 2018;118(9):1622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirkpatrick SI, Reedy J, Krebs-Smith SM, Pannucci TRE, Subar AF, Wilson MM, Lerman JL, Tooze JA. Applications of the Healthy Eating Index for surveillance, epidemiology, and intervention research: considerations and caveats. J Acad Nutr Diet. 2018;118(9):1603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krebs-Smith SM, Pannucci TRE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aljuraiban GS, Gibson R, Oude Griep LM, Okuda N, Steffen LM, Van Horn L, Chan Q. Perspective: the application of a priori diet quality scores to cardiovascular disease risk—a critical evaluation of current scoring systems. Adv Nutr. 2020;11:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JGet al. . Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–23. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Hruby A, Bernstein AM, Ley SH, Wang DD, Chiuve SE, Sampson L, Rexrode KM, Rimm EB, Willett WCet al. . Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol. 2015;66:1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mensink RP. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva (Switzerland): World Health Organization; 2016. [Google Scholar]
- 11. National Center for Health Statistics, Centers for Disease Control and Prevention . About NHANES. [Internet]. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control and Prevention; [updated 2017 Sep 15; cited 2020 Jul 6]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Google Scholar]
- 12. National Center for Health Statistics, Centers for Disease Control and Prevention . NHANES survey methods and analytic guidelines. [Internet]. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control and Prevention; [cited 2020 Jul 6]. Available from: https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx#plan-and-operations. [Google Scholar]
- 13. National Center for Health Statistics, Centers for Disease Control and Prevention . Ethics Review Board (ERB) approval. [Internet]. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control and Prevention; [updated 2017 Nov 29; cited 2020 Jan 5]. Available from: https://www.cdc.gov/nchs/nhanes/irba98.htm. [Google Scholar]
- 14. National Center for Health Statistics, Centers for Disease Control and Prevention . NHANES questionnaire data. [Internet]. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control; [cited 2020 Jul 6]. Available from: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Questionnaire. [Google Scholar]
- 15. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LAet al. . The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–32. [DOI] [PubMed] [Google Scholar]
- 16. National Center for Health Statistics, Centers for Disease Control and Prevention . NHANES dietary data. [Internet]. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control; [cited 2020 Jul 6]. Available from: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Dietary. [Google Scholar]
- 17. USDA . Food and Nutrient Database for Dietary Studies. [Internet]. Beltsville (MD): Agricultural Research Service, US Department of Agriculture; [cited 2020 Jul 6]. Available from: https://data.nal.usda.gov/dataset/food-and-nutrient-database-dietary-studies-fndds. [Google Scholar]
- 18. Agricultural Research Service, USDA . Food Patterns Equivalents Database. [Internet]. Beltsville (MD): Agricultural Research Service, US Department of Agriculture; [cited 2020 Jun 8]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fped-overview/. [Google Scholar]
- 19. National Cancer Institute . SAS code. [Internet]. Bethesda (MD): Division of Cancer Control and Population Sciences, National Cancer Institute; [cited 2019 Dec 27]. Available from: https://epi.grants.cancer.gov/hei/sas-code.html. [Google Scholar]
- 20. National Cancer Institute . Developing the Healthy Eating Index. [Internet]. Bethesda (MD): Division of Cancer Control and Population Sciences, National Cancer Institute; [cited 2020 Jul 6]. Available from: https://epi.grants.cancer.gov/hei/developing.html. [Google Scholar]
- 21. Food and Nutrition Service, USDA.. How the HEI is scored. [Internet]. Alexandria (VA): Food and Nutrition Service, US Department of Agriculture; 2018; [cited 2019 Jul 1]. Available from: https://www.fns.usda.gov/how-hei-scored. [Google Scholar]
- 22. National Center for Health Statistics, Centers for Disease Control and Prevention . NHANES 2015–2016 procedure manuals. [Internet]. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control and Prevention; [cited 2020 Jul 6]. Available from: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/manuals.aspx?BeginYear=2015. [Google Scholar]
- 23. National Center for Health Statistics, Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey: 2015–2016 blood pressure data documentation, codebook, and frequencies. [Internet]. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control and Prevention; 2017; [cited 2020 Jul 6]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/BPX_I.htm. [Google Scholar]
- 24. National Center for Health Statistics, Centers for Disease Control and Prevention . NHANES laboratory data. [Internet]. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control and Prevention; [cited 2020 Jul 6]. Available from: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.Aspx?Component=laboratory. [Google Scholar]
- 25. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 26. SAS [computer program] . Version 9.4. Cary (NC): SAS Institute; 2018. [Google Scholar]
- 27. Nicklas TA, O'Neil CE, Fulgoni VL. Diet quality is inversely related to cardiovascular risk factors in adults. J Nutr. 2012;142(12):2112–8. [DOI] [PubMed] [Google Scholar]
- 28. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JWet al. . 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation. 2019;140:e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dietary Guidelines Advisory Committee . Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services. [Internet]. Washington (DC): Dietary Guidelines Advisory Committee; 2020; [cited 2020 Sep 30]. Available from: https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf. [Google Scholar]
- 30. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, Giovannucci EL. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176:1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jayedi A, Shab-Bidar S. Fish consumption and the risk of chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Adv Nutr. 2020;11:1123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rimm EB, Appel LJ, Chiuve SE, Djoussé L, Engler MB, Kris-Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138:e35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwingshackl L, Bogensberger B, Benčič A, Knüppel S, Boeing H, Hoffmann G. Effects of oils and solid fats on blood lipids: a systematic review and network meta-analysis. J Lipid Res. 2018;59:1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maki KC, Lawless AL, Kelley KM, Kaden VN, Geiger CJ, Dicklin MR. Corn oil improves the plasma lipoprotein lipid profile compared with extra-virgin olive oil consumption in men and women with elevated cholesterol: results from a randomized controlled feeding trial. J Clin Lipidol. 2015;9:49–57. [DOI] [PubMed] [Google Scholar]
- 36. Maki KC, Lawless AL, Kelley KM, Kaden VN, Geiger CJ, Palacios OM, Dicklin MR. Corn oil intake favorably impacts lipoprotein cholesterol, apolipoprotein and lipoprotein particle levels compared with extra-virgin olive oil. Eur J Clin Nutr. 2017;71:33–8. [DOI] [PubMed] [Google Scholar]
- 37. Shan Z, Rehm CD, Rogers G, Ruan M, Wang DD, Hu FB, Mozaffarian D, Zhang FF, Bhupathiraju SN. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999–2016. JAMA. 2019;322:1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicklas TA, Qu H, Hughes SO, He M, Wagner SE, Foushee HR, Shewchuk RM. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am J Clin Nutr. 2011;94:191–8. [DOI] [PubMed] [Google Scholar]
- 39. Hodges JK, Cao S, Cladis DP, Weaver CM. Lactose intolerance and bone health: the challenge of ensuring adequate calcium intake. Nutrients. 2019;11:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCarthy KS, Parker M, Ameerally A, Drake SL, Drake MA. Drivers of choice for fluid milk versus plant-based alternatives: what are consumer perceptions of fluid milk?. J Dairy Sci. 2017;100:6125–38. [DOI] [PubMed] [Google Scholar]
- 41. Bir C, Widmar NO, Wolf C, Delgado MS. Traditional attributes moo-ve over for some consumer segments: relative ranking of fluid milk attributes. Appetite. 2019;134:162–71. [DOI] [PubMed] [Google Scholar]
- 42. Harnack LJ, Cogswell ME, Shikany JM, Gardner CD, Gillespie C, Loria CM, Zhou X, Yuan K, Steffen LM. Sources of sodium in US adults from 3 geographic regions. Circulation. 2017;135:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drewnowski A, Rehm CD. Sodium intakes of US children and adults from foods and beverages by location of origin and by specific food source. Nutrients. 2013;5:1840–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herrick KA, Rossen LM, Parsons R, Dodd KW. Estimating usual dietary intake from National Health and Nutrition Examination Survey data using the National Cancer Institute Method. Vital Health Stat. 2018;2(178):1–63. [PubMed] [Google Scholar]
- 45.National Cancer Institute. Choosing a method. [Internet]. Bethesda (MD): Division of Cancer Control and Population Sciences, National Cancer Institute; [cited 2020 Mar 18]. Available from: https://epi.grants.cancer.gov/hei/tools.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


