ABSTRACT
Background
Neuropsychiatric symptoms in Parkinson's disease (PD) may increase dementia (PDD) risk. The predictive value of these symptoms, however, has not been compared to clinical and demographic predictors of future PDD.
Objectives
Determine if neuropsychiatric symptoms are useful markers of PDD risk.
Methods
328 PD participants completed baseline neuropsychiatric and MDS‐Task Force‐Level II assessments. Of these, 202 non‐demented individuals were followed‐up over a four‐years period to detect conversion to PDD; 51 developed PDD. ROC analysis tested associations between baseline neuropsychiatric symptoms and future PDD. The probability of developing PDD was also modeled as a function of neuropsychiatric inventory (NPI)‐total score, PD Questionnaire (PDQ)‐hallucinations, PDQ‐anxiety, and contrasted to cognitive ability, age, and motor function. Leave‐one‐out information criterion was used to evaluate which models provided useful information when predicting future PDD.
Results
The PDD group experienced greater levels of neuropsychiatric symptoms compared to the non‐PDD groups at baseline. Few differences were found between the PD‐MCI and PD‐N groups. Six neuropsychiatric measures were significantly, but weakly, associated with future PDD. The strongest was NPI‐total score: AUC = 0.66 [0.57–0.75]. There was, however, no evidence it contained useful out‐of‐sample predictive information of future PDD (delta ELPD = 1.8 (SD 2.5)); Similar results held for PDQ‐hallucinations and PDQ‐anxiety. In contrast, cognitive ability (delta ELPD = 36 (SD 8)) and age (delta ELPD = 11 (SD 5)) provided useful predictive information of future PDD.
Conclusions
Cognitive ability and age strongly out‐performed neuropsychiatric measures as markers of developing PDD within 4 years. Therefore, neuropsychiatric symptoms do not appear to be useful markers of PDD risk.
Keywords: longitudinal, neuropsychiatric symptoms, Parkinson's dementia, PDD risk, prediction
The presence of neuropsychiatric symptoms in Parkinson's disease (PD) may represent early markers of progression to dementia (PDD). There is evidence that visual hallucinations (OR = 3–10), illusions (OR = 8), thought disorders, 1 , 2 depression (OR = 2.3), 1 and apathy (HR = 7.2; OR = 1.1) 1 , 3 are associated with future PDD.
Neuropsychiatric symptoms may, however, provide minimal predictive information compared to older age and cognitive decline, both established risk factors for PDD. 4 The former includes associations between older age per se (OR = 2.3; HR = 1.1) and older age at PD onset (OR = 2.2) with risk of PDD. 4 , 5 The latter factor includes baseline PD‐MCI status (OR = 10.2; HR = 3.5–11.3), subjective cognitive complaints (OR = 2.6), and lower cognitive ability (OR = 0.8–10.1). 1 , 4 , 5 Motor features, such as gait impairment (OR = 1.2), falls (OR = 3.0), freezing (OR = 2.6), UPDRS III (HR = 1.0), and mixed tremor/akinetic subtype (OR = 3.3) are also associated with PDD risk. 1 , 2 , 4 Awareness of the importance of neuropsychiatric features to the risk of PDD therefore requires an understanding of their contribution compared to age, cognition, and clinical motor features. These comparisons have not been reported previously.
Using a well‐characterized, longitudinal cohort of PD participants who have been assessed using Level II Movement Disorder Society‐Task Force (MDS‐TF) PD‐MCI criteria, we (1) compared neuropsychiatric symptoms across three cognitive status groups (ie PD‐N, PD‐MCI and PDD). Then we (2) examined the association of neuropsychiatric symptoms and conversion to PDD in a large sample of non‐demented PD participants who were followed up over a four‐year period. We then (3) determined the clinical out‐of‐sample predictive utility of a subset of neuropsychiatric symptoms compared to age, cognitive ability, and motor scores.
Methods
Participants
A baseline convenience sample of 328 PD participants (180 PD‐N, 108 PD‐MCI and 40 PDD) completed MDS‐TF Level II neuropsychological assessments, 6 along with neuropsychiatric and clinical measures (Fig. 1). There were 202 PD participants who were non‐demented at baseline (ie PD‐N and PD‐MCI) and their conversion to PDD within a 4‐year window could be assessed. Individuals were classified as a converter if they had an assessment within 4.5 years post‐baseline where they were classified as PDD; otherwise they were classified as a non‐converter if they had an assessment 3.5 years or later post‐baseline where they were classified as PD‐N or PD‐MCI. At study entry, two PDD participants did not receive the Geriatric Depression Scale (GDS) and one of those also did not receive the Parkinson's Disease Questionnaire (PDQ). Two PD‐N, two PD‐MCI and three PDD participants did not have their motor symptoms evaluated at baseline. The comprehensive neuropsychological assessments, PD diagnostic procedure and exclusion criteria are detailed in Wood et al. (2016). 7 The mean duration of motor symptoms at study entry was 7.7 years (SD 5.5 years). All participants took their usual medications on the day of testing. The study was approved by the Upper South B Regional Ethics Committee of the New Zealand Ministry of Health. At the beginning of each neuropsychological assessment, participants each read and sign a consent form after discussing any questions or concerns they may have. All participants gave informed written consent.
FIG. 1.
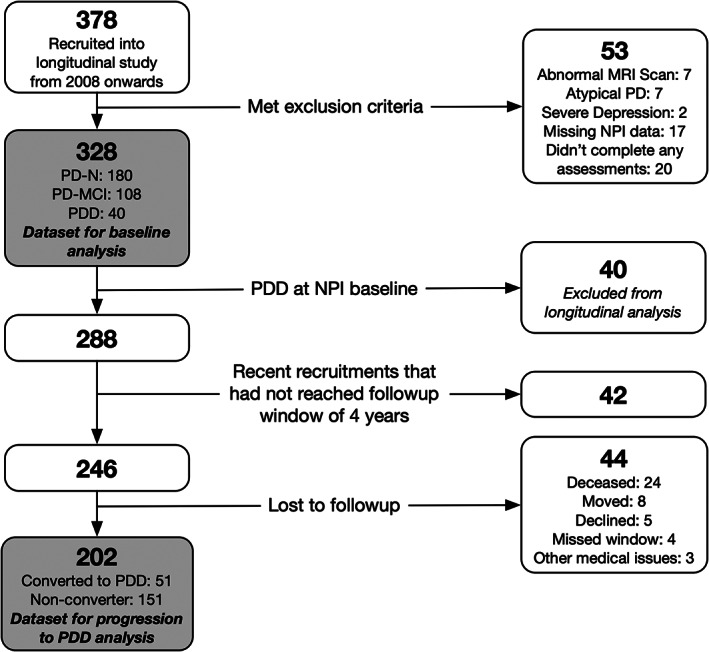
Participant recruitment, exclusions and total followed over 4 years. NPI = neuropsychiatric inventory; PD = Parkinson's disease; PDD = participants who met level II criteria for Parkinson's disease with dementia; PD‐MCI = participants who met level II criteria for Parkinson's disease with mild cognitive impairment; PD‐N = the remaining participants who did not meet criteria for PDD or PD‐MCI were classified as having normal cognition.a Assessments were conducted at baseline and then again every 1 to 2 years subsequently; participants with dementia were not followed further.
Determination of Cognitive Status
PD participants were classified as PDD using established MDS‐TF PDD criteria. 8 The remaining participants were classified at baseline as either PD‐MCI (using a criterion of at least two test impairments at least −1.5SD below norms in a single cognitive domain)), 7 consistent with MDS‐TF Level II PD‐MCI guidelines, 6 or PD‐N (not meeting either PD‐MCI or PDD criteria).
Neuropsychiatric Assessments
These were as follows: (i) The Neuropsychiatric Inventory (NPI), reported by a PD participant's significant other (SO), 9 , 10 ; (ii) 15‐item GDS (Geriatric Depression Scale) 11 , 12 ; (iii) the hallucination item, distressing dreams item, and the four‐items from the emotional well‐being sub‐section of the PDQ (Parkinson's Disease Questionnaire);13 and (iv) two items indicative of hallucinations and depression extracted from Part I of the Unified PD Rating Scale (UPDRS). 14 , 15 The earliest‐recruited patients were assessed on the then‐current UPDRS, with the revised MDS‐UPDRS scale used in the latter part of the study. The original UPDRS Part III scores were therefore scaled to MDS‐UPDRS scores to allow for comparison with the majority of the sample. 16
Analysis
This was conducted in three parts, in the R statistical environment (v.3.6.3). 17 First, baseline demographic and neuropsychological profiles of the three PD groups were compared using Kruskal‐Wallis tests followed by pairwise Wilcoxon rank sum tests. The baseline neuropsychiatric symptoms were then compared pairwise across the PD‐N, PD‐MCI and PDD groups using receiver operator characteristic (ROC) tests to determine the discrimination between baseline groups based on neuropsychiatric symptoms. The ROC analysis included boot‐strapped confidence intervals (pROC). 18 Second, we examined the association between baseline neuropsychiatric measures and conversion or not to PDD using ROC tests in the 202 non‐demented PD participants. Lastly, we evaluated the clinical utility of the selected statistically significant neuropsychiatric measures identified by the ROC analysis along with other previously‐proposed predictors (baseline cognitive ability, age, and motor impairment) using Bayesian regression models (brms (v2.9.0)). 19 We applied the regression models to 198 non‐demented participants (four participants were excluded as they were not administered the UPDRS at baseline). The null model consisted only of an intercept, this was used for comparison. Six models then examined each of six predictors separately: three neuropsychiatric measures from the ROC analysis, global cognitive score (expressed as an aggregate z score derived from averaging performance from measures conducted across the five cognitive domains), age, and motor score. The dependent variable was binary: progression or non‐progression to PDD. The leave‐one‐out information criterion (LOO‐IC), 20 which approximates the expected log pointwise predictive density (ELPD), was used to estimate the predictive accuracy of models. The difference in ELPD of the six models to the null model was calculated, and a delta ELDP of greater than twice the SE of the estimate was taken as evidence that a model was an improvement over the null model. Finally, a model, comprising all six measures, was updated to determine the conditional effects of each predictor.
Results
Group Differences on Baseline Neuropsychiatric Symptoms and Cognitive Status
Table 1 depicts the baseline demographic, neuropsychological, and neuropsychiatric profiles of the three PD groups. Education did not differ between cognitive groups. The PDD group was older, had more advanced PD, greater motor burden, and, of course, showed increased everyday functional‐impairments than the other PD groups at baseline. The PDD group also had higher Levodopa Equivalent Daily Dose (LEDD) than the PD‐N group. The PD‐MCI group showed intermediate mean values compared to the PD‐N and PDD groups, except symptom duration was similar between the two non‐dementing groups. The results from the Kruskal‐Wallis test used for comparisons made in Table 1 are shown in Table S1. Table 2 summarizes medications used by each cognitive group at study entry. Selective serotonin reuptake inhibitors were the most commonly used neuropsychiatric medication across all three groups. A larger number of PDD participants at study entry were also taking quetiapine compared to non‐demented participants.
TABLE 1.
Demographics, neuropsychological and neuropsychiatric measures at study entry [mean (SD)]
| PD‐N | PD‐MCI | PDD | |
|---|---|---|---|
| Demographics | |||
| Sample size (n) | 180 | 108 | 40 |
| Converted to PDD (n) | 8 | 43 | N/A |
| Sex, M:F | 117:63 | 82:26 | 30:10 |
| Age | 68 (8) | 70 (7) a | 74 (7) a , b |
| Education (y) | 13 (3) | 13 (3) | 13 (3) |
| PD‐Specific Measures | |||
| Symptom duration (y) | 7 (5) | 8 (5) | 12 (7) a , b |
| Hoehn and Yahr stage | 2 (0.5) | 2 (0.7) a | 3 (0.7) a , b |
| UPDRS (Motor) | 29 (13) | 39 (15) a | 57 (19) a , b |
| LEDD | 527 (431) | 675 (565) | 723 (408) a |
| Activities of Daily Living | |||
| CDR | 0.1 (0.2) | 0.4 (0.2) a | 1.3 (0.5) a , b |
| Reisberg IADL | 0.5 (0.5) | 0.8 (0.5) a | 2.1 (0.6) a , b |
| Cognitive Assessments | |||
| Premorbid IQ (WTAR) | 111 (8) | 108 (10) | 107 (11) |
| DRS‐2 (AESS) | 12 (2) | 9 (3) a | 5 (3) a , b |
| ADAS‐Cog | 6.6 (2.8) | 10.8 (3.9) a | 22.5 (8.6) a , b |
| MoCA | 27 (2) | 23 (3) a | 17 (4) a , b |
| Global Z score | 0.15 (0.4) | −0.78 (0.5) | −1.85 (0.5) |
| NPI measures | |||
| NPI Total score | 4.0 (6.9) | 4.2 (6.8) | 10.8 (8.2) |
| NPI Hallucinations | 0.1 (0.8) | 0.3 (1.2) | 1.3 (2.8) |
| NPI Depression | 0.8 (1.7) | 0.9 (2.0) | 1.7 (3.0) |
| NPI Anxiety | 1.0 (2.3) | 1.2 (2.0) | 1.9 (2.4) |
| NPI Aggression | 0.3 (1.3) | 0.2 (1.1) | 1.2 (2.1) |
| NPI Apathy | 0.7 (1.8) | 0.7 (1.7) | 2.1 (2.9) |
| NPI Disinhibition | 0.2 (1.0) | 0.1 (0.5) | 0.6 (1.9) |
| NPI Delusions | 0.2 (1.3) | 0.0 (0.1) | 0.8 (2.4) |
| NPI Euphoria | 0.0 (0.2) | 0.0 (0.3) | 0.0 (0.0) |
| NPI Irritability and Liability | 0.5 (1.8) | 0.4 (1.5) | 0.6 (1.6) |
| NPI Aberrant Motor Behavior | 0.2 (1.1) | 0.4 (1.3) | 0.6 (1.7) |
| NPI Sleep and Night time Behavior Disorders | 1.7 (3.0) | 2.0 (3.2) | 1.5 (2.7) |
| NPI Appetite and Eating Disorders | 0.5 (1.7) | 0.5 (1.8) | 0.7 (2.1) |
| PDQ measures | |||
| PDQ Hallucinations | 0.4 (0.8) | 0.6 (1.0) | 1.1 (1.3) |
| PDQ Depression | 0.7 (1.0) | 0.8 (1.0) | 1.3 (1.2) |
| PDQ Anxiety | 1.1 (1.0) | 1.1 (1.0) | 1.6 (1.2) |
| PDQ Aggression | 0.3 (0.6) | 0.5 (0.8) | 0.8 (1.0) |
| PDQ Isolated and Lonely | 0.4 (0.7) | 0.5 (0.8) | 1.0 (0.9) |
| PDQ Weepy and Tearful | 0.6 (0.9) | 0.7 (0.9) | 0.7 (0.7) |
| PDQ Worried about future | 1.1 (1.1) | 1.1 (1.2) | 1.1 (1.0) |
| PDQ Distressing dreams | 0.5 (0.9) | 0.6 (1.0) | 1.3 (1.4) |
| UPDRS measures | |||
| UPDRS Hallucinations | 0.3 (0.6) | 0.5 (0.9) | 1.3 (1.2) |
| UPDRS Depression | 0.5 (0.8) | 0.6 (0.9) | 1.2 (1.0) |
| GDS | 0.1 (0.4) | 0.1 (0.3) | 0.5 (0.6) |
Significantly different from PD‐N, Kruskal‐Wallis followed by pairwise Wilcoxon rank sum tests, P < 0.05.
Significantly different from PD‐MCI, Kruskal‐Wallis followed by pairwise Wilcoxon rank sum tests, P < 0.05.
Abbreviations: PDD, Participants who met Level II criteria for Parkinson's disease with dementia; PD‐MCI, Participants who met Level II criteria for Parkinson's disease with mild cognitive impairment; PD‐N, The remaining Participants who did not meet criteria for PDD or PD‐MCI were classified as having normal cognition; ADAS‐Cog, Alzheimer's Dementia Assessment Scale‐Cognitive; CDR, Clinical Dementia Rating; DRS‐2 (AESS), Dementia Rating Scale‐2 (Age and Education Scaled Score); GDS, Geriatric Depression Score; Global Z score, mean derived from attention, executive function, visuospatial and episodic memory domains; IADL, Instrumental Activities of Daily Living; LEDD, Levodopa Equivalent Daily Dose; MoCA, Montreal Cognitive Assessment; NPI, Neuropsychiatric Inventory; PDQ = Parkinson's disease Questionnaire; UPDRS, Unified Parkinson's Disease Rating Scale; WTAR, Wechsler Test of Adult Reading for premorbid IQ.
TABLE 2.
Neuropsychiatric drug information study entry
| PD‐N | PD‐MCI | PDD | |
|---|---|---|---|
| Sedatives/Hypnotics | |||
| Zopiclone | 5 | 4 | 3 |
| Temazepam | 0 | 1 | 0 |
| Triazolam | 0 | 2 | 0 |
| Nitrazepam | 1 | 1 | 0 |
| Melatonin | 0 | 0 | 0 |
| Antipsychotics | |||
| Quetiapine | 4 | 4 | 10 |
| Clozapine | 1 | 0 | 1 |
| Olanzapine | 0 | 1 | 0 |
| Risperidone | 0 | 0 | 0 |
| Antidepressants | |||
| Selective serotonin reuptake inhibitors (SSRIs) | |||
| Citalopram | 10 | 13 | 10 |
| Escitalopram | 2 | 2 | 0 |
| Paroxetine | 1 | 1 | 0 |
| Fluoxetine | 9 | 3 | 1 |
| Cyclic and related agents | |||
| Nortriptyline | 1 | 6 | 0 |
| Amitriptyline | 6 | 6 | 2 |
| Dothiepin | 1 | 1 | 0 |
| Other antidepressants | |||
| Venlafaxine (SNRI) | 3 | 0 | 1 |
| Moclobemide (MAOI‐A) | 1 | 1 | 0 |
| Mirtazapine | 3 | 3 | 1 |
| Anxiolytics | |||
| Clonazepam | 7 | 3 | 3 |
| Diazepam | 0 | 0 | 0 |
| Lorazepam | 3 | 3 | 2 |
| Oxazepam | 0 | 0 | 0 |
| Anticholinesterase inhibitor | |||
| Donepezil | 0 | 2 | 5 |
| Rivastigmine | 1 | 1 | 2 |
Abbreviations: MAOI‐A, Monoamine oxidase inhibitors—Type A; PDD, Participants who met Level II criteria for Parkinson's disease with dementia; PD‐MCI, Participants who met Level II criteria for Parkinson's disease with mild cognitive impairment; PD‐N, The remaining Participants who did not meet criteria for PDD or PD‐MCI were classified as having normal cognition; SNRI, Serotonin‐noradrenaline reuptake inhibitors.
Figure 2 depicts the baseline AUC values for each neuropsychiatric symptom. Significantly greater levels of hallucinations, depression, anxiety, and aggression in PDD versus PD‐N were present in multiple measures from the NPI, PDQ, UPDRS, and GDS scales. Sub‐components of the NPI and PDQ also indicated greater levels of apathy, loneliness, and distressing dreams in PDD versus PD‐N. When PDD and PD‐MCI groups were compared, a similar overall pattern was seen, but the AUC values were generally smaller; additionally, there was no evidence of a difference in anxiety scores, but there was evidence of a difference in the level of delusions between these groups. There were few significant differences between the PD‐N and PD‐MCI groups, with marginally higher levels of NPI hallucinations, UPDRS hallucinations, and NPI aberrant motor behavior item scores in the PD‐MCI group compared to the PD‐N group.
FIG. 2.
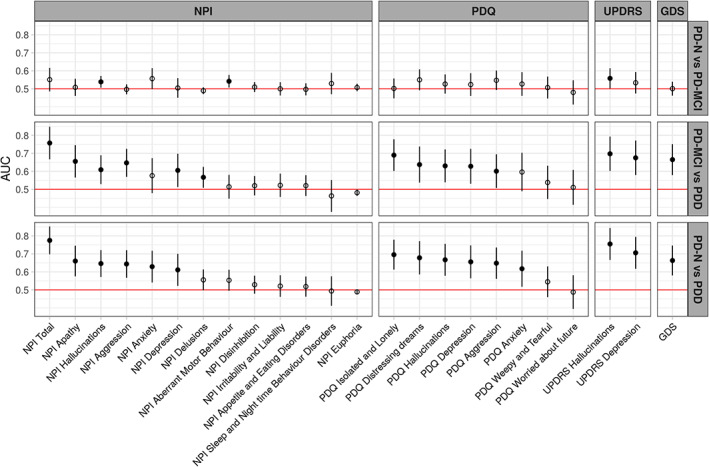
AUC values represent the probability of a classifier using individual behavioral symptom scores to rank a randomly chosen participant from one diagnostic group higher than a participant from another group. The dots represent the estimated AUC values, lines represent 95% CI. Filled dots indicate values significantly greater than 0.5 (chance performance). Items are from the NPI, PDQ, MDS‐UPDRS, and GDS scales. Items within each scale are sorted from right to left by the AUC value for classifying PDD from PD‐N.
Baseline Neuropsychiatric Symptoms Associated with Progression to PDD within 4 Years
Table 3 shows the baseline demographic, neuropsychological, and neuropsychiatric profiles of the subset of 202 participants who were followed over 4 years. The longitudinally‐followed PD‐MCI participants were significantly older, exhibited greater motor impairment, had higher LEDD, a lower level of education, lower premorbid IQ, and showed increased everyday functional‐impairments than the PD‐N participants. PD symptom duration however was similar. Table S2 displays the Kruskal‐Wallis results for the comparisons made in Table 3.
TABLE 3.
Demographics, neuropsychological and neuropsychiatric measures at study entry [mean (SD)] for the 202 non‐dementing PD participants followed‐up over four‐years
| PD‐N | PD‐MCI | |
|---|---|---|
| Demographics | ||
| Sample size (n) | 121 | 91 |
| Converted to PDD (n) | 8 | 43 |
| Sex, M:F | 81:40 | 66:25 |
| Age | 67 (8) | 71 (7) a |
| Education (y) | 13 (3) | 13 (3) |
| PD‐Specific Measures | ||
| Symptom duration (y) | 7 (5) | 8 (5) |
| Hoehn and Yahr stage | 2 (0.5) | 2 (0.7) a |
| UPDRS (Motor) | 27 (12) | 38 (15) a |
| LEDD | 501 (440) | 655 (500) a |
| Activities of Daily Living | ||
| CDR | 0.1 (0.2) | 0.4 (0.2) a |
| Reisberg IADL | 0.5 (0.5) | 0.8 (0.6) a |
| Cognitive Assessments | ||
| Premorbid IQ (WTAR) | 111 (8) | 108 (9) a |
| DRS‐2 (AESS) | 12 (2) | 9 (3) a |
| ADAS‐Cog | 7 (3) | 11 (4) a |
| MoCA | 27 (2) | 23 (3) a |
| Global Z score | 0.18 (0.4) | −0.78 (0.5) |
| NPI measures | ||
| NPI Total score | 3.6 (6.6) | 4.8 (7.5) |
| NPI Hallucinations | 0.1 (0.9) | 0.4 (1.4) |
| NPI Depression | 0.6 (1.6) | 1.1 (2.2) |
| NPI Anxiety | 0.9 (2.2) | 1.3 (2.1) |
| NPI Aggression | 0.3 (1.5) | 0.3 (1.2) |
| NPI Apathy | 0.7 (1.8) | 0.7 (1.7) |
| NPI Disinhibition | 0.1 (0.6) | 0.1 (0.3) |
| NPI Delusions | 0.2 (1.1) | 0.0 (0.1) |
| NPI Euphoria | 0.0 (0.3) | 0.0 (0.2) |
| NPI Irritability and Liability | 0.5 (1.8) | 0.4 (1.6) |
| NPI Aberrant Motor Behavior | 0.2 (1.2) | 0.5 (1.5) |
| NPI Sleep and Night time Behavior Disorders | 1.6 (2.8) | 1.8 (3.1) |
| NPI Appetite and Eating Disorders | 0.5 (1.8) | 0.4 (1.7) |
| PDQ measures | ||
| PDQ Hallucinations | 0.4 (0.9) | 0.6 (1.1) |
| PDQ Depression | 0.6 (0.9) | 0.9 (1.1) |
| PDQ Anxiety | 1.0 (1.0) | 1.2 (1.0) |
| PDQ Aggression | 0.3 (0.7) | 0.5 (0.8) |
| PDQ Isolated and Lonely | 0.4 (0.7) | 0.4 (0.8) |
| PDQ Weepy and Tearful | 0.6 (0.9) | 0.8 (1.0) |
| PDQ Worried about future | 1.0 (1.1) | 1.1 (1.2) |
| PDQ Distressing dreams | 0.4 (0.9) | 0.6 (0.9) |
| UPDRS measures | ||
| UPDRS Hallucinations | 0.4 (0.6) | 0.6 (0.9) |
| UPDRS Depression | 0.4 (0.8) | 0.6 (0.9) |
| GDS | 0.1 (0.4) | 0.1 (0.3) |
Significantly different from PD‐N, Kruskal‐Wallis, P < 0.05.
Abbreviations: PD‐MCI, Participants who met Level II criteria for Parkinson's disease with mild cognitive impairment; PD‐N, The remaining Participants who did not meet criteria for PDD or PD‐MCI were classified as having normal cognition; ADAS‐Cog, Alzheimer's Dementia Assessment Scale‐Cognitive; CDR, Clinical Dementia Rating; DRS‐2 (AESS), Dementia Rating Scale‐2 (Age and Education Scaled Score); GDS, Geriatric Depression Score; Global Z score, mean derived from the five cognitive domains; IADL, Instrumental Activities of Daily Living; LEDD, Levodopa Equivalent Daily Dose; MoCA, Montreal Cognitive Assessment; NPI, Neuropsychiatric Inventory; PDQ, Parkinson's disease Questionnaire; UPDRS, Unified Parkinson's Disease Rating Scale; WTAR, Wechsler Test of Adult Reading for premorbid IQ.
In the non‐demented PD participants, baseline neuropsychiatric symptoms were examined for differences between those who converted to PDD (n = 51; 43 from PD‐MCI and 8 from PD‐N) and those who did not (n = 151). Six neuropsychiatric symptoms had significant AUC values (Fig. 3). NPI total score produced the largest but nonetheless weak association with conversion to PDD (AUC = 0.66 [0.57–0.75]). The PDQ hallucinations item (AUC = 0.62 [0.55–0.70]), NPI anxiety item (AUC = 0.61 [0.53–0.70]), NPI aberrant motor behavior item (AUC = 0.56 [0.51–0.62]), PDQ anxiety item (AUC = 0.60 [0.51–0.69]), and UPDRS hallucination item (AUC = 0.58 [0.50,0.67]) also had weak associations with conversion to PDD. No other neuropsychiatric measures were associated with conversion to PDD. We selected the NPI total score, PDQ hallucination, and PDQ anxiety items for subsequent analysis. The NPI anxiety and aberrant motor behavior items were not used, although they were both weakly associated with future PDD by themselves, because the NPI total score is a summation of all the NPI components. The UPDRS hallucination item was also not selected as it also measured hallucinations, like the PDQ hallucinations item, but was only marginally significantly associated with future PDD.
FIG. 3.
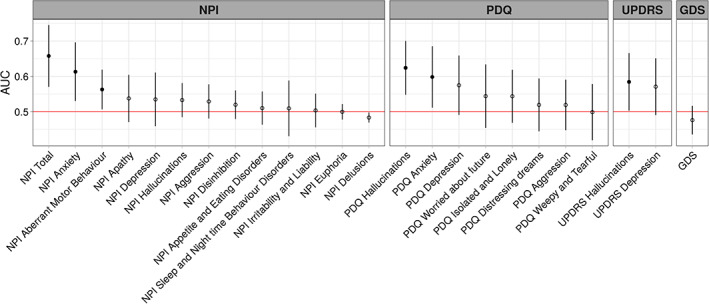
AUC values for conversion to PDD across neuropsychiatric measures. The dots represent AUC values, lines represent 95% CI and filled dots indicate values significantly greater than 0.5.
Determining Useful Predictors of Future Progression to PDD
The improvement in the model fit compared to a null model with only the intercept for each of the three separate neuropsychiatric measure models was very weak (NPI total score, ELPD = 1.8 (SD 2.5); hallucinations, delta ELPD = 2.9 (SD 2.9); anxiety, delta ELPD = 1.9 (SD 2.5)). That is, none of these neuropsychiatric measures provided reliably useful information to predict future dementia. In contrast, independent models containing global cognitive score only (delta ELPD = 36 (SD 8)) and age only (delta ELPD = 11 (SD 5)) had much stronger associations with future PDD. Motor function had minimal association with the probability of future PDD (delta ELPD = 5 (SD 3)). For ease of comparison with previous studies, odds ratios were calculated for each of the three neuropsychiatric measures. At baseline, NPI total score, PDQ hallucinations, and PDQ anxiety were all weakly associated with progression to PDD within 4 years (estimated odds ratio, NPI total score, 1.1 [1.0–1.1]; hallucinations, 1.6 [1.1, 2.1]; anxiety, 1.5, [1.1–2.1]).
The neuropsychiatric measures and previously‐proposed predictors (age, cognitive ability, and motor function) were then included in a single model with the conditional effects of developing PDD 4 years later shown in Figure 4. Along the x‐axis of each of the six plots displayed in Figure 4 are the range of possible scores for each predictor, while the probability of PDD conversion is displayed on the y‐axis. Overall, in the combined model of global cognitive score, age, NPI total score, PDQ hallucinations, PDQ anxiety, and UPDRS motor score, only cognition and age contributed useful predictive information of conversion to PDD within 4 years.
FIG. 4.
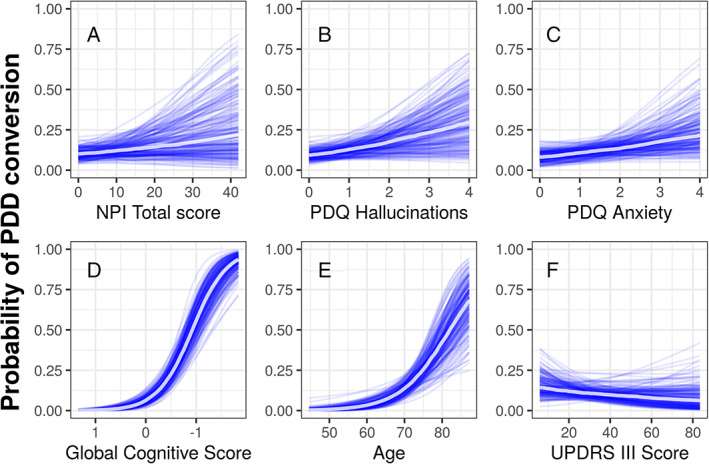
Conditional probability (with other variables held at their mean value) of conversion to PDD within 4 years as a function of (A) NPI Total score, (B) PDQ hallucinations, (C) anxiety (D) global cognitive score with scores worsening from left to right, (E) age, and (F) UPDRS III motor score. Each blue line represents one sample from the posterior, and the central white line represents the mean of those posterior samples. The mean values of each predictor was NPI Total score = 4.1, PDQ hallucinations = 0.5, PDQ anxiety = 1.1, global cognitive score = −0.2, age = 68.2, and UPDRS III motor score = 31.7.
Discussion
At baseline, we found that PDD participants experienced greater neuropsychiatric symptom burden than did non‐demented PD. Over the four‐year follow‐up period, of the neuropsychiatric measures, baseline NPI total score, PDQ hallucinations, PDQ anxiety, UPDRS hallucinations, NPI aberrant motor behavior and NPI anxiety all had weak associations with PDD risk. However, when the NPI total score, PDQ hallucination and PDQ anxiety were examined in a Bayesian regression model and the out‐of‐sample predictive ability compared to age, cognitive ability, and motor function, none of the neuropsychiatric symptoms were useful when predicting future PDD.
Only six neuropsychiatric symptoms were significantly associated with progression to PDD 4 years later. However, global cognitive ability and age, rather than neuropsychiatric symptoms, were better predictors of progression to PDD. Unlike previous studies, we did not find an association between baseline apathy measures and future dementia risk 3 , 21 or baseline depression measures and progression to PDD four‐years later. 1 Nevertheless, we did find an association between total NPI score, hallucinations, anxiety, and aberrant motor behavior and progression to PDD. The relationship between future PDD and hallucinations has been reported by others. 1 , 2 , 22 These studies vary in sample size from 80–224 PD participants (followed for 4‐8 years) and all rated the presence of hallucinations using a single hallucination measure from the UPDRS, whereas we included three hallucination measures in this study (UPDRS, PDQ, and NPI). We found the odds ratio of developing PDD for participants who experienced hallucinations versus those who did not was 1.6 [1.1, 2.1]. This was much lower than previous studies (OR = 3.1–10.2). 1 , 2 However, strengths of our study were the additional comparisons made to determine whether hallucinations provide useful information compared to previously‐proposed risk factors for PDD, which have not been evaluated in previous studies. These suggested that while neuropsychiatric symptoms are common in non‐demented PD 23 , 24 and have a negative impact on quality of life, 25 , 26 they provide no predictive information about PDD development, at least over a 4 year period.
Surprisingly the severity of motor symptoms did not provide any useful predictive information. Many previous studies have found an association between a variety of PD motor symptoms, including freezing, gait impairment, and falls, and increased PDD risk. 1 , 2 , 4 In this study however, motor impairment, as measured by the UPDRS III score, did not add any useful predictive value once age and cognitive ability were known. This finding is supported by two recent multinational studies which both found that when UPDRS III scores were accounted for, cognitive impairment independently increased PDD risk. 4 , 27
Only the NPI hallucinations, aberrant motor behavior, and UPDRS hallucinations items differed significantly when comparing neuropsychiatric symptoms between PD‐MCI and PD‐N. Previous studies have found mixed results. Broeders et al. (2013) 28 found that PD‐MCI patients reported higher rates of depression and anxiety compared to PD‐N, whereas other studies have found no differences in depression, 29 , 30 hallucinations, 30 and NPI total scores. 29 These mixed findings could be due to differences in the tests employed to measure each of these symptoms. We used multiple tests to measure depression (NPI, PDQ, UPDRS, and GDS) and anxiety (NPI and PDQ), whereas Broeders et al. (2013) 28 used the Hospital Anxiety and Depression Scale (HADS). Both the GDS and HADS have clinical utility in detecting depression in PD 31 but could be capturing different aspects of depression symptomatology. Variations in the tests employed to measure each neuropsychiatric symptom might therefore explain the differences between these studies.
At baseline, the PDD group presented with more severe hallucinations, depression, anxiety, apathy, loneliness, distressing dreams, and aggression compared to PD‐N. A similar overall pattern was seen when the PDD group was compared to PD‐MCI. No difference in anxiety levels, however, was found between PDD and PD‐MCI. These cross‐sectional findings at baseline align with previous studies examining the prevalence of neuropsychiatric symptoms, with those with PDD having greater prevalence than those with PD‐MCI and PD‐N. 29 , 30 , 32 Thus, it is clear from this and other studies that the development of dementia in PD brings with it a greater burden of neuropsychiatric symptoms.
There were few participants who experienced certain neuropsychiatric symptoms, such as delusions (n = 15), disinhibition (n = 19), and euphoria (n = 8) as measured by the NPI. This could be due to the scales lacking the sensitivity to detect these symptoms, or that they are uncommon in PD. Aside from the GDS, the other neuropsychiatric items used in this study were not specifically designed to be used as single measures, separate from the overall scales within which they sit. Thus, those items may not be as sensitive in determining the true prevalence of these symptoms in PD populations. The use of single items extracted from larger scales is a limitation of this study, although most other studies examining the relationship between hallucinations and future PDD have also used single measures (from the UPDRS). 1 , 2 , 22 We recommend that future studies employ more specifically‐targeted neuropsychiatric symptom scales (such as the Psychosis and Hallucinations Questionnaire 33 , 34 or HADS), to determine the true predictive value of each neuropsychiatric symptom.
In summary, we found that six neuropsychiatric symptoms present at baseline in non‐demented PD patients were weakly associated with progression to PDD four‐years later, NPI total score, PDQ hallucinations, UPDRS hallucinations, PDQ anxiety, NPI anxiety, and NPI aberrant motor behavior. We also confirmed that participants with PDD experienced more neuropsychiatric symptoms than those with those with either PD‐MCI or PD‐N, with few differences in their prevalence between the two non‐dementing groups. When NPI total score, PDQ hallucinations, PDQ anxiety, and motor function were examined alongside cognitive impairment and age, they did not have any useful predictive value, which suggests that they are not useful markers of future PDD risk but instead evolve in parallel with the disease process.
Author Roles
1) Research Project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
K‐LH: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B;
MRM: 1A, 1B, 2A, 2B, 2C, 3B;
DJM: 1A, 2A, 2B, 2C, 3B;
LL: 1B, 1C, 3B;
SG: 1C, 3B;
MP: 1C, 3B;
BY: 1C, 3B;
RS: 2B, 2C, 3B;
TRM: 2C, 3B;
TLP: 1B, 1C, 3B;
TJA: 1A, 1B, 2C, 3B;
JCD‐A: 1A, 1B, 2A, 2C, 3B.
Disclosures
Ethical Compliance Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The study was approved by the New Zealand Health & Disability Ethics Committee. At the beginning of each neuropsychological assessment, participants each read and signed a consent form after discussing any questions or concerns they may have. All participants gave informed written consent.
Funding Sources and Conflicts of Interest
The research conducted in this article was funded by research support from the New Zealand Health Research Council, Brain Research New Zealand—Rangahau Roro Aotearoa, University of Otago, University of Canterbury, Neurological Foundation of New Zealand, Canterbury Medical Research Foundation, and the New Zealand Brain Research Institute. The authors of this article have no financial relationships relevant to this article to disclose or conflicts of interest.
Financial Disclosures for the previous 12 months
Kyla‐Louise Horne is employed by the University of Otago and New Zealand Brain Research Institute. She received funding from Brain Research New Zealand—Rangahau Roro Aotearoa and the New Zealand Brain Research Institute over the past 12 months. Michael R. MacAskill is employed by the University of Otago. He received research support from the Canterbury Medical Research Foundation, Neurological Foundation of New Zealand, and the New Zealand Brain Research Institute over the past 12 months. Daniel J. Myall is employed by the New Zealand Brain Research Institute. He received support from the Canterbury Medical Research Foundation, New Zealand Brain Research Institute, and Brain Research New Zealand—Rangahau Roro Aotearoa over the past 12 months. Leslie Livingston is employed by the University of Otago. She received support from the Canterbury Medical Research Foundation, Health Research Council of New Zealand, the University of Otago Research Grant and the New Zealand Brain Research Institute over the past 12 months. Sophie Grenfell is employed at the New Zealand Brain Research Institute. She received support from the New Zealand Brain Research Institute over the past 12 months. Maddie Pascoe is employed at the New Zealand Brain Research Institute. She received support from the Neurological Foundation of New Zealand, the New Zealand Brain Research Institute and Cure Kids over the past 12 months. Bob Young was employed at the New Zealand Brain Research Institute. He received support from the New Zealand Brain Research Institute over the past 12 months. Reza Shoorangiz is employed at the New Zealand Brain Research Institute. He received support from the New Zealand Brain Research Institute and Brain Research New Zealand—Rangahau Roro Aotearoa and the Neurological Foundation of New Zealand over the past 12 months. Tracy R. Melzer is employed by the University of Otago. He received support from the Canterbury Medical Research Foundation, the Health Research Council of New Zealand, the Neurological Foundation of New Zealand, a University of Otago Research Grant, the New Zealand Brain Research Institute and Brain Research New Zealand—Rangahau Roro Aotearoa over the past 12 months. Toni L. Pitcher is employed by the University of Otago. She received research support from the Neurological Foundation of New Zealand, Brain Research New Zealand—Rangahau Roro Aotearoa and the New Zealand Brain Research Institute over the past 12 months. Dr Pitcher also received an honorarium from STADA Australia. Tim J. Anderson is employed by the University of Otago and Anderson Neurology Ltd. He received research support from the Health Research Council of New Zealand and Brain Research New Zealand—Rangahau Roro Aotearoa over the past 12 months. John C. Dalrymple‐Alford is employed by the University of Canterbury. He received research support from the University of Canterbury, Canterbury Medical Research Foundation, Health Research Council of New Zealand, Neurological Foundation of New Zealand, Lotteries Health Research, and Brain Research New Zealand—Rangahau Roro Aotearoa over the past 12 months.
[Correction added on February 25, 2021 after first online publication: Reference 7, 8, 28‐34 have been renumbered in the reference list.]
Supporting information
Table S1. Kruskal‐Wallis results for demographics, neuropsychological measures presented in Table 1 at study entry [mean (SD)]
Table S2. Kruskal‐Wallis results for demographics, neuropsychological measures presented in Table 3 at study entry [mean (SD)] for the 202 non‐dementing PD participants followed‐up over four‐years[Correction added on February 25, 2021 after first online publication: Supporting Information Tables S1 and S2 have been updated.]
Acknowledgments
We would like to acknowledge individuals who have collected data, including Saskia van Stockum, Charlotte Graham, Krysta Callander, Megan Livingstone and Beth Elias. We would also like to acknowledge Sam Harrison for helping collate data used in this paper.
References
- 1. Anang JB, Gagnon J‐F, Bertrand J‐A, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83(14):1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aarsland D, Andersen K, Larsen JP, Lolk A. Prevalence and characteristics of dementia in Parkinson disease: an 8‐year prospective study. Arch Neurol 2003;60(3):387–392. [DOI] [PubMed] [Google Scholar]
- 3. Fitts W, Weintraub D, Massimo L, et al. Caregiver report of apathy predicts dementia in Parkinson's disease. Parkinsonism Relat Disord 2015;21(8):992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoogland J, Boel JA, de Bie RM, et al. Mild cognitive impairment as a risk factor for Parkinson's disease dementia. Mov Disord 2017;32(7):1056–1065. [DOI] [PubMed] [Google Scholar]
- 5. Factor S, Feustel P, Friedman J, et al. Longitudinal outcome of Parkinson's disease patients with psychosis. Neurology 2003;60(11):1756–1761. [DOI] [PubMed] [Google Scholar]
- 6. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement Disorder Society task force guidelines. Mov Disord 2012;27(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wood K‐L, Myall DJ, Livingston L, et al. Different PD‐MCI criteria and risk of dementia in Parkinson's disease: 4‐year longitudinal study. npj Parkinson's Dis 2016;2(1):15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22(12):1689–1707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- 9. Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology 1997;48(5 Suppl 6):10S–16S. [DOI] [PubMed] [Google Scholar]
- 10. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 11. D'ath P, Katona P, Mullan E, Evans S, Katona C. Screening, detection and management of depression in elderly primary care attenders. I: the acceptability and performance of the 15 item geriatric depression scale (GDS15) and the development of short versions. Fam Pract 1994;11(3):260–266. [DOI] [PubMed] [Google Scholar]
- 12. Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15‐item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry 2006;14(2):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res 1995;4(3):241–248. [DOI] [PubMed] [Google Scholar]
- 14. Fahn S, Elton RL. UPDRS program members. Unified Parkinsons disease rating scale. Recent developments in Parkinson's disease 1987;2:153–163. [Google Scholar]
- 15. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): Scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 16. Goetz CG, Stebbins GT, Tilley BC. Calibration of unified Parkinson's disease rating scale scores to Movement Disorder Society‐unified Parkinson's disease rating scale scores. Mov Disord 2012;27(10):1239–1242. [DOI] [PubMed] [Google Scholar]
- 17. Team RC . R: A Language and Environment for Statistical. Comput Secur 2013. [Google Scholar]
- 18. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J‐C, Müller M. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinf 2011;12(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bürkner P‐C. Brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 2017;80(1):1–28. [Google Scholar]
- 20. Vehtari A, Gelman A, Gabry J. Practical Bayesian model evaluation using leave‐one‐out cross‐validation and WAIC. Stat Comput 2017;27(5):1413–1432. [Google Scholar]
- 21. Pedersen KF, Alves G, Aarsland D, Larsen JP. Occurrence and risk factors for apathy in Parkinson disease: a 4‐year prospective longitudinal study. J Neurol Neurosurg Psychiatry 2009;80(11):1279–1282. [DOI] [PubMed] [Google Scholar]
- 22. Aarsland D, Andersen K, Larsen JP, Perry R, Wentzel‐Larsen T, Lolk A, Kragh‐Sørensen P. The rate of cognitive decline in Parkinson disease. Arch Neurol 2004;61(12):1906–1911. [DOI] [PubMed] [Google Scholar]
- 23. Kulisevsky J, Pagonabarraga J, Pascual‐Sedano B, García‐Sánchez C, Gironell A, TG Study . Prevalence and correlates of neuropsychiatric symptoms in Parkinson's disease without dementia. Mov Disord 2008;23(13):1889–1896. [DOI] [PubMed] [Google Scholar]
- 24. Lees A, Hardy J, T R. Parkinson's disease. Lancet 2009;373(9680):2055–2066. [DOI] [PubMed] [Google Scholar]
- 25. Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population‐based, prospective study. J Am Geriatr Soc 2000;48(8):938–942. [DOI] [PubMed] [Google Scholar]
- 26. Marsh L. Neuropsychiatric aspects of Parkinson's disease. Psychosomatics 2000;41(1):15–23. [DOI] [PubMed] [Google Scholar]
- 27. Hoogland J, Boel JA, de Bie RM, et al. Risk of Parkinson's disease dementia related to level I MDS PD‐MCI. Mov Disord 2019;34(3):430–435. [DOI] [PubMed] [Google Scholar]
- 28. Broeders M, de Bie RMA, Velseboer DC, Speelman JD, Muslimovic D, Schmand B. Evolution of mild cognitive impairment in Parkinson disease. Neurology. 2013;81(4):346–352. 10.1212/wnl.0b013e31829c5c86. [DOI] [PubMed] [Google Scholar]
- 29. Dalrymple‐Alford J, MacAskill M, Nakas C, et al. The MoCA: well‐suited screen for cognitive impairment in Parkinson disease. Neurology 2010;75(19):1717–1725. [DOI] [PubMed] [Google Scholar]
- 30. Hobson P, Meara J. Mild cognitive impairment in Parkinson's disease and its progression onto dementia: a 16‐year outcome evaluation of the Denbighshire cohort. Int J Geriatr Psychiatry 2015;30(10):1048–1055. [DOI] [PubMed] [Google Scholar]
- 31. Schrag A, Barone P, Brown RG, et al. Depression rating scales in Parkinson's disease: critique and recommendations. Mov Disord 2007;22(8):1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bronnick K, Aarsland D, Larsen J. Neuropsychiatric disturbances in Parkinson's disease clusters in five groups with different prevalence of dementia. Acta Psychiatr Scand 2005;112(3):201–207. [DOI] [PubMed] [Google Scholar]
- 33. Shine JM, Qiu J, et al. Validation of the psychosis and hallucinations questionnaire in non‐demented patients with Parkinson's disease. Mov Disord Clin Pract 2015;2(2):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muller AJ, Mills JM, O'Callaghan C, Naismith SL, Clouston PD, Lewis SJ, Shine JM. Informant‐and self‐appraisals on the psychosis and hallucinations questionnaire (PsycH‐Q) enhances detection of visual hallucinations in Parkinson's disease. Mov Disord Clin Pract 2018;5(6):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Kruskal‐Wallis results for demographics, neuropsychological measures presented in Table 1 at study entry [mean (SD)]
Table S2. Kruskal‐Wallis results for demographics, neuropsychological measures presented in Table 3 at study entry [mean (SD)] for the 202 non‐dementing PD participants followed‐up over four‐years[Correction added on February 25, 2021 after first online publication: Supporting Information Tables S1 and S2 have been updated.]


