Myorhythmia is a hyperkinetic movement disorder characterized by slow, repetitive, rhythmic, cranial and limb contractions, typically disappearing during sleep. Infectious, autoimmune or vascular lesions involving brainstem or diencephalic structures are the most common etiologies. 1
Anti‐IgLON5 disease was originally reported as a progressive neurological syndrome characterized by a preeminent sleep disorder, variably associated with bulbar dysfunctions, gait instability, oculomotor abnormalities and cognitive decline. 2 , 3 Since initial descriptions, several hyperkinetic movement disorders have been described, including oro‐facial myorhythmia in few cases. 4 , 5 We describe a case of anti‐IgLON5 disease‐related myorhythmia involving both facial and limb muscles, persisting during physiological and pathological sleep.
Case Report
A 68‐year‐old African‐Caucasian female was admitted for a 4‐year progressive history of disequilibrium and involuntary bilateral limb jerks, which impaired standing and walking, associated with apathy and mood depression. Neurological examination showed repetitive sustained involuntary contractions at rest and during voluntary action involving facial, cervical, upper and lower limb muscles, resulting in a kiss fashion movement, flexion at elbow with extension of hands and abduction of thighs with extension of feet. Contractions affected the oro‐pharyngeal region as well, resulting in lingual retraction, elevation of soft palate, associated with a swallowing‐like movement and guttural sound.
A 24 hours video‐polysomnography (VPSG) documented repetitive, rhythmic, low frequency (0.6 Hz) muscle contractions synchronous on explored muscles both in relaxed and activated wakefulness as well as in all sleep stages [Fig. 1A, Video 1]. These findings were consistent with the diagnosis of myorhythmia. Sleep structure showed a prevalence of light stages; REM sleep disclosed normal atonia; no pathological behavior or breathing disorder were recorded [Fig. 1B].
FIG. 1.
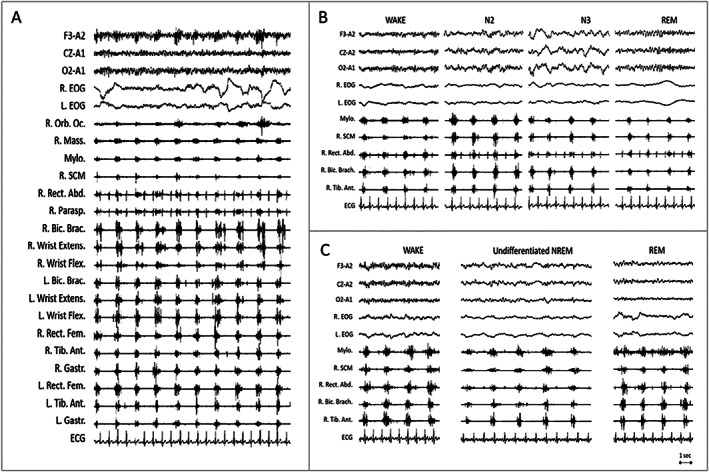
Polygraphic recording of myorhythmia during wakefulness and sleep. (A) Myorhythmia during relaxed wakefulness. Excerpt of 24‐hour polysomnographic recording shows rhythmic, low frequency (0.6 Hz) muscle contractions, synchronous on all explored muscles. Contractions lasted between 300 and 700 ms affecting agonist and antagonist muscle in a co‐contraction pattern. (B) During the same 24 hours polysomnographic recording showed in (A) myorhythmia persists in NREM (N2; N3) and REM sleep stages. REM sleep shows the physiological muscle atonia. (C) Excerpts of the second nocturnal polysomographic recording 1 year later. Myorhythmia appears unchanged since the year before, persisting during wakefulness and sleep. Physiological NREM sleep figures, such as sleep spindles and K‐complexes, were no longer detectable (undifferentiated NREM). During REM sleep muscle tone persists. EEG (F3‐A2; Cz‐A2; O2‐A1); R, right; L, left; EOG, electroculogram; Orb. Oc., orbicularis oculi; Mass., masseter; Mylo., mylohyoideus; SCM, sternocleidomastoideus; Rect. Abd., rectus abdominis; Parasp., paraspinalis; Bic. Brac., biceps brachii; Extens., extensor; Flex., flexor; Rect. Fem., rectus femoris; Tib. Ant., tibialis anterior; Gastr., Gastrocnemius; ECG, electrocardiogram.
FIG. 2.
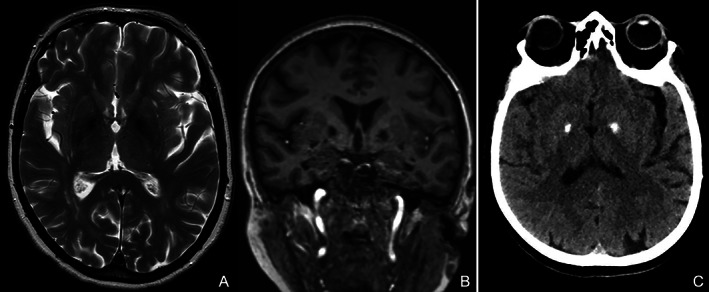
Brain MRI in axial T2 (A) and coronal T1 (B) weighted sequences showed bilateral and symmetrical mineralization of pallidal nuclei. Brain CT (C) demonstrated hyperdensity of pallidal nuclei indicative for calcification.
Brain imaging (MRI and CT) showed bilateral calcification limited to pallidal nuclei [Fig. 2]. Cerebrospinal fluid (CSF) analysis disclosed mild pleocytosis (7 cells/cc), oligoclonal bands were negative. Serum anti‐neuronal antibodies and surface neuronal antibodies (NMDAR, LGI1, CASPR2, AMPAR, GABA A R, GABA B R, mGluR1, mGluR2, mGluR5, DPPX, Neurexin‐3‐alpha) were negative. Total‐body fluorodeoxyglucose‐PET ruled malignancy out. Histologic examination of jejunal biopsies and PCR for Tropheryma whipplei on CSF excluded Whipple's disease (WD). Symptomatic treatment with pramipexole was ineffective.
Video 1.
Video excerpt showing myorhythmia during sleep.
At 1‐year follow‐up, intense perspiration episodes and vocalizations during sleep were reported. Neurological examination disclosed fragmented ocular pursuit, limb dysmetria, diffuse hyperreflexia with lower limb spasticity. Neuropsychological assessment showed a mild cognitive impairment with prevalent verbal memory dysfunction. On nocturnal‐VPSG, NREM sleep figures were no longer detectable and undifferentiated NREM (U‐NREM) sleep occupied 75% of total sleep time.
A 5‐minute episode with vocalizations and simple/finalistic movements (pointing or looking for something) arose from U‐NREM. REM sleep lost the normal muscle atonia, but no REM sleep behavior episodes were recorded. Myorhythmia appeared unchanged on polygraphic recording, occurring both in wakefulness, U‐NREM and REM sleep [Fig. 1C]. Because of progressive and severe sleep disorder, anti‐IgLON5 antibodies were tested and found positive in both serum and CSF collected during the first admission, with similar proportion of IgG1 and IgG4 isotype. HLA DRB1*10:01 and HLA‐DQB1*05:01 were positive. Intravenous immunoglobulins and therapeutic plasma exchange were ineffective. The patient died few weeks later at the age of 70 during sleep.
Discussion
We described a case of anti‐IgLON5 disease‐related myorhythmia that involves both cranial and limb muscles. Previously only oro‐facial myorhythmia was reported, associated with a progressive supranuclear palsy–like phenotype or combined with sleep disorders. 4 , 5 Like these previous descriptions, 4 , 5 also in our case the contraction pattern spares ocular involvement, which represents a hallmark of WD instead. 1
Moreover, in our patient myorhythmia displayed an unusual occurrence persisting during both wakefulness and sleep, as opposed to the state‐dependent pattern described in most well‐known etiologies, that typically desist in sleep. 1 Persistence of movements during sleep was also reported in the early descriptions of WD, even though without documentation from a specific neurophysiological study. 6 , 7 Subsequently, a report evaluating wake–sleep occurrence of myorhythmia through 24 hours VPSG highlighted that the apparent sleep‐persisting myorhythmia in WD was due to the inability to maintain a continuous and stable sleep stage. 8 Here we clearly demonstrate the persistence of myorhythmia through NREM and REM sleep stages, even before the development of the progressive and severe sleep dysfunction that characterized anti‐IgLON5 disease.
Both the synchronous cranial‐limb pattern of myorhythmia and the disruption of the physiological sleep modulation on the motor system suggest a diencephalic or brainstem dysfunction. Despite the lack of post‐mortem examination in our case, this hypothesis is supported by the evidence that anti‐IgLON5 disease is associated with neuronal loss and hyperphosphorylated‐tau deposition in brainstem and hypothalamus. 3
Further descriptions are needed to confirm if the persistence of myorhythmia during sleep could be considered a distinctive pattern of anti‐IgLON5 disease.
Anti‐IgLON5 disease is a novel clinical entity whose clinical spectrum is probably wider than what described so far as our case shows. Better knowledge of the disease is essential to achieve rapid recognition and promptly undertake potentially disease‐modifying therapy.
Author Roles
(1) Research project: A. Study concept and design, B. Acquisition of data, C. Analysis and interpretation of data, D. Supervision; (2) Manuscript: A. Writing of the first draft, B. Review and revision.
G.M.A.: 1A, 1B, 1C, 2A
G.C.B.: 1A, 1C, 1D, 2B
V.M.: 1B, 1C, 2B
G.P.: 1B, 1C
C.G.: 1C, 2B
J.S.: 1C, 2B
P.C.: 1C, 1D, 2B
F.P.: 1A, 1B, 1C, 1D, 2B
Disclosures
Ethical Compliance Statement
The authors confirm that approval of an institutional review board was not required for this work. Informed written consent for publication was obtained from patient's relatives. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The authors report no relevant disclosures or conflicts of interest for this manuscript.
Financial Disclosures for the Previous 12 Months
The authors report no financial disclosure for the previous 12 months other than employment from the respective affiliations.
Acknowledgments
We thank Annagrazia Cecere for her invaluable help in editing the video and figures, and patient's relatives for their willingness and support.
References
- 1. Baizabal‐Carvallo JF, Cardoso F, Jankovic J. Myorhythmia: Phenomenology, etiology, and treatment. Mov Disord 2015;30:171–179. [DOI] [PubMed] [Google Scholar]
- 2. Gaig C, Graus F, Compta Y, et al. Clinical manifestations of the anti‐IgLON5 disease. Neurology 2017;88:1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabater L, Gaig C, Gelpi E, et al. A novel non‐rapid‐eye movement and rapid‐eye movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: A case series, characterisation of the antigen, and post‐mortem study. Lancet Neurol 2014;13:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Honorat JA, Komorowski L, Josephs KA, et al. IgLON5 antibody: Neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm 2017;4:e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morales‐Briceño H, Cruse B, Fois AF, et al. IgLON5‐mediated neurodegeneration is a differential diagnosis of CNS Whipple disease. Neurology 2018;90:1113–1115. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz MA, Selhorst JB, Ochs AL, et al. Oculomasticatory myorhythmia: A unique movement disorder occurring in Whipple's disease. Ann Neurol 1986;20:677–683. [DOI] [PubMed] [Google Scholar]
- 7. Xia C, Duquette A, Frucht S, Lafontaine AL. Whipple's disease presenting with segmental myoclonus and hypersomnia. Mov Disord 2012;27:1216–1217. [DOI] [PubMed] [Google Scholar]
- 8. Calandra‐Buonaura G, Provini F, Guaraldi P, et al. Oculomasticatory myorhythmia and agrypnia excitata guide the diagnosis of Whipple disease. Sleep Med 2013;14:1428–1430. [DOI] [PubMed] [Google Scholar]


