ABSTRACT
Background
Palatal tremor (PT) is an uncommon movement disorder that may be classified into symptomatic (SPT) or essential (EPT). The etiology of SPT is varied, with involvement of the Guillain‐Mollaret triangle (GMT) and inferior olivary hypertrophy. EPT is associated with ear clicks and normal imaging and may have a functional basis.
Objectives
This study aims to explore the clinical and radiological features of a large cohort of patients with PT.
Methods
This is a retrospective chart review of patients with PT who were evaluated by the movement disorders subspeciality of the neurology department. Demographic, clinical, and imaging details of patients with PT were documented.
Results
A total of 22 patients with PT comprising 17 with SPT and 5 with EPT were included in this study. No patient was aware of the PT. Ear clicks were reported in 2 patients with SPT and in 3 patients with EPT. The most common etiology for SPT was vascular, followed by degenerative conditions. Patients with SPT had associated features such as tremor (70.6%), ataxia (64.7%), dystonia (52.9%), myoclonus (17.6%), and eye movement abnormalities (75%). Lesions involving the GMT were found in 82% of patients with SPT. Apart from PT, patients with EPT had no other motor symptoms, and imaging was normal. Of the patients with EPT, 2 had additional functional movement disorders.
Conclusion
PT has significant etiological heterogeneity and can be easily missed because of the lack of awareness by patients. Involvement of the inferior olivary nucleus may not be necessarily observed. A functional etiology should be considered in cases of EPT.
Keywords: palatal tremor, Guillain‐Mollaret triangle, inferior olivary nucleus, symptomatic, essential
Palatal tremor (PT), first described by Politzer in 1878, is a rare movement disorder characterized by rhythmic 0.5 to 5 Hz 1 movements of the soft palate. The nosology of PT has been variable, and several terms such as cephalic murmur, rhythmic palatal myoclonus, focal dyskinesia, and chorea of soft palate have been previously used to describe the movement disorder. 2
PT may be classified as essential palatal tremor (EPT) and symptomatic palatal tremor (SPT). EPT is an isolated focal tremor, 1 and patients may report associated ear clicks. Typically, there are no other neurological symptoms or signs, and imaging is normal. The pathophysiology of EPT is uncertain and controversial. Recently, a functional basis has been suggested for EPT and demonstrated by electrophysiological studies. 3 It has been suggested that functional PT is an underrecognized entity, and a large number of patients with EPT may have a functional origin. 4 SPT occurs secondary to lesions involving the Guillain‐Mollaret Triangle (GMT) and has a variable etiology that includes vascular events, demyelination, infections, trauma, drugs, and several degenerative conditions. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 SPT is associated with eye signs, tremor, and ataxia attributed to the involvement of the brainstem and spinal motor neurons, 1 and imaging often shows inferior olivary (IO) hypertrophy in addition to the primary lesion. 5
PT is a rare disorder with a vast etiological spectrum. There are relatively few studies that have described the varied clinical, etiological, and radiological features of PT. The present study describes the clinical profile, associated movement disorders, and radiological features of a large, single‐center cohort of patients with PT.
Patients and Methods
Subject Recruitment and Clinical Evaluation
This is a retrospective chart review of patients with PT who were evaluated by the Parkinson's Disease and Movement Disorders Subspeciality of the Department of Neurology at the National Institute of Mental Health and Neurosciences, Bangalore. Patients were identified by reviewing our video database from January 2005 to 2019 by using the search terms “palatal tremor” or “palatal myoclonus.” Hence only those patients who had a video were included in this study. Most of these patients had presented with various neurological symptoms and on examination were found to have palatal tremor. Demographic, clinical, and imaging details were documented from the hospital case records. An Institute Ethics Committee waiver was obtained for the present study (no. NIMHANS/IEC/2019–20), and informed consent for video recording and publication had been obtained from the patients. One patient with EPT (Video 5) has been previously reported. 15
Video 5.
Essential palatal tremor. (Note: This case has been previously published. 15 )
Statistical Analysis
Data were expressed using descriptive statistics, where the mean and standard deviation were used for continuous variables, and categorical variables were described using frequencies and percentages.
Results
Demographic Data
A total of 22 patients were included in this study, of which the majority of patients had SPT (77.3%, n = 17), followed by EPT in 5 patients. There was no significant difference in sex between the groups (SPT: male:female, 10:7; EPT: male:female, 3:2). Patients with SPT were unaware of the presence of PT, owing to which the mean age at onset (AAO) does not refer to the AAO of PT, rather, it refers to the onset of other symptoms secondary to the causative etiology. In patients with SPT, the mean AAO was 37.0 ± 17.2 years, whereas the mean AAO of symptoms in EPT was 22.6 ± 11.4 years. Patients with EPT were evaluated at a significantly earlier age in comparison with patients with SPT (24.6 ± 10.0 and 41.5 ± 15.4, respectively; P = 0.03). Although not statistically significant, patients with EPT had a relatively shorter duration of illness in comparison with patients with SPT (4.4 ± 7.6 and 2.1 ± 2.0, respectively).
Symptomatic Palatal Tremor
Etiology
A wide variety of etiologies were observed in patients with SPT (Table 1). A vascular event was the most common underlying etiology (29.4%, n = 5), of which 4 were bleeds and 1 was an infarct. All of these patients had strokes involving the cerebellum, midbrain, or both (Table 1, Video 1). A genetic etiology was suspected based on clinical evaluation and imaging in 17.6% of patients (n = 3): probable Alexander disease (Video 2), probable autosomal recessive cerebellar ataxia, and myoclonus dystonia. A total of 3 patients (17.6%) had a suspected degenerative etiology, that is, progressive ataxia and palatal tremor (PAPT). Of the patients, 2 (11.7%) had a postinfective etiology, that is, encephalitis. A posttraumatic etiology was reported in 2 patients (11.7%) who had sustained head injuries during road traffic accidents. Postradiation pontomedullary syndrome was observed in a patient with a pontine arteriovenous malformation (Video 3). Finally, a probable tardive etiology was suspected in a patient with a schizotypal disorder with tardive dystonia who had a history of consumption of flupenthixol before the onset of dystonia (Video 4).
TABLE 1.
Summary of clinical and imaging characteristics in patients with symptomatic palatal tremor
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y/Sex | 62/M | 53/M | 35/F | 19/M | 32/M | 63/F | 32/M | 39/F | 50/F | 21/M | 34/M | 27/F | 43/M | 21/F | 46/M | 60/M | 69/F |
| AAO, y | 62 | 52 | 33 | 7 | 29 | 57 | 31 | 5 | 50 | 20 | 34 | 27 | 42 | 41 | 45 | 60 | 54 |
| Etiology | V | V | D | G | G | D | PR | G | I | PT | I | V | Td | V | PT | V | D |
| Ear clicks | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Tremor | − | − | H, T, UL | T | T | H, UL (H) | H | − | H | − | − | UL | UL | UL | UL (H) | H, UL (H) | H |
| Dystonia | − | Fa | C | Fa, R UL | − | UL | − | − | − | R UL, R LL | − | − | C | C | − | C | C |
| Myoclonus | − | − | Fa | UL | − | − | − | − | − | − | − | − | − | − | − | − | UL |
| Ataxia | + | − | + | + | + | + | − | + | − | + | + | + | − | − | + | − | + |
| GMT lesion | + | + | + | − | + | + | + | − | − | + | + | + | + | + | + | + | + |
| IO | HI, HT | HI, HT | HI, HT | − | HI | HI, HT | HI, HT | − | − | HI, HT | HI | − | HI, HT | HI, HT | HI, HT | HI, HT | HI, HT |
| DN | + | + | + | − | − | − | − | − | − | − | − | − | − | + | − | + | + |
| RN | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − |
| SCP | − | − | − | − | + | + | + | − | − | − | + | − | − | + | + | + | + |
| CTT | + | − | − | − | + | + | + | − | − | + | + | + | − | − | + | + | − |
| Cerebellar atrophy | − | + | + | − | − | + | + | + | − | − | − | − | − | − | − | − | + |
| Brainstem | − | + | − | − | + | − | + | − | − | − | + | − | − | − | − | − | + |
| Supratentor‐ial | + | + | − | − | − | − | − | − | + | − | − | − | − | + | − | − | − |
M, male; F, female; AAO, age at onset; V, vascular; D, degenerative; G, genetic; PR, postradiation; I, infective; PT, posttraumatic; Td, tardive; H, head; T, tongue; UL, upper limb; UL (H), upper limb Holmes tremor; Fa, facial; C, cervical; R, right; LL, lower limb; GMT, Guillain‐Mollaret triangle; IO, inferior olive; HI, hyperintensity; HT, hypertrophy; DN, dentate nucleus; RN, red nucleus; SCP, superior cerebellar peduncle; CTT, central tegmental tract.
Video 1.
Palatal tremor in a patient with midbrain infarct. The patient had cervical dystonia, a no‐no type of head tremor, and left upper limb Holmes tremor. Head and truncal tremor worsened upon standing, and he patient needed support to walk.
Video 2.
Palatal tremor with associated flaring of alae nasi in a patient with suspected Alexander disease.
Video 3.
Primary gaze nystagmus, head tremor, and palatal tremor in a patient with postradiation therapy for pontine arteriovenous malformation.
Video 4.
Palatal tremor observed in a patient with tardive dystonia secondary to flupenthixol consumption. The patient also had cervical dystonia.
Clinical Features
None of the patients were aware of the presence of PT, and was identified on clinical examination rather than through history. Only 2 patients (11.7%) with SPT reported ear clicks. All patients with SPT had at least 1 additional movement disorder (AMD), that is, tremor (other than the PT), dystonia, ataxia, or myoclonus (Fig. 1). A single AMD was observed in 6 patients (35.3%), among whom ataxia was the most prevalent (50%, n = 3), followed by tremor (33.3%, n = 2) and dystonia (16.6%, n = 1). A total of 2 AMDs were observed in 7 patients (41.1%), among whom tremor and dystonia (42.8%, n = 3) and tremor and ataxia (42.8%, n = 3) were the most common followed by dystonia and ataxia (14.3%, n = 1). One patient had a triad of tremor, dystonia, and ataxia (5.8%). All 4 AMDs were observed in 3 patients (35.3%).
FIG. 1.

Venn diagram showing combinations of additional movement disorders observed in patients with symptomatic palatal tremor.
Pyramidal signs were present in 9 (52.9%) patients, and eye movement abnormalities were observed in 75% of patients (n = 13). Gaze‐evoked nystagmus was the predominant abnormality (61.5%, n = 8), followed by saccadic abnormalities (62.5%, n = 5), that is, slow saccades (50%, n = 4), hypermetric saccades (12.5%, n = 1), and finally, extraocular muscle palsy (25%, n = 2). Speech abnormalities were found in 88.2% of patients (n = 15), and scanning speech was the most common abnormality (47%, n = 7), followed by hypophonic speech (17.6%, n = 3), spastic speech (11.7%, n = 2), and mixed dysarthria (5.8%, n = 1) and anarthria (5.8%, n = 1).
Imaging
Of the 17 patients with SPT, 1 patient with myoclonus dystonia was found to have normal magnetic resonance imaging (MRI), and abnormalities were identified in 16 patients (94.1%; Table 1, Figs. 2 and 3). Of the patients, 14 (87.5%) were found to have lesions involving structures of the GMT (Table 1, Figs. 2, 3, 4).
FIG. 2.
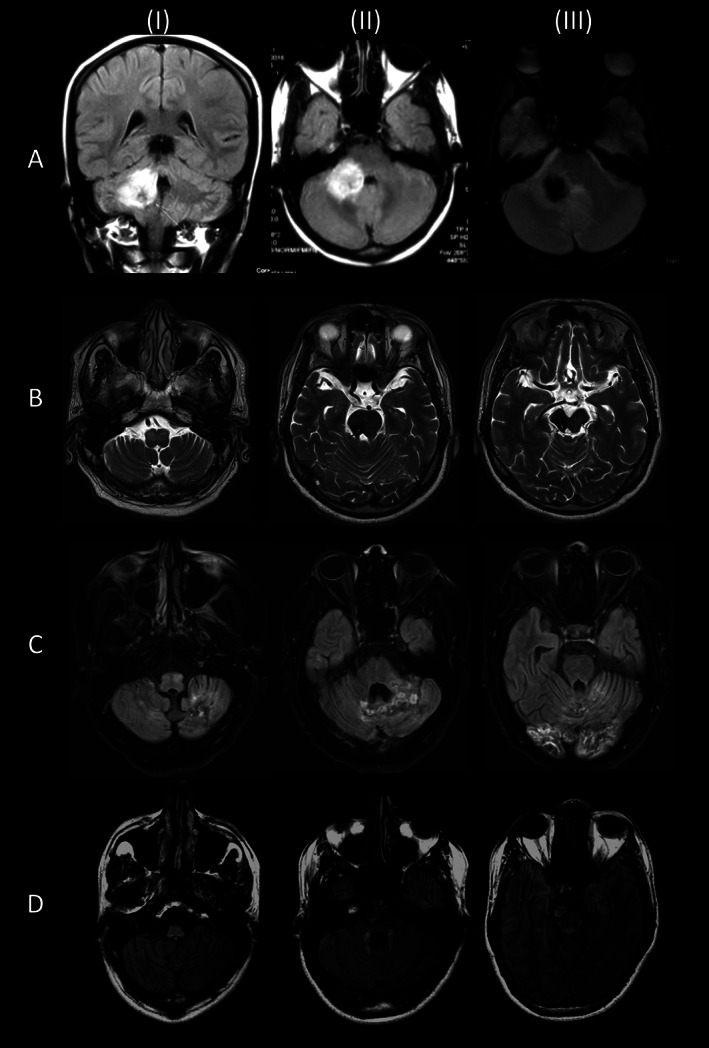
(A) Magnetic resonance imaging in a patient with symptomatic palatal tremor secondary to a large cerebellar bleed (I, II: T1‐weighted scans; III: susceptibility‐weighted image). There was no inferior olivary hypertrophy. (B) Bilateral olivary hypertrophy and hyperintensity secondary to a midbrain infarct (T2‐weighted scans). (C) Bilateral olivary hypertrophy and hyperintensity secondary to cerebellar bleeds in a case of antiphospholipid antibody syndrome (fluid‐attenuated inversion recovery scan). (D) Hyperintense lesions involving the medulla, peri‐fourth ventricle, and midbrain in a case of suspected Alexander disease (fluid‐attenuated inversion recovery scan).
FIG. 3.
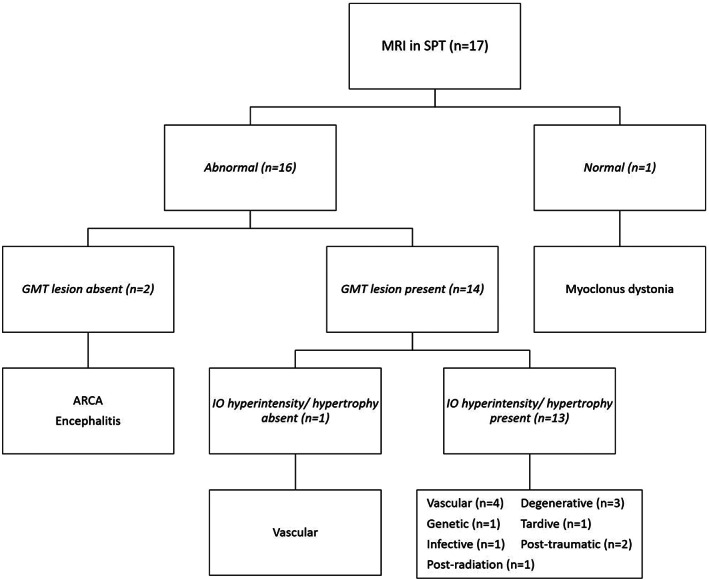
MRI changes in patients with symptomatic palatal tremor. ARCA, autosomal recessive cerebellar ataxia; GMT, Guillain‐Mollaret triangle; IO, inferior olive; MRI, magnetic resonance imaging; SPT, symptomatic palatal tremor.
FIG. 4.
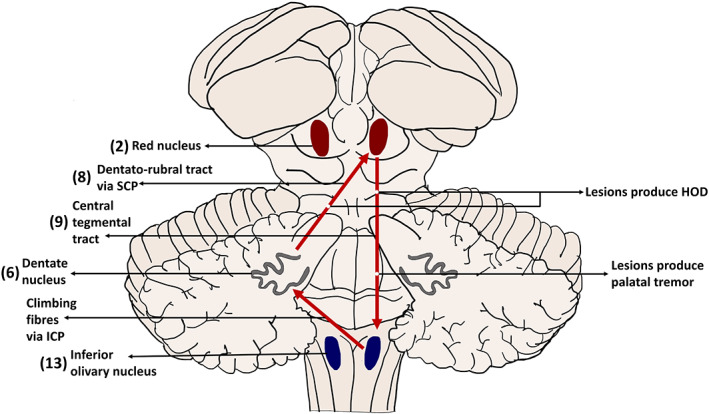
Schematic representation of the Guillain‐Mollaret triangle. Numbers in parentheses indicate the number of patients with a lesion involving the structure. HOD, hypertrophic olivary degeneration; ICP, inferior cerebellar peduncle; SCP, superior cerebellar peduncle.
Almost all patients (n = 13) with a GMT lesion had either hyperintensity or hypertrophy of the IO. A total of 2 patients had isolated hyperintensity of the IO, whereas the rest of the patients had both hyperintensity and hypertrophy of the IO. The single patient without an IO lesion, who had a vascular etiology, was found to have a central tegmental tract (CTT) lesion. A total of 2 patients with abnormal MRIs did not have GMT lesions; one was a case of viral encephalitis who had temporal and insular diffusion restriction, and the other was a case of autosomal recessive cerebellar ataxia who had cerebellar atrophy.
Treatment and Follow‐Up
Treatment offered to the patients with SPT was primarily for the AMD rather than the PT because it was not a cause a concern in any of the patients as all were unaware of the PT, and the 2 patients who had ear clicks were not disturbed by the sound. A therapeutic trial of clonazepam was given in 15 of the 16 patients, but there was no improvement in all of the 13 patients who returned for follow‐up. In 3 patients, propranolol and primidone were advised for the limb tremor, but these drugs also failed to reduce the PT. A total of 2 patients with a Holmes tremor underwent thalamic lesioning, and although reduction of the upper limb tremor was obtained, there was no change in the PT. In 1 patient with viral encephalitis, the PT spontaneously resolved within 3 days of onset, suggesting the possibility of an epileptic origin. Her electroencephalogram was unfortunately scheduled for and performed after the resolution of the PT.
Essential Palatal Tremor
A total of 5 patients had EPT, among whom 3 presented with ear clicks; however, they were unaware of the PT. In comparison to SPT, patients with EPT (Video 5) have significant and visible pharyngeal involvement. A total of 2 female patients presented with functional movement disorders and were incidentally observed to have a PT. Neither of the them reported the presence of PT or clicks. Of these 2 patients, 1 patient presented with irregular jerking of limbs and flinging of shoulders (Video 6), and the other patient presented with ataxia and astasia abasia. All of the patients with EPT had a normal brain MRI. Botulinum toxin injection was injected to the tensor veli palatini muscle in the 3 patients who reported ear clicks. All 3 patients were observed to have good improvement lasting 3 to 6 months. A total of 2 patients could not continue with botulinum toxin therapy because of financial constraints. The third patient, who was discussed in a previous article, 15 was a child who developed palatal palsy with dysphagia following the second injection. As a result, his parents were hesitant and deferred future injections.
Video 6.
Functional palatal tremor. The patient also had significant additional functional movement disorders in the form of a jerky variable head tremor, grimacing, jaw opening and closing, intermittent jerky movements of the upper limbs, and astasia abasia.
Discussion
PT is a rare movement disorder with relatively few studies reporting the varied etiological, clinical, and radiological features. The present study adds to the currently limited database of PT. We described the clinical features, associated movement disorders, and radiological profile of 22 cases with PT. In the present study, we observed that SPT was the most common variant of PT. Patients with SPT typically present with other symptoms secondary to a primary disease, rather than the PT, and usually do not complain of a PT. These patients may often have AMDs such as tremor, dystonia, myoclonus, and ataxia, which could be secondary to lesions produced by the primary disease. Patients with EPT often report ear clicks rather than the sensation of involuntary movements in the throat. It is imperative to examine these patients thoroughly to avoid missing a PT.
The exact AAO of PT, especially SPT, is often uncertain as patients are unaware of the existence of the PT, and the PT is incidentally observed on examination. In several cases, the causal association between a possible etiology and PT is presumptive, as a significant latency may exist between the insult and development of PT. For instance, the latency between the stroke and onset of PT is highly variable and may range anywhere between 1 to 6 months after the initial disease, but may even occur 30 months after the stroke. 16 In our cohort, we observed that the AAO of SPT was much later than in EPT. This is in concurrence with previous reports by Deuschl et al, 6 who reported the AAO of SPT at 45.1 ± 17.3 years and EPT at 24.8 ± 12.9 years, and Stamelou et al, 4 who reported the AAO of SPT at 53.6 ± 5.2 years and EPT at 37.2 ± 7.4 years.
There is significant etiological heterogeneity in PT, especially in SPT wherein the PT occurs secondary to a wide range of conditions that may damage the GMT. These include vascular, inflammation, demyelination, genetic, infectious, neoplastic, neurodegenerative, and miscellaneous causes such as traumatic brain injury, tardive, amyotrophic lateral sclerosis, and so on. 17 Degenerative and genetic causes reported in the literature primarily include PAPT, polymerase γ–related mutations, neuroferritinopathy, spinocerebellar ataxia type 20, and so on. 1 , 7 , 18 Recent reports of PAPT have demonstrated the presence of 3‐repeat and 4‐repeat tau‐positive inclusions in the IO, midbrain, pons, and thalamus, and these results suggest the possibility of PAPT being a tauopathy. 19 , 20 Although there are numerous case reports that describe various etiologies, there are limited large series of cases or systematic reviews that provide the prevalence of different etiologies (Table 2). In the present study, vascular lesions were the most common etiology, followed by degenerative, probable genetic, posttraumatic, postinfective, postradiation, and tardive. In a large review by Deuschl et al, 6 a vascular etiology was the most common, whereas in studies by Stamelou et al 4 and Nagappa et al, 7 a genetic etiology was the most common. The former study 4 reported 5 cases of PAPT, wherein 2 were negative for a GFAP mutation. Nagappa et al 7 reported variations in POLG, WDR81, NDUFS8, TENM4, and EEF2. However, the prevalence of genetic etiologies in their study may have been biased as a result of patients being selected from a cohort evaluated for mitochondrial and neurogenetic disorders. We had 1 case of PT secondary to postradiation ponto‐medullary syndrome, and this is commonly reported and frequently occurs in cases with brainstem arteriovenous malformations. 21
TABLE 2.
Etiology of palatal tremor in previous case series and the present study
| Study, Year | Type of Article | Sample Size | SPT:EPT | Etiology of SPT | % (n) |
|---|---|---|---|---|---|
| Deuschl et al, 1990 6 | Review article | 287 | 210:77 | Vascular | 54.86 (115) |
| Trauma | 10.95 (23) | ||||
| Tumor | 9.04 (19) | ||||
| Multiple sclerosis | 4.28 (9) | ||||
| Degenerative | 3.33 (7) | ||||
| Encephalitis | 2.38 (5) | ||||
| Other | 15.23 (32) | ||||
| Stamelou et al, 2012 4 | Case series | 17 | 7:10 a | Genetic | 71.43 (5) |
| Vascular | 14.29 (1) | ||||
| Not specified | 14.29 (1) | ||||
| (Diaphragmatic myoclonus) | |||||
| Nagappa et al, 2018 7 | Case series | 27 | 26:1 | Genetic | 34.65 (9) |
| Vascular | 23.07 (6) | ||||
| Trauma | 11.53 (3) | ||||
| Infections | 7.69 (2) | ||||
| Atypical demyelination | 7.69 (2) | ||||
| Posterior fossa tumor | 7.69 (2) | ||||
| Sarcoidosis | 3.84 (1) | ||||
| Present study, 2020 | Case series | 22 | 17:5 | Vascular | 29.41 (5) |
| Degenerative | 17.64 (3) | ||||
| Probably genetic | 17.64 (3) | ||||
| Posttraumatic | 11.76 (2) | ||||
| Postinfective | 11.76 (2) | ||||
| Postradiation | 5.88 (1) | ||||
| Tardive syndrome | 5.88 (1) |
SPT, symptomatic palatal tremor; EPT, essential palatal tremor.
Psychogenic palatal tremor, 7 patients; palatal tics, 2 patients; primary EPT, 1 patient.
In the present study, all patients with SPT were found to have at least 1 AMD. Although several patterns of combinations were observed, no specific correlations were apparent between these patterns and the imaging abnormalities. A larger number of AMDs did not suggest a greater extent of imaging abnormality. Tremor, that is, other than PT, and ataxia were the most common AMDs. This could be secondary to the involvement of the cerebellum and its connections. Dystonia was also observed, and the majority of these patients had cervical dystonia. However, not all patients with dystonia had a lesion involving the basal ganglia, owing to which the cause of the dystonia is uncertain. Finally, myoclonus was relatively less prevalent and was always observed in combination with tremor, dystonia, and ataxia. From an etiological perspective, MRI lesions, correlated with suspected etiology, and all patients with suspected PAPT had tremor and ataxia in addition to the PT.
The pathophysiological basis for both SPT and EPT is enigmatic. In SPT, based on the presence of additional signs and symptoms and abnormalities on MRI, involvement of the GMT has been implicated. The GMT triangle is formed by the dentate nucleus, red nucleus, and IO nucleus (ION; Fig. 4). Fibers from the contralateral dentate nucleus ascend through the superior cerebellar peduncle and cross the midline in the brachium conjunctivum to reach the ipsilateral red nucleus. From the red nucleus, fibers pass through the central tegmental tract to the ipsilateral ION, and finally fibers from the ION cross the midline to end in the contralateral dentate nucleus. 22 Although ION changes, either hyperintensity or hypertrophy, are commonly associated with PT, it is imperative to know that these changes are not the cause of the PT, that is, olivary changes occur secondary to lesions involving structures that produce PT. In several cases, hypertrophic olivary degeneration (HOD) is suggested to occur because of deafferentation of the ION, and only pathologies that affect the dentato‐rubral or dentato‐olivary pathways lead to HOD. 23 Isolated lesions of the CTT may cause a PT without HOD. It has been hypothesized that lesions of the CTT may lead to denervation of the nucleus ambiguus, which supplies the soft palate, and this may present as a PT. 24 Furthermore, HOD may develop several months after the onset of the PT or the primary disease. This could be the reason why a few cases within our cohort did not show an IO lesion on the MRI. In addition, as demonstrated in Alexander disease, 25 and rarely in medullary infarcts, 26 PT may occur despite the profound atrophic degeneration of the IO without HOD. This suggests that HOD‐induced olivo‐cerebellar hypersynchrony may not be the only mechanism underlying PT. ION neurons have been suggested to possess a spontaneous oscillatory activity with a frequency of 0.5 to 12 Hz. Dendrito‐dendritic gap junctions exist between ION neurons and lead to electrotonic coupling, and this coupling in turn leads to clusters of synchronized activity. T type calcium channels exist in ION neurons, and the abnormal modulation of these also leads to synchronization. 27 A recent study demonstrated an increase in randomness of oculopalatal tremor with hyperventilation, which supports the role of calcium channels in PT. 28 As stated previously, lesions in the GMT lead to transsynaptic deafferentation, a reduction in inhibitory afferents, and a subsequent increase in electrotonic coupling attributed to a compensatory increase in the presynaptic terminals of the deafferented ION. Based on these properties of the ION, the “dual mechanism” theory suggests the role of ION as the “pacemaker” and involvement of the deep cerebellar nuclei in the genesis of PT. 24 , 27 Hence, in several cases, although the lesions on MRI do not directly appear to involve the ION, patients may develop PT. Interestingly, 1 patient with a suspected tardive etiology was found to have ION abnormalities, and the cause of this anomaly is a conundrum. It is plausible that the patient had a different underlying etiology that was not evident at the time of examination.
The theories behind the pathophysiology of EPT include central, peripheral, voluntary, or functional. 17 A few studies using centrally acting drugs such as lamotrigine, sodium valproate, and flunarizine have reported alleviation of the PT and suggested the possibility of a central generator for EPT. 2 In several cases with EPT, local inflammation in the oral and nasal cavities has been reported, and pressure changes in the ear canal have been suggested to modulate PT, and in a few other cases EPT has been found to improve after tonsillectomy. 2 Based on these cases, a possible peripheral etiology has been suggested. In very few case reports, patients have been shown to demonstrate voluntary control over the PT and were able to control the frequency of tremor. It has been postulated that such patients have the ability to activate a central generator that permits them to modulate PT frequency. 2 Furthermore, a differential involvement of muscles had been suggested in SPT and EPT based on the higher presence of ear clicks in EPT. 29 The levator veli palatini is said to be involved in SPT, whereas the tensor veli palatini is involved in EPT. This also supports the clinically visible difference between SPT and EPT that was observed in our study. A functional basis also has been attributed as a possible cause for a majority of patients with EPT. 2 , 4 A recent study by Vial et al 3 used Bereitschaftspotential and demonstrated the functional nature of EPT in 2 cases. This observation is helpful as establishing a functional basis for the PT will prevent unnecessary medical interventions.
The treatment of EPT is far from satisfactory, and various drugs have been tried in the treatment of PT. Benzodiazepines, anticholinergics, calcium channel blockers, 5HT agonists, and nootropics (piracetam) have been tried with minimal benefit. 30 Gabapentin and memantine have been reported to produce modest benefits in oculopalatal tremor. 24 Botulinum toxin injections to the tensor veli palatine muscle are the most effective modality of treatment patients with PT, specifically EPT. 31 The improvement typically lasts for 3 to 6 months. However, despite the benefits, caution should be exercised when injecting for PT as dysphagia may commonly occur following injection as a result of palatal palsy. 15 SPT may not often undergo this intervention as patients seldom report any discomfort attributed to the PT. In our cohort, 2 patients underwent thalamic lesioning for the purpose of reduction of Holmes tremor, but there was no improvement in PT in either of them.
There are limitations to this study. This cohort of patients with PT was highly selective and obtained from within the movement disorder subspeciality, owing to which patients with other causes of PT such as demyelination, epileptic, genetic, and so on may have been missed. Hence, the current study does not represent a complete picture of causes of PT that may be seen at a referral center. We were unable to mention the exact AAO of the PT or the relationship with the onset of baseline neurological illness as patients were unaware of the PT, and this was observed only on examination. A genetic etiology was considered on the basis of clinical and imaging findings, as genetic studies were not performed for these patients. Few patients were lost to follow‐up; hence we were unable to ascertain if the PT persisted or subsided over time. Finally, owing to the retrospective nature of this study, we were unable to comment on how many patients with EPT could have had a functional etiology, as entrainability, variability, and sustainability of the PT were not specifically looked for in these patients.
In conclusion, PT is a rare condition with significant etiological heterogeneity. It can be easily missed owing to the lack of awareness by patients, and thorough clinical examination is necessary to ensure recognition of this condition. Although involvement of the ION is often considered pathognomonic of a palatal tremor, it may not be necessarily observed at the time of imaging and may develop later in the course of illness. A functional etiology should be considered in cases of EPT.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
B.K.S.: 1A, 1B, 1C, 2A, 2B, 3A
S.P.: 1A, 1B, 1C, 2A, 2B, 3B
V.V.H.: 1B, 1B, 1C, 3B
K.N.: 1C, 2C, 3B
N.K.: 1C, 2C, 3B
M.N.: 1C, 2C, 3B
R.Y.: 1A, 1C, 3B
P.K.P.: 1A, 1B, 2B, 2C, 3B
Disclosures
Ethical Compliance Statement: The authors confirm that the approval of an Institutional Review Board was not required for this work. Informed written consent was obtained from the patient. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: The authors declare that there are no additional disclosures to report.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018;33(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zadikoff C, Lang A, Klein C. The ‘essentials’ of essential palatal tremor: a reappraisal of the nosology. Brain 2006;129(4):832–840. [DOI] [PubMed] [Google Scholar]
- 3. Vial F, Akano E, Attaripour S, McGurrin P, Hallett M. Electrophysiological evidence for functional (psychogenic) essential palatal tremor. Tremor Other Hyperkinet Mov (N Y) 2020;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stamelou M, Saifee TA, Edwards MJ, Bhatia KP. Psychogenic palatal tremor may be underrecognized: reappraisal of a large series of cases. Mov Disord 2012;27(9):1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deuschl G, Toro C, Valls‐Solé J, Zeffiro T, Zee DS, Hallett M. Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain 1994;117(Pt 4):775–788. [DOI] [PubMed] [Google Scholar]
- 6. Deuschl G, Mischke G, Schenck E, Schulte‐Mönting J, Lücking CH. Symptomatic and essential rhythmic palatal myoclonus. Brain 1990;113(Pt 6):1645–1672. [DOI] [PubMed] [Google Scholar]
- 7. Nagappa M, Bindu PS, Sinha S, et al. Palatal tremor revisited: disorder with nosological diversity and etiological heterogeneity. Can J Neurol Sci 2018;45(2):243–247. [DOI] [PubMed] [Google Scholar]
- 8. Cazals X, Omoumi P, Agnard P, et al. Reversible metronidazole‐induced encephalopathy and hypertrophic olivary degeneration. J Radiol 2010;91(3 Pt 1):304–306. [DOI] [PubMed] [Google Scholar]
- 9. Mahasuar R, Kuruvilla A, Jacob K. Palatal tremor after lithium and carbamazepine use: a case report. J Med Case Reports 2010;4(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung YF, Wong WWY, Tang KW, Chan JHM, Li PCK. Ciprofloxacin‐induced palatal tremor. Mov Disord 2007;22(7):1038–1043. [DOI] [PubMed] [Google Scholar]
- 11. Johansen KK, Bindoff LA, Rydland J, Aasly JO. Palatal tremor and facial dyskinesia in a patient with POLG1 mutation. Mov Disord 2008;23(11):1624–1626. [DOI] [PubMed] [Google Scholar]
- 12. Samuel M, Torun N, Tuite PJ, Sharpe JA, Lang AE. Progressive ataxia and palatal tremor (PAPT) clinical and MRI assessment with review of palatal tremors. Brain 2004;127(6):1252–1268. [DOI] [PubMed] [Google Scholar]
- 13. Wills A, Sawle G, Guilbert P, Curtis A. Palatal tremor and cognitive decline in neuroferritinopathy. J Neurol Neurosurg Psychiatry 2002;73(1):91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thyagarajan D, Chataway T, Li R, Gai WP, Brenner M. Dominantly‐inherited adult‐onset leukodystrophy with palatal tremor caused by a mutation in the glial fibrillary acidic protein gene. Mov Disord 2004;19(10):1244–1248. [DOI] [PubMed] [Google Scholar]
- 15. Pal PK, Lakshmi PS, Nirmala M. Efficacy and complication of botulinum toxin injection in palatal myoclonus: experience from a patient. Mov Disord 2007;22(10):1484–1486. [DOI] [PubMed] [Google Scholar]
- 16. Nishie M, Yoshida Y, Hirata Y, Matsunaga M. Generation of symptomatic palatal tremor is not correlated with inferior olivary hypertrophy. Brain 2002;125(Pt 6):1348–1357. [DOI] [PubMed] [Google Scholar]
- 17. Bhattacharjee S. Palatal tremor‐pathophysiology, clinical features, investigations, management and future challenges. Tremor Other Hyperkinet Mov (N Y) 2020;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knight MA, Gardner RJ, Bahlo M, et al. Dominantly inherited ataxia and dysphonia with dentate calcification: spinocerebellar ataxia type 20. Brain 2004;127(Pt 5):1172–1181. [DOI] [PubMed] [Google Scholar]
- 19. Gao AF, Faust‐Socher A, Al‐Murshed M, Del Bigio MR, Lang AE, Munoz DG. Progressive ataxia and palatal tremor: two autopsy cases of a novel tauopathy. Mov Disord 2017;32(10):1465–1473. [DOI] [PubMed] [Google Scholar]
- 20. Mari Z, Halls AJ, Vortmeyer A, et al. Clinico‐pathological correlation in progressive ataxia and palatal tremor: a novel tauopathy. Mov Disord Clin Pract 2014;1(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yun JH, Ahn JS, Park JC, Kwon DH, Kwun BD, Kim CJ. Hypertrophic olivary degeneration following surgical resection or gamma knife radiosurgery of brainstem cavernous malformations: an 11‐case series and a review of literature. Acta Neurochir 2013;155(3):469–476. [DOI] [PubMed] [Google Scholar]
- 22. Murdoch S, Shah P, Jampana R. The Guillain–Mollaret triangle in action. Pract Neurol 2016;16(3):243–246. [DOI] [PubMed] [Google Scholar]
- 23. Elnekiedy A, Naguib N, Hamed W, Mekky J, HHM H. MRI and Neurological presentation of hypertrophic olivary degeneration. Egypt J Radiol Nucl Med 2016;47(3):1019–1029. [Google Scholar]
- 24. Shaikh AG, Hong S, Liao K, et al. Oculopalatal tremor explained by a model of inferior olivary hypertrophy and cerebellar plasticity. Brain 2010;133(3):923–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pareyson D, Fancellu R, Mariotti C, et al. Adult‐onset Alexander disease: a series of eleven unrelated cases with review of the literature. Brain 2008;131(9):2321–2331. [DOI] [PubMed] [Google Scholar]
- 26. Kattah JC, Elble RJ, De Santo J, Shaikh AG. Oculopalatal tremor following sequential medullary infarcts that did not cause hypertrophic olivary degeneration. Cerebellum Ataxias 2020;7(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borruat F‐X. Oculopalatal tremor: current concepts and new observations. Curr Opin Neurol 2013;26(1):67–73. [DOI] [PubMed] [Google Scholar]
- 28. Theeranaew W, Kim H‐J, Loparo K, Kim J‐S, Shaikh AG. Hyperventilation increases the randomness of ocular palatal tremor waveforms. Cerebellum 2020. 10.1007/s12311-020-01171-1. [DOI] [PubMed] [Google Scholar]
- 29. Deuschl G, Toro C, Hallett M. Symptomatic and essential palatal tremor. 2. Differences of palatal movements. Mov Disord 1994;9(6):676–678. [DOI] [PubMed] [Google Scholar]
- 30. Pandurangi AA, Nayak RB, Bhogale GS, Patil NM, Chate SS, Chattopadhaya S. Clonazepam in the treatment of essential palatal tremors. Indian J Pharmacol 2012;44(4):528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slengerik‐Hansen J, Ovesen T. Botulinum toxin treatment of objective tinnitus because of essential palatal tremor: a systematic review. Otol Neurotol 2016;37(7):820–828. [DOI] [PubMed] [Google Scholar]


