Synkinesis is a subset of motor overflow in which voluntary movements of one part of the body are accompanied by involuntary activation of other, non‐mirroring muscles. 1 This disorder has been observed in neurodegenerative diseases such as Parkinson's disease, 2 Creutzfeldt–Jakob disease, 3 and other parkinsonian disorders. 2 , 4 Moreover, it is also observed in healthy older subjects. 2 Their occurrence in patients with corticobasal syndrome (CBS) might be expected, but this phenomenon is still scarcely described in the literature. Here, we report 2 cases of patients with probable CBS 5 presenting ipsilateral and contralateral foot‐hand synkinesis and distinct amyloid imaging biomarkers results.
A 52‐year‐old man (patient 1) was referred to our service with a 2‐year history of progressive language impairment and limb rigidity. Neurological examination disclosed hypomimia, asymmetrical right‐sided parkinsonism, cervical dystonia, ideomotor apraxia worse on the right side, right arm levitation, and nonfluent aphasia. A diagnosis of CBS was made. It was noteworthy that when we asked him to move his right foot, he started involuntarily to move his left hand, with a similar pattern and pace, presenting a contralateral and ipsilateral foot–hand synkinesis (Video 1, Segment 1).
Video 1.
Segment 1: patient diagnosed with corticobasal syndrome is asked to move his right foot when, simultaneously, he performed involuntary movements in his left hand, with a similar pattern and pace. Despite explaining to him to only move his feet, seconds later both hands were involuntarily moving. The patient also presented bradykinesia and rigidity at both arms (worse on the right side) and bilateral ideomotor apraxia (also worse in the right arm). Segment 2: patient diagnosed with probable CBS developed involuntary movements in his right hand when asked to move his right foot and later demonstrated the same movements in his left hand. In this patient, myoclonus in both upper limbs is also notable (worse on the right side).
Patient 2 was a 65‐year‐old man with a 4‐year history of progressive cognitive impairment and asymmetric rigidity. His neurological examination demonstrated right‐sided parkinsonism, bilateral myoclonus, and ideomotor apraxia (both worse on the right), and cortical sensory deficits. He was diagnosed with probable CBS. When we asked him to move his right foot, he developed involuntary movements in his right hand and later in his left hand (Video 1, Segment 2). Voluntary movements from the hands did not elicit synkinesis in both patients.
Both patients underwent a comprehensive investigation that included magnetic resonance imaging (MRI), fluorodeoxyglucose (FDG)‐positron emission tomography (PET), and Pittsburgh compound‐B (PIB)‐PET. Patient 1 showed an asymmetrical hypometabolism at the frontoparietal region contralateral to the affected side at FDG‐PET (Fig. 1A) and negative PIB‐PET (Fig. 1B). Conversely, patient 2 showed an asymmetrical hypometabolism predominantly at posterior temporoparietal regions at FDG‐PET (Fig. 1D) and positive cortical amyloid deposition at PIB‐PET (Fig. 1E).
FIG. 1.
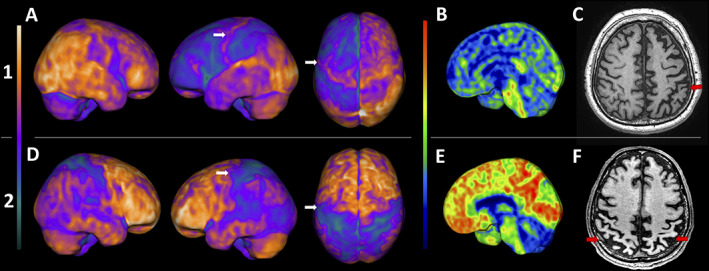
Upper row (1) patient 1: (A) FDG‐PET 3D‐stereotactic surface projection (3D‐SSP, software cortex ID suite, GE Healthcare): asymmetric frontoparietal hypometabolism, including the sensory and motor cortex, worst on the left side. PIB‐PET 3D‐SSP (B) negative for amyloid deposition, and T1‐weighted MRI (C) with asymmetric frontoparietal atrophy, also worst on the left (red arrows). Lower row (2) patient 2: (C) FDG‐PET 3D‐SSP: asymmetric posterior temporoparietal hypometabolism, also including the sensory and motor cortex, worst on the left side. PIB‐PET 3D‐SSP (E) positive for diffuse cortical amyloid deposition, and T1‐weighted MRI (F) showing bilateral parietal cortical atrophy (red arrows). The white arrows on A and D point to the supplementary motor cortex, probably related to the synkinesis.
Patient 1 was diagnosed as CBS probably related to tauopathy, whereas patient 2 was diagnosed as CBS probably related to Alzheimer's disease. Both patients also revealed hypometabolism at the supplementary motor area (SMA) and premotor cortex, contralateral to the affected side where synkinesis occurred.
Although the brain networks involved in synkinesis are poorly understood, they are likely related to dysfunction in the secondary motor areas, such as the premotor cortex, SMA, cingulate, and their connections to the primary motor cortex. 1 A previous study using functional magnetic resonance imaging (fMRI) data in patients with hand–foot synkinesis showed that the SMA was activated during hand movements besides the foot motor cortex region. Therefore, the SMA might orchestrate the coordination of involuntary movements, probably being anatomically correlated with synkinesis. 4 Consonant with this, our cases revealed hypometabolism in this area (Fig. 1).
We demonstrate that synkinesis might be a motor finding of CBS and synkinesis are probably not related to its underlying pathology. Instead, synkinesis occur because of dysfunction of secondary motor areas, mainly the SMA, a typical anatomical affected site in CBS.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review, and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review, and Critique.
J.B.P.: 1A, 1B, 1C, 3A
S.M.D.B.: 3B
A.M.C.: 1A, 1B, 1C, 3B
R.N.: 3B
Disclosures
Ethical Compliance Statement
The ethical committee of the University of Sao Paulo approved the investigation procedure and informed consent under protocol number 2.046.113. All patients or caregivers provided written informed consent for the study. We confirm that we have read the Journal's position on ethical publication issues and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
This report is part of research supported by the São Paulo Research Foundation (FAPESP) in Brazil, reference number 2017/10033‐4. The authors report no conflicts.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no disclosures to report.
References
- 1. Kojović M, Bhatia KP. Bringing order to higher order motor disorders. J Neurol 2019;266:797–805. [DOI] [PubMed] [Google Scholar]
- 2. Salazar G, Casas E, Oliveras D, Rando A, Sergio P. Ocular‐jaw synkinesia in normal, Parkinson's disease, and multiple system atrophy subjects: clinical and electrophysiological findings. Clin Neurophysiol 2010;121:94–97. [DOI] [PubMed] [Google Scholar]
- 3. Park IS, Song IU, Lee SB, Lee KS, Kim HT, Kim JS. Mirror movements and involuntary homolateral limb synkinesis in a patient with probable Creutzfeldt‐Jakob disease. Clin Neurol Neurosurg 2009;111:380–383. [DOI] [PubMed] [Google Scholar]
- 4. Salardini A, Narayanan NS, Arora J, Constable T, Jabbari B. Ipsilateral synkinesia involves the supplementary motor area. Neurosci Lett 2012;523:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]


