Abstract
Pyogenic liver abscess (PLA) is a rarely encountered condition in the emergency department (ED) that necessitates a timely diagnosis by the emergency physician. An early ED diagnosis is challenging as the presenting symptoms of PLA are often variable and nonspecific. The rapid bedside diagnosis of PLA with point‐of‐care ultrasound (POCUS) performed by emergency physicians has not been investigated thoroughly. This case report describes the expeditious identification and ED management of PLA by implementing emergency physician‐performed POCUS as the initial diagnostic modality.
Keywords: emergency department, emergency ultrasound, hepatic abscess, point‐of‐care ultrasound, pyogenic liver abscess, ultrasonography
1. INTRODUCTION
Pyogenic liver abscess (PLA) is defined as an infectious liver lesion of bacterial etiology, predominantly Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, or Streptococcus species. 1 , 2 About 50% to 75% of PLAs have a solitary abscess, which are more likely to be polymicrobial, although they also are more often cryptogenic compared with multiple abscesses. 1 , 3 PLA is an uncommon entity with an annual incidence of only 3.6 cases per 100,000 population in the United States. 1 , 2 The average incidence is escalating at a rate of 4% per year in the United States, which may be attributed to an increased prevalence of predisposing factors, such as diabetes mellitus, abdominal surgery, cardiopulmonary disease, cirrhosis, chronic renal failure, immunosuppression, and malignancy. 1 , 2 , 4 Underlying hepatobiliary disease is the major etiology of PLAs, with the remainder of cases caused by direct extension of a focal infection, penetrating trauma, or portal venous system and/or systemic bacteremia. 1 , 3 , 5 In‐hospital mortality rates exceeding 30% have previously been described for patients with PLA; however, more recent fatality data ranges between 0.9% to 5.6%. 1 , 2 , 3 , 5 , 6 It should be noted that patients with advanced age and/or 3 or more comorbidities have increased mortality. 2 PLA is infrequently seen in the emergency department (ED) but nonetheless requires prompt recognition to prevent morbidity and mortality.
Point‐of‐care ultrasound (POCUS) is an invaluable bedside tool for the ED evaluation of numerous abdominal emergencies. Radiology‐ or gastroenterology‐performed ultrasonography has been a first‐line imaging modality for the initial diagnosis of PLAs with a sensitivity ranging from 67% to 92%, with a more recent ED‐based retrospective study reporting a sensitivity of 86%. 1 , 7 Computed tomography (CT) imaging is the typical first‐line, gold standard imaging modality in the ED setting, with a diagnostic sensitivity superior to POCUS for detecting smaller PLAs. 1 , 7 There is a notable scarcity of literature describing emergency physician‐performed POCUS to identify PLAs. 8 , 9 , 10 This case report highlights the critical role of emergency physician‐performed POCUS for the early ED diagnosis of the rare but potentially life‐threatening condition of PLA.
2. CASE PRESENTATION
A 52‐year‐old female with a past history of hypertension, chronic back pain, and gastric bypass presented by ambulance to the ED with a chief complaint of intermittent nausea and non‐bloody, non‐bilious vomiting for ≈6 weeks. She endorsed occasional, self‐limiting episodes of gradual‐onset, vaguely characterized, moderate severity right upper quadrant abdominal pain with radiation to the back. The patient also reported several episodes of non‐bloody watery diarrhea, decreased appetite and oral intake, and weight loss in the past several weeks. She denied fevers, chills, headache, myalgias, chest pain, palpitations, shortness of breath, syncope, hematuria, dysuria, urinary frequency and urgency, or any other associated symptoms. She denied any recent travel, history of intravenous drug use, drinking water from a contaminated source, or known sick contacts. Upon arrival, the patient was afebrile, blood pressure of 122/71 mmHg, heart rate of 100 beats per minute, respiratory rate of 18 breaths per minute, and oxygen saturation of 99% on room air. On physical examination, she was non‐toxic appearing, although in moderate discomfort, a mild degree of clinical dehydration was appreciated, and the abdomen was diffusely tender to palpation without rebound, guarding, or peritoneal signs. Scleral icterus and jaundice were absent. The remainder of the examination was unremarkable.
The clinical presentation was concerning for an emergent intraabdominal process, with the differential diagnosis including but not limited to a gallbladder pathology (ie, biliary colic and cholecystitis) or abdominal aortic aneurysm. Abdominal POCUS examinations were immediately performed by emergency medicine residents and an ultrasound fellowship‐trained emergency physician with a GE Venue (GE Healthcare, Chicago, IL) using a C1‐5 curvilinear transducer. The patient was scanned at the bedside in a semirecumbent position of maximal comfort; however, POCUS examination was technically challenging because of the patient's body habitus, abdominal tenderness, and bowel gas artifacts. POCUS confirmed a normal gallbladder and the abdominal aorta did not demonstrate aortic aneurysm or dissection. Notably, POCUS evaluation of the right upper quadrant in the coronal plane at the anterior axillary line incidentally revealed a large septated hypoechoic cavity with internal debris and posterior acoustic enhancement consistent with an abscess in the right hepatic lobe (Figure 1). Based on the POCUS findings, blood cultures and intravenous antibiotics (vancomycin, cefepime, and metronidazole) were ordered for empiric coverage. CT scan of the abdomen and pelvis was subsequently expedited and confirmed a large (12.4 × 11.0 cm) multiloculated thick walled septated fluid collection/abscess present in the right hepatic lobe extending to the dome of the liver (Figure 2). CT imaging also noted a trace right pleural effusion. Laboratory analysis was significant only for mild elevations of aspartate aminotransferase (AST) at 118 (15–37 units/L) and total bilirubin at 1.2 (0.2–1.0 mg/dL); bloodwork was conspicuously unremarkable for white blood cell count of 10.5 (4.0–10.5 10∧3/uL), lactic acid of 1.6 mmol/L, alanine aminotransferase (ALT) of 46 (13–56 units/L), and alkaline phosphatase of 86 (45–117 units/L).
FIGURE 1.
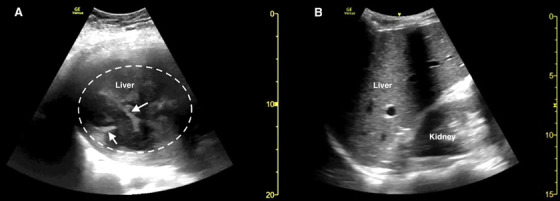
Point‐of‐care ultrasound of the right upper quadrant in the coronal plane demonstrating (A) multiple hypoechoic collections and septations (white arrows) within the liver parenchyma consistent with a pyogenic liver abscess (dotted white circle) and (B) a representative example of normal liver parenchyma echotexture
FIGURE 2.
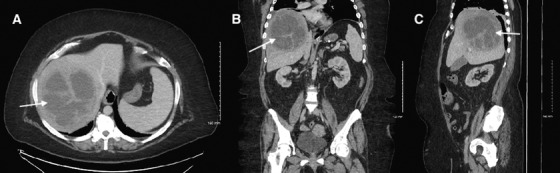
Computed tomography of abdomen/pelvis demonstrating a pyogenic liver abscess (white arrows) in axial (A), coronal (B), and sagittal (C) planes
The patient was admitted for further management by gastroenterology and additional intravenous antibiotics. CT‐guided percutaneous drainage of the PLA was performed with aspiration of foul‐smelling pus. Infectious disease was consulted for continued intravenous antibiotic therapy. Fluid cultures from the hepatic collection were positive for Streptococcus intermedius; however, there was no growth of anaerobic organisms. The patient was discharged home on hospital day 10 with plan for a continuation of ceftriaxone (based on culture sensitivities) via peripherally inserted central catheter for a total of 4 weeks and outpatient gastroenterology follow‐up.
3. DISCUSSION
The diagnosis of PLA is notoriously challenging in the emergent and acute care setting. Retrospective studies have observed that of admitted patients with an ultimate diagnosis of PLA, only about 20% of cases were correctly diagnosed in the ED. 4 , 6 The difficulty in identifying a PLA likely occurs because the presenting symptoms are often non‐specific and variable. 1 , 4 The classic triad of PLA symptoms (fever, jaundice, and right upper quadrant tenderness) is present in 7% to 43% of patients. 1 , 3 , 4 , 5 , 7 Notably, abdominal pain was present in only 26% of PLA patients in one study. 7 Fever is almost ubiquitous (79% to 100% of cases) and other constitutional symptoms (malaise, fatigue, anorexia, and weight loss) are usually present. 1 , 3 , 4 , 5 , 6 , 7 In this case, the patient did not exhibit fever or jaundice (symptoms included in the classic triad) but rather abdominal pain with a constellation of more non‐specific symptoms (nausea, vomiting, diarrhea, anorexia). Leukocytosis and elevated alkaline phosphatase levels are the most common laboratory abnormalities associated with PLA; however, many cases typically have other laboratory markers (ALT, AST, and bilirubin) that are minimal and/or absent. 1 , 3 , 5 Our case patient did not have the distinctive abnormalities in the white blood cell or alkaline phosphatase levels, further confirming the inherent diagnostic challenge of PLA in the ED. The consequences of a missed or delayed diagnosis of PLA are substantial; therefore, it is essential that emergency physicians have a high index of clinical suspicion for those patients with significant risk factors.
PLA should be highly considered in the differential diagnosis in cases of fever of unknown origin. 5 , 6 , 7 Patients with PLA who present with criteria for systemic inflammatory response syndrome are more likely to have the correct diagnosis ascertained in the ED. 6 , 7 The Mortality in Emergency Department Sepsis score offers valuable prognostic information of mortality outcomes and intensive care admission rates for PLA patients. 11 Rapid emergency physician identification of the infectious source is crucial as delayed PLA diagnosis is associated with the progression to septic shock and multiorgan failure. 7 POCUS may have a decisive role for identifying the source of infection in suspected septic patients when implemented into the ED diagnostic algorithm. 12 It is interesting to note that POCUS performed in the ED has also been associated with a higher percentage of PLA patients correctly diagnosed compared to those misdiagnosed (∼34% and ∼3% respectively), although the diagnostic accuracy of emergency physician‐performed POCUS for PLA is still unknown. 6 Furthermore, the utility of POCUS for the diagnosis of PLAs and other similar infectious conditions in resource‐limited settings has noteworthy implications. 13 , 14 This case illustrates another promising application of emergency physician‐performed POCUS as the first‐line imaging modality to support an early ED diagnosis of PLA.
4. CONCLUSIONS
PLA is a rare but emerging condition that carries a high mortality burden if misdiagnosed and untreated. Prompt emergency physician identification of PLA with POCUS harnesses the potential to expedite management and timely interventions in the ED. Further studies are required to establish the diagnostic accuracy and ideal role of emergency physician‐performed POCUS for PLA.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
This research was supported in part by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
McClure MB, Patel K, Cabrera G, Kalivoda EJ. Point‐of‐care ultrasound diagnosis of a pyogenic liver abscess in the emergency department. JACEP Open. 2021;2:e12412.
Supervising Editor: Michael Blaivas, MD, MBA
Funding and support: ByJACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
REFERENCES
- 1. Johannsen EC, Sifri CD, Madoff LC. Pyogenic liver abscesses. Infect Dis Clin North Am. 2000;14(3):547‐563. [DOI] [PubMed] [Google Scholar]
- 2. Meddings L, Myers RP, Hubbard J, et al. A population‐based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105(1):117‐124. [DOI] [PubMed] [Google Scholar]
- 3. Rahimian J, Wilson T, Oram V, et al. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39(11):1654‐1659. [DOI] [PubMed] [Google Scholar]
- 4. Hernandez JL, Ramos C. Pyogenic hepatic abscess: clues for diagnosis in the emergency room. Clin Microbiol Infect. 2001;7(10):567‐570. [DOI] [PubMed] [Google Scholar]
- 5. Liu L, Chen W, Lu X, et al. Pyogenic liver abscess: a retrospective study of 105 cases in an emergency department from East China. J Emerg Med. 2017;52(4):409‐416. [DOI] [PubMed] [Google Scholar]
- 6. Chia DWJ, Kuan WS, Ho WH, et al. Early predictors for the diagnosis of liver abscess in the emergency department. Intern Emerg Med. 2019;14(5):783‐791. [DOI] [PubMed] [Google Scholar]
- 7. Lin AC, Yeh DY, Hsu YH, et al. Diagnosis of pyogenic liver abscess by abdominal ultrasonography in the emergency department. Emerg Med J. 2009;26(4):273‐275. [DOI] [PubMed] [Google Scholar]
- 8. Pearl R, Pancu D, Legome E. Hepatic abscess. J Emerg Med. 2005;28(3):337‐339. [DOI] [PubMed] [Google Scholar]
- 9. McKaigney C. Hepatic abscess: case report and review. West J Emerg Med. 2013;14(2):154‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iradukunda D, Lee J, Kim DJ. Unexpected source of fever: liver abscess on point‐of‐care ultrasound. CJEM. 2018;20(5):802‐803. [DOI] [PubMed] [Google Scholar]
- 11. Kuo SH, Lee YT, Li CR, et al. Mortality in emergency department sepsis score as a prognostic indicator in patients with pyogenic liver abscess. Am J Emerg Med. 2013;31(6):916‐921. [DOI] [PubMed] [Google Scholar]
- 12. Cortellaro F, Ferrari L, Molteni F, et al. Accuracy of point of care ultrasound to identify the source of infection in septic patients: a prospective study. Intern Emerg Med. 2017;12(3):371‐378. [DOI] [PubMed] [Google Scholar]
- 13. Gupta S, Kim SH, Choy G. Chapter 6: liver. In: Shah S, Price D, eds. Partners in Health Manual of Ultrasound for Resource‐Limited Settings. Boston, MA: Partners in Health; 2011. https://www.pih.org/practitioner-resource/manual-of-ultrasound-for-resource-limited-settings. [Google Scholar]
- 14. Sippel S, Muruganandan K, Levine A, et al. Review article: use of ultrasound in the developing world. Int J Emerg Med. 2011;4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]


