Abstract
Background
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases worldwide. AD pathogenesis is multifactorial, involving environmental and genetic factors. IL-13 stands out as one of the main cytokines in the pathophysiology of AD. Currently, dupilumab, which targets both IL-4 and IL-13 signalling, is the only biologic agent approved for the treatment of moderate-to-severe AD. New targeted biologic therapies are being developed, such as lebrikizumab and tralokinumab, two selective IL-13 inhibitors. This article reviews the role of IL-13 in AD and the most recent data on lebrikizumab and tralokinumab.
Methods
A narrative review of the literature was written after retrieving relevant articles in the PubMed database (up until December 2020) using the following keywords present in the title, abstract or body: atopic dermatitis; interleukin 13; IL-13; tralokinumab; lebrikizumab, biologic therapy.
Discussion
A phase IIb trial showed that all three dosing regimens evaluated (lebrikizumab 125 mg every 4 weeks (Q4W), 250 mg Q4W or 250 mg every 2 weeks) achieved rapid and dose-dependent efficacy concerning the signs and symptoms of AD, with a statistically significant improvement, at week 16. Tralokinumab was studied in three phase III clinical trials and reached its primary endpoints at week 16 (ECZTRA 1 and 2 in monotherapy and ECZTRA 3 with concomitant topical corticosteroids), with response maintained over time. Both lebrikizumab and tralokinumab exhibited good safety profiles in AD trials, with adverse effects usually being comparable between the control and treatment groups.
Conclusion
The evidence supports the hypothesis that selective antagonism of IL-13 is sufficient to control AD, providing an improvement in the patient’s quality of life. Therefore, the development of lebrikizumab and tralokinumab represents a new and exciting phase in the management of AD.
Keywords: atopic dermatitis, interleukin-13, lebrikizumab, IL-13, tralokinumab
Introduction
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases worldwide,1 with an increasing incidence in recent years, especially in industrialized countries and urban areas.2 The estimated prevalence is 15% in children and 2–10% in adults. In the adult population, the frequency of AD appears to be increasing and is probably underdiagnosed.3
AD is characterized by the presence of eczema lesions (erythema, oozing and desquamation) and intense pruritus, which can lead to excoriations, secondary bacterial and viral infections, and lichenification in chronic cases.4,5 Moreover, it is associated with other atopic comorbidities such as asthma and allergic rhinitis.6 Longitudinal studies have shown that the persistence of AD in adulthood is related to severe forms of presentation, an earlier age of onset of symptoms, a family history of AD and early sensitization to allergens.7–9
AD has a significant impact on a patient’s quality of life due to its chronicity and the presence of subjective symptoms10 associated with decreased mental health scores compared to the general population.11 Some of the contributing factors are sleep disturbances, reduced school/work productivity, interpersonal problems, social isolation, reduced self-esteem, development of mental disorders (anxiety, depression), and suicidal ideation in the most severe cases.12–16
The pathogenesis of AD is multifactorial, involving genetic factors and the interaction between dysfunction of the epidermal barrier, immune dysregulation and changes in the skin microbiome.17 The immunomediated mechanisms are characterized by an inappropriate activation of type 2 T helper cells (TH2) and type 2 innate lymphoid cells (ILC2), with an increased expression of inflammatory cytokines, particularly interleukins IL-4 and IL-13.18,19 IL-13 stands out as one of the main cytokines in the pathophysiology of AD6,20 through its increasingly prominent role in the production and maintenance of the inflammatory process as well as in epidermal barrier dysfunction.21
Long-term therapy is often required given the chronic and recurrent nature of AD.22–24 In fact, moderate-to-severe forms of AD account for 20% of all cases25 and the conventional systemic therapies, such as cyclosporine, corticosteroids, methotrexate or azathioprine, often present limited efficacy and/or long-term toxicity,14,18,26 making control of AD a challenge for both clinician and patient.27
In the last decade, as a result of deeper knowledge about AD pathophysiology, particularly of the cytokines and receptors involved in inflammation, great advances have been made, with the emergence of new pharmacological options.17,18,28 Currently, dupilumab (anti-IL4Rα) is the only biologic drug approved by the US Food and Drug Administration and by the European Medicines Agency for the treatment of moderate-to-severe forms of AD.21,29,30 New targeted biologic therapies are being developed, including lebrikizumab and tralokinumab, two selective IL-13 inhibitors.18
Numerous randomized controlled trials have been or are currently evaluating the safety and efficacy of tralokinumab and lebrikizumab for AD treatment.31,32 This article reviews the role of IL-13 in AD and the most recent data on lebrikizumab and tralokinumab.
Methods
A search in the PubMed database (up until December 2020) for articles with the specific keywords “atopic dermatitis”, “interleukin 13”, “IL-13”, “tralokinumab”, “lebrikizumab” and “biologic therapy” present in the title, abstract or body was performed. The reference lists of those articles were examined to retrieve other studies that were considered relevant and contributed to the scientific purpose of the present review but had not been retrieved by the database search.
Review
AD pathogenesis and the role of IL-13
AD is a complex, multifactorial disease caused by the interaction between multiple environmental and genetic factors.22 Several mechanisms contribute to AD, including genetic predisposition, epidermal barrier dysfunction, immune dysregulation, changes in the skin microbiome and an abnormal pruritic response.18,33,34
Patients with AD present a skin barrier dysfunction in both lesional and nonlesional skin.35,36 Dysfunction of the epidermal barrier is due to abnormalities in the formation of structural proteins and/or in their lipid metabolism33 — the first due to mutations of the genes that encode the formation of the structural key proteins of the epidermal barrier and immune dysfunction;37,38 the second because of the disorganized lipid matrix of the epidermal barrier, with a decrease in the number of long-chain and very-long-chain ceramides and an increase in the number of free fatty acids.39 As a result, there is a marked transepidermal water loss that facilitates penetration by potential allergens, irritants or pathogenic microorganisms.21,40
As an immunomediated disease, AD is characterized by the inappropriate and excessive activation of TH2 and ILC2 cells, with an increase in the production of proinflammatory molecules33 such as type 2 cytokines,41 particularly IL-4 and IL-13, but also IL-31 and IL-22.18 IL-4 and IL-13 have been highlighted as the central mediators of AD.14 In addition to their effects on the TH2 inflammatory response, they have numerous multifaceted impacts on the pathogenesis of AD, particularly on the dysfunction of the epidermal barrier and on the pruritis.14,42,43
In their signalling cascades, IL-4 and IL-13 share a heterodimeric receptor composed of IL-4Rα and IL-13Rα1, known as the type 2 receptor (of IL-4) (Figure 1).14 Despite this, these interleukins have distinct functions in atopic inflammation.36,44 TH2 cytokines negatively regulate the expression of antimicrobial peptides (AMPs).35,45 The disruption of the skin’s epidermal barrier, associated with this AMP deficiency, causes a greater propensity to colonization and infection by Staphylococcus aureus.35,45,46 In fact, in AD, the skin microbiome is altered compared to healthy and normal skin. There is an increase in the abundance of S. aureus and a decrease in bacterial diversity.47 Thus, the modified epidermal barrier promotes the colonization by S. aureus, which worsens, in a self-amplifying loop, the epidermal barrier’s rupture.46,48
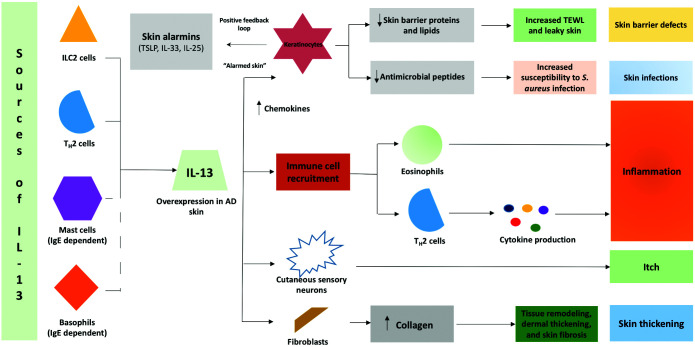
Figure 1.
The role of IL-13 in atopic dermatitis.
Adapted from Bieber T.21
TEWL, transepidermal water loss.
In AD, itching is not mediated by histamine.45 This symptom occurs mainly due to IL-31, a cytokine produced by TH2 cells.35,49 IL-31 favours the sensory nerve’s ramification and elongation, causing sensitization to minimal stimuli and sustained itching in AD patients.35,45,46
IL-13 is a pleiotropic cytokine predominantly produced by TH2 cells and ILC2 but also, to a lesser extent, by mast cells, basophils, eosinophils, natural killer cells, macrophages, dendritic cells and monocytes (Figure 2).24,50 Free IL-13 binds to the α1 subunit of the IL-13 receptor (IL-13Rα1) in all cells of the human body, but particularly to monocytes and B cells. In a cascade reaction, this binding favours the recruitment of IL-4Rα, inducing, by dimerization, the formation of a signal transducer that activates Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2), leading to the phosphorylation of signal transducer and activator of transcription 6 (STAT6), a transcription factor that promotes TH2 differentiation, and to class-switching to IgE14,51,52 (Figure 1). Additionally, it was demonstrated that STAT6 suppresses the activity of regulatory T cells, essential to the maintenance of tolerance to the antigens themselves and to the prevention of excessive inflammation.53 IL-13 also has the ability to bind with high affinity to the α2 subunit of the IL-13 receptor (IL-13Rα2); however, this receptor has no significant cytoplasmic domain and does not seem to function as a signal mediator. It is believed to act as a decoy receptor that internalizes the IL-13 found in excessive circulating levels.18 Recent studies showed that a different ligand, CHI3L1/YKL-40, which is overexpressed in AD patients, could activate IL-13Rα2 and, through the IL-13Rα2–TMEM219 axis, could promote the production of TGFβ, collagen deposition and remodelling of fibrotic tissue.54
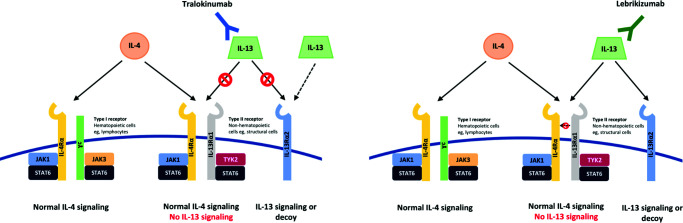
Figure 2.
Tralokinumab binds to the IL-13 cytokine in an epitope that overlaps with the binding site of the IL-13Rα receptors, preventing IL-13 from binding to both IL-13Rα1 and IL-13Rα2. However, the binding affinity of IL-13 to the IL-13Rα2 receptor is higher than for tralokinumab; therefore, unbound IL-13 can still bind to the receptor. Lebrikizumab exerts its activity by binding to the IL-13 cytokine at an epitope that overlaps with the binding site of the IL-4Rα receptor, preventing heterodimerization of the IL-4Rα/IL-13Rα1 subunits. IL-13 can still bind to IL-13Rα2.
Adapted from Bieber T.21
IL-13 plays a pivotal role in the production and maintenance of the TH2 inflammatory reaction and in the dysfunction of the epidermal barrier.6,21 The overexpression of this cytokine reduces the integrity of the epithelial barrier by leading to the down-regulation of its key components, particularly its proteins, such as filaggrin, loricrin and involucrin,6,21,42,43 amongst others, and of its lipids.55 IL-13-mediated tissue inflammation promotes fibrotic skin remodelling and skin thickening through the recruitment of fibroblasts and a subsequent increase in collagen deposition.55 By decreasing the expression of antimicrobial peptides, the overexpression of IL-13 in AD leads to an increased susceptibility to skin infections, particularly from S. aureus.56 At the same time, IL-13 seems to be directly linked to pruritis, as it sensitizes sensitive neurons considered to be pruritogenic.57
In skin biopsies of patients with AD, there is an overexpression of IL-13 in lesional57 and nonlesional58 skin as compared to healthy individuals as well as high levels of IL-13-producing T cells.59,60 In addition, the severity of AD is directly related to increased IL-13 levels,61,62 whilst a decrease in its concentration has been shown to correlate to improved clinical outcomes.58
At a lesional level, a difference between the expression of IL-4 and IL-13 in skin lesions of patients with AD has been found, with evidence of overexpression of IL-13 and a nearly undetectable expression of IL-4,6,62 supporting the fact that AD is a disease mainly dominated by IL-13.20,21,62
Treatment of AD
In patients with mild forms of AD, treatment with topical agents, such as corticosteroids and calcineurin inhibitors, together with emollient agents, is often sufficient for clinical improvement.23,63 However, in patients with moderate-to-severe AD, these options may not be effective, requiring the use of systemic therapy to control AD.23,26,63,64
Conventional immunosuppressive systemic therapies, such as systemic corticosteroids, cyclosporine and methotrexate, have been shown to be effective in improving the signs and symptoms of AD.26,65 However, none of these agents targets a specific component of the pathogenesis of AD,13,66 with their use being limited due to their long-term adverse effects and toxicities.26 Thus, these systemic therapeutic options have proved to be insufficient, making control of AD a challenge, both for the clinician and the patient.13,27
In recent years, more in-depth knowledge about the pathogenesis of AD has enabled important advances in therapeutic development through the emergence of new pharmacological options such as new biologic immunomodulatory drugs capable of specifically blocking some individual inflammatory mediators.67 To date, dupilumab is the only biologic drug approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of moderate-to-severe forms of AD.21,30 This drug is a human monoclonal antibody that inhibits the signalling of both IL-4 and IL-13 by blocking IL-4Rα, their common receptor, thereby preventing the downstream inflammatory reaction. This drug has already demonstrated dose-dependent improvements in the clinical responses of patients with moderate-to-severe AD.68,69
IL-13 inhibitors
As IL-13 is a central mediator of the pathogenesis of AD,6 the emergence of drugs that target this cytokine may maximize the efficacy and limit the toxicity associated with treatment.70 Thus, new targeted therapeutics have emerged, including the selective IL-13 inhibitors: lebrikizumab and tralokinumab.
Lebrikizumab
Lebrikizumab, a fully human IgG4κ monoclonal antibody, specifically binds to soluble IL-13 in an epitope that overlaps firmly with the IL-4Rα binding site, avoiding signalling through the IL-4Rα/IL-13Rα1 heterodimeric receptor.18,71 It does not prevent IL-13 from binding to IL-13Rα2, thus leaving intact this endogenic mechanism of regulation (Figure 1).18,72
A 12-week randomized, multicentre, double-blind, placebo-controlled, phase II clinical study (TREBLE) included 209 adults with moderate-to-severe forms of AD.70 This study evaluated the effectiveness and safety of lebrikizumab in different doses versus placebo, in combination with topical corticosteroid (TCS) use70 (Table 1). Patients aged between 18 and 75 years with moderate-to-severe AD (Eczema Area and Severity Index (EASI) ≥14, Investigator Global Assessment (IGA) ≥3 in the screening and at the end of the run-in period, affected body surface area (BSA) ≥10% and a visual analogue scale for pruritus ≥3 in the screening) with inadequate response to TCS and regular use of emollients were included.70 The primary endpoint was the achievement of a reduction of ≥50% in the EASI (EASI 50) from baseline at week 12. The secondary endpoints included the percentage of patients who achieved an EASI 75 response, an IGA of 0 or 1, and a reduction of 50% or more in the Scoring Atopic Dermatitis (SCORAD) tool (SCORAD 50) from baseline at week 12.70 An initial 2-week period was conducted with TCS. During the clinical trial period, the participants also used medium potency TCS twice daily on the lesional skin.70 Patients were randomized 1:1:1:1 to placebo or lebrikizumab injection in: lebrikizumab 125 mg single dose (group 1), lebrikizumab 250 mg single dose (group 2), lebrikizumab 125 mg every 4 weeks (Q4W) (group 3) and placebo Q4W (group 4).70
Table 1.
Anti-IL-13 monoclonal antibodies in the treatment of atopic dermatitis.
| Study design | Endpoints | Results | Main adverse events | |
|---|---|---|---|---|
| Lebrikizumab Phase II (TREBLE) (NCT02340234)70 |
Randomized, PC, DB -209 patients -12 weeks -continuous use of TCS and 2-week run-in with TCS (bid) Randomized 1:1:1:1 125 mg SD 250 mg SD 125 mg Q4WQ4W Placebo Q4WQ4W via SC |
Primary: % of patients EASI 50at week 12 Secondary: % of patients EASI 75; IGA 0/1; SCORAD 50 at week 12 |
EASI 50 achieved in 82.4% of patients in the 125 mg Q4WQ4W group vs 62.3% in the placebo group (p=0.026) at week 12 EASI 75 achieved in the 125 mg Q4WQ4W group (54.9%, p=0.036) vs placebo (34%) Higher percentage of participants in the 125 mg Q4W group achieved IGA 0/1 (p=0.098) vs placebo SCORAD 50 achieved in the 125 mg Q4W and 250 mg SD groups (47.2%, p=0.030; 51%, p=0.012) vs placebo (26.4%) Greater reduction of BA effected in the 125 mg Q4W group (↓ 57.7%) |
Upper respiratory tract infections Nasopharyngitis Headaches Pain at the injection site Reactions at the injection site Herpes infections Conjunctivitis |
| Lebrikizumab Phase IIb (NCT03443024)72 |
Randomized, PC, DB, parallel group -280 patients -16 weeks Randomized 3:3:3:2 125 mg Q4W (250 mg LD) 250 mg Q4W (500 mg LD) 250 mg Q2W (500 mg LD baseline + at week 2) Placebo Q2W - via SC |
Primary: % change in EASI from baseline to week 16 Secondary: % patients achieving EASI 50, EASI 75, EASI 90; IGA 0/1; % change in the NRS score |
All the groups that received leb: significant improvements in the % change EASI (MLS): 125 mg, Q4W (−62.3%, p=0.2), 250 mg Q4W (−69.2%, p=0.002), 250 mg Q2W (−72.1%, p<0.001) vs placebo (41.1%) at week 16 Significantly more patients from the 250 mg Q4W and 250 mg Q2W groups achieved EASI 50, EASI 75, EASI 90 and IGA 0/1 at week 16 (77%, 56.1%, 36.1%, 33.7%; 81%, 60.6%, 44%, 44.6%) vs placebo (45.8%, 24.3%, 11.4%, 15.3%) All groups that received leb improved in the NRS pruritus score (125 mg Q4W: 36.9%; 250 mg Q4W: 48.6%; 250 mg Q2W: 61.8%) vs placebo (worsening 6.8%) |
|
| Tralokinumab Phase IIb (NCT02347176)76 |
Randomized, PC, DB -204 patients -12 weeks -Continuous use of TCS and 2-week run-in with TCS Randomized 1:1:1:1 45 mg Q2W 150 mg Q2W 300 mg Q2W Placebo Q2W - via SC |
Primary: % change in EASI, patients with IGA response 0/1 + reduction ≥2 points, from baseline to week 12 Secondary: change in EASI and SCORAD from baseline to week 22; % of patients achieving EASI 50 and SCORAD 50 at week 12, % of patients achieving IGA IGA response up to week 22; change in the NRS and DLQI scores up to week 12 |
Significant reduction in EASI in groups 150 and 300 mg (mean adjusted difference of −4.4, p=0.03; −4.9, p=0.01) vs placebo at week 12 Significantly more patients in the 300 mg group vs placebo achieved EASI 50 (73.4 vs 51.9%, p=0.03) and EASI 75 (42.5% vs 15.5%, p=0.003) at week 12 SCORAD improvements in 150 and 300 mg groups (p=0.003, p=0.002) Higher percentage of participants with SCORAD 50 in the 150 mg group (44.2%, p=0.008) and in the 300 mg group (44.1%, p=0.009) vs placebo (19.5%) at week 12 Higher percentage of patients treated with 300 mg achieved an IGA response vs placebo (26.7% vs 11.8%, p=0.06) Significant improvements in the DLQI (300 mg group, p=0.006) and pruritis (45 mg and 300 mg groups; p=0.04, p=0.002) |
Nasopharyngitis Upper respiratory tract infections Headaches |
| Tralokinumab (ECZTRA 1 and 2) Phase III (NCT03131648; NCT03160885)31 |
Randomized, PC, DB 52 weeks ECZTRA 1: Tralokinumab Q2W: 603 Placebo: 199 ECZTRA 2: Tralokinumab Q2W: 593 Placebo: 201 Randomized 3:1 300 mg Q2W placebo Q2W If clinical response achieved at week 16: 300 mg Q2W 300 mg Q4W placebo Q2W SC route |
Primary: IGA 0/1 and/or EASI 75 at week 16 Secondary: Weekly average of worst daily pruritus NRS ≥4 and SCORAD and DLQI score changes from baseline to week 16 Additionally, proportion of patients achieving 50% or 90% improvement in EASI, change in EASI, change in worst daily pruritus NRS and its reduction by ≥ 3 points, change in POEM, and reduction of DLQI by ≥4 points IGA score 0/1 and EASI 75 at week 52 in patients who had achieved the considered measure at week 16 |
Significantly higher achievement of clinical response with tralokinumab in comparison with placebo at week 16:
Worst daily pruritus NRS ≥4 points achieved by 20.0% and 10.3% in ECZTRA 1 (p=0.002) and by 25.0% and 9.5% in ECZTRA 2 (p<0.001) Mean change in SCORAD was −25.2 vs −14.7 in ECZTRA 1 (p<0.001) and −28.1 vs −14.0 in ECZTRA 2 (p<0.001) Adjusted mean change in DLQI was −7.1 vs −5.0 in ECZTRA 1 (p=0.002) and −8.8 vs −4.9 in ECZTRA 2 (p<0.001) Early improvements in pruritus, sleep interference, DLQI, SCORAD and POEM were observed At week 52, as maintenance endpoints: ECZTRA 1: IGA 0/1 was maintained by 51.3% with tralokinumab Q2W vs 47.4% with placebo (p=0.68) and by 38.9% with tralokinumab Q4W (p=0.50); EASI 75 was maintained by 59.6% with tralokinumab Q2W vs 33.3% with placebo (p=0.056) and by 49.1% with tralokinumab Q4W (p=0.27) ECZTRA 2: IGA 0/1 was maintained by 59.3% with tralokinumab Q2W vs 25.0% with placebo (p=0.004) and by 44.9% with tralokinumab Q4W (p=0.084); EASI 75 was maintained by 55.8% with tralokinumab Q2W vs 21.4% with placebo (p<0.001) and by 51.4% with tralokinumab Q4W (p=0.001) |
Atopic dermatitis Viral upper respiratory tract infections Upper respiratory infections Conjunctivitis Keratoconjunctivitis Keratitis Skin infections, including those requiring systemic treatment Malignancies Eczema herpeticum Pruritus Headache |
| Tralokinumab Phase III (NCT03363854)32 |
Randomized, PC, DB 32 weeks Tralokinumab Q2W: 253 Placebo: 127 (TCS as needed) Randomized 2:1 300 mg Q2W placebo Q2W Rerandomized 1:1 tralokinumab Q2W tralokinumab Q4W (TCS as needed) SC route |
Primary: IGA 0/1 and/or EASI 75 at week 16 Secondary: Changes from baseline to week 16 in SCORAD, weekly average of worst daily pruritus NRS ≥4 and DLQI score Additionally, proportion of patients achieving 50% or 90% improvement in EASI, change in EASI, reduction of DLQI ≥4 points, POEM, worst daily pruritus NRS and TCS use; IGA score 0/1 and EASI 75 at week 52 in patients who had achieved the considered measure at week 16 |
Significantly higher achievement of clinical response with tralokinumab Q2W in comparison with placebo at week 16: IGA 0/1 achieved by 38.9% vs 26.2%, respectively (p=0.015); EASI 75 achieved by 56.0% vs 35.7% (p<0.001) Considering changes from baseline to week 16 in patients treated with tralokinumab vs placebo: SCORAD: −37.7 vs −26.8 (p<0.001) Worst daily pruritus NRS ≥4: 45.4% vs 34.1% patients (p=0.037)) DLQI: −11.7 vs −8.8 (p<0.001) Cumulative corticosteroid use lower for tralokinumab-treated patients (p=0.004) Maintenance endpoints at week 32 in patients treated with tralokinumab Q2W vs Q4W: IGA 0/1: 89.6% vs 77.6% EASI 75: 92.5% vs 90.8% |
Atopic dermatitis Viral upper respiratory tract infections Upper respiratory infections Conjunctivitis, keratoconjunctivitis, keratitis Skin infections, including those requiring systemic treatment, malignancies, eczema herpeticum Injection site reaction Headache |
BA, body area; DB, double-blind; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EASI 50, improvement of ≥50% in the EASI from baseline; EASI 75, improvement of ≥75% in the EASI from baseline; EASI 90, improvement of ≥90% in the EASI from baseline; IGA, Investigator’s Global Assessment; IGA 0/1, ‘free/almost free’; LD, loading-dose; leb, lebrikizumab; MLS, mean least squares; NRS, Numeric Rating Scale; PC, placebo-controlled; POEM, Patient-Oriented Eczema Measure; Q2W, every 2 weeks; Q4W, every 4 weeks; SC, subcutaneous; SCORAD, Scoring of Atopic Dermatitis; SCORAD 50, reduction of ≥50 in the SCORAD from baseline; SD, single dose; TCS, topical corticosteroids.
At week 12, group 4 had significantly more patients reaching EASI 50 (82.4% versus 62.3%, respectively; p=0.026) and EASI 75 (54.9% versus 34.0%, respectively; p=0.036) versus placebo, confirming the efficacy of this dosage.22,70 In groups 1 and 2, the percentages were 69.2% and 69.8%, respectively, with no statistically significant improvements, suggesting a possible dose–response relationship.70,73 Furthermore, group 3 achieved a statistically significant IGA 0/1 response compared with the placebo group (33.3% versus 18.9%, p=0.098) and experienced the greatest reduction in the affected BSA (reduction of 57.7%). In groups 2 and 3, significantly more patients (51.0%, p=0.012 and 47.2%, p=0.030, respectively) achieved a SCORAD 50 at week 12 versus placebo (26.4%).70
Regarding safety, lebrikizumab was well tolerated.22,70 All the events that occurred were mild and lasted a median of 1–3 days. The events associated with eosinophilia occurred only in the group of patients who received lebrikizumab but they were infrequently reported (n=5, 3.2%), not severe and were not associated with signs/symptoms, which resulted in dose reductions and therapy discontinuation.22,70 Injection site reactions occurred infrequently (1.3% in all lebrikizumab groups and 1.9% in the placebo group).68 The rate of conjunctivitis was 13%, 10%, 6% and 8% in groups 1, 2, 3 and 4, respectively. There were no deaths, anaphylactic reactions, neoplasms, or parasitic or intracellular infections.70
Lebrikizumab was recently studied as monotherapy in a phase IIb randomized, dose-ranging, double-blind, parallel-group, placebo-controlled clinical trial (NCT03443024) (Table 1).72 This trial consisted of 16 weeks of treatment, followed by 16 weeks of safety follow-up.66,72 The inclusion criteria were a diagnosis of AD for at least 1 year, an EASI of ≥16, an IGA of 3 or 4, and an affected BSA of ≥10% at screening and baseline.72 The primary endpoint was the achievement of a percentage change in baseline EASI at week 16. The secondary endpoints included the percentage of patients who, at week 16, achieved EASI 50, EASI 75 and EASI 90; IGA of 0 or 1; a Numeric Rating Scale (NRS) improvement of ≥4 points; a percentage change in the total involvement of the affected body area; a change in the Patient-Oriented Eczema Measure; and a change in the Dermatology Life Quality Index (DLQI).71 Patients were randomized 3:3:3:2 for the placebo (Q2W) or subcutaneous lebrikizumab injections into the following groups: initial dose/loading dose (LD) of 250 mg followed by 125 mg Q4W; (2) LD of 500 mg followed by 250 mg Q4W; (3) LD of 500 mg at baseline and at week 2, followed by 250 mg Q2W; and (4) placebo at baseline and Q2W.72
All lebrikizumab groups showed statistically significant dose-dependent improvements in the primary endpoint versus placebo at week 16 (mean least squares of the percent change in the EASI: lebrikizumab 125 mg Q4W (−62.3%, p<0.05), 250 mg Q4W (−69.2%, p<0.01) and 250 mg Q2W (−72.1%, p<0.001) versus placebo (41.1%)).72 Significantly more patients belonging to the lebrikizumab 250 mg Q4W (group 2) and lebrikizumab 250 mg Q2W (group 3) groups achieved EASI 50, EASI 75, EASI 90 and IGA 0/1 at week 16, as compared to placebo, with favourable results from week 4 in all severity scores (group 2: 77.0%, 56.1%, 36.1%, 33.7%; group 3: 81.0%, 60.6%, 44.0%, 44.6%; placebo: 45.8%, 24.3%, 11.4%, 15.3%, respectively).66,72 All groups that received lebrikizumab reported statistically significant improvements in the NRS score versus placebo (percentage changes from baseline: group 1: 36.9%; group 2: 48.6%; group 3: 61.8%; mean worsening of 6.8% in the placebo group).72 The drug also showed rapid and consistent effectiveness in this reduction (decrease starting at treatment day 2 in patients who received high doses of lebrikizumab).
Regarding the safety of the drug, adverse events (AEs) were reported in 57.5%, 48.8% and 61.3% of the lebrikizumab 125 mg Q4W, 250 mg Q4W, and 250 mg Q2W groups, respectively, versus 46.2% in the placebo group. In the groups that received lebrikizumab, the AEs were all mild to moderate, the most common being upper respiratory tract infections (2.7–11.3%), nasopharyngitis (2.5–12.0%), headaches (1.3–5.3%) and pain at the injection site (0.0–5.3%).71 None of these led to discontinuation of therapy. Regarding AEs of clinical interest, these occurred in a few patients who received the drug (2.7–9.3%, 2.7–5.0% and 1.4–3.8%, respectively, for lebrikizumab 125 mg Q4W, 250 mg Q4W and 250 mg Q2W groups). The rates of development of conjunctivitis were low (2.7%, 3.8%, 1.4% and 0.0% for groups 1, 2, 3 and 4, respectively) and of mild-to-moderate severity, not leading to the discontinuation of therapy or inclusion in the study.72 The main results are summarized in Table 1.
Tralokinumab
Tralokinumab competitively blocks the binding of IL-13 to two different receptors: IL-13Rα1 and IL-13Rα2, a decoy receptor that mediates the endogenous regulation of IL-13 (Figure 2).74,75
Phase II trials
A phase IIb randomized, double-blind, placebo-controlled, dose-ranging trial (NCT02347176) evaluated tralokinumab in different doses in 204 moderate-to-severe AD patients with concomitant TCS use (Table 1).76 The included patients were between 18 and 75 years of age, with SCORAD ≥25, EASI ≥12, BSA ≥10% and IGA ≥3.76 The patients were randomized 1:1:1:1 into the following groups after an early period of 2 weeks with TCS (run-in): placebo and tralokinumab in 45, 150 or 300 mg doses Q2W for 12 weeks.76 The primary outcomes included a reduction of ≥2 points in EASI from baseline by week 12.76 The secondary endpoints included changes in SCORAD and EASI from baseline per visit until week 22, a SCORAD reduction of ≥50% and an EASI reduction of ≥50% at week 12, and the percentage of participants who achieved an IGA response by week 22. Tralokinumab 150 and 300 mg dose groups starting at week 4 had critical clinical improvements in EASI score.76 At week 12, the same groups had significantly reduced EASI scores compared to placebo (mean adjusted difference of −4.4, p=0.03 and −4.9, p=0.01, respectively).76 Concerning an IGA response, no significant differences were observed though there were some dose-dependent improvements, with the 300 mg group achieving the highest percentage of response (26.7% versus 11.8%; p=0.06).76 SCORAD improvements were achieved in the groups treated with 150 and 300 mg of tralokinumab (p=0.003 and p=0.002, respectively) versus placebo. From week 2, SCORAD improvements were recognized in all tralokinumab doses and maintained until week 12. At week 12, the percentage of participants with SCORAD 50 was superior in the 150 mg and 300 mg groups compared to placebo (44.2%, p=0.008 and 44.1%, p=0.009 versus 19.5%).76 Additionally, at week 12, the participants treated with tralokinumab 300 mg demonstrated an improvement in the NRS (mean adjusted difference, −1.14, p=0.002) and an improvement in DLQI (p=0.006).
The subgroups with larger baseline levels of periostin and DPP-4 (which are upregulated by IL-13 and their levels indicate an increase in IL-13 activity) scored better responses with tralokinumab.76 The tralokinumab groups had an AE profile similar to that of the placebo group. The most common AEs were nasopharyngitis (19%) and upper respiratory tract infections (9%).76,77
Phase III trials
ECZTRA 1 (NCT03131648) and ECZTRA 2 (NCT03160885)31 are both phase III, randomized, double-blind, 52-week, placebo-controlled trials. After a 600 mg LD on day 0, patients were randomized (3:1) to placebo or subcutaneous tralokinumab 300 mg Q2W for 16 weeks. After 16 weeks, the patients who achieved a clinical response (EASI 75 or IGA 0/1) were rerandomized 2:2:1 to placebo or tralokinumab 300 mg Q2W or Q4W for 36 weeks; the patients who obtained a clinical response with placebo maintained the placebo but were not incorporated in the analysis after week 16; the patients who did not obtain a clinical response were shifted to open-label tralokinumab 300 mg Q2W with TCS optional.
A closing safety follow-up was performed. Through the maintenance time, patients who experienced a drug effect decline were conveyed to open-label tralokinumab.31 Before randomization, patients underwent a washout period for topical treatments for 2 weeks and for 4 weeks for systemic therapies. Rescue treatment was managed to control unbearable symptoms with no patient withdrawal from open-label or randomized treatment. Still, these patients were considered nonresponders in the primary analyses.
These trials included AD adult patients who were suitable for systemic therapy. The inclusion criteria were an IGA score of ≥3 at screening, an EASI of ≥12 and ≥16 at screening and baseline, respectively, a BSA of ≥10% at baseline, and an NRS score of ≥4 during the week before baseline.31
The primary endpoints were IGA 0/1 and EASI 75 at week 16. Secondary endpoints included SCORAD and DLQI score changes from baseline and an NRS reduction of at least 4 points to week 16. At week 52, maintenance endpoints covered EASI 75 and IGA scores 0/1 in patients randomized initially to tralokinumab, with the corresponding measures obtained at week 16 without rescue medicine.31
At week 16, patients on tralokinumab achieved significantly higher rates of IGA 0/1 and EASI 75 compared with those on placebo: in ECZTRA 1: IGA 0/1 in 15.8% versus 7.1% (p=0.002), EASI 75 achieved by 25.0% versus 12.7% patients (p<0.001); in ECZTRA 2: 22.2% versus 10.9% (p<0.001) and 33.2% versus 11.4% (p<0.001), respectively.31 For all the secondary endpoints, meaningful improvements were also recognized: a reduction in NRS of ≥4 points in 20.0% and 10.3% with tralokinumab and placebo, respectively, in ECZTRA 1 (p=0.002), and in 25.0% and 9.5% in ECZTRA 2 (p<0.001); the mean change in SCORAD was −25.2 versus −14.7 (p<0.001) in ECZTRA 1 and −28.1 versus −14.0 (p<0.001) in ECZTRA 2; finally, the adjusted mean change in DLQI was −7.1 versus −5.0 (p=0.002) in ECZTRA 1 and −8.8 versus −4.9 (p<0.001) in ECZTRA 2.31
At week 16, in ECZTRA 1, patients who achieved IGA 0/1 maintained their response at 51.3% versus 47.4% (p=0.68) versus 38.9% (p=0.50), with tralokinumab Q2W, placebo and tralokinumab Q4W, respectively; in ECZTRA 2, patients who reached IGA 0/1 maintained their response at 59.3% with tralokinumab Q2W versus 25.0% with placebo (p=0.004) and 44.9% with tralokinumab Q4W (p=0.084). In ECZTRA 1, EASI 75 was maintained at 59.6% with tralokinumab Q2W versus 33.3% with placebo (p=0.056) and 49.1% with tralokinumab Q4W (p=0.27); in ECZTRA 2, EASI 75 was maintained at 55.8% with tralokinumab Q2W versus 21.4% with placebo (p<0.001) and 51.4% with tralokinumab Q4W (p=0.001). ECZTRA 2 exhibited a higher difference between placebo and tralokinumab than ECZTRA 1, which may be explained by more use of TCS in ECZTRA 1 (35.8%) compared to ECZTRA 2 (22.8%).
In ECZTRA 1 and ECZTRA 2, tralokinumab showed a higher decrease in eczema herpeticum events and lesional skin S. aureus colonization. ECZTRA 2 showed a lower frequency of skin infections requiring treatment.31
The percentage of AEs in the initial treatment period was comparable between arms, with most being mild or moderate in severity. The most prevalent AEs were conjunctivitis and upper respiratory tract infections, which occurred most frequently with tralokinumab, whilst skin infections and flares of AD occurred most often with placebo.31 In the maintenance period, AEs occurred more frequently in the Q2W group than in the Q4W group, with a low number of events leading to permanent interruption.31 The incidence of conjunctivitis was higher with tralokinumab than placebo, being mild in most cases. The majority of conjunctivitis resolved at the end of the treatment period except for one case, where it led to withdrawal from the study.31 A more significant number of patients managed with tralokinumab exhibited eosinophilia at the beginning of the treatment period, which reverted to baseline in the continuation period.31 In all trials, the neutralizing antibody presence did not alter the safety and efficacy of tralokinumab and was detected in only 3 and 8 patients treated with tralokinumab (in ECZTRA 1 and 2, respectively).31
ECZTRA 3 (NCT03363854) was a randomized, double-blind, multicentre, placebo plus optional TCS controlled trial.32 This trial included adult patients with a diagnosis of AD for ≥1 year with an unsatisfactory response with topical medications or with documented systemic therapy in the past year. The inclusion criteria were an IGA score of ≥3, EASI score of ≥12 at screening and ≥16 at baseline, BSA ≥10% at screening and baseline, and NRS average score of ≥4 during the week before baseline. The included patients were randomized 2:1 to tralokinumab 300 mg Q2W associated with TCS as wanted. Patients who obtained clinical response criteria (IGA 0/1 or EASI 75) at week 16 were rerandomized 1:1 to tralokinumab Q2W or Q4W. Patients from the placebo or tralokinumab groups who did not achieve a clinical response initiated tralokinumab Q2W plus TCS as needed.32
The primary endpoints were EASI 75 and IGA 0/1 at week 16. Secondary endpoints included a DLQI score, a weekly average of worst daily pruritus NRS ≥4 and SCORAD changes from baseline to week 16, the proportion of patients achieving 50% or 90% EASI improvement, a change in Patient-Oriented Eczema Measure, EASI or worst daily pruritus, improvement in DLQI ≥4 points, and TCS use. Maintenance endpoints (tralokinumab Q2W plus TCS and tralokinumab Q4W plus TCS at week 32) were EASI 75 and IGA 0/1 in patients who had accomplished these responses at week 16.32
At week 16, 38.9% of patients on tralokinumab Q2W achieved IGA 0/1 versus 26.2% with placebo (p=0.015), and EASI 75 was achieved by 56.0% versus 35.7% (p<0.001). Acknowledging responders at week 16, IGA 0/1 was sustained by 89.6% with tralokinumab Q2W and by 77.6% with tralokinumab Q4W, whereas 92.5% and 90.8% of patients maintained EASI 75, respectively, at week 32. Of patients who did not respond with tralokinumab Q2W at week 16, 30.5% and 55.8% reached IGA 0/1 and EASI 75, respectively, at week 32.32 Furthermore, 45.4% versus 34.1% of patients achieved a reduction of ≥4 points in the NRS pruritus score (p=0.037) with tralokinumab versus placebo, respectively; an improvement in the total DLQI score of −11.7 versus −8.8 (p<0.001) and an improvement in the SCORAD score of −37.7 versus −26.8 (p<0.001).32 More patients treated with tralokinumab achieved EASI 50 or EASI 90 at week 16 and a reduction in weekly average of the worst NRS for daily pruritus and EASI score. Cumulative TCS use was more limited for patients treated with tralokinumab (p=0.004).32 In this study, 84 (66.7%) and 180 (71.4%) patients in the placebo and tralokinumab groups had AEs; most were mild or moderate in severity. The most common AEs related to tralokinumab were conjunctivitis, upper respiratory tract infection, headache and an injection site reaction. Due to AEs, 6 patients discontinued, although none of the AEs were severe.32
In the initial treatment period, all conjunctivitis cases were mild or moderate, but there was discontinuation in one case. Skin infections requiring systemic treatment were more frequent in the placebo group than in patients treated with tralokinumab Q2W. There were no differences in the number of cases of herpetic eczema. During the continuation of treatment, there was no increase in the frequency of AEs. The pattern of AEs was similar to that of tralokinumab Q2W in the initial treatment period. Events were reported less frequently in patients given tralokinumab Q4W than with tralokinumab Q2W. During this period, 2 AEs related to malignant diseases were diagnosed. Four patients stopped treatment with tralokinumab, two due to AD worsening, one because of herpetic eczema and one because of prostate cancer. Additionally, 13 serious AEs were recorded, with no differences between groups or treatment periods, 6 in the initial period and 7 in the maintenance period. The main results are summarized in Table 1.
Discussion
AD is a complex skin inflammatory disease with TH2/TH22 polarization and, depending on the disease phenotype, has variable contributions from the TH1 and TH17 signalling pathways.78 Other important AD features are dysbiosis, skin barrier dysfunction and pruritus.35 IL-13 has been shown to be a central cytokine in AD, contributing to AD pruritus (both directly or indirectly through IL-31)49 and epidermal barrier dysfunction,79 inducing the differentiation of TH2 cells,18 decreasing AMP synthesis, favouring skin infections and promoting fibrosis.18
Both IL-4 and IL-13 share the IL-4Rα/IL-13Rα1 heterodimeric receptor (type 2 receptor) in their signalling cascades. However, IL-13 participates preferentially in peripheral tissues, including skin. In fact, skin lesioned by AD is clearly dominated by IL-13, with very low levels of IL-4. In addition, the levels of circulating IL-13 and IL-13-producing T cells are increased in patients with AD.58 Therefore, from the analysis of the pathophysiology of AD, IL-13 seems to play a more relevant role than IL-4.80
Currently, dupilumab, which targets both IL-4 and IL-13 signalling, is the only biologic agent approved for the treatment of moderate-to-severe AD. Recently, both lebrikizumab and tralokinumab, two selective IL-13 inhibitors, showed a favourable efficacy and safety profile.71 Lebrikizumab showed, at week 12, in the phase II trial, that adult patients with moderate-to-severe AD treated with lebrikizumab 125 mg Q4W and TCS achieved greater EASI 50 than those treated with placebo.70 Subsequently, a phase IIb trial showed that all three dosing regimens evaluated (lebrikizumab 125 mg Q4W, 250 mg Q4W or 250 mg Q2W) achieved rapid and dose-dependent efficacy concerning the signs and symptoms of AD, with a statistically significant improvement at week 16 in the average percentage difference in EASI compared to placebo.72 In general, it was well tolerated.70,72
Tralokinumab was studied in three phase III clinical trials and reached its primary endpoints at week 16 in all trials (ECZTRA 1 and 2 in monotherapy and ECZTRA 3 with concomitant TCS), with response maintained over time. Significant improvements in secondary outcomes, such as pruritus and quality of life, were also shown.31,32
Both agents differ in their binding epitopes and their ability to block one or both receptors of IL-13; however, it is unknown whether these differences are associated with clinical implications. Due to differences in the study design (mainly in the study duration, population size, whether corticosteroids were permitted during the study, the dose regimens used and participant selection criteria), it is difficult to compare results between the agents and establish direct comparisons of the findings.18
Also difficult is the comparison of these results with those of dupilumab because no head-to-head studies have been conducted and data from phase III lebrikizumab studies are not yet available. The average difference in EASI scores appears numerically higher for dupilumab81 but the placebo-adjusted EASI 75 response is similar for lebrikizumab (37%) and dupilumab (32–36%), whilst that of tralokinumab is slightly lower (12–22%), which may also be partially explained by differences in the study designs (namely the population size, the duration of the studies, criteria for corticosteroid usage and the selection criteria of the participants). In a comparison of safety and tolerability issues in AD between dupilumab and the selective IL-13 inhibitors, dupilumab presented conjunctivitis as a considerable side effect, with high rates in phase III studies (SOLO 1 and 2 and CHRONOS).69,82–84 In contrast, in lebrikizumab studies, there were low conjunctivitis rates, but slightly higher than the placebo group, with no apparent dose–response relationship.70,72 Tralokinumab showed similar results,77 suggesting that selective IL-13 inhibition may be associated with a mild increased risk of conjunctivitis in AD but lower than dupilumab.72 Phase III studies for lebrikizumab and real-world studies are needed to complement these findings.
This possible difference may be explained by the effect of IL-4 signalling blockade on promoting a TH1 response.72 A TH1 response leads to goblet cell apoptosis mediated by IFNγ and to a reduction in mucin production,85–87 which, in turn, leads to the development of dry eye and conjunctivitis.88 Biopsies from AD patients treated with dupilumab who developed conjunctivitis were analysed and showed a substantial shortage of intraepithelial goblet cells, corroborating this hypothesis.89 Certainly, additional data are needed to evaluate the mechanisms underlying dupilumab-induced conjunctivitis.
Finally, despite the developments observed in recent years in the treatment of AD, with the appearance of several new agents of different classes (such as IL-4/IL-13 inhibitors, IL-13 inhibitors and JAK inhibitors), there remain many patients who do not respond to treatment, probably due to the heterogeneity of AD and the existence of several endotypes. The identification of biomarkers that allow the identification of patients who are more likely to benefit from certain treatments will be very important. Interestingly, periostin and DPP-4, the levels of which indicate an increase in IL-13 activity, have shown to be associated with a better response to tralokinumab and may function as biomarkers of efficacy response to this agent. In the era of personalized medicine, the identification of these biomarkers will be essential and therefore further studies are needed.76
Ongoing phase III studies evaluating lebrikizumab and tralokinumab will further elucidate its potential role in AD treatment (Table 2).
Table 2.
Ongoing trials evaluating the safety and efficacy of tralokinumab and lebrikizumab for atopic dermatitis treatment.
| Drug, sponsor | Clinical trial | Phase | Estimated enrolment (number of participants) | Status | Estimated study completion date |
|---|---|---|---|---|---|
| Lebrikizumab, Eli Lilly and Company | NCT04392154 | III | 900 | Recruiting | May 30, 2024 |
| Lebrikizumab, Eli Lilly and Company | NCT04626297 | III | 240 | Not yet recruiting | November 5, 2021 |
| Lebrikizumab, Eli Lilly and Company | NCT04250350 | III | 200 | Recruiting | May 31, 2022 |
| Lebrikizumab, Eli Lilly and Company | NCT04146363 | III | 400 | Recruiting | May 9, 2022 |
| Lebrikizumab, Eli Lilly and Company | NCT04250337 | III | 225 | Recruiting | October 13, 2021 |
| Tralokinumab | NCT04556461 | II | 16 | Recruiting | March 2022 |
| Tralokinumab, LEO Pharma | NCT03587805 | III | 1125 | Enrolling by invitation | September 13, 2021 |
| Tralokinumab, LEO Pharma | NCT04587453 | III | 100 | Recruiting | September 2021 |
| Tralokinumab, LEO Pharma | NCT03556592 | I | 40 | Completed | |
| Tralokinumab, LEO Pharma | NCT03526861 | III | 299 | Active, not recruiting | February 20, 2021 |
Conclusion
Dermatology is increasingly moving along the path towards the emergence of directed, effective and safe therapies. AD is one of the chronic inflammatory skin diseases that is following this path due to a thorough investigation that explores the unique and complex immune fingerprint of AD. In recent years, the greater knowledge of AD pathogenesis led to improved therapeutic strategies through the emergence of new immunomodulator drugs targeting these key elements.
IL-13 is believed to be the main mediator of the pathophysiology of AD, mediating its effects at the tissue level, resulting in dysfunction of the epidermal barrier, pruritus, thickening of the skin and skin inflammation. Thus, selective IL-13 inhibitors, such as lebrikizumab and tralokinumab, have been developed for the treatment of AD, showing a favourable safety profile and effectiveness in the treatment of moderate-to-severe AD. These data support the hypothesis that selective antagonism of IL-13 is sufficient to control AD, providing an improvement in patient quality of life. Therefore, the development of lebrikizumab and tralokinumab represents a new and exciting phase in the management of AD.
Acknowledgements
None.
Footnotes
Contributions: All the authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: Francisca Gonçalves has no conflicts of interest. Egídio Freitas has no conflicts of interest. Tiago Torres is a scientific consultant/speaker/clinical study investigator for AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Biocad, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Fresenius Kabi Pharma, Janssen, Leo Pharma, MSD, Mylan, Novartis, Pfizer, Samsung-Bioepis, Sandoz, Sanofi, UCB and Viatris. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/03/dic.2021-1-7-COI.pdf
Funding declaration: No sources of funding were used to conduct this study or prepare this manuscript.
Correct attribution: Copyright © 2021 Gonçalves F, Freitas E, Torres T. https://doi.org/10.7573/dic.2021-1-7. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/selective-il-13-inhibitors-for-the-treatment-of-atopic-dermatitis
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233(5):344–357. doi: 10.1159/000484406. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrocini G, Napolitano M, Megna M, Balato N, Patruno C. Treatment of atopic dermatitis with biologic drugs. Dermatol Ther. 2018;8(4):527–538. doi: 10.1007/s13555-018-0258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 4.DaVeiga SP. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc. 2012;33(3):227–234. doi: 10.2500/aap.2012.33.3569. [DOI] [PubMed] [Google Scholar]
- 5.Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children. Immunol Allergy Clin North Am. 2015;35(1):161–183. doi: 10.1016/j.iac.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsoi LC, Rodriguez E, Degenhardt F, et al. Atopic dermatitis Is an IL-13–dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139(7):1480–1489. doi: 10.1016/j.jid.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters AS, Kellberger J, Vogelberg C, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126(3):590–595.e3. doi: 10.1016/j.jaci.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113(5):925–931. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 9.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Li K, Seo SJ, et al. Quality of life and disease severity are correlated in patients with atopic dermatitis. J Korean Med Sci. 2012;27(11):1327. doi: 10.3346/jkms.2012.27.11.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maksimovic N, Jankovic S, Marinkovic J, Sekulovic LK, Zivkovic SV. Health-related quality of life in patients with atopic dermatitis. J Dermatol. 2012;39:42–47. doi: 10.1111/j.1346-8138.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 12.Rønnstad ATM, Halling-Overgaard A-S, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448–456.e30. doi: 10.1016/j.jaad.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Renert-Yuval Y, Guttman-Yassky E. What’s new in atopic dermatitis. Dermatol Clin. 2019;37(2):205–213. doi: 10.1016/j.det.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg JI, Kantor R. The Role of Interleukins 4 and/or 13 in the Pathophysiology and treatment of atopic dermatitis. Dermatol Clin. 2017;35(3):327–334. doi: 10.1016/j.det.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131(2):428–433. doi: 10.1016/j.jaci.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker AM, Wang AR, Li W-Q, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Napolitano M, Marasca C, Fabbrocini G, Patruno C. Adult atopic dermatitis: new and emerging therapies. Expert Rev Clin Pharmacol. 2018;11(9):867–878. doi: 10.1080/17512433.2018.1507734. [DOI] [PubMed] [Google Scholar]
- 18.Moyle M, Cevikbas F, Harden JL, Guttman-Yassky E. Understanding the immune landscape in atopic dermatitis: the era of biologics and emerging therapeutic approaches. Exp Dermatol. 2019;28(7):756–768. doi: 10.1111/exd.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szegedi K, Lutter R, Res PC, et al. Cytokine profiles in interstitial fluid from chronic atopic dermatitis skin. J Eur Acad Dermatology Venereol. 2015;29(11):2136–2144. doi: 10.1111/jdv.13160. [DOI] [PubMed] [Google Scholar]
- 21.Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75(1):54–62. doi: 10.1111/all.13954. [DOI] [PubMed] [Google Scholar]
- 22.Dattola A, Bennardo L, Silvestri M, Nisticò SP. What’s new in the treatment of atopic dermatitis? Dermatol Ther. 2019;32(2):e12787. doi: 10.1111/dth.12787. [DOI] [PubMed] [Google Scholar]
- 23.Megna M, Napolitano M, Patruno C, et al. Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther. 2017;7(1):1–23. doi: 10.1007/s13555-016-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Napolitano M, Megna M, Patruno C, Gisondi P, Ayala F, Balato N. Adult atopic dermatitis: a review. G Ital Dermatol Venereol. 2016;151(4):403–411. http://www.ncbi.nlm.nih.gov/pubmed/25658440. [PubMed] [Google Scholar]
- 25.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70(7):836–845. doi: 10.1111/all.12619. [DOI] [PubMed] [Google Scholar]
- 26.Silverberg JI. Atopic dermatitis treatment: current state of the art and emerging therapies. Allergy Asthma Proc. 2017;38(4):243–249. doi: 10.2500/aap.2017.38.4054. [DOI] [PubMed] [Google Scholar]
- 27.Deleanu D, Nedelea I. Biological therapies for atopic dermatitis: an update (Review) Exp Ther Med. 2019;17:1061–1067. doi: 10.3892/etm.2018.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nezamololama N, Fieldhouse K, Metzger K, Gooderham M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: a review of abrocitinib, baricitinib, and upadacitinib. Drugs Context. 2020;9:2020-8-5. doi: 10.7573/dic.2020-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gooderham MJ, Hong HC, Eshtiaghi P, Papp KA. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3):S28–S36. doi: 10.1016/j.jaad.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Chang HY, Nadeau KC. IL-4Rα inhibitor for atopic disease. Cell. 2017;170(2):222. doi: 10.1016/j.cell.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br J Dermatol. doi: 10.1111/bjd.19574. Published online December 30, 2020:bjd.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450–463. doi: 10.1111/bjd.19573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misery L, Huet F, Gouin O, Ständer S, Deleuran M. Current pharmaceutical developments in atopic dermatitis. Curr Opin Pharmacol. 2019;46:7–13. doi: 10.1016/j.coph.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 34.McPherson T. Current understanding in pathogenesis of atopic dermatitis. Indian J Dermatol. 2016;61(6):649. doi: 10.4103/0019-5154.193674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii M. Current understanding of pathophysiological mechanisms of atopic dermatitis: interactions among skin barrier dysfunction, immune abnormalities and pruritus. Biol Pharm Bull. 2020;43(1):12–19. doi: 10.1248/bpb.b19-00088. [DOI] [PubMed] [Google Scholar]
- 36.Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158(4):281–286. doi: 10.1111/imm.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaniboni MC, Samorano LP, Orfali RL, Aoki V. Skin barrier in atopic dermatitis: beyond filaggrin. An Bras Dermatol. 2016;91(4):472–478. doi: 10.1590/abd1806-4841.20164412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. 2017;278(1):116–130. doi: 10.1111/imr.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janssens M, van Smeden J, Gooris GS, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res. 2012;53(12):2755–2766. doi: 10.1194/jlr.P030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonness S, Bieber T. Molecular basis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2007;7(5):382–386. doi: 10.1097/ACI.0b013e3282a643c3. [DOI] [PubMed] [Google Scholar]
- 41.Brunner PM, Guttman-Yassky E, Leung DYM. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4):S65–S76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124(3):R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Kim BE, Leung DYM, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126(3):332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranasinghe C, Trivedi S, Wijesundara DK, Jackson RJ. IL-4 and IL-13 receptors: roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor Rev. 2014;25(4):437–442. doi: 10.1016/j.cytogfr.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40(2):84–92. doi: 10.2500/aap.2019.40.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furue M, Ulzii D, Vu YH, Tsuji G, Kido-Nakahara M, Nakahara T. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16(2):97–107. doi: 10.22034/IJI.2019.80253. [DOI] [PubMed] [Google Scholar]
- 47.Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82(1):222–228. doi: 10.1016/j.jaad.2019.08.078. [DOI] [PubMed] [Google Scholar]
- 48.Geoghegan JA, Irvine AD, Foster TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 2018;26(6):484–497. doi: 10.1016/j.tim.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228.e13. doi: 10.1016/j.cell.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hershey GKK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111(4):677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 51.Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res. 2011;50(1):87–96. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCormick SM, Heller NM. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine. 2015;75(1):38–50. doi: 10.1016/j.cyto.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorsey NJ, Chapoval SP, Smith EP, Skupsky J, Scott DW, Keegan AD. STAT6 Controls the number of regulatory T cells in vivo, thereby regulating allergic lung inflammation. J Immunol. 2013;191(4):1517–1528. doi: 10.4049/jimmunol.1300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwak EJ, Hong JY, Kim MN, et al. Chitinase 3-like 1 drives allergic skin inflammation via Th2 immunity and M2 macrophage activation. Clin Exp Allergy. 2019;49(11):1464–1474. doi: 10.1111/cea.13478. [DOI] [PubMed] [Google Scholar]
- 55.Berdyshev E, Goleva E, Bronova I, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight. 2018;3(4):e98006. doi: 10.1172/jci.insight.98006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171(6):3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 57.Miron Y, Miller PE, Ghetti A, Ramos M, Hofland H, Cevikbas F. LB1511 Neuronal responses elicited by interleukin-13 in human neurons. J Invest Dermatol. 2018;138(9):B8. doi: 10.1016/j.jid.2018.06.042. [DOI] [Google Scholar]
- 58.Ungar B, Garcet S, Gonzalez J, et al. An Integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol. 2017;137(3):603–613. doi: 10.1016/j.jid.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 59.La Grutta S, Richiusa P, Pizzolanti G, et al. CD4 + IL-13 + cells in peripheral blood well correlates with the severity of atopic dermatitis in children. Allergy. 2005;60(3):391–395. doi: 10.1111/j.1398-9995.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 60.Czarnowicki T, Gonzalez J, Shemer A, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136(1):104–115.e7. doi: 10.1016/j.jaci.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 61.Choy DF, Hsu DK, Seshasayee D, et al. Comparative transcriptomic analyses of atopic dermatitis and psoriasis reveal shared neutrophilic inflammation. J Allergy Clin Immunol. 2012;130(6):1335–1343.e5. doi: 10.1016/j.jaci.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tazawa T, Sugiura H, Sugiura Y, Uehara M. Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch Dermatol Res. 2004;295(11):459–464. doi: 10.1007/s00403-004-0455-6. [DOI] [PubMed] [Google Scholar]
- 63.Snast I, Reiter O, Hodak E, Friedland R, Mimouni D, Leshem YA. Are biologics efficacious in atopic dermatitis? a systematic review and meta-analysis. Am J Clin Dermatol. 2018;19(2):145–165. doi: 10.1007/s40257-017-0324-7. [DOI] [PubMed] [Google Scholar]
- 64.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis. J Am Acad Dermatol. 2014;71(1):116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133(2):429–438. doi: 10.1016/j.jaci.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 66.Loh TY, Hsiao JL, Shi VY. Therapeutic potential of lebrikizumab in the treatment of atopic dermatitis. J Asthma Allergy. 2020;13:109–114. doi: 10.2147/JAA.S211032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361(9352):151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 68.Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 69.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 70.Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE) J Am Acad Dermatol. 2018;78(5):863–871.e11. doi: 10.1016/j.jaad.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Ultsch M, Bevers J, Nakamura G, et al. Structural basis of signaling blockade by anti-IL-13 antibody lebrikizumab. J Mol Biol. 2013;425(8):1330–1339. doi: 10.1016/j.jmb.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 72.Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis. JAMA Dermatol. 2020;156(4):411. doi: 10.1001/jamadermatol.2020.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu J, Guttman-Yassky E. Efficacy of biologics in atopic dermatitis. Expert Opin Biol Ther. 2020;20(5):525–538. doi: 10.1080/14712598.2020.1722998. [DOI] [PubMed] [Google Scholar]
- 74.Popovic B, Breed J, Rees DG, et al. Structural characterisation reveals mechanism of IL-13-neutralising monoclonal antibody tralokinumab as inhibition of binding to IL-13Rα1 and IL-13Rα2. J Mol Biol. 2017;429(2):208–219. doi: 10.1016/j.jmb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Hussein YM, Ahmad AS, Ibrahem MM, et al. Interleukin 13 receptors as biochemical markers in atopic patients. J Investig Allergol Clin Immunol. 2011;21(2):101–107. [PubMed] [Google Scholar]
- 76.Wollenberg A, Howell MD, Guttman-Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J Allergy Clin Immunol. 2019;143(1):135–141. doi: 10.1016/j.jaci.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 77.Wollenberg A, Howell MD, Guttman-Yassky E, et al. A Phase 2b dose-ranging efficacy and safety study of tralokinumab in adult patients with moderate to severe atopic dermatitis. Ski J Cutan Med. 2018;2:S29. doi: 10.25251/skin.2.supp.28. [DOI] [Google Scholar]
- 78.Li R, Hadi S, Guttman-Yassky E. Current and emerging biologic and small molecule therapies for atopic dermatitis. Expert Opin Biol Ther. 2019;19(4):367–380. doi: 10.1080/14712598.2019.1573422. [DOI] [PubMed] [Google Scholar]
- 79.Karo-Atar D, Bitton A, Benhar I, Munitz A. Therapeutic targeting of the interleukin-4/interleukin-13 signaling pathway: in allergy and beyond. BioDrugs. 2018;32(3):201–220. doi: 10.1007/s40259-018-0280-7. [DOI] [PubMed] [Google Scholar]
- 80.Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015;75(1):25–37. doi: 10.1016/j.cyto.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drucker AM, Ellis AG, Bohdanowicz M, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis. JAMA Dermatol. 2020;156(6):659. doi: 10.1001/jamadermatol.2020.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 83.Akinlade B, Guttman-Yassky E, Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459–473. doi: 10.1111/bjd.17869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferreira S, Torres T. Conjunctivitis in patients with atopic dermatitis treated with dupilumab. Drugs Context. 2020;9:2020-2-3. doi: 10.7573/dic.2020-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tukler Henriksson J, Coursey TG, Corry DB, De Paiva CS, Pflugfelder SC. IL-13 stimulates proliferation and expression of mucin and immunomodulatory genes in cultured conjunctival goblet cells. Investig Opthalmol Vis Sci. 2015;56(8):4186. doi: 10.1167/iovs.14-15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.García-Posadas L, Hodges RR, Diebold Y, Dartt DA. Context-dependent regulation of conjunctival goblet cell function by allergic mediators. Sci Rep. 2018;8(1):12162. doi: 10.1038/s41598-018-30002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pflugfelder SC, Corrales RM, de Paiva CS. T helper cytokines in dry eye disease. Exp Eye Res. 2013;117:118–125. doi: 10.1016/j.exer.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maudinet A, Law-Koune S, Duretz C, Lasek A, Modiano P, Tran THC. Ocular surface diseases induced by dupilumab in severe atopic dermatitis. Ophthalmol Ther. 2019;8(3):485–490. doi: 10.1007/s40123-019-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bakker DS, Ariens LFM, Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180(5):1248–1249. doi: 10.1111/bjd.17538. [DOI] [PMC free article] [PubMed] [Google Scholar]