Umberto Ianni, MD and Marco Zimarino, MD, PhD
Main findings of the ISCHEMIA trial
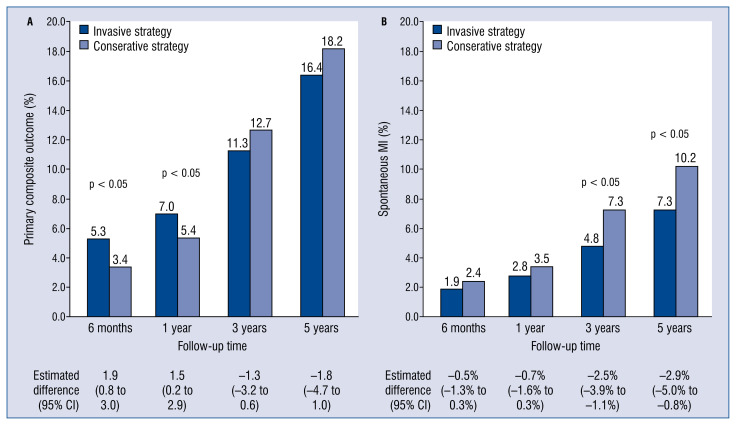
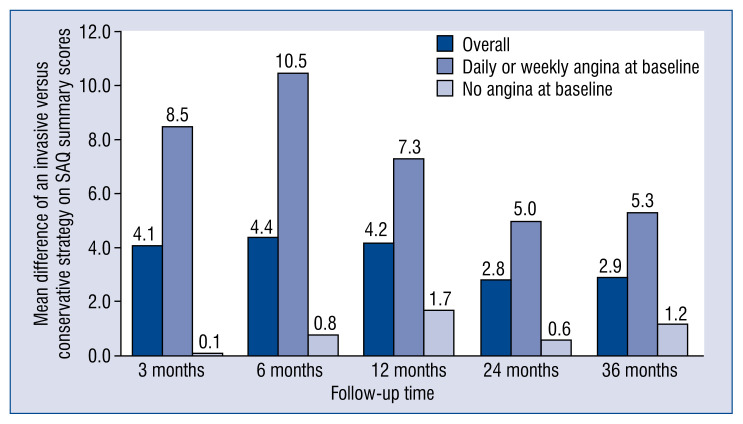
The long-awaited results of the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial were recently made available in the ‘New England Journal of Medicine’ [1, 2]. Two main manuscripts detailed on a composite endpoint of death from cardiovascular causes, myocardial infarction (MI), hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest [1] and on quality of life assessment [2]. Overall, the ISCHEMIA trial encompassing 5179 randomized patients with chronic coronary syndromes (CCS), documented that the risk of the primary composite endpoint is similar for an initial invasive strategy with coronary angiography and revascularization, either by percutaneous coronary intervention (PCI) or by coronary artery bypass grafting (CABG) and an initial conservative strategy with optimal medical therapy (OMT) (Fig. 1A) [1]. An initial invasive strategy clearly produced a greater improvement in angina-related health status (Fig. 2) [2]. As for the occurrence of adverse events, when compared with an initial conservative approach, the invasive strategy was associated with an early increased risk, seemingly derived from periprocedural events, that waned and even inverted in the long term, showing a trend towards a protective effect [1].
Figure 1.
Clinical endpoints differences between treatment groups across a 5-year follow-up and relative estimated difference (95% confidence interval [CI]); A. Primary composite outcome; B. Spontaneous myocardial infarction (MI).
Figure 2.
Effect of the invasive strategy on symptoms, estimated as the mean difference of invasive versus conservative strategy on Seattle Angina Questionnaire (SAQ) summary scores, according to baseline angina burden during 36 month follow-up.
Such results were in line with previous findings in patients with CCS from COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) [3] and ORBITA (Objective Randomized Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina) [4] trials. However, the idea that sicker patients, with more disease or more ischemia, would do better with revascularization has not been questioned, as the high-risk population — patients with unprotected left main disease, impaired systolic function, heart failure, recent acute coronary syndromes and/or Canadian Cardiovascular Society class III or IV angina of recent onset — were quite reasonably excluded from the trial.
Amendments and limitations of eligibility criteria
The initial plan was to conduct an international, multi-center randomized trial in 8000 CCS patients, comparing early invasive management followed by routine revascularization with OMT and revascularization only in case of persistent symptoms or MI [5].
This trial design was elegant and designed originally with hard primary endpoints, which would have offered a definitive answer. Randomization to conservative or invasive strategies was carried out after coronary computed tomographic angiographic (CCTA), before coronary angiography, thus preventing physicians from a withdrawing-bias in patients with lesions suitable for immediate stenting. The trial was started in July 2012, but investigators soon realized that the recruitment was slow and event rates lower than anticipated. Consequently, several substantial changes were applied to both the design and the primary endpoint to complete the trial.
The definition of the ischemic burden was central to the primary hypothesis, that extent and severity of ischemia are related to adverse outcomes in CCS, as documented in the COURAGE trial nuclear substudy [6], where patients with ischemia reduction had a lower risk of death or MI, mainly if baseline ischemia was moderate-to-severe (≥ 10% myocardium). The laudable intention that moved the ISCHEMIA investigators to enroll only patients with moderate-to-severe ischemia, having rigorous documentation by stress imaging assessment as single-photon emission computed tomography or stress-echocardiography or stress-cardiac magnetic resonance, was later aborted forcefully. In order to expedite recruitment, patients with a lesser amount of ischemia (≥ 5% myocardium) or with documentation of ischemia on the sole electrocardiogram-exercise test — a diagnostic modality with a much lower diagnostic sensibility — were deemed eligible, reaching the minimal threshold for the predefined power but tarnishing the original ambitious aim. Arguably, the severity of ischemia cannot be measured without imaging, and the lack of standardized grading and inconsistency in reporting of the extent and severity of ischemia across different stress imaging modalities raise major concerns on the accuracy of ischemic burden quantification and patient selection [7, 8]. Furthermore, according to the main study protocol, almost one-third of randomized patients with estimated glomerular filtration rate (eGFR) < 60 mL/min with a “high likelihood” of significant left main disease based on the results of the stress test did not undergo CCTA due to the risk of contrast-induced nephropathy. In this case, trial eligibility relied on the physician determination, carrying a subjective component in the diagnostic pathway and ultimately introducing another potential source of selection bias.
There is also a major concern about the over-interpretation of the ISCHEMIA trial design in clinical practice, where a problematic reading may result in extrapolating beyond the data provided in the study [9]. This is the case when advocating CCTA as the first-line strategy in all patients with chest pain, regardless of the pre-test likelihood of coronary artery disease (CAD) and demonstration of ischemia. In the ISCHEMIA trial, CCTA was primarily aimed at ruling out the presence of significant unprotected left main disease — but surprisingly not a proximal three-vessel disease — and to confirm the presence of obstructive CAD in patients with at least moderate ischemia. Claiming that CCTA should be performed at a population level where the prevalence of the left main disease is predictably low has the potential of exposing a significant number of patients to ionizing radiation and nephrotoxic contrast agents, when alternative and more cost-effective options are available [10, 11].
Finally, about 14% of patients enrolled failed to meet eligibility criteria for ischemia at a later core laboratory evaluation, but they were part of the study population anyway; this cohort showed a trend towards an increased benefit from the conservative strategy, further flawing the main findings of the study.
Changing the primary endpoint: A contingency plan
The ISCHEMIA trial was conducted in accordance with the most rigorous clinical trial standards, and investigators should be commended for preparing a contingency plan in case of lower than expected event rates for the original primary endpoint of cardiovascular death or MI. This pre-specified back-up strategy was vital in avoiding a common pitfall of other trials, namely lower-than-projected power because of lower than anticipated event rates. It led to the 5-component endpoint, including cardiovascular death, MI, hospitalization for unstable angina, resuscitated cardiac arrest, or heart failure, as per the original grant proposal awarded by National Heart, Lung, and Blood Institute (NHLBI) in 2011 [8].
Accordingly, projections in 2015, using updated assumptions for the randomization rate and demonstrating an enrollment rate lagging behind timelines and lower than expected adverse events, suggested that the intended goals needed better calibration in favor of a more reasonable and pragmatic target. The sample size was therefore lowered by nearly 35% and the mean follow-up duration by 25% (from 4 to 3.2 years). However, activation of the contingency plan had a price to pay. Indeed, by dropping the prespecified power threshold below 90%, investigators were facing two alternatives: either reporting an underpowered trial with a negative prespecified primary outcome, or a potentially false-positive trial with “re-engineered” alternative outcomes [12].
For the multinational ISCHEMIA trial, conducted at 320 sites in 37 countries, investigators obtained 108 million USD from NHLBI, based on the recognition that trial results would have allowed saving of > 500 million USD/year from the reduction of unnecessary revascularization, clearly exceeding the public fund earmarked [12].
The publication of the trial spurred controversy among the scientific community [13].
As claimed by Antman and Braunwald [14], the most obvious conclusion is that the two strategies seem to have similar efficacy. At the same time, the patients in the invasive-strategy group reported substantially fewer angina symptoms than those in the conservative approach. However, the magnitude of this benefit depended on angina frequency at baseline, with 35% of cases being asymptomatic. Possible reasons for such similar findings are the low-risk of the study population and the potential effect of practice patterns that may have excluded more symptomatic patients. In the main trial, the incidence of the primary outcome was sensitive to the definition of MI, with a substantial prevalence of periprocedural MIs in the early follow-up favoring a conservative strategy and higher incidence of spontaneous MIs in a later course, when the invasive approach showed a protective effect. Investigators adjudicated MI employing a primary or secondary definition, the latter using references from the assay manufacturer’s package insert and including contingencies to allow diagnosing MI despite whether they had various elements of the medical record missing. For spontaneous MI, investigators adopted the third universal definition of MI (types 1, 2, 4b, 4c), while for procedural MI (types 4a, 5) creatine kinase myocardial band (CK-MB) was the preferred biomarker and higher thresholds were used: (i) post-PCI, a rise in CK-MB > 5-fold the upper limit of normal (ULN) or a rise in troponin > 35-fold the ULN post-PCI; (ii) post-CABG, a rise in CK-MB > 10-fold the ULN or a rise in troponin > 70-fold the ULN post-PCI. Investigators adopted such thresholds with the aim of compensating for the reduced prognostic relevance of periprocedural as compared to spontaneous MI [15]. A similar controversy recently animated the presentation of the 5-year outcomes of the EXCEL (Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trial [16]. Finally, the adoption of a definition of periprocedural MI for both PCI and CABG based on CK rise > 10-fold the ULN or > 5-fold the ULN with new Q-waves, angiographic vessel occlusion, or loss of myocardium on imaging was strongly associated with increased 3-year mortality even after controlling for potential confounders [17].
In the ISCHEMIA trial, the invasive strategy was associated with a trend towards a reduced incidence of death from cardiovascular causes or MI (16.5% vs. 14.9%), as was the primary definition of the study, and a significant absolute risk reduction of 2.9% of spontaneous MI at 5 years (Fig. 1B) [1]. Antman and Braunwald [14] hypothesized as well that ISCHEMIA might have ended before a substantial difference in favor of the invasive strategy had emerged. As therapeutic benefits tend to wane at longer follow-up in aging populations, authors herein, respectfully disagree that prolonging of the study would have unveiled such a difference; nevertheless, by considering a calculated event rate of 3.3 per 100 person-years in the invasive group and of 3.8 per 100 person-years, with a type I error of 5% (α = 0.05) and a statistical power of 80%, the study population should have been followed-up for 6.5 years overall. Alternatively, the same difference in the hard-end points would have been detected with a population of about 8000 patients per group, obviously requiring a far larger resource allocation for the trial.
Group comparisons were performed according to the intention-to-treat principle that counts crossovers within originally assigned groups, regardless of the treatment actually received. However, no adjustment for non-adherence to the randomized treatment strategy, such as censoring at the time of crossover, was considered as deemed susceptible to bias as per the study protocol. After randomization, 16% of patients in the invasive arm did not receive any revascularization, while 21% of patients in the conservative-strategy group underwent revascularization, therefore resulting in a bidirectional crossover likely driving potential underestimation of the actual long-term benefit of the invasive approach. However, the prespecified aim of the study was to test the initial management strategy, and, in this view, the intensity of crossover was acceptable despite an invasive approach, which was later pursued in more than one-fourth of the study population. Nevertheless, the intense crossover rate observed in ISCHEMIA, along with slow enrollment, would suggest that keeping an ischemic patient away from revascularization for quite a while can be somewhat tricky in the absence of a sham group as in ORBITA [4].
Special subgroups
Chronic kidney disease
Patients with advanced chronic kidney disease (CKD) have been systematically excluded or only marginally included in most trials on cardiovascular disease, thus preventing a confident estimation of treatment benefits [18]. Patients with advance CKD, defined as an eGFR < 30 mL/min/1.73 m2 of the body surface area or on dialysis, and moderate or severe myocardial ischemia were investigated in the ISCHEMIA-CKD (Management of Coronary Disease in Patients with Advanced Kidney Disease) trial [19]. In contrast with the main trial, the use of CCTA angiography was not recommended as a screening test because of the potential risk of acute kidney injury. Therefore, no core-laboratory was used for validation. After a median of 2.2-year follow-up, the primary endpoint — a composite of death and non-fatal MI — was similar in the two groups. The invasive arm experienced a higher incidence of non-procedural stroke and new dialysis initiation. Overall, ISCHEMIA findings have been confirmed even in the CKD population, pending a few considerations. Firstly, the exclusion of patients with reduced ejection fraction and/or refractory angina could have prevented the potential benefits of revascularization. Secondly, the selection of frail and comorbid patients with compromised status could be itself a proxy for futility, where mortality was high (27% at 3 years) in both subgroups, and coronary angiography not performed in 15% of the patients allocated in the invasive arm. Thirdly, selection criteria poorly identified ischemic patients amenable to revascularization, as in the invasive strategy group only 50% of patients underwent revascularization, and the absence of obstructive CAD was documented in one-quarter of the cohort. Beyond confirming the poor outcome of patients with CKD, ISCHEMIA-CKD results highlighted the modest positive predictive value of stress testing for the detection of obstructive epicardial CAD, while suggesting a high prevalence and prognostic significance of coronary microvascular disease in CKD patients with CCS [20].
Ischemia with no obstructive coronary artery disease
Almost 20% of screened patients were excluded from the ISCHEMIA trial for the absence of clear imaging evidence of obstructive coronary disease by CCTA. If they were complaining of ischemic symptoms, they merged into the aligned CIAO-ISCHEMIA (Changes in Ischemia and Angina over One year among ISCHEMIA) trial. Although not yet available in a full-length manuscript, these data were recently presented at the American College of Cardiology 2020 Scientific Session [21], enquire an interesting target population, with a high prevalence of women (66% in CIAO-ISCHEMIA compared with 26% in ISCHEMIA with obstructive CAD, p < 0.001). Despite controversial data, such patients now seem to portend a heterogeneous but in general benign prognosis, similar to that of the general population, with the presence of “some” coronary atherosclerosis being the main outcome determinant [22]. However, CIAO-ISCHEMIA was designed to test changes in ischemia and angina symptoms in the population of ischemia and no obstructive coronary artery disease (INOCA) and is not powered to test hard cardiovascular events. At 1 year, in patients in CIAO-ISCHEMIA, stress echocardiograms became normal in about half of patients, and in 45% of cases, they were the same as at baseline or worse. Angina symptoms improved in 42% and worsened in 14% of patients, and the number of medications to control angina on average remained the same. Interestingly, the change in stress test findings and the change in symptoms over 1 year were not related. These findings seem to reject the hypothesis that the extent of myocardial ischemia is responsible for angina in INOCA patients, suggesting that unmeasured determinants would contribute to patient symptoms, possibly including sensory, emotional, autonomic, motor, cognitive and other sex-related components. Again, the association among angina, coronary atherosclerosis and myocardial ischemia has confirmed to be exceedingly elusive.
The extent of revascularization in multivessel coronary artery disease
Since the benefit of revascularization is directly proportional to the extent of ischemia and of the atherothrombotic burden provoking it, then the management of patients with multivessel CAD should be prioritized. In this cohort, there is extensive evidence that complete revascularization confers a more considerable clinical benefit [23], mostly when performed with state-of-the-art techniques, namely second or third generation drug-eluting stent (DES) and extensive use of arterial conduits for CABG [24]. However, while complete revascularization has outlined a relevant advantage in the setting of an acute coronary syndrome, such benefit has not yet been proven in CCS. The ISCHEMIA trial would have been a milestone in this view. A comprehensive definition of the adequacy of myocardial revascularization should take into account the size of the vessel, the severity of the lesion, the ischemic burden caused by the lesion, and the viability of the depending myocardial territory [25]. The quality of the design in the ISCHEMIA trial is also evidenced by the availability of two separate flow-charts, to guide the invasive strategy in both imaging and non-imaging subgroups. The use of fractional flow reserve was strongly recommended [26, 27], and most of the revascularization procedures were performed according to best practice evidence, with > 90% last-generation DES for PCI and internal mammary arteries for CABG [1]. The benefit of the invasive approach, albeit still not significant, was proportional to the extent of CAD. Unfortunately, details regarding the adequacy and methods of revascularization are yet not available, and the scientific community yearningly awaits such data for further argumentations. As an example, it would be interesting to know the prevalence of bifurcations and chronic total occlusions on the total amount of PCI performed in both conservative and invasive strategy groups [28], as lesion complexity seems to affect periprocedural and long-term outcomes [29, 30], and to have a relevant implication on the subsequent dual antiplatelet therapy [31, 32] as well. Similarly, the complexity of the revascularization treatment might have affected the outcome [33].
In this view, the dataset deriving from the ISCHEMIA trial would be extremely valuable to explore the impact of lesion complexity and the optimal duration of antiplatelet therapy on the outcome.
Conclusions
The ISCHEMIA trial highlighted many challenges that cardiologists experience during their daily practice in the diagnosis and management of CCS, and it also delivered reassurance that for patients with at least moderate ischemia and acceptable symptoms who meet the trial criteria, invasive management may be reasonably deferred during optimal titration of OMT. While an extended follow-up of the ISCHEMIA trial would be highly informative and as new cardiac imaging techniques for fully-quantitative assessment of myocardial perfusion appear on the horizon, further prospective research should investigate whether longitudinal changes in adverse coronary plaque characteristics and whether ischemic burden associated with more intensive treatment would reduce the risk of ischemic events.
While guidelines continue to suggest that revascularization should be offered at an earlier stage to patients with the highest ischemia burden [34], the question of whether ischemia truly matters and how revascularization affects outcomes remains unsolved [35].
The view herein, holds that the legacy of the ISCHEMIA trial is two-fold: firstly, reiterating the crucial value of randomized controlled trials, which must be done before hypotheses become false certainties; secondly, recalling the “courage” to follow a pathway, once it has been designed, without hesitation, regardless the expected effects.
Footnotes
Conflict of interest: None declared
References
- 1.Maron D, Hochman J, Reynolds H, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382(15):1395–1407. doi: 10.1056/nejmoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spertus J, Jones P, Maron D, et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N Engl J Med. 2020;382(15):1408–1419. doi: 10.1056/nejmoa1916370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden WE, O’Rourke RA, Teo KK, et al. COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 4.Al-Lamee R, Thompson D, Dehbi HM, et al. ORBITA investigators. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391(10115):31–40. doi: 10.1016/S0140-6736(17)32714-9. [DOI] [PubMed] [Google Scholar]
- 5.Maron DJ, Hochman JS, O’Brien SM, et al. ISCHEMIA Trial Research Group. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J. 2018;201:124–135. doi: 10.1016/j.ahj.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw LJ, Berman DS, Maron DJ, et al. COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LJ, Berman DS, Picard MH, et al. Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging. 2014;7(6):593–604. doi: 10.1016/j.jcmg.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maron D, Harrington R, Hochman J. Planning and Conducting the ISCHEMIA Trial. Circulation. 2018;138(14):1384–1386. doi: 10.1161/circulationaha.118.036904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw L, Nagel E, Salerno M, et al. Cardiac Imaging in the Post ISCHEMIA Trial Era - A Multi Society Viewpoint. JACC: Cardiovasc Imaging. 2020 doi: 10.1016/j.jcmg.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Jang JJ, Bhapkar M, Coles A, et al. PROMISE Investigators. Predictive model for high-risk coronary artery disease. Circ Cardiovasc Imaging. 2019;12(2):e007940. doi: 10.1161/CIRCIMAGING.118.007940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge Y, Pandya A, Steel K, et al. Cost-Effectiveness analysis of stress cardiovascular magnetic resonance imaging for stable chest pain syndromes. JACC Cardiovasc Imaging. 2020;13(7):1505–1517. doi: 10.1016/j.jcmg.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Murthy VL, Eagle KA. ISCHEMIA: A Search for clarity and why we may not find it. Am Heart J. 2018;203:82–84. doi: 10.1016/j.ahj.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortés C, Johnson TW, Silber S, et al. ISCHEMIA trial: The long-awaited evidence to confirm our prejudices. Cardiol J. 2020;27(4):336–341. doi: 10.5603/CJ.2020.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antman EM, Braunwald E. Managing stable ischemic heart disease. N Engl J Med. 2020;382(15):1468–1470. doi: 10.1056/NEJMe2000239. [DOI] [PubMed] [Google Scholar]
- 15.Koskinas KC, Ndrepepa G, Räber L, et al. Prognostic impact of periprocedural myocardial infarction in patients undergoing elective percutaneous coronary interventions. Circ Cardiovasc Interv. 2018;11(12):e006752. doi: 10.1161/CIRCINTERVENTIONS.118.006752. [DOI] [PubMed] [Google Scholar]
- 16.Stone G, Kappetein A, Sabik J, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381(19):1820–1830. doi: 10.1056/nejmoa1909406. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Yehuda O, Chen S, Redfors B, et al. Impact of large periprocedural myocardial infarction on mortality after percutaneous coronary intervention and coronary artery bypass grafting for left main disease: an analysis from the EXCEL trial. Eur Heart J. 2019;40(24):1930–1941. doi: 10.1093/eurheartj/ehz113. [DOI] [PubMed] [Google Scholar]
- 18.Zannad F, Rossignol P. Cardiovascular outcome trials in patients with advanced kidney disease: time for action. Circulation. 2017;135(19):1769–1771. doi: 10.1161/CIRCULATIONAHA.117.027338. [DOI] [PubMed] [Google Scholar]
- 19.Bangalore S, Maron DJ, O’Brien SM, et al. ISCHEMIA-CKD Research Group. Management of coronary disease in patients with advanced kidney disease. N Engl J Med. 2020;382(17):1608–1618. doi: 10.1056/NEJMoa1915925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajaj NS, Singh A, Zhou W, et al. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in patients with chronic kidney impairment. Circulation. 2020;141(1):21–33. doi: 10.1161/CIRCULATIONAHA.119.043916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds HR. Natural history of symptoms and stress echo findings in patients with moderate or severe ischemia and no obstructive CAD (INOCA): the NHLBI-funded CIAO ancillary study to the ISCHEMIA trial. ACC. 2020 March 30;2020 [Google Scholar]
- 22.Radico F, Zimarino M, Fulgenzi F, et al. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J. 2018;39(23):2135–2146. doi: 10.1093/eurheartj/ehy185. [DOI] [PubMed] [Google Scholar]
- 23.Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62(16):1421–1431. doi: 10.1016/j.jacc.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 24.Zimarino M, Ricci F, Romanello M, et al. Complete myocardial revascularization confers a larger clinical benefit when performed with state-of-the-art techniques in high-risk patients with multivessel coronary artery disease: A meta-analysis of randomized and observational studies. Catheter Cardiovasc Interv. 2016;87(1):3–12. doi: 10.1002/ccd.25923. [DOI] [PubMed] [Google Scholar]
- 25.Zimarino M, Curzen N, Cicchitti V, et al. The adequacy of myocardial revascularization in patients with multivessel coronary artery disease. Int J Cardiol. 2013;168(3):1748–1757. doi: 10.1016/j.ijcard.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Lee JM, Nam CW, et al. Consensus document for invasive coronary physiologic assessment in Asia-Pacific countries. Cardiol J. 2019;26(3):215–225. doi: 10.5603/CJ.a2019.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez-Chico JL, Chen Y, Yu W, et al. Diagnostic accuracy and reproducibility of optical flow ratio for functional evaluation of coronary stenosis in a prospective series. Cardiol J. 2020;27(4):350–361. doi: 10.5603/CJ.a2020.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutiérrez-Chico JL, Louvard Y. DECISION-CTO: A „negative” clinical trial? Really? Cardiol J. 2017;24(3):231–233. doi: 10.5603/CJ.a2017.0049. [DOI] [PubMed] [Google Scholar]
- 29.Zimarino M, Briguori C, Amat-Santos IJ, et al. Mid-term outcomes after percutaneous interventions in coronary bifurcations. Int J Cardiol. 2019;283:78–83. doi: 10.1016/j.ijcard.2018.11.139. [DOI] [PubMed] [Google Scholar]
- 30.Werner GS, Martin-Yuste V, Hildick-Smith D, et al. EUROCTO trial investigators. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39(26):2484–2493. doi: 10.1093/eurheartj/ehy220. [DOI] [PubMed] [Google Scholar]
- 31.Giustino G, Chieffo A, Palmerini T, et al. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J Am Coll Cardiol. 2016;68(17):1851–1864. doi: 10.1016/j.jacc.2016.07.760. [DOI] [PubMed] [Google Scholar]
- 32.Zimarino M, Renda G, De Caterina R. Optimal duration of antiplatelet therapy in recipients of coronary drug-eluting stents. Drugs. 2005;65(6):725–732. doi: 10.2165/00003495-200565060-00001. [DOI] [PubMed] [Google Scholar]
- 33.Zimarino M, Corcos T, Favereau X, et al. Rotational coronary atherectomy with adjunctive balloon angioplasty for the treatment of ostial lesions. Cathet Cardiovasc Diagn. 1994;33(1):22–27. doi: 10.1002/ccd.1810330106. [DOI] [PubMed] [Google Scholar]
- 34.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 35.Al-Lamee R, Jacobs AK. The ISCHEMIA Trial: Was it Worth the Wait? Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.045007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]