Abstract
Background
Abdominal aortic aneurysm (AAA) and coronary atherosclerosis share common risk factors. In this study, a single-center management experience of patients with a coexistence of AAA and coronary artery disease (CAD) is presented.
Methods
271 consecutive patients who underwent elective AAA repair were reviewed. Coronary imaging in 118 patients was considered suitable for exploration of AAA coexistence with CAD.
Results
Significant coronary stenosis (> 70%) were found in 65.3% of patients. History of cardiac revascularization was present in 26.3% of patients, myocardial infarction (MI) in 31.4%, and 39.8% had both. In a subgroup analysis, prior history of percutaneous coronary intervention (PCI) (OR = 6.9, 95% CI 2.6–18.2, p < 0.001) and patients’ age (OR = 1.1, 95% CI 1.0–1.2, p = 0.007) were independent predictors of significant coronary stenosis. Only 52.0% (40/77) of patients with significant coronary stenosis underwent immediate coronary revascularization prior to aneurysm repair: PCI in 32 cases (4 drug-eluting stents and 27 bare metal stents), coronary artery bypass graft in 8 cases. Patients undergoing revascularization prior to surgery had longer mean time from coronary imaging to AAA repair (123.6 vs. 58.1 days, p < 0.001). Patients undergoing coronary artery evaluation prior to AAA repair had shorter median hospitalization (7 [2–70] vs. 7 [3–181] days, p = 0.007) and intensive care unit stay (1 [0–9] vs. 1 [0–70] days, p = 0.014) and also had a lower rate of major adverse cardiovascular events or multiple organ failure (0% vs. 3.9%, p = 0.035). A total of 11.0% of patients had coronary artery aneurysms.
Conclusions
Patients with AAA might benefit from an early coronary artery evaluation strategy.
Keywords: abdominal aortic aneurysm, coronary artery aneurysm, coronary arteriography, coronary artery disease
Introduction
Abdominal aortic aneurysm (AAA) is the local pathologic dilation of the abdominal aorta and is defined as an aorta size greater than 30 mm or a local dilation of abdominal aorta of more than 50%, as compared to aortic diameter measured distally to dilatation [1]. The prevalence of AAA in the general population ranges from 1.0% to 1.3% among females and 3.9–7.2% in males, with an upward trend observed in older populations [2, 3]. Age, male gender, personal history of coronary artery disease (CAD), smoking, and hypertension are associated with the presence of AAA [4, 5]. The open surgical or endovascular aneurysm repair (EVAR) methods are the treatments of choice, and no pharmacological management is available to effectively limit the disease progression [1, 2, 6, 7]. AAA and coronary atherosclerosis share common risk factors [8]. Both the prevalence of CAD in patients with AAA and the AAA prevalence in CAD patients are significantly higher relative to the general population [9]. The frequency of CAD in patients with AAA was estimated to be as high as 65% [10]. Comorbid CAD increases the perioperative risk of death and myocardial ischemia during AAA repair, as well as long-term clinical outcomes [11, 12]. Intuitively, the preoperative evaluation of coronary atherosclerosis, subsequent percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) and myocardial revascularization should enhance postoperative prognosis [13, 14]. The preoperative evaluation of the existence of concomitant coronary stenosis appears to be reasonable [15, 16]. However, the optimal treatment of patients with both AAA and CAD remains controversial. Some studies show no benefits of prophylactic coronary interventions before major vascular surgery [15, 17], while others recommended this approach for the prevention of perioperative complications [18–20]. Current European Society of Cardiology and European Society of Anesthesiology Joint Task Force Guidelines advocate similar indications for coronary artery angiography (CAG) as in non-surgical settings and recommend CAG prior to non-urgent noncardiac surgery in patients with proven myocardial ischemia or unstable chest pain (Canadian Cardiovascular Society class III or IV) despite pharmacological treatment (recommendation class I C). These recommendations also suggest that preoperative CAG might be considered in stable patients scheduled for carotid endarterectomy (class IIb B), which is an intermediate-risk intervention, while disregarding the high-risk AAA patients [21].
In this study, experience in the management of patients with concomitant AAA and CAD is presented. The specific aim of current study was to re-analyze the coexistence of AAA and CAD in a contemporary sample, and finally to evaluate the complication rates associated with different approaches. One of the leading hypotheses is that patients with AAA might benefit from an early coronary artery evaluation strategy and receiving optimal CAD management/therapy prior to AAA repair.
Methods
Two hundred and seventy-one consecutive patients were retrospectively reviewed (247 males, 24 females) who underwent an elective AAA open surgery or EVAR from January 2008 through December 2015 at John Paul II Hospital in Krakow, Poland. CAG or multiple-slice coronary computed tomography (CT) angiography was available in 144 patients, out of whom 115 patients had undergone coronary imaging within 1 year prior to AAA repair, 1 patient underwent CAG simultaneously with EVAR procedure, and 2 patients were subjected to CAG within 2 months postoperatively. Coronary imaging in these 118 patients (106 males, 12 females) were deemed suitable for exploration of AAA coexistence with coronary pathology. Among those patients, classical CAG was performed in 89.8% (106/118), in the remaining 10.2% of patients (12/118), Electrocardiogram (ECG)-gated multi-row contrast-enhanced CT was performed using 64- to 256-row scanners.
To analyze factors associated with the presence of significant coronary stenosis, patients with prior CABG were additionally excluded, who inherently all had significant stenosis (n = 17). Clinical data and postoperative complications were recorded for all 271 patients.
To sum up, three subsets of analyses were performed: logistic regression of predictors of coronary stenosis (n = 101), coexistence and management of significant stenosis (n = 118), and finally an analysis of complication rates in all patients (n = 271) dichotomized by the facts of then, current preoperative coronary evaluation.
The AAA repair was performed either using open surgery or EVAR technique. Each patient was qualified for EVAR or classic repair by a vascular team comprising vascular surgeons, angiologists, interventional cardiologists, and anesthesiologists. The treatment decisions were based on the procedural guidelines, depending on the anatomy of the lesion, required scope of the procedure, perioperative risk, and comorbid conditions, including contraindications to general anesthesia, as well as the overall state of health and prognosis. The conducting physician and vascular team also decided whether preoperative coronary artery evaluation was necessary. Demographics, past medical history, information on treatments, and results of laboratory and imaging tests of all patients from the study group were collected from the patient medical records. The study complied with local bioethics committee regulations according to the Declaration of Helsinki.
Statistical analysis
Data are presented as percentages, mean values with corresponding standard deviations or medians and ranges as appropriate. The Shapiro-Wilk was used test to determine if quantitative data were normally distributed. The Levene test was relied upon to verify variance homogeneity. The Student t-test and Mann-Whitney U test were used to statistically compare the two groups, as appropriate. Analysis of variance (ANOVA) was performed if a discriminating factor categorized quantitative variables into more than two groups and variance homogeneity assumption was fulfilled. In post-hoc analyses, we relied on the Tukey test of multiple comparisons. A multiple logistic regression model was built by the inclusion of all univariately significant predictors along all available established risk factors and subsequent backward elimination (α for inclusion and elimination was set at 0.05). Additionally, graphs were generated to illustrate these analyses. Statistical analyses were conducted using STATISTICA v12 (StatSoft Inc., 2014). A p-value of less than 0.05 was considered significant.
Results
Patients characteristics
Clinical characteristics of all enrolled patients are summarized in Table 1. No death during the AAA repair procedure was observed, and only one in-hospital death was observed due to massive bleeding and cardiac arrest in an urgent patient without coronary artery evaluation prior to AAA open repair. History of cardiac revascularization (PCI or CABG) was present in 26.3% of cases and history of MI in 31.4%, while 39.8% of patients had both.
Table 1.
Clinical characteristics of enrolled patients.
| Patients who underwent AAA repair (n = 271) | Patients who underwent AAA repair and coronary artery imaging (n = 118) | Patients who underwent AAA repair and coronary artery imaging without prior CABG (n = 101) | |
|---|---|---|---|
| Clinical features | |||
| Age [years] | 68.9 ± 7.7 | 68.9 ± 6.6 | 69.3 ± 6.5 |
| Male sex | 91.1% | 89.8% | 89.1% |
| Hypertension | 87.8% | 92.4% | 92.1% |
| Dyslipidemia | 74.3% | 79.7% | 81.2% |
| Diabetes or prediabetes* | 24.0% | 31.4% | 29.7% |
| Smoking | 33.2% | 33.9% | 34.7% |
| COPD | 10.0% | 8.5% | 7.9% |
| Atrial fibrillation | 11.4% | 11.9% | 10.9% |
| Peripheral atherosclerosis** | 48.0% | 55.1% | 57.4% |
| History of MI | 31.7% | 32.2% | 28.7% |
| History of PCI | 27.7% | 41.5% | 46.5% |
| History of CABG | 18.5% | 14.4% | 0% |
| Heart failure | 21.0% | 23.7% | 17.8% |
| EVAR | 42.8% | 32.2% | 31.7% |
| Symptomatic AAA | 26.2% | 23.7% | 23.8% |
| AAA diameter, median (range) [mm] | 59.0 (32–91) | 58.0 (38–90) | 58.0 (38–90) |
| Complications | |||
| Perioperative MI | 0.7% | 0.8% | 1.0% |
| Cardiac arrest | 0.4% | 0% | 0% |
| Perioperative stroke | 0.4% | 0% | 0% |
| Perioperative TIA | 0.7% | 0% | 0% |
| MACE | 1.8% | 0.8% | 1.0% |
| MOF | 1.5% | 0% | 0% |
| MACE or MOF | 2.2% | 0.8% | 1.0% |
| Reoperation need | 3.7% | 2.5% | 3.0% |
| Red blood cell transfusion | 17.0% | 19.5% | 18.8% |
| Plasma transfusion | 8.9% | 11.0% | 10.9% |
| ICU stay median (range) [days] | 1 (0–70) | 1 (0–9) | 1 (0–9) |
| In-hospital stay, median (range) [days] | 7 (2–181) | 7 (2–70) | 7 (2–22) |
Prediabetes includes impaired fasting glucose and impaired glucose tolerance;
Peripheral atherosclerosis encompasses significant lesions in carotid and lower extremity arteries; AAA — abdominal aortic aneurysm; CABG — coronary artery bypass grafting; COPD — chronic obstructive pulmonary disease; EVAR — endovascular aneurysm repair; ICU — intensive care unit; MACE — major adverse cardiovascular event (MI or cardiac arrest or stroke or TIA); MI — myocardial infarction; MOF — multiple organ failure; PCI — percutaneous coronary intervention; TIA — transient ischemic attack
Coronary artery stenosis in AAA patients
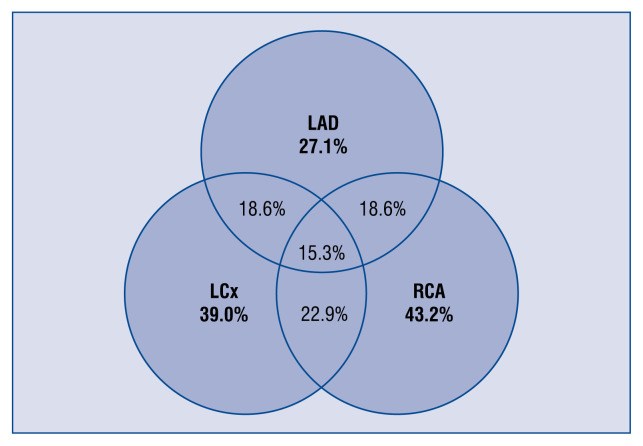
In patients who underwent coronary artery imaging, significant coronary stenosis (> 70%) were found in 65.3% (77/118). The distribution of significant stenosis between major coronary branches is shown in Figure 1. Overall, the single-vessel disease was found in 34.7% of patients, two-vessel in 11.9%, and three-vessel in 11.0%. In 7.6% of all cases, there was significant stenosis of the left main coronary artery. Almost all patients had visible atherosclerotic coronary lesions and in only 2.5% (3) of patients had no atherosclerotic evidence found in standard CAG.
Figure 1.
The distribution of significant stenosis (> 70%) between major coronary branches (n = 118). LCx — left circumflex artery; LAD — left anterior descending artery; RCA — right coronary artery.
Predictors of coronary stenoses in AAA patients
The presence of significant coronary lesions in multiple logistic regression analysis was assessed in a subgroup of patients with coronary artery imaging but without a prior history of CABG (n = 101), who inherently all presented with significant lesions (n = 17). Factors univariately associated with the presence of significant atherosclerosis included prior history of PCI (odds ratio [OR] = 6.1, 95% confidence interval [CI] 2.5–15.2, p < 0.001), MI (OR = 2.8, 95% CI 1.1–7.4, p = 0.036), and age (OR = 1.1, 95% CI 1.0–1.2, p = 0.017). The model (area under the curve [AUC] = 0.78, 95% CI 0.7–0.8; R2 Nagelkerka = 0.30) was built by additionally adjusting for sex and sum of modifiable cardiovascular risk factors (which were univariately insignificant). Independent predictors of significant stenosis comprised the only history of prior PCI (OR = 6.9, 95% CI 2.6–18.2, p < 0.001) and age (OR = 1.1, 95% CI 1.0–1.2, p = 0.007). The proportion of patients with significant coronary artery stenosis reached 80.9% (38/47), if there was a PCI in anamnesis, however, as much as 40.7% (22/54) of patients with AAA and no history of PCI or the CABG had significant coronary lesions.
Management of significant stenosis in AAA patients
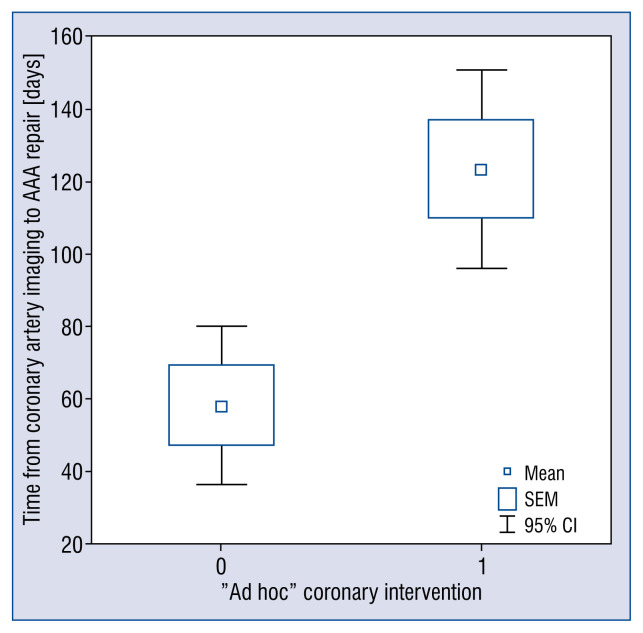
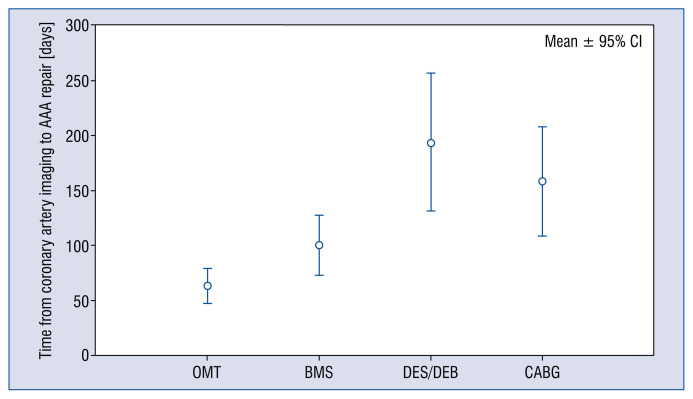
Only 52.0% (40/77) of patients with significant coronary stenosis underwent immediate coronary revascularization prior to aneurysm repair: ad hoc PCI in 32 cases and urgent CABG in 8 cases. Patients undergoing revascularization prior to surgery had longer mean time from coronary artery imaging to AAA repair, compared with the remaining patients with significant coronary stenosis (123.6 vs. 58.1 days, p < 0.001; Fig. 2), although there was no difference in mean AAA diameter (60.3 vs. 60.3 mm, p = 0.996). ANOVA and the Tukey post hoc analyses revealed that PCI with drug-eluting stent or drug-eluting balloon (DES or DEB), as well as CABG, were interventions that significantly postponed AAA repair, as shown in Figure 3 [194.0 days; p = 0.02, and 158.3 days, p = 0.04, respectively vs. 63.0 days for conservative therapy; p < 0.001 for the model). Interestingly, out of 32 patients qualified to ad hoc PCI revascularization, only four were implanted with DES, and one restenosis was treated with DEB, while 27 patients (84.4% of PCI patients) were implanted with bare metal stents (BMS) due to imminent surgery. Nevertheless, complete anatomical revascularization (defined as the absence of significant lesions or chronic occlusion that are not secured by a bypass) prior to AAA repair was achieved in only 52.5% of patients (62/118).
Figure 2.
Mean time from coronary artery imaging to abdominal aortic aneurysm (AAA) repair for patients with significant coronary stenosis who did not have coronary intervention (Group 0) and who underwent ad hoc revascularization prior to surgery (Group 1) (p < 0.001); CI — onfidence interval; SEM — standard error of the mean.
Figure 3.
Comparison of mean time from coronary artery imaging to abdominal aortic aneurysm (AAA) repair for patients with optimal medical therapy (OMT) of coronary artery disease and patients with different types of interventions; BMS — bare metal stent; CABG — coronary artery bypass graft; DEB — drug-eluting balloons; DES — drug-eluting stents; CI — confidence interval.
Coronary artery evaluation prior to AAA and postoperative outcomes
Patients who had undergone coronary artery evaluation (and treatment if indicated) prior to aneurysm repair had shorter median stay in the intensive care unit and hospitalization time (Table 2). They also tended to have lower major adverse cardiovascular event (MACE) rate, which reached statistical significance after grouping together with multiple organ failure (MOF) (Table 2). These better outcomes were observed despite the higher incidence of cardiovascular risk factors in this group, such as impaired lipid or glucose metabolism, history of prior PCI or presence of peripheral atherosclerosis (Table 2). Also, these patients were less frequently undergoing EVAR, and yet the need for transfusion of red blood cell concentrates and plasma were similar (Table 2). Based on the present retrospective data, the approximate number needed to treat in avoiding one major complication through preoperative coronary evaluation is 26.
Table 2.
Clinical features and comparisons of patients who had undergone coronary artery imaging within 12 months prior to abdominal aortic aneurysm (AAA) repair and remaining subjects undergoing elective AAA repair.
| Patients who had undergone coronary artery imaging within 12 months prior to AAA repair (n = 115) | Remaining subjects undergoing elective AAA repair (n = 156) | P | |
|---|---|---|---|
| Clinical features | |||
| Age [years] | 69.0 ± 6.7 | 68.9 ± 8.4 | 0.907 |
| Male sex | 90.4% | 91.7% | 0.724 |
| Hypertension | 92.2% | 84.6% | 0.060 |
| Dyslipidemia | 80.7% | 69.5% | 0.038 |
| Diabetes or prediabetes* | 31.3% | 18.6% | 0.015 |
| Smoking | 34.6% | 36.9% | 0.702 |
| COPD | 8.7% | 11.0% | 0.538 |
| Atrial fibrillation | 12.3% | 11.0% | 0.739 |
| Peripheral atherosclerosis** | 56.1% | 42.9% | 0.031 |
| History of MI | 31.3% | 32.1% | 0.896 |
| History of PCI | 41.7% | 17.4% | 0.000 |
| History of CABG | 14.8% | 21.3% | 0.173 |
| Heart failure | 24.8% | 18.8% | 0.241 |
| EVAR | 32.2% | 51.3% | 0.002 |
| Symptomatic AAA | 23.6% | 33.1% | 0.104 |
| AAA diameter, median (range) [mm] | 58.5 (38–90) | 59.0 (32–91) | 0.562 |
| Complications | |||
| Perioperative MI | 0% | 1.3% | 0.330 |
| Cardiac arrest | 0% | 0.6% | 0.576 |
| Perioperative stroke | 0% | 0.6% | 0.576 |
| Perioperative TIA | 0% | 1.3% | 0.330 |
| MACE | 0% | 3.2% | 0.061 |
| MOF | 0% | 2.6% | 0.108 |
| MACE or MOF | 0% | 3.9% | 0.035 |
| Reoperation need | 2.6% | 4.5% | 0.628 |
| Red blood cell transfusion | 20.0% | 14.7% | 0.254 |
| Plasma transfusion | 11.3% | 7.1% | 0.223 |
| In-hospital death | 0% | 0.6% | 0.576 |
| ICU stay, median (range) [days] | 1 (0–9) | 1 (0–70) | 0.014 |
| In-hospital stay, median (range) [days] | 7 (2–70) | 7 (3–181) | 0.007 |
Prediabetes includes impaired fasting glucose and impaired glucose tolerance;
Peripheral atherosclerosis encompasses significant lesions in carotid and lower extremity arteries; AAA — abdominal aortic aneurysm; CABG — coronary artery bypass grafting; COPD — chronic obstructive pulmonary disease; EVAR — endovascular aneurysm repair; ICU — intensive care unit; MACE — major adverse cardiovascular event (MI or cardiac arrest or stroke or TIA); MI — myocardial infarction; MOF — multiple organ failure; PCI — percutaneous coronary intervention; TIA — transient ischemic attack
Coexistence of AAA and other vascular pathologies
Abdominal aortic aneurysm were accompanied by common iliac artery aneurysms in 25.4% (30/118), and in another 27.1% of patients (32/118), non-aneurysmatic iliac ectasia was present. A significant proportion of patients (11.0%, 13/118) also presented with aneurysmatic lesions in coronary arteries, which were equally distributed between right and left coronary artery trees. According to the classification of Markis et al. [22], 1 patient was identified with type I aneurysm (0.9%) and 2 patients with type II aneurysms (1.7%), of whom 1 had already undergone surgical removal of the proximal right coronary artery aneurysm followed by its chronic occlusion. Additionally, 5 patients with type III and 5 with type IV aneurysms were detected (4.2% each).
Discussion
Among patients undergoing noncardiac surgery, major vascular surgery is associated with a high risk of perioperative MI. The highest incidence of periprocedural MI is observed in patients undergoing open AAA repair (3.7%). Moreover, patients with MI had higher overall complication rates and mortality, emphasizing the necessity of preventing this morbid complication [23]. The current study confirms that the prevalence of CAD in a contemporary sample of patients with AAA is considerably high — two-thirds of patients undergoing AAA repair had significant coronary stenosis. This is similar to previous reports reaching 65%, despite better risk factor management and modern pharmacotherapy available [10, 15, 24, 25]. Even patients without any prior history of cardiac revascularization had significant lesions (found in 2 of every 5 subjects), and those already after PCI had significant lesions in as much as 4 in every 5 patients. It is still unclear whether this high association between the presence of AAA and atherosclerosis is causal or simply due to shared risk factors, as well as which risk factors contribute most to this phenomenon [8, 26, 27].
Coronary revascularization is an established method of reducing cardiovascular events, but interestingly, a randomized trial carried out in patients undergoing major vascular surgery failed to demonstrate the benefits of prophylactic CAD treatment for the clinical outcomes in patients with angiographically determined coronary artery stenosis [17, 28]. Current guidelines indicate that there is no known benefit for elective coronary revascularization of asymptomatic lesions prior to AAA repair [17]. Also, Hosokawa et al. [15] show that in patients undergoing AAA open repair and coronary artery intervention, the cardiac event-free rate was comparable with that of other groups, although mortality was higher. On the other hand, Kordowicz et al. [29] conclude that simultaneous open repair of AAA and cardiac surgery is a feasible option for patients with CAD. Similar conclusions were reached by Sumin et al. [20], where CAG and preventive revascularization before AAA surgery were associated with less perioperative complications, MIs, and lower mortality. Moreover, Sun et al. [18], after retrospective analysis of 368 Chinese patients with AAA, concluded that myocardial evaluation and subsequent revascularization before AAA surgery could improve the clinical outcome in patients with severe CAD.
Because elective open surgical aneurysm repair is considered high-risk surgery, when AAA and symptomatic CAD are detected, coronary artery revascularization (PCI or CABG) should be performed before AAA open repair [30]. However, the prevalence of asymptomatic, significant CAD in patients with AAA was found to be as high as 61% and, moreover, 31% of these patients fulfilled indications for coronary revascularization [31]. There is, therefore, a significant group of patients with asymptomatic but severe CAD that do not undergo coronary artery evaluation and necessary revascularization before high-risk AAA surgery. In the current study, 65.3% of all patients with AAA who underwent coronary evaluation have significant coronary stenosis (≥ 70% diameter), with 40.7% of those were without prior history of PCI or CABG. Only 52% of those patients underwent immediate coronary revascularization prior to AAA repair, and the intervention was associated with postponement of surgery or EVAR. Furthermore, revascularization was performed using optimal techniques (CABG or PCI with DES/DEB) only in 32.5% of cases, and BMS was implanted in the remaining 67.5% of patients (because of a need for an immediate AAA repair). We also found that patients who underwent coronary artery evaluation (and treatment if indicated) prior to AAA repair had shorter median intensive care unit stay and whole hospitalization time, as well as lower MACE or MOF rate (Table 2). Interestingly, better outcomes in patients with coronary imaging were observed despite a higher prevalence of cardiovascular risk factors in this group, which could be explained by the fact that they were probably more carefully diagnosed, and pharmacotherapy was better optimized, subject to the individual approach of the conducting physicians. Furthermore, patients with prior coronary imaging were less frequently undergoing EVAR, and there was no statistically significant difference in the need for red blood cell concentrates and plasma transfusion, despite probably more frequent current or recent dual antiplatelet therapy (exact pharmacotherapy was not recorded in the current study). These observations lead us to conclude that coronary artery evaluation should be performed in all patients with diagnosed AAA at the earliest possible stage.
The overall incidence of coronary artery aneurysm is estimated to be from 0.3% to 5.3% [32]. The present study brought significantly increased prevalence of the coronary artery aneurysms, that was found in 11.0% of all patients. Other authors also noted that there is a high incidence (of up to 17%) of coronary aneurysms in patients with aortic aneurysms in both thoracic and abdominal segments [33]. However, pathophysiological mechanisms behind coronary artery aneurysms remain debatable; the phenomenon of their co-occurrence with the AAAs could have several reasonable explanations. First, the most common cause of coronary artery aneurysms is CAD, which is also the main risk factor for AAA development [34]. Second, hereditary connective tissue disorders and mutations in matrix metalloproteinases genes can result in both aortic and coronary aneurysms [32, 35]. Moreover, the coronary artery aneurysms might have an iatrogenic origin and occur as a complication after balloon angioplasty or stent implantation, interventions that were frequent in the present study population [32]. Finally, chronic inflammation might be associated with the coexistence of the AAAs, CAD, and coronary artery aneurysms [36]. Patients with aneurysmal coronary disease are usually asymptomatic and, even in the absence of obstructive CAD, have an increased risk of MI and mortality rate similar to patients suffering from three-vessel obstructive CAD [37, 38]. In this light, patients with AAA might additionally benefit from early coronary artery evaluation [39].
Limitations of the study
The current study is not without limitations. This study was a retrospective, cross-sectional, single-center study, which might contribute to selection bias. However, results were comparable to other reports. A relatively large sample size was presented for the field, but were too small to reliably assess any rare observations [15, 24, 25]. The qualification criteria for coronary artery evaluation were subject to the individual practice of the conducting physician and not a randomization process. Hence, it was only possible to retrospectively approximate, rather than directly compare, the differences in outcomes between patients undergoing preoperative coronary evaluation and those entering the procedure without such preprocedural assessment. The identified factors influencing the decision to evaluate coronary arteries before surgery included: history of hypercholesterolemia, diabetes, prior PCI, and qualification for classical AAA open repair, as indicated in Table 2. Moreover, due to the retrospective nature of the study, complications were derived from medical records and not proactively assessed throughout hospitalization, for instance, by routine monitoring of cardiac biomarkers [40]. Due to the structure of the database, it was not possible to identify patients that were scheduled for aneurysm repair and died before hospital admission. This is a major limitation. However, the probability of such a scenario is low, because all patients were scheduled and managed based on an individualized risk-benefit approach, and patients with a high risk of rupture were treated urgently. Moreover, coronary imaging performed outside of the center might have been missed and was not indicated in the medical history. However, this seems unlikely. Patients that had undergone coronary imaging more than 1 year prior to AAA repair (n = 26) from the analysis of coexistence were also arbitrary excluded. On the other hand, only relevant, current CAG were analyzed, which is a major advantage of this study, and allowed a demonstration of the importance of preoperative evaluation of CAD.
Conclusions
In conclusion, major findings from the current study are: (1) despite advancements in risk factor management, still 2 of every 3 patients undergoing coronary artery evaluation prior to AAA repair have significant coronary lesions; (2) prior history of PCI and patient age are independent predictors of significant coronary stenoses; (3) patients undergoing “last-minute” coronary imaging receive suboptimal, conservative therapy or PCI with BMS; (4) nevertheless, patients subjected to preoperative coronary evaluation and treatment had a lower incidence of composite end-point comprising MACE and MOF; and (5) patients with AAA have a higher probability of the presence of coronary artery aneurysms. It can be concluded that patients with AAA might benefit from an early coronary artery evaluation strategy.
Footnotes
Conflict of interest: None declared
References
- 1.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 2.Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Dereziński T, Fórmankiewicz B, Migdalski A, et al. Występowanie tętniaków aorty brzusznej w populacji miejsko-wiejskiej w centralnej Polsce — Gniewkowo Aortic Study. Kardiol Pol. 2017;75(7):705–710. doi: 10.5603/kp.a2017.0071. [DOI] [PubMed] [Google Scholar]
- 4.Golledge J, Muller J, Daugherty A, et al. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26(12):2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 5.Ye Zi, Bailey KR, Austin E, et al. Family history of atherosclerotic vascular disease is associated with the presence of abdominal aortic aneurysm. Vasc Med. 2016;21(1):41–46. doi: 10.1177/1358863X15611758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antiochos P, Monney P, Fournier S, et al. Endovascular management of heavily calcified abdominal aorta dissection during transcatheter aortic valve implantation. Cardiol J. 2016;23(6):655–656. doi: 10.5603/CJ.2016.0107. [DOI] [PubMed] [Google Scholar]
- 7.Treptau J, Ebnet J, Akin M, et al. Angiographic detection of fatal acute aortic dissection Stanford type A under resuscitation. Cardiol J. 2016;23(6):620–622. doi: 10.5603/CJ.2016.0103. [DOI] [PubMed] [Google Scholar]
- 8.Van Kuijk JP, Flu WJ, Dunckelgrun M, et al. Coronary artery disease in patients with abdominal aortic aneurysm: a review article. J Cardiovasc Surg (Torino) 2009;50(1):93–107. [PubMed] [Google Scholar]
- 9.Elkalioubie A, Haulon S, Duhamel A, et al. Meta-Analysis of abdominal aortic aneurysm in patients with coronary artery disease. Am J Cardiol. 2015;116(9):1451–1456. doi: 10.1016/j.amjcard.2015.07.074. [DOI] [PubMed] [Google Scholar]
- 10.Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199(2):223–233. doi: 10.1097/00000658-198402000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukai S, Yao H, Miyamoto T, et al. The long-term follow-up results of elective surgical treatment for abdominal aortic aneurysms. Ann Thorac Cardiovasc Surg. 2002;8(1):38–41. [PubMed] [Google Scholar]
- 12.Roger VL, Ballard DJ, Hallett JW, et al. Influence of coronary artery disease on morbidity and mortality after abdominal aortic aneurysmectomy: a population-based study, 1971–1987. J Am Coll Cardiol. 1989;14(5):1245–1252. doi: 10.1016/0735-1097(89)90423-3. [DOI] [PubMed] [Google Scholar]
- 13.Rinckenbach S, Hassani O, Thaveau F, et al. Current outcome of elective open repair for infrarenal abdominal aortic aneurysm. Ann Vasc Surg. 2004;18(6):704–709. doi: 10.1007/s10016-004-0114-6. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki Y, Isobe F, Kinugasa S, et al. Influence of coronary artery disease on operative mortality and long-term survival after abdominal aortic aneurysm repair. Surg Today. 2004;34(4):313–317. doi: 10.1007/s00595-003-2708-y. [DOI] [PubMed] [Google Scholar]
- 15.Hosokawa Y, Takano H, Aoki A, et al. Management of coronary artery disease in patients undergoing elective abdominal aortic aneurysm open repair. Clin Cardiol. 2008;31(12):580–585. doi: 10.1002/clc.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borioni R, Tomai F, Pederzoli A, et al. Coronary risk in candidates for abdominal aortic aneurysm repair: a word of caution. J Cardiovasc Med (Hagerstown) 2014;15(11):817–821. doi: 10.2459/JCM.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 17.McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351(27):2795–2804. doi: 10.1056/NEJMoa041905. [DOI] [PubMed] [Google Scholar]
- 18.Sun T, Cheng Yt, Zhang Hj, et al. Severe coronary artery disease in Chinese patients with abdominal aortic aneurysm: prevalence and impact on operative mortality. Chin Med J (Engl) 2012;125(6):1030–1034. [PubMed] [Google Scholar]
- 19.Wolff T, Baykut D, Zerkowski HR, et al. Combined abdominal aortic aneurysm repair and coronary artery bypass: presentation of 13 cases and review of the literature. Ann Vasc Surg. 2006;20(1):23–29. doi: 10.1007/s10016-005-9324-9. [DOI] [PubMed] [Google Scholar]
- 20.Sumin AN, Korok EV, Panfilov SD, et al. [Myocardial revascularization before abdominal aortic surgery in patients with ischemic heart disease]. Kardiologiia. 2013;53(4):62–68. [PubMed] [Google Scholar]
- 21.Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA) Eur Heart J. 2014;35(35):2383–2431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 22.Markis JE, Joffe CD, Cohn PF, et al. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976;37(2):217–222. doi: 10.1016/0002-9149(76)90315-5. [DOI] [PubMed] [Google Scholar]
- 23.Sutzko DC, Andraska EA, Obi AT, et al. Risk factors associated with perioperative myocardial infarction in major open vascular surgery. Ann Vasc Surg. 2018;47:24–30. doi: 10.1016/j.avsg.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kioka Y, Tanabe A, Kotani Y, et al. Review of coronary artery disease in patients with infrarenal abdominal aortic aneurysm. Circ J. 2002;66(12):1110–1112. doi: 10.1253/circj.66.1110. [DOI] [PubMed] [Google Scholar]
- 25.Kishi K, Ito S, Hiasa Y. Risk factors and incidence of coronary artery lesions in patients with abdominal aortic aneurysms. Intern Med. 1997;36(6):384–388. doi: 10.2169/internalmedicine.36.384. [DOI] [PubMed] [Google Scholar]
- 26.Hernesniemi JA, Vänni V, Hakala T. The prevalence of abdominal aortic aneurysm is consistently high among patients with coronary artery disease. J Vasc Surg. 2015;62(1):232–240.e3. doi: 10.1016/j.jvs.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Takagi H, Umemoto T ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Coronary artery disease and abdominal aortic aneurysm growth. Vasc Med. 2016;21(3):199–208. doi: 10.1177/1358863X15624026. [DOI] [PubMed] [Google Scholar]
- 28.Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49(17):1763–1769. doi: 10.1016/j.jacc.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 29.Kordowicz A, Ghosh J, Baguneid M. A single centre experience of simultaneous open abdominal aortic aneurysm and cardiac surgery. Interact Cardiovasc Thorac Surg. 2010;10(1):63–66. doi: 10.1510/icvts.2009.219105. [DOI] [PubMed] [Google Scholar]
- 30.Jaffery Z, Grant A. Multisystem revascularization. Ochsner J. 2009;9(4):211–219. [PMC free article] [PubMed] [Google Scholar]
- 31.Marsico F, Giugliano G, Ruggiero D, et al. Prevalence and severity of asymptomatic coronary and carotid artery disease in patients with abdominal aortic aneurysm. Angiology. 2015;66(4):360–364. doi: 10.1177/0003319714540319. [DOI] [PubMed] [Google Scholar]
- 32.Abou Sherif S, Ozden Tok O, Taşköylü Ö, et al. Coronary artery aneurysms: a review of the epidemiology, pathophysiology, diagnosis, and treatment. Front Cardiovasc Med. 2017;4:24. doi: 10.3389/fcvm.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balderston JR, Giri J, Kolansky DM, et al. Coronary artery aneurysms associated with ascending aortic aneurysms and abdominal aortic aneurysms: pathophysiologic implications. Catheter Cardiovasc Interv. 2015;85(6):961–967. doi: 10.1002/ccd.25726. [DOI] [PubMed] [Google Scholar]
- 34.Cohen P, O’Gara PT. Coronary artery aneurysms: a review of the natural history, pathophysiology, and management. Cardiol Rev. 2008;16(6):301–304. doi: 10.1097/CRD.0b013e3181852659. [DOI] [PubMed] [Google Scholar]
- 35.Lamblin N, Bauters C, Hermant X, et al. Polymorphisms in the promoter regions of MMP-2, MMP-3, MMP-9 and MMP-12 genes as determinants of aneurysmal coronary artery disease. J Am Coll Cardiol. 2002;40(1):43–48. doi: 10.1016/s0735-1097(02)01909-5. [DOI] [PubMed] [Google Scholar]
- 36.Kuivaniemi H, Platsoucas CD, Tilson MD. Aortic aneurysms: an immune disease with a strong genetic component. Circulation. 2008;117(2):242–252. doi: 10.1161/CIRCULATIONAHA.107.690982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.al-Harthi SS, Nouh MS, Arafa M, et al. Aneurysmal dilatation of the coronary arteries: diagnostic patterns and clinical significance. Int J Cardiol. 1991;30(2):191–194. doi: 10.1016/0167-5273(91)90094-6. [DOI] [PubMed] [Google Scholar]
- 38.Baman TS, Cole JH, Devireddy CM, et al. Risk factors and outcomes in patients with coronary artery aneurysms. Am J Cardiol. 2004;93(12):1549–1551. doi: 10.1016/j.amjcard.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Mariscalco G, Mantovani V, Ferrarese S, et al. Coronary artery aneurysm: management and association with abdominal aortic aneurysm. Cardiovasc Pathol. 2006;15(2):100–104. doi: 10.1016/j.carpath.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Botto F, Alonso-Coello P, Chan MTV, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564–578. doi: 10.1097/ALN.0000000000000113. [DOI] [PubMed] [Google Scholar]