Abstract
Objective
To evaluate changes in demographics, clinical practices and long-term clinical outcomes of patients with ST segment-elevation myocardial infarction (STEMI) before and beyond 2010.
Design
Multicentre retrospective cohort study.
Setting
The Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) AMI Registries Wave-1 (2005–2007, 26 centres) and Wave-2 (2011–2013, 22 centres).
Participants
9001 patients with STEMI who underwent coronary revascularisation (Wave-1: 4278 patients, Wave-2: 4723 patients).
Primary and secondary outcome measures
The primary outcome was all-cause death at 3 years. The secondary outcomes were cardiovascular death, cardiac death, sudden cardiac death, non-cardiovascular death, non-cardiac death, myocardial infarction, definite stent thrombosis, stroke, hospitalisation for heart failure, major bleeding, target vessel revascularisation, ischaemia-driven target vessel revascularisation, any coronary revascularisation and any ischaemia-driven coronary revascularisation.
Results
Patients in Wave-2 were older, more often had comorbidities and more often presented with cardiogenic shock than those in Wave-1. Patients in Wave-2 had shorter onset-to-balloon time and door-to-balloon time, were more frequently implanted drug-eluting stents, and received guideline-directed medication than those in Wave-1. The cumulative 3-year incidence of all-cause death was not significantly different between Wave-1 and Wave-2 (15.5% and 15.7%, p=0.77). The adjusted risk of all-cause death in Wave-2 relative to Wave-1 was not significant at 3 years (HR 0.92, 95% CI 0.83 to 1.03, p=0.14), but lower beyond 30 days (HR 0.86, 95% CI 0.75 to 0.98, p=0.03). The adjusted risks of Wave-2 relative to Wave-1 were significantly lower for definite stent thrombosis (HR 0.59, 95% CI 0.43 to 0.81, p=0.001) and for any coronary revascularisation (HR 0.75, 95% CI 0.69 to 0.81, p<0.001), but higher for major bleeding (HR 1.34, 95% CI 1.20 to 1.51, p=0.005).
Conclusions
We could not demonstrate improvement in 3-year mortality risk from Wave-1 to Wave-2, but we found reduction in mortality risk beyond 30 days. We also found risk reduction for definite stent thrombosis and any coronary revascularisation, but an increase in the risk of major bleeding from Wave-1 to Wave-2.
Keywords: myocardial infarction, coronary intervention, coronary heart disease
Strengths and limitations of this study.
Evaluating changes of demographics, clinical practices and long-term clinical outcomes between patients with ST segment-elevation myocardial infarction enrolled beyond 2010 and those enrolled before 2010.
Multicentre registry with large sample size enrolled consecutive patients who underwent revascularisation for acute myocardial infarction.
Systematic differences between two cohorts in the selection of patients and collection of events.
Introduction
The early mortality of patients with ST segment-elevation myocardial infarction (STEMI) has been steadily declining over the past several decades.1–5 This trend appears to have been driven by many factors, including demographic change, better pharmacological management, widespread distribution of thrombolysis and/or primary percutaneous coronary intervention (PCI), shorter door-to-balloon time and improvement in secondary prevention.4 6–10 Several large studies had demonstrated improvement of early mortality for patients with STEMI from 1990s to 2000s.1–3 10 Treatment based on the updated guidelines might have further improved the clinical outcomes of patients with STEMI beyond 2000s.11 12 It is currently unknown whether the changes in the guidelines have contributed to change real-world clinical practice and to improve clinical outcomes; in particular, there is a few data evaluating the long-term clinical outcomes in patients with STEMI enrolled beyond 2010 compared with those enrolled before 2010, when the new-generation DES was approved in Japan.10 13–15 Therefore, we sought to evaluate changes in demographics, clinical practices, and long-term clinical outcomes of patients with STEMI using data from two large Japanese cohorts of patients with acute myocardial infarction (AMI) enrolled in 2005–2007 and 2011–2013.
Methods
Study population
The Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) AMI Registries Wave-1 and Wave-2 are a series of physician-initiated, non-company sponsored, multicentre registry enrolling consecutive patients with AMI who underwent coronary revascularisation, either PCI or isolated coronary artery bypass grafting (CABG), within 7 days of the onset of symptoms. Wave-1 enrolled patients between January 2005 and December 2007 among 26 centres (both PCI and CABG available: 20 centres, and only PCI available: 6 centres) in Japan after the introduction of drug-eluting stents (DESs) in 2004 (online supplemental appendix A).16 Wave-2 enrolled patients between January 2011 and December 2013 among 22 centres (both PCI and CABG available: 16 centres, and only PCI available: 6 centres) in Japan after approval of the new-generation DES in 2010 (online supplemental appendix A). We made a historical comparison on demographics, clinical practices and long-term clinical outcomes of patients with STEMI between Wave-1 and Wave-2.
bmjopen-2020-043683supp001.pdf (406.2KB, pdf)
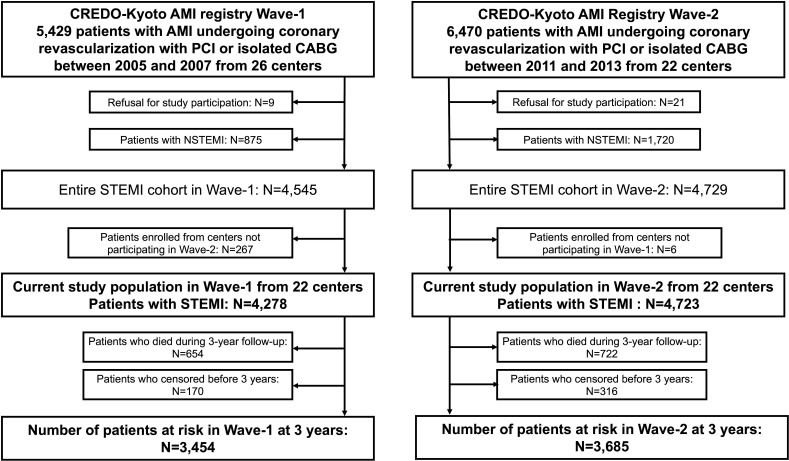
We enrolled a total of 11 899 consecutive patients with AMI who had undergone coronary revascularisation with PCI or isolated CABG within 7 days from onset from Wave-1 (n=5429) and Wave-2 (n=6470). In the present study, we excluded patients with refusal for study participation (Wave-1: n=9 and Wave-2: n=21) and non-ST segment-elevation myocardial infarction (NSTEMI) (Wave-1: n=875 and Wave-2: n=1720). To make Wave-1 and Wave-2 comparable, we further excluded 267 patients in Wave-1 who were enrolled from four cardiology divisions and five cardiovascular surgery divisions not participating in Wave-2 and 6 patients in Wave-2 who were enrolled from one cardiovascular surgery division not participating in Wave-1. Finally, the current study population was 9001 patients with STEMI (Wave-1: 4278 patients and Wave-2: 4723 patients) from 22 centres (both PCI and CABG available: 15 centres and only PCI available: 7 centres) (figure 1).
Figure 1.
Study flowchart. AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CREDO-Kyoto, Coronary Revascularization Demonstrating Outcome Study in Kyoto; NSTEMI, non-ST segment-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST segment-elevation myocardial infarction.
Definitions and clinical outcome measures
Patients with STEMI were defined by the electrocardiograms as patients with ≥0.1 mV of ST-segment elevation in ≥2 limb leads or ≥0.2 mV in ≥2 contiguous precordial leads, accompanied by chest pain lasting at least 30 min or increased serum levels of cardiac biomarkers such as troponin and/or creatine kinase MB fraction. Baseline clinical, angiographic and procedural characteristics were collected by the experienced clinical research coordinators from the independent clinical research organisation (Research Institute for Production Development, Kyoto, Japan; online supplemental appendix B) from the hospital charts or hospital databases according to the prespecified definitions.
Diabetes was defined as treatment with oral hypoglycaemic agents or insulin, prior clinical diagnosis of diabetes, glycated haemoglobin level of ≥6.5% or non-fasting blood glucose level of ≥200 g/L. Left ventricular ejection fraction was measured either by contrast left ventriculography or echocardiography. Prior stroke was defined as ischaemic or haemorrhagic stroke with neurological symptoms lasting >24 hours. Peripheral vascular disease was regarded as present when carotid, aortic or other peripheral vascular diseases were being treated or scheduled for surgical or endovascular interventions. Renal function was expressed as estimated glomerular filtration rate calculated by the Modification of Diet in Renal Disease formula modified for Japanese patients.17
The primary outcome measure of this study was all-cause death at 3 years. The secondary outcome measures were cardiovascular death, cardiac death, sudden cardiac death, non-cardiovascular death, non-cardiac death, myocardial infarction, definite stent thrombosis, stroke, hospitalisation for heart failure, major bleeding, target vessel revascularisation, ischaemia-driven target vessel revascularisation, any coronary revascularisation and ischaemia-driven any coronary revascularisation. The definition of death was described in detail previously.18 19 Myocardial infarction was defined according to the definition in the Arterial Revascularisation Therapy Study,20 and only Q-wave myocardial infarction was regarded as myocardial infarction when it occurred within 7 days of the index procedure.21 Definite stent thrombosis was defined according to the Academic Research Consortium (ARC) definition.22 Stroke during follow-up was defined as ischaemic or haemorrhagic stroke requiring hospitalisation with symptoms lasting >24 hours. Hospitalisation for heart failure was defined as hospitalisation due to worsening heart failure requiring intravenous drug therapy. Major bleeding was defined as the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries moderate/severe bleeding.21 23 Target vessel revascularisation (TVR) was defined as either PCI or CABG related to the original target vessel. Any coronary revascularisation was defined as either PCI or CABG for any reason. Scheduled staged coronary revascularisation procedures performed within 3 months of the initial procedure were not regarded as follow-up events, but included in the index procedure. Duration of dual antiplatelet therapy (DAPT) was left to the discretion of each attending physician. Persistent discontinuation of DAPT was defined as withdrawal of either thienopyridines or aspirin for at least 2 months.
Data collection and follow-up
The methods for collecting follow-up information were described in detail previously.24 Follow-up started at the time of revascularisation for STEMI and were censored at 3 years after the index procedure to ensure >90% of clinical follow-up rate in both Wave-1 and Wave-2. Complete 3-year follow-up information was obtained for 96.2% of patients in Wave-1 and 93.2% of patients in Wave-2, respectively. Death, myocardial infarction, stroke and major bleeding were adjudicated by the clinical event committee (online supplemental appendix C).
Statistical analysis
We expressed continuous variables as mean±SD or median with IQR and used Student’s t-test or Wilcoxon’s rank-sum test based on their distributions for comparing continuous variables. We expressed categorical variables as frequencies and percentages and used χ2 test for comparing categorical variables. To calculate the survival functions, follow-up periods were separately calculated for each outcome with censoring due to death or the last visit. The non-fatal outcomes other than the analysed outcomes in the survival analyses were ignored. Cumulative incidence was estimated by the Kaplan-Meier method and differences were assessed with the log-rank test. To estimate the overall and cause-specific HR and their 95% CIs of Wave-2 compared with Wave-1, we used multivariable Cox proportional hazard models by incorporating the 17 clinically relevant factors listed in table 1. The variables did not include the factors related to management during the index hospitalisation because differences in management converged into the changes between Wave-1 and Wave-2. Continuous risk-adjusting variables were dichotomised according to the clinically meaningful reference values to make proportional hazard assumptions robust and to be consistent with previous reports.24 25 We assessed proportional hazard assumptions for the risk-adjusting variables on the plots of log (time) versus log (−log (survival)) stratified by the variable and verified the assumptions were acceptable for all variables. The missing values for the risk-adjusting variables were imputed as ‘normal’ in the binary classification because data should have been available if abnormalities were suspected. We performed subgroup analysis for major bleeding stratified by the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria.26 We conducted landmark analyses for all-cause death and major bleeding within and beyond 30 days to distinguish perioperative and non-perioperative events.
Table 1.
Baseline characteristics comparing between Wave-1 and Wave-2
| Wave-1 | Wave-2 | P value | |
| (n=4278) | (n=4723) | ||
| Clinical characteristics | |||
| Age (years) | 67.6±12.2 | 68.8±12.5 | <0.001 |
| Age≥75 years* | 1336 (31%) | 1694 (36%) | <0.001 |
| Men* | 3156 (74%) | 3538 (75%) | 0.23 |
| Body Mass Index (kg/m2) | 23.6±3.5 | 23.7±3.6 | 0.40 |
| Body Mass Index<25.0 kg/m2* | 3058 (72%) | 3269 (69%) | 0.02 |
| Hypertension* | 3343 (78%) | 3768 (80%) | 0.06 |
| Diabetes mellitus* | 1395 (33%) | 1664 (35%) | 0.009 |
| On insulin therapy | 205 (4.8%) | 270 (5.7%) | 0.06 |
| Current smoking* | 1730 (40%) | 1702 (36%) | <0.001 |
| Heart failure* | 1350 (32%) | 1566 (33%) | 0.11 |
| LVEF | 52.5±12.9 | 53.8±12.4 | <0.001 |
| LVEF≤40% | 596 (18%) | 595 (14%) | <0.001 |
| Prior PCI | 364 (8.5%) | 523 (11%) | <0.001 |
| Prior CABG | 53 (1.2%) | 59 (1.2%) | 1.00 |
| Prior myocardial infarction* | 381 (8.9%) | 427 (9.0%) | 0.85 |
| Prior stroke (symptomatic)* | 394 (9.2%) | 521 (11%) | 0.005 |
| Peripheral vascular disease* | 138 (3.2%) | 209 (4.4%) | 0.004 |
| eGFR<30 mL/min/1.73 m2, without haemodialysis* | 202 (4.7%) | 288 (6.1%) | 0.005 |
| Hemodialysis* | 73 (1.7%) | 131 (2.8%) | 0.001 |
| eGFR <30 mL/min/1.73 m2 or haemodialysis | 275 (6.4%) | 419 (8.9%) | <0.001 |
| Atrial fibrillation | 418 (9.8%) | 419 (8.9%) | 0.15 |
| Anaemia (haemoglobin<11.0 g/L)* | 438 (10%) | 531 (11%) | 0.13 |
| Thrombocytopenia (platelet<100×109/L) | 84 (2.0%) | 102 (2.2%) | 0.56 |
| Chronic obstructive pulmonary disease | 140 (3.3%) | 173 (3.7%) | 0.34 |
| Liver cirrhosis | 101 (2.4%) | 101 (2.1%) | 0.52 |
| Malignancy* | 337 (7.9%) | 516 (11%) | <0.001 |
| Presentation | |||
| Living alone | 509 (13%) | 780 (17%) | <0.001 |
| Direct admission | 2215 (54%) | 2603 (57%) | 0.02 |
| Interfacility transfer | 1866 (44%) | 1983 (42%) | 0.12 |
| Killip class III/IV | 725 (17%) | 915 (19%) | 0.003 |
| Cardiogenic shock | 596 (14%) | 757 (16%) | 0.005 |
| Cardiopulmonary arrest* | 142 (3.3%) | 193 (4.1%) | 0.06 |
| Maximum CK | 2133 (1002–4077) | 1836 (767–3663) | <0.001 |
| Angiographic characteristics | |||
| Infarct related artery location | |||
| Left anterior descending coronary artery* | 1979 (46%) | 2191 (46%) | 0.91 |
| Left circumflex coronary artery | 443 (10%) | 479 (10%) | 0.76 |
| Right coronary artery | 1732 (40%) | 1898 (40%) | 0.78 |
| Left main coronary artery | 107 (2.5%) | 172 (3.6%) | 0.002 |
| Coronary artery bypass graft | 19 (0.4%) | 24 (0.5%) | 0.77 |
| Multivessel disease | 2222 (52%) | 2655 (56%) | <0.001 |
| Procedural characteristics | |||
| Onset-to-balloon time (hours) | 4.2 (2.8–7.2) | 4.0 (2.7–6.6) | <0.001 |
| Door-to-balloon time (min) | 90 (60–132) | 79 (59–110) | <0.001 |
| Intra-aortic balloon pump use | 738 (17%) | 994 (21%) | <0.001 |
| Percutaneous cardiopulmonary support use | 116 (2.7%) | 149 (3.2%) | 0.24 |
| PCI* | 4180 (98%) | 4625 (98%) | 0.48 |
| Transradial approach | 498 (12%) | 733 (16%) | <0.001 |
| Transfemoral approach | 3432 (82%) | 3640 (79%) | <0.001 |
| IVUS use for the culprit lesion | 1260 (30%) | 2653 (57%) | <0.001 |
| Stent use for the culprit lesion | 3739 (89%) | 4241 (92%) | <0.001 |
| Bare metal stent | 2946 (79%) | 1735 (41%) | <0.001 |
| DES | 793 (21%) | 2506 (59%) | <0.001 |
| Staged PCI | 932 (22%) | 1018 (22%) | 0.77 |
| Stent use including staged PCI | 3802 (91%) | 4295 (93%) | 0.001 |
| Bare metal stent | 2542 (67%) | 1490 (35%) | <0.001 |
| DES | 1260 (33%) | 2805 (65%) | <0.001 |
| First-generation DES use | 1257 (99%) | 47 (1.7%) | <0.001 |
| Sirolimus-eluting stent (CYPHER) | 1174 (93%) | 27 (57%) | |
| Paclitaxel-eluting stent (TAXUS) | 115 (9.1%) | 21 (45%) | |
| New-generation DES use | – | 2776 (99%) | |
| Everolimus-eluting stent (XIENCE) | – | 2054 (74%) | |
| Everolimus-eluting stent (PROMUS) | – | 1616 (58%) | |
| Biolimus-eluting stent (NOBORI) | – | 725 (26%) | |
| Zotarolimus-eluting stent (RESOLUTE) | – | 255 (9.2%) | |
| Zotarolimus-eluting stent (ENDEAVOR) | – | 49 (1.8%) | |
| CABG | 98 (2.3%) | 98 (2.1%) | 0.48 |
| Off pump | 34 (35%) | 43 (44%) | 0.19 |
| ITA use | 82 (84%) | 80 (82%) | 0.71 |
| Baseline medications | |||
| Antiplatelet therapy | |||
| Thienopyridine | 3993 (93%) | 4521 (96%) | <0.001 |
| Ticlopidine | 3652 (85%) | 124 (2.6%) | <0.001 |
| Clopidogrel | 340 (7.9%) | 4339 (92%) | <0.001 |
| Aspirin | 4209 (98%) | 4636 (98%) | 0.45 |
| Cilostazol | 1501 (35%) | 116 (2.5%) | <0.001 |
| Statins | 2281 (53%) | 3885 (82%) | <0.001 |
| High-intensity statin therapy† | 67 (1.6%) | 78 (1.7%) | 0.81 |
| Beta blockers | 1747 (41%) | 2555 (54%) | <0.001 |
| ACE inhibitors/ARB | 3040 (71%) | 3554 (75%) | <0.001 |
| Nitrates | 1269 (30%) | 832 (18%) | <0.001 |
| Calcium channel blockers | 885 (21%) | 970 (21%) | 0.88 |
| Nicorandil | 1198 (28%) | 966 (20%) | <0.001 |
| Warfarin | 495 (12%) | 591 (13%) | 0.18 |
| DOAC | – | 61 (1.3%) | – |
| Proton pump inhibitors | 1470 (34%) | 3505 (74%) | <0.001 |
| Histamine type 2 receptor blockers | 1393 (33%) | 553 (12%) | <0.001 |
Continuous variables were expressed as mean±SD or median (IQR). Categorical variables were expressed as number (percentage).
There were missing values for Body Mass Index in 341 patients (Wave-1: 232 (5.4%) and Wave-2: 109 (2.3%)), for LVEF in 1385 patients (Wave-1: 951 (22%) and Wave-2: 434 (9.2%)), for eGFR in 94 patients (Wave-1: 80 (1.9%) and Wave-2: 14 (0.3%)), for haemoglobin level in 110 patients (Wave-1: 99 (2.3%) and Wave-2: 11 (0.2%)), for platelet count in 47 patients (Wave-1: 29 (0.7%) and Wave-2: 18 (0.4%)), for max CK in 91 patients (Wave-1: 39 (0.9%) and Wave-2: 52 (1.1%)). The numbers of missing values for Body Mass Index, eGFR, haemoglobin level and platelet count were negligibly small. The missing values for these variables were imputed as ‘normal’ in the binary classification because data should have been available if abnormalities were suspected. On the other hand, the missing values for LVEF were not imputed in the categorical classification because the numbers of missing values were substantial for these variables. Onset-to-balloon time and door-to-balloon time were analysed only for patients who underwent PCI within 24 hours of the onset of symptoms excluding nosocomial onset (onset-to-balloon time: 3271 patients in Wave-1 and 3372 patients in Wave-2; door-to-balloon time: 3228 patients in Wave-1 and 3242 patients in Wave-2).
*Risk-adjusting variables for the Cox proportional hazard models.
†High-intensity statin therapy in this study was defined as the statin doses greater than or equal to atorvastatin 20 mg, pitavastatin 4 mg or rosuvastatin 10 mg.
ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CK, creatine kinase; DES, drug-eluting stent; DOAC, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; ITA, internal thoracic artery; IVUS, intravascular ultrasound; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
All analyses were performed using R V.3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). All reported p values were two-tailed, and p values less than 0.05 were considered statistically significant.
Patient and public involvement
In this study, patients were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
Clinical and procedural characteristics
Patients in Wave-2 were older and were more often living alone than those in Wave-1. Patients in Wave-2 more often had diabetes, end-stage renal failure, prior stroke, peripheral vascular disease, prior PCI and malignancy, and less often had ejection fractions ≤40% and current smoking than those in Wave-1 (table 1).
Regarding presentation, Wave-2 as compared with Wave-1 included more patients who directly admitted to the participating centres without interfacility transfer, and who presented with cardiogenic shock and/or Killip class III/IV. Regarding angiographic characteristics, the prevalence of left anterior descending artery culprit was not different between Wave-1 and Wave-2. Patients in Wave-2 more often had multivessel disease than those in Wave-1 (table 1).
Regarding procedural characteristics, onset-to-balloon time and door-to-balloon time were significantly shorter in Wave-2 than in Wave-1. Prevalence of transradial approach increased significantly, but only slightly, from Wave-1 to Wave-2. Prevalence of DES use was much higher in Wave-2 than in Wave-1, with new-generation DES use in the vast majority of DES cases in Wave-2 (table 1). Intra-aortic balloon pumping was more often used in Wave-2 than in in Wave-1 (table 1).
In terms of baseline medications, patients in Wave-2 more often took thienopyridine, statins, beta blockers, ACE inhibitors/angiotensin receptor blockers and proton pump inhibitors than those in Wave-1, while patients in Wave-2 less often took cilostazol than those in Wave-1. The prevalence of high-intensity statin therapy was very low in both Wave-1 and Wave-2. Regarding the kind of thienopyridine, the vast majority of patients in Wave-1 took ticlopidine, while the vast majority of patients in Wave-2 took clopidogrel (table 1).
Clinical outcomes
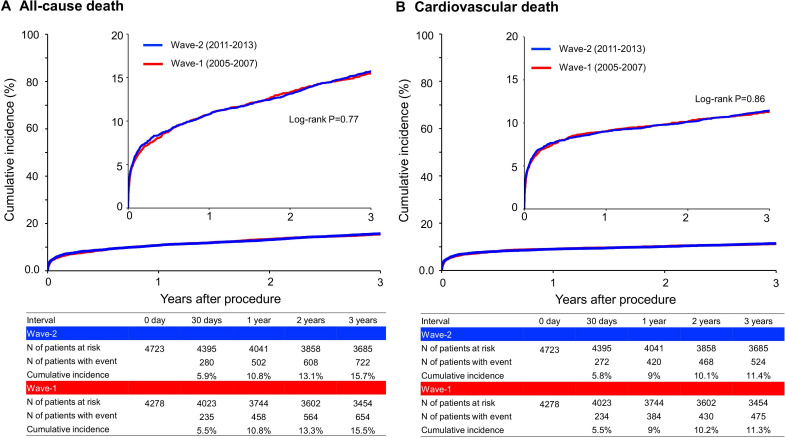
The cumulative 3-year incidence of all-cause death was not significantly different between Wave-1 and Wave-2 (15.5% vs 15.7%, log-rank p=0.77) (figure 2A and table 2). The adjusted risk of Wave-2 relative to Wave-1 remained insignificant for all-cause death (HR 0.92, 95% CI 0.83 to 1.03, p=0.14) (table 2). In the 30-day landmark analysis, cumulative incidence of all-cause death was not significantly different between Wave-1 and Wave-2 both within 30 days (5.5% vs 5.9%, log-rank p=0.37) and beyond 30 days (10.6% vs 10.4%, log-rank p=0.74). However, after adjusting confounders, the lower mortality risk of Wave-2 relative to Wave-1 was significant beyond 30 days after index procedure (HR 0.86, 95% CI 0.75 to 0.98, p=0.03), although it was not significant within 30 days (HR 1.04, 95% CI 0.87 to 1.23, p=0.69) (online supplemental figure 1). The results of the 30-day landmark analysis were consistent in patients with and without cardiogenic shock (online supplemental figure 1).
Figure 2.
Kaplan-Meier curves (A) for all-cause death and (B) for cardiovascular death comparing between Wave-1 and Wave-2.
Table 2.
Clinical outcomes comparing between Wave-1 and Wave-2
| Endpoints | Wave-1 | Wave-2 | Crude HR | P value | Adjusted HR | P value | ||||
| (n=4278) | (n=4723) | |||||||||
| Patients with event (n) | (95% CI) | (95% CI) | ||||||||
| (Cumulative 3-year incidence) | ||||||||||
| All-cause death | 654 | (15.5%) | 722 | (15.7%) | 1.02 | (0.91 to 1.13) | 0.77 | 0.92 | (0.83 to 1.03) | 0.14 |
| Cardiovascular death | 475 | (11.3%) | 524 | (11.4%) | 1.01 | (0.89 to 1.15) | 0.86 | 0.93 | (0.82 to 1.06) | 0.26 |
| Cardiac death | 448 | (10.7%) | 489 | (10.7%) | 1.00 | (0.88 to 1.14) | 1.00 | 0.93 | (0.81 to 1.05) | – |
| Sudden cardiac death | 47 | (1.2%) | 45 | (1.1%) | 0.88 | (0.59 to 1.33) | 0.54 | 0.76 | (0.50 to 1.15) | – |
| Non-cardiovascular death | 179 | (4.7%) | 198 | (4.8%) | 1.03 | (0.84 to 1.26) | 0.80 | 0.90 | (0.73 to 1.10) | 0.29 |
| Non-cardiac death | 206 | (5.4%) | 233 | (5.7%) | 1.05 | (0.87 to 1.27) | 0.61 | 0.91 | (0.75 to 1.10) | – |
| Myocardial infarction | 169 | (4.3%) | 202 | (4.8%) | 1.10 | (0.90 to 1.35) | 0.36 | 1.04 | (0.85 to 1.28) | 0.72 |
| Definite stent thrombosis* | 81 | (2.3%) | 60 | (1.5%) | 0.65 | (0.47 to 0.91) | 0.01 | 0.59 | (0.43 to 0.81) | 0.001 |
| Stroke | 191 | (4.9%) | 243 | (5.7%) | 1.17 | (0.97 to 1.42) | 0.10 | 1.09 | (0.90 to 1.31) | 0.40 |
| Hospitalisation for heart failure | 267 | (7.0%) | 305 | (7.4%) | 1.06 | (0.90 to 1.25) | 0.50 | 0.97 | (0.82 to 1.14) | 0.68 |
| Major bleeding | 492 | (12.0%) | 741 | (16.5%) | 1.39 | (1.25 to 1.56) | <0.001 | 1.34 | (1.20 to 1.51) | 0.005 |
| Target vessel revascularisation | 1017 | (26.3%) | 816 | (19.5%) | 0.70 | (0.64 to 0.77) | <0.001 | 0.69 | (0.63 to 0.76) | – |
| Ischaemia-driven target vessel revascularisation | 353 | (9.1%) | 364 | (8.7%) | 0.94 | (0.81 to 1.09) | 0.43 | 0.92 | (0.79 to 1.06) | – |
| Any coronary revascularisation | 1277 | (33.0%) | 1112 | (26.6%) | 0.76 | (0.70 to 0.83) | <0.001 | 0.75 | (0.69 to 0.81) | – |
| Ischaemia-driven any coronary revascularisation | 472 | (12.3%) | 522 | (12.6%) | 1.02 | (0.90 to 1.15) | 0.80 | 0.99 | (0.87 to 1.12) | – |
The risk of Wave-2 relative to Wave-1 was expressed as HR with 95% CI. The covariates for the multivariate Cox proportional hazard models are indicated in table 1.
Myocardial infarction was based on the ARTS definition.
Major bleeding was defined as GUSTO moderate/severe bleeding.
*Definite stent thrombosis was based on the ARC definition and was analysed only for patients who underwent PCI with stent implantation (3739 patients in Wave-1 and 4241 patients in Wave-2).
ARC, Academic Research Consortium; ARTS, Arterial Revascularisation Therapy Study; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; PCI, percutaneous coronary intervention.
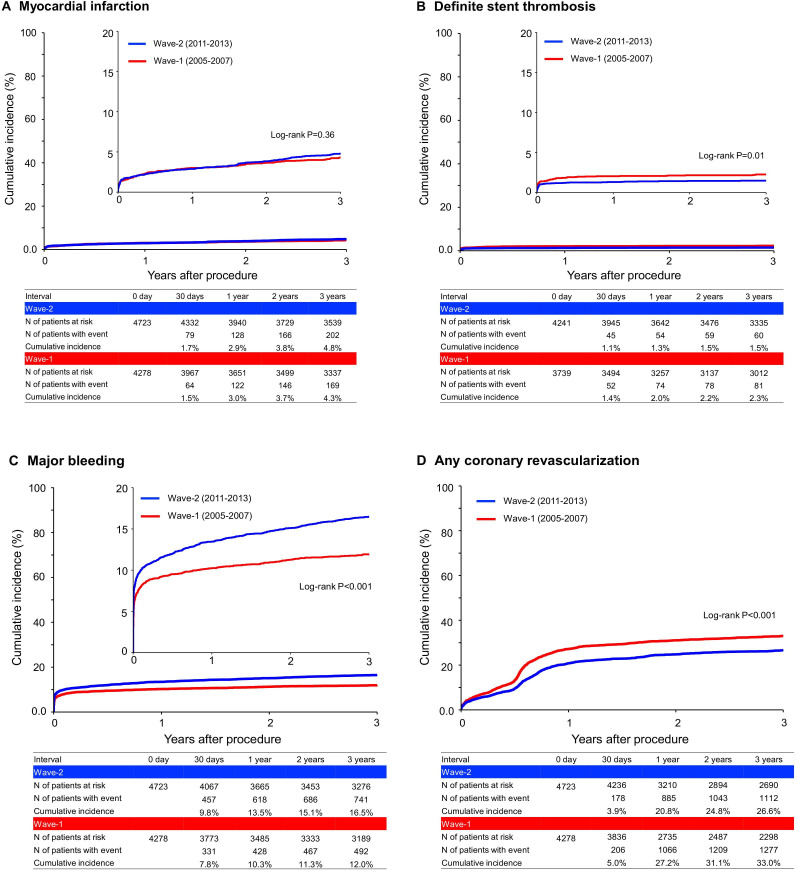
The lower crude and adjusted risks of Wave-2 relative to Wave-1 were significant for definite stent thrombosis and any coronary revascularisation, while those were insignificant for cardiovascular death, myocardial infarction and stroke (figures 2B and 3 and table 2).
Figure 3.
Kaplan-Meier curves comparing between Wave-1 and Wave-2 for (A) myocardial infarction, (B) definite stent thrombosis, (C) major bleeding and (D) any coronary revascularisation. Definite stent thrombosis was based on the ARC definition and was analysed only for patients who underwent PCI with stent implantation (3739 patients in Wave-1 and 4241 patients in Wave-2). Major bleeding was defined as GUSTO moderate/severe bleeding. ARC, Academic Research Consortium; GUSTO, Global Utilisation of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries.
Meanwhile, the cumulative 3-year incidence of major bleeding was significantly higher in Wave-2 than in Wave-1 (16.5% and 12.0%, log-rank p<0.001) (figure 3 and table 2). The excess adjusted risk of Wave-2 relative to Wave-1 remained significant for major bleeding (HR 1.34, 95% CI 1.20 to 1.51, p=0.005) (table 2). In the 30-day landmark analysis, the excess crude and adjusted risks of Wave-2 relative to Wave-1 for major bleeding were significant both within 30 days and beyond 30 days (online supplemental figure 2). In the subgroup analysis, the higher risk of Wave-2 relative to Wave-1 for major bleeding was consistent in patients with and without ARC-HBR (online supplemental figure 3). The cumulative incidence of persistent DAPT discontinuation was significantly lower in Wave-2 than in Wave-1, indicating significantly longer DAPT duration in Wave-2 than in Wave-1 (online supplemental figure 4).
Discussion
The main findings of this study were as follows: (1) regarding demographics, patients with STEMI in Wave-2 were older, more often had comorbidities and more often presented with serious haemodynamic conditions than those in Wave-1; (2) regarding clinical practice, patients in Wave-2 had shorter onset-to-balloon time and door-to-balloon time, were more frequently treated with DES, more often received guideline-directed medical therapy at baseline, and had longer duration of DAPT during follow-up than those in Wave-1; (3) The 3-year adjusted risks of patients in Wave-2 relative to those in Wave-1 were not significantly different for all-cause death, myocardial infarction and stroke, and significantly lower for definite stent thrombosis and any coronary revascularisation, but significantly higher for major bleeding; (4) we witnessed a lower adjusted mortality risk of Wave-2 relative to Wave-1 beyond 30 days but not within 30 days.
There was scarcity of data evaluating demographics, clinical practices and long-term clinical outcomes in patients with STEMI enrolled beyond 2010 compared with those enrolled before 2010.10 27 In the present study, we could not demonstrate significant improvement in mortality risk from Wave-1 to Wave-2. The mortality rates at 30 days were still around 5%–6% in both Wave-1 and Wave-2, which was in line with the previous studies.28 29 It was true that patients in Wave-2 were older and sicker than those in Wave-1. However, even the adjusted analysis did not suggest improvement in 30-day morality risk from Wave-1 to Wave-2. We did observe significantly shorter onset-to-balloon time and door-to-balloon time with less frequent interfacility transfer and more frequent use of DES in Wave-2 than in Wave-1. However, these changes in clinical practice did not lead to improvement in 30-day mortality rate. Further shortening of onset-to-balloon time, more widespread use of transradial approach and improved management of cardiogenic shock might be important to improve 30-day mortality rate.16 30–37
On the other hand, beyond 30 days after the index procedure, we found a significantly lower adjusted mortality risk of patients in Wave-2 relative to those in Wave-1. The changes in clinical practices that might have contributed to lower mortality risk in Wave-2 relative to Wave-1 included shorter onset-to-balloon time, introduction of new-generation DES and higher prevalence of guideline-directed medications use, particularly statins. Indeed, in the present study, the rates of definite stent thrombosis and any coronary revascularisation were significantly lower in Wave-2 than in Wave-1, which was in line with the previous study comparing new-generation DES with first-generation DES.38 Moreover, we did find substantial increase in the prevalence of statins use. Nevertheless, the prescription rate of high-intensity statin therapy was extremely low in both Wave-1 and Wave-2. The efficacy of high-intensity statin therapy has been firmly established in preventing cardiovascular events in patients with coronary artery disease.39 40 We should make every effort to promote wider penetration of high-intensity statin therapy in Japan.
Meanwhile, we have demonstrated that the cumulative 3-year incidence of major bleeding was significantly higher in Wave-2 than in Wave-1. Patients in Wave-2 were older and sicker than those in Wave-1. However, even after adjusting confounders, the excess risk of Wave-2 relative to Wave-1 remained significant for major bleeding. Moreover, the excess bleeding risk of Wave-2 relative to Wave-1 was significant regardless of ARC-HBR. Furthermore, the excess bleeding risk of Wave-2 relative to Wave-1 was significant both within 30 days and beyond 30 days. One of the reasons for the higher bleeding risk within 30 days in Wave-2 than in Wave-1 might be the different types of thienopyridine used in Wave-1 and Wave-2. In Wave-1, the vast majority of patients took ticlopidine 100 mg two times per day as the standard dose in Japan, which was much lower than the dose used globally (250 mg two times per day), while in Wave-2, the vast majority of patients took clopidogrel 75 mg once per day, which was the the dose used globally. The 30-day rate of major bleeding in Wave-2 was substantial (entire cohort: 9.8%, ARC-HBR: 14.8% and non-ARC-HBR: 5.4%), warranting to explore the optimal antiplatelet regimen in patients with STEMI minimising bleeding events while maintaining efficacy in preventing thrombotic events. For the higher bleeding risk beyond 30 days in Wave-2 than in Wave-1, one of the reasons in addition to the difference in the types of thienopyridine might be the longer DAPT duration in Wave-2 than in Wave-1. Recent studies have suggested clinical benefit with very short DAPT after PCI in reducing major bleeding without increase in cardiovascular events, although patients with STEMI constituded only a small proportion in the Short and Optimal duration of Dual Antiplatelet Therapy after Everolimus-eluting Cobalt-Chromium Stent-2 trial, and were excluded in the Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention trial.41 42 We should continue to pursue the optimal DAPT duration and optimal maintenance antithrombotic regimen in patients with STEMI. Our study, which was based on the multicentre registry with large sample size, enrolled consecutive patients who underwent revascularisation for AMI, and the follow-up rate was high enough. Therefore, we believe our findings should be applicable in Japan or other similar settings outside Japan, but the changes in clinical pictures of STEMI should be investigated in other settings with different healthcare systems.
Limitations
There are several limitations of this study. First, historical comparison should result in differences in selection of patients and collection of events, although we were careful in using data only from those centres that participated in both Wave-1 and Wave-2, standardising the follow-up duration at 3 years, and adopting the identical methodology for baseline and follow-up data collection, and definitions of baseline characteristics and clinical outcome measures in Wave-1 and Wave-2. We could not deny the possibility of ascertainment bias for myocardial infarction, although we adopted the identical definition of myocardial infarction in Wave-1 and Wave-2. The less widespread use of troponin for the diagnosis of myocardial infarction in Wave-1 compared with Wave-2 might have underestimated the incidence of myocardial infarction in Wave-1, as reflected by the fact that there were much larger number of patients with NSTEMI in Wave-2 than in Wave-1. Moreover, we could not deny the possibility of ascertainment bias for major bleeding, although we adopted the identical definition in Wave-1 and Wave-2. It could be possible that more major bleeding events were recorded in the hospital charts due to the growing interest in bleeding events in later time period. Second, the incidence of various endpoints during the 3-year follow-up is probably overestimated because not accounting for competing risks. Third, we chose several outcomes as secondary outcomes carrying the risk of multiple comparisons. Fourth, we only included patients who underwent coronary revascularisation, which might have lead to selection bias. However, it is quite rare for a patient with STEMI not undergoing primary PCI. Finally, residual unmeasured confounders might exist.
Conclusions
We could not demonstrate improvement in 3-year mortality risk from Wave-1 to Wave-2, but we found significant reduction in mortality risk beyond 30 days. We also found a significant risk reduction for definite stent thrombosis and any coronary revascularisation but an increase in the risk of major bleeding from Wave-1 to Wave-2.
Supplementary Material
Acknowledgments
We appreciate the support and collaboration of the coinvestigators participating in the
Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) PCI/CABG Registry Wave-1 and the CREDO-Kyoto PCI/CABG Registry Wave-2. We are indebted to the outstanding effort of the clinical research coordinators for data collection.
Footnotes
Contributors: TKi conceptualised the Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) AMI Registry. YT prepared the original draft of the manuscript. HSh, TM and TKi reviewed and edited the the original draft of the manuscript. YT, HSh, YYo, YM-N, KYamam and KYamaj curated data. YT, TM and TKi constructed methodology for this study. YT and TM performed the statistical analysis. HSh, TM, RT, KYamaj, JT, HW, SS, MI, TTak, MS, NE, KI, TI, TTam, TO, ES, TY, HSa, KA, YS, YF, YS, YN, KK, TKo, KM and TKi are investigators of the CREDO-Kyoto AMI Registry. YT, HSh, YY, YM-N, KYam, ETK, EY, YYa, MF, HW, HY and KN assessed and validated events within the CREDO-Kyoto AMI Registry. TKi is the guarantor.
Funding: This study was supported by an educational grant from the Research Institute for Production Development (Kyoto, Japan) and the Pharmaceuticals and Medical Devices Agency in Japan (Tokyo, Japan). Grant numbers were not applicable.
Competing interests: All authors have completed the Unified Competing Interest form and declare the following: HSh reports personal fees from Abbott Vascular, Boston Scientific and Daiichi Sankyo. TM reports lecturer's fees from Bayer, Daiichi Sankyo, Japan Lifeline, Kyocera, Mitsubishi Tanabe, Novartis and Toray; the manuscript fees from Bristol-Myers Squibb and Kowa; serving on advisory boards for Asahi Kasei, Boston Scientific, Bristol-Myers Squibb and Sanofi. ETK reports grant from Ono Pharmaceutical and reports personal fees from Daiichi Sankyo, AstraZeneca, Bristol-Myers Squibb, Tanabe-Mitsubishi Pharma, Ono Pharmaceutical, MSD KK and Pfizer. NE reports personal fees from Abbott Vascular, Medtronic, Terumo, Bayer, Boston Scientific, Daiichi-Sankyo, Edwards Lifescience, Pfizer, Bristol Myers Squibb, Takeda and Boehringer Ingelheim. YF reports personal fees from Daiichi Sankyo, Bayer, Sanofi, Kowa, Pfizer, Bristol-Myers Squibb, Otsuka Parmaceutical, Sumitomo Dainippon Pharma, Takeda and Ono Pharmaceutical. YN reports grant from Abbott Vascular and Boston Scientific, and reports personal fees from Abbott Vascular, Bayer, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo. TKi reports personal fees from Abbott Vascular, MSD, Eisai, Edwards Lifescience, Ono Pharmaceutical, Tsumura, Medical Review, Kowa, Sanofi, Daiichi Sankyo, Takeda Pharmaceutical, Pharmaceuticals and Medical Devices Agency, Abiomed, Bayer, Bristol-Myers Squibb, Boston Scientific, Lifescience, Toray, Astellas Amgen Biopharma, Astellas, AstraZeneca, Otsuka Parmaceutical, OrbusNeich, MSD Life Science Foundation, Public Health Research Foundation, Chugai Pharmaceutical, Boehringer Ingelheim, Japan Society for the Promotion of Science, Interscience, Philips, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, Terumo, Novartis Pharma and Sumitomo Dainippon Pharma.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol for CREDO-Kyoto AMI Registry Wave-1 and Wave-2 were approved by the human research ethics committees of the Kyoto University Graduate School of Medicine (E42, E2400). The relevant institutional review boards at all participating hospitals approved the study protocols. We waived written informed consent for both registries because of the retrospective nature of the study; however, we excluded those patients who refused participation in the study when contacted at follow-up, which is concordant with the guidelines of the Japanese Ministry of Health, Labor and Welfare.
References
- 1. Fox KAA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA 2007;297:1892–900. 10.1001/jama.297.17.1892 [DOI] [PubMed] [Google Scholar]
- 2. Rogers WJ, Frederick PD, Stoehr E, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of myocardial infarction from 1990 to 2006. Am Heart J 2008;156:1026–34. 10.1016/j.ahj.2008.07.030 [DOI] [PubMed] [Google Scholar]
- 3. Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987-2008. Circulation 2012;125:1848–57. 10.1161/CIRCULATIONAHA.111.047480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. 10.1056/NEJMoa0908610 [DOI] [PubMed] [Google Scholar]
- 5. Puymirat E, Simon T, Steg PG, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA 2012;308:998–1006. 10.1001/2012.jama.11348 [DOI] [PubMed] [Google Scholar]
- 6. Stolt Steiger V, Goy J-J, Stauffer J-C, et al. Significant decrease in in-hospital mortality and major adverse cardiac events in Swiss STEMI patients between 2000 and December 2007. Swiss Med Wkly 2009;139:453–7. doi:smw-12607 [DOI] [PubMed] [Google Scholar]
- 7. Puymirat E, Schiele F, Steg PG, et al. Determinants of improved one-year survival in non-ST-segment elevation myocardial infarction patients: insights from the French FAST-MI program over 15 years. Int J Cardiol 2014;177:281–6. 10.1016/j.ijcard.2014.09.023 [DOI] [PubMed] [Google Scholar]
- 8. Jernberg T, Johanson P, Held C, et al. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011;305:1677–84. 10.1001/jama.2011.522 [DOI] [PubMed] [Google Scholar]
- 9. Gale CP, Allan V, Cattle BA, et al. Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003-2010: a multilevel and relative survival analysis for the National Institute for cardiovascular outcomes research (NICOR). Heart 2014;100:582–9. 10.1136/heartjnl-2013-304517 [DOI] [PubMed] [Google Scholar]
- 10. Puymirat E, Simon T, Cayla G, et al. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI program (French registry of acute ST-elevation or non-ST-elevation myocardial infarction) 1995 to 2015. Circulation 2017;136:1908–19. 10.1161/CIRCULATIONAHA.117.030798 [DOI] [PubMed] [Google Scholar]
- 11. O'Gara PT, Kushner FG, Ascheim DD. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. J Am Coll Cardiol 2013;2013:485–510. [DOI] [PubMed] [Google Scholar]
- 12. Ibanez B, James S, Agewall S. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of cardiology (ESC). Eur Heart J 2017;2018:119–77. [DOI] [PubMed] [Google Scholar]
- 13. Eagle KA, Goodman SG, Avezum A, et al. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: findings from the global registry of acute coronary events (grace). Lancet 2002;359:373–7. 10.1016/S0140-6736(02)07595-5 [DOI] [PubMed] [Google Scholar]
- 14. Fox KAA, Goodman SG, Anderson FA, et al. From guidelines to clinical practice: the impact of hospital and geographical characteristics on temporal trends in the management of acute coronary syndromes. the global registry of acute coronary events (GRACE). Eur Heart J 2003;24:1414–24. 10.1016/S0195-668X(03)00315-4 [DOI] [PubMed] [Google Scholar]
- 15. Carruthers KF, Dabbous OH, Flather MD, et al. Contemporary management of acute coronary syndromes: does the practice match the evidence? The global registry of acute coronary events (GRACE). Heart 2005;91:290–8. 10.1136/hrt.2003.031237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shiomi H, Nakagawa Y, Morimoto T, et al. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: observational study. BMJ 2012;344:e3257. 10.1136/bmj.e3257 [DOI] [PubMed] [Google Scholar]
- 17. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–92. 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 18. Natsuaki M, Morimoto T, Shiomi H, et al. Application of the modified high bleeding risk criteria for Japanese patients in an all-comers registry of percutaneous coronary intervention from the CREDO-kyoto registry cohort-3. Circ J 2020. 10.1253/circj.CJ-20-0836 [DOI] [PubMed] [Google Scholar]
- 19. Matsumura-Nakano Y, Shiomi H, Morimoto T, et al. Comparison of outcomes of percutaneous coronary intervention versus coronary artery bypass grafting among patients with Three-Vessel coronary artery disease in the new-generation drug-eluting stents era (from CREDO-Kyoto PCI/CABG registry Cohort-3). Am J Cardiol 2021;391. 10.1016/j.amjcard.2020.12.076 [DOI] [PubMed] [Google Scholar]
- 20. Serruys PW, Unger F, Sousa JE, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med 2001;344:1117–24. 10.1056/NEJM200104123441502 [DOI] [PubMed] [Google Scholar]
- 21. Kimura T, Morimoto T, Furukawa Y, et al. Long-term safety and efficacy of sirolimus-eluting stents versus bare-metal stents in real world clinical practice in Japan. Cardiovasc Interv Ther 2011;26:234–45. 10.1007/s12928-011-0065-0 [DOI] [PubMed] [Google Scholar]
- 22. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 23. GUSTO investigators . An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–82. 10.1056/NEJM199309023291001 [DOI] [PubMed] [Google Scholar]
- 24. Takeji Y, Shiomi H, Morimoto T, et al. Demographics, practice patterns and long-term outcomes of patients with non-ST-segment elevation acute coronary syndrome in the past two decades: the CREDO-Kyoto Cohort-2 and Cohort-3. BMJ Open 2021;11:e044329. 10.1136/bmjopen-2020-044329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimura T, Morimoto T, Furukawa Y, et al. Long-term outcomes of coronary-artery bypass graft surgery versus percutaneous coronary intervention for multivessel coronary artery disease in the bare-metal stent era. Circulation 2008;118:S199–209. 10.1161/CIRCULATIONAHA.107.735902 [DOI] [PubMed] [Google Scholar]
- 26. Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation 2019;140:240–61. 10.1161/CIRCULATIONAHA.119.040167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szummer K, Wallentin L, Lindhagen L, et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995-2014. Eur Heart J 2017;38:3056–65. 10.1093/eurheartj/ehx515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menees DS, Peterson ED, Wang Y, et al. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med 2013;369:901–9. 10.1056/NEJMoa1208200 [DOI] [PubMed] [Google Scholar]
- 29. Biswas S, Duffy SJ, Lefkovits J, et al. Australian trends in procedural characteristics and outcomes in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol 2018;121:279–88. 10.1016/j.amjcard.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 30. Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA 2000;283:2941–7. 10.1001/jama.283.22.2941 [DOI] [PubMed] [Google Scholar]
- 31. De Luca G, Suryapranata H, Zijlstra F, et al. Symptom-onset-to-balloon time and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol 2003;42:991–7. 10.1016/S0735-1097(03)00919-7 [DOI] [PubMed] [Google Scholar]
- 32. McNamara RL, Wang Y, Herrin J, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2006;47:2180–6. 10.1016/j.jacc.2005.12.072 [DOI] [PubMed] [Google Scholar]
- 33. Maeng M, Nielsen PH, Busk M, et al. Time to treatment and three-year mortality after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction-a Danish trial in acute myocardial Infarction-2 (DANAMI-2) substudy. Am J Cardiol 2010;105:1528–34. 10.1016/j.amjcard.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 34. Rollando D, Puggioni E, Robotti S, et al. Symptom onset-to-balloon time and mortality in the first seven years after STEMI treated with primary percutaneous coronary intervention. Heart 2012;98:1738–42. 10.1136/heartjnl-2012-302536 [DOI] [PubMed] [Google Scholar]
- 35. Park J, Choi KH, Lee JM, et al. Prognostic implications of door-to-balloon time and Onset-to-Door time on mortality in patients with ST -Segment-Elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Heart Assoc 2019;8:e012188. 10.1161/JAHA.119.012188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakatsuma K, Shiomi H, Morimoto T, et al. Inter-Facility transfer vs. Direct admission of patients with ST-segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ J 2016;80:1764–72. 10.1253/circj.CJ-16-0204 [DOI] [PubMed] [Google Scholar]
- 37. Gandhi S, Kakar R, Overgaard CB. Comparison of radial to femoral PCI in acute myocardial infarction and cardiogenic shock: a systematic review. J Thromb Thrombolysis 2015;40:108–17. 10.1007/s11239-014-1133-y [DOI] [PubMed] [Google Scholar]
- 38. Toyota T, Shiomi H, Morimoto T, et al. Meta-analysis of long-term clinical outcomes of everolimus-eluting stents. Am J Cardiol 2015;116:187–94. 10.1016/j.amjcard.2015.03.059 [DOI] [PubMed] [Google Scholar]
- 39. Taguchi I, Iimuro S, Iwata H, et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation 2018;137:1997–2009. 10.1161/CIRCULATIONAHA.117.032615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40., Baigent C, Blackwell L, et al. , Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watanabe H, Domei T, Morimoto T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA 2019;321:2414–27. 10.1001/jama.2019.8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med 2019;381:2032–42. 10.1056/NEJMoa1908419 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-043683supp001.pdf (406.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.