Abstract
Background
Immune-related adverse events (irAEs) are a serious side effect of immune checkpoint inhibitor (ICI) therapy for patients with advanced cancer. Currently, predisposing risk factors are undefined but understanding which patients are at increased risk for irAEs severe enough to require hospitalization would be beneficial to tailor treatment selection and monitoring.
Methods
We performed a retrospective review of patients with cancer treated with ICIs using unidentifiable claims data from an Aetna nationwide US health insurance database from January 3, 2011 to December 31, 2019, including patients with an identified primary cancer and at least one administration of an ICI. Regression analyses were performed. Main outcomes were incidence of and factors associated with irAE requiring hospitalization in ICI therapy.
Results
There were 68.8 million patients identified in the national database, and 14 378 patients with cancer identified with at least 1 administration of ICI in the study period. Patients were followed over 19 117 patient years and 504 (3.5%) developed an irAE requiring hospitalization. The incidence of irAEs requiring hospitalization per patient ICI treatment year was 2.6%, rising from 0% (0/71) in 2011 to 3.7% (93/2486) in 2016. Combination immunotherapy (OR: 2.44, p<0.001) was associated with increased odds of developing irAEs requiring hospitalization, whereas older patients (OR 0.98 per additional year, p<0.001) and those with non-lung cancer were associated with decreased odds of irAEs requiring hospitalization (melanoma OR: 0.70, p=0.01, renal cell carcinoma OR: 0.71, p=0.03, other cancers OR: 0.50, p<0.001). Sex, region, zip-code-imputed income, and zip-code unemployment were not associated with incidence of irAE requiring hospitalization. Prednisone (72%) and methylprednisolone (25%) were the most common immunosuppressive treatments identified in irAE hospitalizations.
Conclusions
We found that 3.5% of patients initiating ICI therapy experienced irAEs requiring hospitalization and immunosuppression. The odds of irAEs requiring hospitalization were higher with younger age, treatment with combination ICI therapy (cytotoxic T lymphocyte-associated 4 and programmed cell death protein 1 (PD-1) or programmed death-ligand 1 (PD-L1)), and lower for other cancers compared with patients on PD-1 or PD-L1 inhibitors with lung cancer. This evidence from the first nationwide study of irAEs requiring hospitalization in the USA identified the real-world epidemiology, risk factors, and treatment patterns of these irAEs which may guide treatment and management decisions.
Keywords: Immunotherapy
Background
Immune checkpoint inhibitors (ICIs) are medications increasingly used to treat cancer that modulate the endogenous immune response. These medications block interaction of checkpoint proteins on tumor cells binding to T-cells, and include inhibition of programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PDL-1), and cytotoxic T lymphocyte-associated 4 (CTLA-4) thus allowing immune activation.1–3 ICIs strengthen antitumor immunity, resulting in enhanced progression free survival and in many cases also overall survival in numerous cancer types.4 Expanding indications for ICIs have rapidly increased ICI use among patients with advanced malignancies, as the percentage of US patients with cancer eligible for ICI therapy has risen from 1.5% in 2011 to 38.5% in 2019.5 6
Activation of the immune system by ICIs can also result in complications of treatment known as immune-related adverse events (irAEs).7 irAEs commonly affect the skin, liver, gastrointestinal tract, and endocrine organs, and although the majority are mild, irAEs may range in severity from mild to life threatening and may require hospitalization with immunosuppression.3 8–13 irAEs are stratified by a clinical grading system which guides treatment decisions including whether to continue therapy, suspend therapy, or treat with immunosuppression.3 14–18 irAEs represent a considerable source of morbidity and mortality for the patient due to the adverse event itself, holding or discontinuing ICI therapy, and possible blunting of the ICI stimulated immune response through systemic immunosuppression.11 19 20
Predicting which patients are at risk of severe irAEs is challenging due to multiple factors: wide variability and lack of standardization in clinical definition of irAEs, lack of a specific International Classification of Disease (ICD) code to denote irAEs, and significant discordance among providers in classifying irAEs in clinical practice.10 21 22 Additionally, the data required to use the Common Terminology Criteria for Adverse Events grading system are frequently unavailable in Insurance Claims databases. Further, reporting of irAEs in clinical trials of ICIs has been shown to be inconsistent and suboptimal, indicating a need for standardized methodology to assess irAEs.23 Understanding which patients are at high risk prior to initiation of therapy would allow for early diagnosis and treatment of irAEs.24 Although there have been efforts to model the risk of irAEs among different classes of ICI therapy, previous studies have focused on clinical trial data,23 used single center data,24 or did not report detailed patient demographics for risk factors.25–27 Here, we propose a definition of irAE requiring hospitalization as hospitalization with new or escalated immunosuppression within 2 years of ICI therapy initiation, and identify factors associated with risk of irAEs requiring hospitalization using an Aetna national claims database from the USA.
Methods
Using unidentifiable Aetna insurance national claims data from January 2011 to December 2019, all patients receiving ICI treatment were identified by Current Procedural Terminology (CPT) procedure codes, and separated into three treatment groups, PD-1 or PD-L1, CTLA-4, and combination (CTLA-4 and PD-1/PD-L1) therapy. Combination therapy was defined as receiving ICI in two classes on the same day. Patient cancer diagnoses were identified using ICD codes, and stratified into major cancer groups: melanoma, lung cancer, renal cell carcinoma (RCC), bladder cancer, colon cancer, head and neck squamous cell carcinoma, Hodgkin’s lymphoma, gastric cancer, liver cancer, cervical cancer, breast cancer, prostate cancer, or Merkel cell carcinoma. ICD codes denoting a “personal history,” “cancers in situ,” or “benign” conditions were removed by searching the ICD code text for those strings. Patients with only one cancer diagnosis in the month preceding their first ICI treatment and who had at least three diagnosis codes with that cancer over the study period were included in the study population to minimize contribution from ICD coding errors. Patients with a primary cancer diagnosis of breast and prostate cancer were excluded, as these indications were not Food and Drug Administration (FDA) approved (except those with microsatellite instability-high) for the majority of the study duration28–30). For the analysis, cancers were grouped into melanoma, lung cancer, RCC, and other, which included bladder cancer, colon cancer, head and neck squamous cell carcinoma, Hodgkin’s lymphoma, gastric cancer, liver cancer, cervical cancer, or Merkel cell carcinoma. Demographic data, including age, gender, region, zip-code-imputed income, and zip-code-imputed unemployment from 2010 census data, were calculated for each patient. Charlson Comorbidity Index (CCI) score without contribution from cancer (as all patients had advanced cancer) was calculated for each patient using ICD codes prior to initiation of immunotherapy.31
irAEs requiring hospitalization were defined as any inpatient hospitalization associated with commencement of a new immunosuppressive drug not present in a claim in the 14 days prior to admission, or a dose escalation of an immunosuppressive medication that was present in a claim in the 14 days prior to admission (list of medications in online supplemental table 1) within 2 years after initiation of ICI. Combination ICI was defined as administration of two ICIs administered on the same day, and were codified into Nivolumab combination therapy (Nivolumab and any other ICI) and pembrolizumab combination therapy (pembrolizumab and any other ICI). All occurrences of combination therapy were with these two medications. Inpatient hospitalization was defined as place of service being coded as inpatient, with length of stay defined as the discharge date minus the admission date. Medication type and dose were identified by Healthcare Common Procedure Coding System (HCPCS) and National Drug Code (NDC) code. For each hospitalization, new immunosuppressive medications or a dose escalation of prior immunosuppressive medications were identified, and the highest treatment dose given of each was codified. When dosing was “up to” a certain dose, the maximum dose was used.
jitc-2020-001935supp001.pdf (81.4KB, pdf)
Study outcomes
The primary study outcome is incidence of irAE, requiring hospitalization as defined as a hospitalization with new or dose escalation of immunosuppression within 2 years of ICI initiation. The secondary outcomes are hospitalizations without immunosuppression, intensive care unit (ICU) admission, and immunosuppressant treatment in irAE hospitalizations.
Statistical analysis
Analyses were performed in R V.3.6.3 (R Statistical Software). Continuous variables were compared with a t-test, and categorical variables were compared with the Pearson’s χ2. Linear regressions were performed on continuous variables, and logistic regressions performed on categorical variables. Where data were non-normal (eg, income), variables were normalized using the scale function in R.
Results
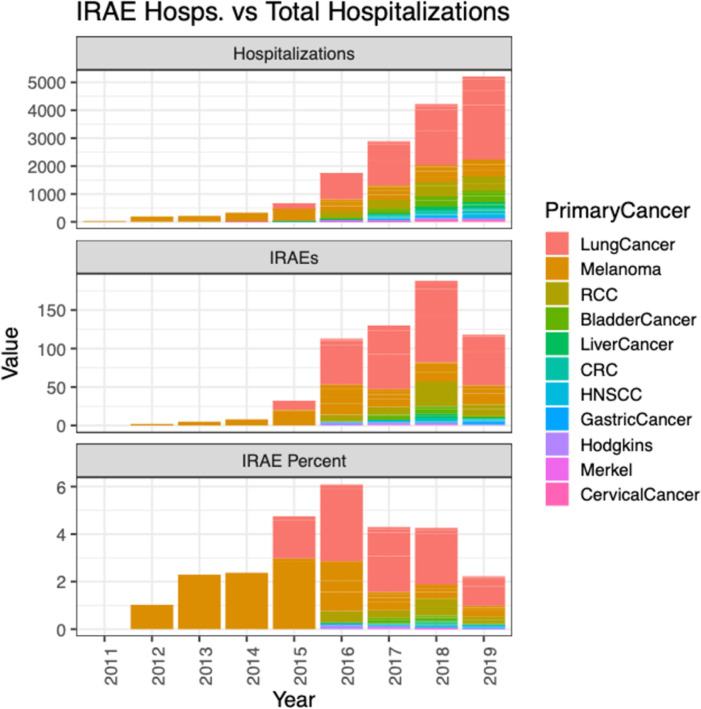
There were 68.8 million patients identified in the national database. Fourteen thousand three hundred seventy-eight patients were identified as having received an ICI administration, an identified primary cancer, and complete demographic information. A summary of demographic characteristic is reported in table 1. The most commonly identified cancer for patients receiving an ICI was lung cancer (N=7393, 51.4%), followed by melanoma (N=3157, 22.0%), RCC (N=1486, 10.3%), and other cancers (N=2342, 16.3%) a group which includes bladder cancer, colon cancer, head and neck squamous cell carcinoma Hodgkin’s lymphoma, gastric cancer, liver cancer, cervical cancer, and Merkel cell carcinoma (table 1). The cumulative follow-up time in years after ICI commencement in the 14 378 ICIs recipients was 19 177 (median follow-up, 927 days for patients with irAE, 736 for patients without irAE), and the total number of irAE admissions identified during those years was 504. The overall incidence rate of irAEs requiring hospital admission per patient year was 2.6%, ranging from 0% in 2011 to 3.7% in 2016 (figure 1, table 2). The most common ICI drug exposure was to pembrolizumab, with 7574 (39.5%) patient years, followed by nivolumab with 7367 (38.4%) patient years, and ipilimumab 1561 (8.1%) patient years (table 2).
Table 1.
Demographic, treatment, and outcome characteristics of patients with and without severe immune-related adverse event (irAE)
| Patients without irAE | Patients with severe irAE | P value | |
| Number (%) | 13 874 (96.5%) | 504 (3.5%) | |
| Men (%) | 8172 (58.9%) | 298 (59.1%) | 0.96 |
| Women (%) | 5702 (41.1%) | 206 (40.9%) | 0.96 |
| Avg age (years) | 66.7 | 63.5 | <0.001 |
| Avg follow-up time (days) | 735.5 | 926.5 | <0.001 |
| Average time to hospitalization (days) | 172.0 | 148.7 | <0.001 |
| Immune checkpoint inhibitor use | |||
| PD-1 | 10 815 (78.0%) | 372 (73.8%) | 0.03 |
| PD-L1 | 1052 (7.6%) | 12 (2.4%) | <0.001 |
| CTLA-4 | 1022 (7.4%) | 42 (8.3%) | 0.47 |
| CTLA4/PD-1 or CTLA-4/PD-L1 combination | 985 (7.1%) | 78 (15.5%) | <0.001 |
| Average ICI treatment Length | 163.0 days | 168.2 days | 0.58 |
| Underlying conditions | |||
| Lung cancer | 7114 (51.3%) | 279 (55.4%) | 0.08 |
| Melanoma | 3036 (21.9%) | 121 (24.0%) | 0.28 |
| Renal cell carcinoma | 1430 (10.3%) | 56 (11.1%) | 0.61 |
| Other cancer | 2294 (16.5%) | 48 (9.5%) | <0.001 |
| Average Charlson Comorbidity Index | 1.5 | 1.5 | 0.90 |
| Secondary outcomes | |||
| Number of hospitalizations | 1 | 3.1 | <0.001 |
| Hospital length (days) | 3.1 | 6 | <0.001 |
| Proportion in ICU | 0.02 | 0.056 | <0.001 |
| Regional information | |||
| Average zip code unemployment | 0.06 | 0.06 | 0.33 |
| Average zip code income | 63 168.1 | 64 332.8 | 0.32 |
| East North Central | 2123 (15.3%) | 80 (15.9%) | 0.77 |
| East South Central | 445 (3.2%) | 14 (2.8%) | 0.68 |
| Mid-Atlantic | 3272 (23.6%) | 110 (21.8%) | 0.39 |
| Mountain | 626 (4.5%) | 24 (4.8%) | 0.88 |
| New England | 661 (4.8%) | 38 (7.5%) | 0.01 |
| Pacific | 1089 (7.8%) | 30 (6.0%) | 0.14 |
| South Atlantic | 3261 (23.5%) | 107 (21.2%) | 0.26 |
| West North Central | 470 (3.4%) | 25 (5.0%) | 0.08 |
| West South Central | 1927 (13.9%) | 76 (15.1%) | 0.49 |
Other cancers include: bladder cancer, colon cancer, head and neck squamous cell carcinoma Hodgkin’s lymphoma, gastric cancer, liver cancer, cervical cancer, and Merkel cell carcinoma.
CTLA-4, cytotoxic T lymphocyte-associated 4; ICI, immune checkpoint inhibitor; ICU, intensive care unit; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
Figure 1.
Incidence of immune-related adverse event (irAE) hospitalizations and total hospitalizations in patients on ICI therapy. CRC, colorectal cancer; HNSCC, head and neck squamous cell carcinoma; Merkel, Merkel Cell Carcinoma; RCC, renal cell carcinoma.
Table 2.
Immune checkpoint inhibitor usage and immune-related adverse event (irAE) incidence over time
| Event | Therapy class | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | Total | |
| Number of patients | 19 177 | % of total | ||||||||||
| Pembrolizumab | PD-1 | – | – | – | – | 113 | 393 | 1341 | 2342 | 3385 | 7574 | 39.5% |
| Nivolumab | PD-1 | – | – | – | – | 422 | 1702 | 1652 | 1922 | 1669 | 7367 | 38.4% |
| Ipilimumab | CTLA-4 | 71 | 193 | 196 | 319 | 306 | 205 | 140 | 74 | 57 | 1561 | 8.1% |
| Nivolumab combination therapy | – | – | – | – | 17 | 129 | 219 | 478 | 566 | 1409 | 7.3% | |
| Atezolizumab | PD-L1 | – | – | – | – | – | 57 | 207 | 278 | 658 | 1200 | 6.3% |
| Avelumab | PD-L1 | – | – | – | – | – | – | 6 | 31 | 26 | 63 | 0.3% |
| Pembrolizumab combination therapy | – | – | – | – | – | – | 2 | 1 | – | 3 | 0.0% | |
| Number of irAEs | 504 | |||||||||||
| Pembrolizumab | PD-1 | – | – | – | – | – | 13 | 36 | 67 | 50 | 166 | 32.9% |
| Nivolumab | PD-1 | – | – | – | – | 10 | 58 | 52 | 54 | 32 | 206 | 40.9% |
| Ipilimumab | CTLA-4 | – | 2 | 4 | 6 | 17 | 6 | 6 | 1 | – | 42 | 8.3% |
| Nivolumab combination therapy | – | – | – | – | – | 16 | 11 | 30 | 21 | 78 | ||
| Atezolizumab | PD-L1 | – | – | – | – | – | – | 4 | 5 | 2 | 11 | |
| Avelumab | PD-L1 | – | – | – | – | – | – | – | 1 | – | 1 | |
| irAE/patient year | 0.0% | 1.0% | 2.0% | 1.9% | 3.1% | 3.7% | 3.1% | 3.1% | 1.7% | 2.6% | ||
CTLA-4, cytotoxic T lymphocyte-associated 4; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
The incidence of irAEs requiring hospitalization as defined by hospitalization with new or escalated immunosuppression within 2 years of ICI initiation was 504/14 378 patients (3.5%) (table 2). irAEs requiring hospitalization were identified in 372 (3.3%) patients on PD-1 therapy, 12 (1.1%) on PD-L1 therapy, 42 (3.9%) on CTLA-4 therapy, and 78 (7.3%) on combination CTLA-4 and PD-1 or PD-L1 therapy. The average time from start date of ICI treatment to irAE requiring hospitalization was 168.2 days (range: 1–722). Seven thousand five hundred eighty-seven (53%) patients were hospitalized in 16 053 hospitalizations within 2 years of immunotherapy initiation. The total number of patients that were hospitalized more than once was 3888 (27%). Patients with irAE requiring hospitalization had more total hospitalizations than those without irAE requiring hospitalization in the 2 years after ICI initiation (3.1 vs 1 hospitalizations, p<0.001). Length of stay was greater for patients with irAEs requiring hospitalization than those without irAE requiring hospitalization (6 vs 3.1 days, p<0.001). Patients with irAE requiring hospitalization were more likely to be in the ICU than those without (0.06 vs 0.02 ICU visits per patient, p<0.001).
Multivariable modeling
Regressions were performed on the incidence of irAEs requiring hospitalization, all cause hospitalizations, and ICU stay (tables 3–5). In each regression, female patients with lung cancer on PD-1/PD-L1 therapy were used as the reference group.
Table 3.
Multivariate regression results for incidence of severe immune-related adverse event
| Estimate | SE | Statistic | P value | OR | OR SE | |
| Patient characteristics | ||||||
| Age | −0.02 | 0.00 | −5.49 | <0.001 | 0.98 | 0.05 |
| Male gender | 0.07 | 0.09 | 0.76 | 0.45 | 1.07 | 0.15 |
| Charlson Comorbidity Index (excluding cancer) | 0.03 | 0.02 | 1.61 | 0.11 | 1.03 | 0.23 |
| Immune checkpoint inhibitor use | ||||||
| CTLA-4 therapy | 0.29 | 0.2 | 1.42 | 0.16 | 1.34 | 0.2 |
| Combination ICI therapy | 0.89 | 0.14 | 6.18 | <0.001 | 2.44 | 0.11 |
| Underlying conditions | ||||||
| Other cancer | −0.69 | 0.16 | −4.29 | <0.001 | 0.5 | 0.03 |
| Melanoma | −0.35 | 0.14 | −2.45 | 0.01 | 0.71 | 0.21 |
| Renal cell carcinoma | −0.35 | 0.16 | −2.17 | 0.03 | 0.71 | 0.11 |
| Regional information | ||||||
| Zip code average income (normalized) | 0.04 | 0.05 | 0.74 | 0.46 | 1.04 | 0.17 |
| Zip code average unemployment (normalized) | −0.02 | 0.05 | −0.39 | 0.7 | 0.98 | 0.16 |
| East South Central | −0.25 | 0.3 | −0.83 | 0.41 | 0.78 | 0.02 |
| Mid-Atlantic | −0.17 | 0.15 | −1.09 | 0.28 | 0.85 | 0.14 |
| Mountain | −0.08 | 0.24 | −0.32 | 0.75 | 0.93 | 0.19 |
| New England | 0.35 | 0.21 | 1.71 | 0.09 | 1.42 | 0.34 |
| Pacific | −0.37 | 0.22 | −1.68 | 0.09 | 0.69 | 0.14 |
| South Atlantic | −0.18 | 0.15 | −1.18 | 0.24 | 0.84 | 0.2 |
| West North Central | 0.23 | 0.24 | 0.96 | 0.34 | 1.26 | 0.12 |
| West South Central | −0.04 | 0.17 | −0.23 | 0.82 | 0.96 | 0.14 |
Note: reference group is female patients on programmed cell death protein 1 or programmed death-ligand 1 therapy with lung cancer in East North Central Region.
Other cancers include: bladder cancer, colon cancer, head and neck squamous cell carcinoma Hodgkin’s lymphoma, gastric cancer, liver cancer, cervical cancer, and Merkel cell carcinoma.
CTLA-4, cytotoxic T lymphocyte-associated 4; ICI, immune checkpoint inhibitor.
Table 4.
Multivariate regression results for incidence of hospitalization
| Variable | Estimate | SE | Statistic | P value | OR | OR SE |
| Patient characteristics | ||||||
| Age | 0.00 | 0.00 | 0.81 | 0.42 | 1.00 | 0.01 |
| Male gender | −0.02 | 0.03 | −0.71 | 0.48 | 0.98 | 0.07 |
| Charlson Comorbidity Index (excluding cancer) | 0.06 | 0.01 | 7.19 | <0.001 | 1.06 | 0.02 |
| Immune checkpoint inhibitor use | ||||||
| CTLA4 therapy | 0.85 | 0.08 | 10.81 | <0.001 | 2.34 | 0.04 |
| Combination ICI therapy | 0.53 | 0.07 | 7.55 | <0.001 | 1.7 | 0.03 |
| Underlying conditions | ||||||
| Other cancer | −0.08 | 0.05 | −1.59 | 0.11 | 0.93 | 0.06 |
| Melanoma | −0.6 | 0.05 | −11.39 | <0.001 | 0.55 | 0.03 |
| Renal cell carcinoma | −0.28 | 0.06 | −4.75 | <0.001 | 0.75 | 0.07 |
| Regional information | ||||||
| Zip code average income (normalized) | 0.04 | 0.02 | 1.94 | 0.05 | 1.04 | 0.01 |
| Zip code average unemployment (normalized) | 0.04 | 0.02 | 2.28 | 0.02 | 1.05 | 0.07 |
| East South Central | ||||||
| Mid-Atlantic | −0.12 | 0.06 | −2.11 | 0.04 | 0.89 | 0.03 |
| Mountain | −0.33 | 0.09 | −3.63 | <0.001 | 0.72 | 0.05 |
| New England | −0.16 | 0.09 | −1.8 | 0.07 | 0.85 | 0.04 |
| Pacific | −0.37 | 0.08 | −4.93 | <0.001 | 0.69 | 0.04 |
| South Atlantic | −0.27 | 0.06 | −4.89 | <0.001 | 0.76 | 0.07 |
| West North Central | −0.19 | 0.1 | −1.87 | 0.06 | 0.83 | 0.04 |
| West South Central | −0.19 | 0.06 | −2.96 | <0.01 | 0.83 | 0.05 |
Note: reference group is female patients on programmed cell death protein 1 or programmed death-ligand 1 therapy with lung cancer in East North Central Region.
Other cancers include: bladder cancer, colon cancer, head and neck squamous cell carcinoma Hodgkin’s lymphoma, gastric cancer, liver cancer, cervical cancer, and Merkel cell carcinoma.
CTLA4, cytotoxic T lymphocyte-associated 4; ICI, immune checkpoint inhibitor.
Table 5.
Multivariate regression results for incidence of intensive care unit admission
| Estimate | SE | Statistic | P value | OR | OR SE | |
| Patient characteristics | ||||||
| Age | −0.03 | 0.00 | −5.41 | <0.001 | 0.97 | 0.00 |
| Male gender | 0.11 | 0.12 | 0.89 | 0.37 | 1.12 | 0.28 |
| Charlson Comorbidity Index (excluding cancer) | 0.06 | 0.03 | 2.36 | 0.02 | 1.06 | 0.07 |
| Immune checkpoint inhibitor use | ||||||
| CTLA4 therapy | 0.36 | 0.25 | 1.47 | 0.14 | 1.44 | 0.29 |
| Combination ICI therapy | 0.21 | 0.22 | 0.96 | 0.34 | 1.23 | 0.46 |
| Underlying conditions | ||||||
| Other cancer | 0.11 | 0.17 | 0.67 | 0.5 | 1.12 | 0.24 |
| Melanoma | −0.07 | 0.19 | −0.38 | 0.7 | 0.93 | 0.19 |
| Renal cell carcinoma | 0.11 | 0.2 | 0.55 | 0.58 | 1.12 | 0.03 |
| Regional information | ||||||
| Zip code average income (normalized) | 0.17 | 0.06 | 2.72 | 0.01 | 1.19 | 0.08 |
| Zip code average unemployment (normalized) | 0.05 | 0.07 | 0.68 | 0.5 | 1.05 | 0.37 |
| East South Central | ||||||
| Mid-Atlantic | −0.08 | 0.2 | −0.39 | 0.7 | 0.92 | 0.16 |
| Mountain | 0.21 | 0.29 | 0.7 | 0.48 | 1.23 | 0.25 |
| New England | −0.58 | 0.37 | −1.56 | 0.12 | 0.56 | 0.21 |
| Pacific | 0.15 | 0.25 | 0.6 | 0.55 | 1.16 | 0.14 |
| South Atlantic | −0.1 | 0.21 | −0.48 | 0.63 | 0.91 | 0.17 |
| West North Central | 0.11 | 0.35 | 0.31 | 0.76 | 1.11 | 0.27 |
| West South Central | 0.22 | 0.22 | 1 | 0.32 | 1.24 | 0.37 |
Note: reference group is female patients on programmed cell death protein 1or programmed death-ligand 1 therapy with Lung Cancer in East North Central Region.
Other cancers include: bladder cancer, colon cancer, head and neck squamous cell carcinoma Hodgkin’s lymphoma, gastric cancer, liver cancer, cervical cancer, and Merkel cell carcinoma.
CTLA4, cytotoxic T lymphocyte-associated 4; ICI, immune checkpoint inhibitor.
Severe irAE incidence regression (table 3): compared with the reference group, combination ICI therapy (anti-PD-1/PD-L1 and CTLA-4) was associated with increased odds of irAE requiring hospitalization (OR: 2.44, p<0.001). Older patients (OR 0.98 per additional year, p<0.001), patients with other cancers (OR 0.50, p<0.001), patients with melanoma (OR 0.71, p=0.01) and patients with RCC (OR 0.71, p=0.03) were associated with decreased odds of a irAE requiring hospitalization than the reference group.
Hospitalization incidence regression (table 4): compared with the reference group, anti-CTLA-4 therapy (OR: 2.34, p<0.001), combination ICI therapy (OR: 1.70, p<0.001), and higher non-cancer CCI (OR: 1.06, p<0.001) were associated with increased odds of all cause hospitalization. Patients with melanoma were associated with decreased odds of all cause hospitalization compared with the reference group (OR: 0.55, p<0.001). Region was also associated with all cause hospitalization, notably decreased in the Pacific region (OR: 0.69, p<0.001).
ICU stay incidence regression (table 5): compared with the reference group, older patients were associated with decreased odds of all cause admission to the ICU (OR: 0.97 per additional year, p<0.001). Patients with an irAE hospitalization (OR: 2.60, p<0.001), higher non-cancer CCI (OR: 1.07, p=0.01), and higher zip code average income (OR: 1.19, p=0.01) were associated with increased odds of all cause ICU admission than the reference group.
Immunosuppressant use in hospitalizations
Of the immunosuppressants identified in online supplemental table 1), prednisone (72%) and methylprednisolone (25%) accounted for the majority of immunosuppressant medications found in irAE hospitalizations. The remaining identified immunosuppressants each accounted for less than 1% immunosuppressive medications identified during an irAE hospitalization: azathioprine, cyclosporine, everolimus, infliximab, methotrexate, mycophenolate, sirolimus, and tacrolimus (table 6).
Table 6.
Number of immune-related adverse events hospitalizations with administration of immunosuppressants
| Immunosuppressant | Number of hospitalizations |
| Prednisone | 454 (71.8%) |
| Methylprednisolone | 160 (25.3%) |
| Mycophenolate | 5 (0.8%) |
| Tacrolimus | 3 (0.5%) |
| Cyclosporine | 2 (0.3%) |
| Everolimus | 2 (0.3%) |
| Infliximab | 2 (0.3%) |
| Sirolimus | 2 (0.3%) |
| Azathioprine | 1 (0.2%) |
| Methotrexate | 1 (0.2%) |
| Basiliximab | 0 |
| IVIG | 0 |
| Leflunomide | 0 |
| Rituximab | 0 |
| Tocilizumab | 0 |
| Vedolizumab | 0 |
IVIG, Intravenous immunoglobulin.
Discussion/conclusion
ICIs have shown great promise in treating a variety of advanced cancers. However, activation of the immune system during treatment frequently leads to immune-related adverse events in many patients. This study describes the experience of ICI use and the incidence of irAEs requiring hospitalization for patients in a national Aetna insurance database, and proposes an algorithm to identify and study irAEs requiring hospitalization using claims data. The results of this study can be used to identify patients at higher risk of being admitted with irAE and enable analysis of other large claims databases to compare experience across payors and institutions.
Our study showed that the absolute incidence of irAEs requiring hospitalization in the study population increased over time from 2011 to 2019, a finding that is consistent with the increased use of ICI over that time period. We demonstrate that combination therapy is associated with increased odds of irAE requiring hospitalization, which is in agreement with prior findings that combination therapy32 and CTLA-4 therapy23 33–35 are associated with higher incidence of irAEs relative to PD-1 or PD-L1 treatment. The incidence of irAEs requiring hospitalization in this study was 3.5% overall, with 3.3% for patients receiving PD-1, 1.1% for patients receiving PD-L1, 3.9% for patients receiving CTLA-4 therapy, and 7.3% for patients on combination therapy. These figures are lower compared with early estimates of clinical trial data, which reported high-grade irAEs in 6.3% of patients with PDL-1, 7.1% of patients taking PD-1, 21.5% of patients taking CTLA-4, and 54.8% in CLTA-4 and PD-1 combination therapy.34 Similarly, a meta-analysis of phases II and III clinical trials showed incidence of grade 3 or 4 immune-related adverse events for atezolizumab, nivolumab, pembrolizumab, and ipilimumab of 15.1%, 14.1%, 19.8%, and 28.6%, respectively.36 However, a recent publication identified a similar rate (3.6%) of high-grade (grade 3+) irAEs in a single-center experience with PD-1 therapy.24 The lower rate found in our population relative to clinical trial data may be representative of less toxic ICI treatment regimens or reflect real-world treatment patterns and identification of high-grade events outside of clinical trial settings. Additionally, our study would not include irAE admissions where immunosuppression is not indicated for treatment, such as endocrinopathies. In our study, average hospitalization length of stay for irAE patients was 6 days, which is consistent with previous study results.32 Our study reports that patients with severe irAEs were more likely to have an ICU admission (OR 2.60, p<0.001), which is not a widely reported outcome in large scale ICI studies.34
As ICI indications have expanded and use of ICIs has continued to grow, the percentage of patients with cancer eligible for ICI therapy has been estimated at up to 38.5% in 2019, representing up to 233 790 potential US ICI patients treated with standard of care therapy alone.6 If, as in our study population, 3.5% of patients receiving ICI therapy experience irAEs requiring hospitalization, that would represent approximately 8200 hospitalizations if all eligible patients received therapy—a population that is expected to grow.6 This increasing incidence of irAEs requiring hospitalization necessitates greater knowledge and training among emergency department, primary care, internal medicine, and specialty providers to accurately diagnose and treat severe immune-related adverse events.37 If these irAEs are not treated appropriately, there is risk of substantially increased morbidity and mortality.26
Additionally, these data show that older age is protective of both irAEs requiring hospitalization and ICU stay. Previous studies have shown mixed results between age and the risk of irAE in ICI recipients, with alternate studies finding no association between age and irAEs,7 higher incidence with older patients38 and higher incidence with younger patients.39 With regards to the likelihood of ICU stay, our findings are similar to a single center study showing that patients <70 years old have a higher likelihood of an ICU stay as well as longer ICU stays than older patients.38 This may be due to relatively less severe irAEs, immune senescence in older patients, differing goals of care, more cautious treatment in the elderly, lower proportion of combination ICI therapy in the elderly, or a combination of all. Male gender was not predictive of irAE, hospitalization, or ICU stay in our study. A study reviewing PD-1 therapy found a greater incidence of all grade irAEs in women, but no gender difference in high-grade irAEs, which is more similar to the irAE definition in this study.40 This study also showed that severe irAEs are more frequent among patients with lung cancer than patients with RCC, melanoma, or other primary cancers. The difference in incidence may be due different provider experience using ICI therapy among these cancer types. Although previous studies have shown differences in type of irAEs for different primary cancers,33 the current analysis of irAEs requiring hospitalization defines the overall likelihood of developing an irAE requiring hospitalization by primary cancer.
We show that by far the most frequent immunosuppressive treatments used with patients on ICIs in this study are prednisone (72%) and methylprednisolone (25%), with limited use of other immunosuppressants. This is consistent with guidance that grade 3+irAEs should be treated with prednisone or methylprednisolone, and other immunosuppressants such as infliximab should be reserved for those who do not respond to initial therapy.3 This result signifies that few (3%) hospitalizations for severe irAEs require immunosuppressive medication other than prednisone or methylprednisolone. These data imply that in our population, refractory irAEs requiring second line immunosuppression are not common, and incidence of such refractory toxicities is likely low.
This analysis is limited by a retrospective study design, lack of comprehensive mortality data, and inability to review chart-level data to determine clinician interpretation of suspected irAEs. As a result, our definition of severe irAEs is likely overly strict and limited to patients with more severe events than what has been previously reported in literature. In particular, this definition of severe irAE used in this analysis would not include low-grade toxicities that did not result in hospitalization with new or escalated immunosuppression, and as such the results may not be generalizable to low-grade irAE and the overall number is likely underestimated. Additionally, the definition of severe irAE in this analysis is impacted by regional, hospital system, and individual provider capabilities and practice patterns; for example, the ability of a hospital to provide emergency care for an irAE in the inpatient setting. Also, as our inclusion criteria involve selecting for patients with cancer diagnosis codes in a post-treat matter, it may incur collider bias. In this first, population-level analysis of immunotherapy toxicities in the USA, we describe a method of querying large, insurance claims databases for suspected high-grade irAEs and identify the incidence, risk factors, and outcomes associated with severe irAEs. We further identified patient factors that can be used to predict the likelihood of severe irAEs and lay the methodological basis for future research on this topic utilizing insurance claims data. Additionally, we described that patients with an identified irAE hospitalization have more total hospitalizations in the 2 years after starting ICI (3.1 vs 1 hospitalizations), and the length of stay for irAE hospitalizations is double that for non-irAE hospitalizations (6 vs 3.1 days). This, coupled with the increasing incidence of ICI use, underscore the importance of understanding the likelihood of and factors related to severe irAEs and large, population-level databases will enable researchers to answer important questions regarding risk factors and outcomes for severe irAEs that cannot be answered in clinical trials or with individual studies. It is critical that we build on this study to further refine queries and that open-access is granted to replicate these important findings across other databases.
Acknowledgments
The authors thank Susan Churchill of the Department of Biomedical Informatics in the Blavatnik Institute at Harvard Medical School and Kathe Fox of Aetna for providing assistance with claims data access.
Footnotes
MK and WM contributed equally.
Contributors: MK and WM contributed equally to this manuscript. KLR and YRS contributed equally to this manuscript. MK, WM, SW, VP, and CL performed analyses, contributed to study design, and drafted the manuscript. K-HY, FW, LZ, VN, AG, SK, KLR, and YRS contributed to study design, and manuscript review.
Funding: This work was supported by the National Institutes of Health: 5T32GM007309, T32GM007753, and F30CA224588 awarded to Wongvibulsin, S and Kalinich, M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Yu, KH is supported in part by the Blavatnik Center for Computational Biomedicine Award.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data are licensed unidentifiable insurance claims data from a national insurer.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Harvard Medical School Institutional Review Board granted blanket approval for the use of the de-identified claims database.
References
- 1.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94. 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer 2018;6:8. 10.1186/s40425-018-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S, Johnson DB. Immune-Related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019;2:e192535. 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open 2020;3:e200423. 10.1001/jamanetworkopen.2020.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ksienski D, Wai ES, Croteau NS, et al. Association of age with differences in immune related adverse events and survival of patients with advanced nonsmall cell lung cancer receiving pembrolizumab or nivolumab. J Geriatr Oncol 2020;11:807–13. 10.1016/j.jgo.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 8.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 9.Kosche C, Stout M, Sosman J, et al. Dermatomyositis in a patient undergoing nivolumab therapy for metastatic melanoma. Melanoma Res. September 2019;1. [DOI] [PubMed] [Google Scholar]
- 10.Sosa A, Lopez Cadena E, Simon Olive C, et al. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol 2018;10:175883591876462–11. 10.1177/1758835918764628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691–7. 10.1200/JCO.2012.41.6750 [DOI] [PubMed] [Google Scholar]
- 13.Inno A, Metro G, Bironzo P, et al. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori 2017;103:405–21. 10.5301/tj.5000625 [DOI] [PubMed] [Google Scholar]
- 14.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. j. immunotherapy cancer 2017;5:1–28. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol 2020;83:1255–68. 10.1016/j.jaad.2020.03.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 2017;35:3807–14. 10.1200/JCO.2017.73.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassel JC, Heinzerling L, Aberle J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev 2017;57:36–49. 10.1016/j.ctrv.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 18.Rudzki JD. Management of adverse events related to checkpoint inhibition therapy. Memo 2018;11:132–7. 10.1007/s12254-018-0416-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. j. immunotherapy cancer 2017;5. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamal S, Hudson M, Fifi-Mah A, et al. Immune-Related adverse events associated with cancer immunotherapy: a review for the practicing rheumatologist. J Rheumatol 2020;47:166–75. 10.3899/jrheum.190084 [DOI] [PubMed] [Google Scholar]
- 21.Hsiehchen D, Watters MK, Lu R, et al. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw Open 2019;2:e1911519. 10.1001/jamanetworkopen.2019.11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–92. 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 23.Chen TW, Razak AR, Bedard PL, et al. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann Oncol 2015;26:1824–9. 10.1093/annonc/mdv182 [DOI] [PubMed] [Google Scholar]
- 24.Eun Y, Kim IY, Sun J-M, et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep 2019;9:1–8. 10.1038/s41598-019-50574-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang P-F, Chen Y, Song S-Y, et al. Immune-Related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol 2017;8:730. 10.3389/fphar.2017.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baxi S, Yang A, Gennarelli RL, et al. Immune-Related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. 10.1136/bmj.k793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komaki Y, Komaki F, Yamada A, et al. Meta-Analysis of the risk of immune-related adverse events with Anticytotoxic T-Lymphocyte-Associated antigen 4 and Antiprogrammed death 1 therapies. Clin Pharmacol Ther 2018;103:318–31. 10.1002/cpt.633 [DOI] [PubMed] [Google Scholar]
- 28.Narayan P, Wahby S, Gao JJ, et al. Fda approval summary: Atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin Cancer Res 2020;26:2284–9. 10.1158/1078-0432.CCR-19-3545 [DOI] [PubMed] [Google Scholar]
- 29.Vaddepally RK, Kharel P, Pandey R, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020;12:738. 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019;2:e192535. 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 32.Balaji A, Zhang J, Wills B, et al. Immune-Related adverse events requiring hospitalization: spectrum of toxicity, treatment, and outcomes. J Oncol Pract 2019;15:e825–34. 10.1200/JOP.18.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377–85. 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- 34.El Osta B, Hu F, Sadek R, et al. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 2017;119:1–12. 10.1016/j.critrevonc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 35.Wang LX, Quach HT, Moodabigil NV, et al. Health care utilization and steroid-refractory toxicities from immune checkpoint inhibitors. Cancer 2020;126:322–8. 10.1002/cncr.32542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C, Chen Y-P, Du X-J, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 2018;7:k4226. 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson DB, Reynolds KL, Sullivan RJ, et al. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol 2020;21:e398–404. 10.1016/S1470-2045(20)30107-8 [DOI] [PubMed] [Google Scholar]
- 38.Ahmed T, Lycan T, Dothard A, et al. Performance status and age as predictors of immunotherapy outcomes in advanced Non–Small-Cell lung cancer. Clin Lung Cancer 2020;21:e286–93. 10.1016/j.cllc.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah KP, Song H, Ye F, et al. Demographic factors associated with toxicity in patients treated with Anti–Programmed cell death-1 therapy. Cancer Immunol Res 2020;8:851–5. 10.1158/2326-6066.CIR-19-0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duma N, Abdel‐Ghani A, Yadav S, et al. Sex differences in tolerability to Anti‐Programmed cell death protein 1 therapy in patients with metastatic melanoma and Non‐Small cell lung cancer: are we all equal? Oncologist 2019;24:e1148. 10.1634/theoncologist.2019-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001935supp001.pdf (81.4KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data are licensed unidentifiable insurance claims data from a national insurer.