Abstract

Heterogeneous catalysis plays an important role in many chemical reactions, especially those applied in industrial processes, and therefore, its theoretical foundations are introduced not only to students majoring in chemical engineering or catalysis but also as part of general chemistry courses. The consideration of catalytic activity of various solids and mechanisms of catalytic reactions requires the introduction of the concept of an active site, which together with the catalyst specific surface area are discussed as key parameters controlling the reaction rate. There are many known demonstrations of heterogeneous catalysis phenomena that can be performed live in a lecture hall, but all of them focus only on the general idea of catalytic processes and are not suitable for quantitative analysis. Therefore, herein we present a simple demonstration of the influence of the specific surface area of a catalyst on the rate of a catalytic reaction. This demonstration is based on a model reaction of hydrogen peroxide decomposition catalyzed by cobalt spinel (Co3O4) calcined at various temperatures. The differences in reaction rates can be monitored visually, and the obtained data can be used directly for a simple kinetic analysis, including comparison of numerical values of the reaction rate constants.
Keywords: First-Year Undergraduate/General, High School/Introductory Chemistry, Demonstrations, Catalysis, Kinetics
Introduction
Heterogeneous catalysis is an important area of chemistry, mainly because of its wide industrial applications and environmental benefits. Technological processes involving solid catalysts have enormous importance for society because of their role in the production of food (to make fertilizers), fuels,1,2 polymers, and various industrial chemicals (methanol, ethylene oxide, nitric acid, sulfuric acid, etc.) as well as in air pollution abatement (e.g., in removal of NOx from automotive and industrial exhaust gases via selective catalytic reduction, catalytic soot oxidation, N2O decomposition).3−6
Heterogeneous catalytic reactions are complex processes that involve interactions of liquid and/or gas reactant(s) with the surface of a solid catalyst.7 The concept of an active site (i.e., a particular surface atom or group of such atoms exhibiting special structure and properties, where the catalyzed transformation takes place) is central to discussion of the catalytic reaction mechanisms. The nature of the active sites of working catalysts may be unraveled with the use of fundamental studies,8,9 and their specific activity can be further improved. However, when an active site reaches the top intrinsic performance, it is the concentration of available active sites per unit volume of a reactor or per unit catalyst mass that determines the efficiency of the catalytic process. Usually, the number of active sites per unit surface area is very similar for materials of a given type, and thus, the extent of exposed surface of a catalyst determines its overall activity. While the reaction kinetics in a catalytic reactor may be influenced by many different factors, such as diffusion kinetics, adsorption–desorption kinetics, and surface reactions equilibria, it is the number of active sites that seems to be the key parameter controlling the reaction rate. Thus, an important strategy for improving the performance of catalysts involves increasing the number of accessible active sites in the reactor. This may be achieved by increasing the specific surface area (SSA) of the catalyst.
It is not easy to make a live demonstration showing the influence of the number of active sites on the catalytic reaction rate during lectures in an ordinary lecture hall. There are several examples of heterogeneous catalytic reactions that are simple enough for such classroom demonstrations, i.e., catalytic oxidation of alcohols,10 ammonia,11,12 acetone,13 and sulfur dioxide;14 reduction of alcohols;15,16 and variations on the decomposition of hydrogen peroxide, such as H2O2 decomposition catalyzed by manganese dioxide,17 transition metals,18 or Cr2O72– adsorbed on solids.19 Those experiments allow demonstration of the heterogeneous catalysis phenomenon but are not suitable for quantitative analysis of the influence of the number of active sites (which is directly proportional to the specific surface area) on the reaction rate. Therefore, our aim was to develop such an experiment suitable for demonstration in an ordinary classroom or lecture hall.
The choice of the decomposition of hydrogen peroxide as a model reaction was dictated by the simplicity of the reaction setup, harmless gaseous product of the reaction, and a common, inexpensive substrate. There are two best-known approaches for demonstrations of hydrogen peroxide decomposition: (1) the reaction catalyzed by potassium iodide solution in the presence of a detergent, called “Elephant’s Toothpaste”,20−26 and (2) the reaction catalyzed by solid manganese oxide, called “Genie in the Bottle” or “Aladdin’s Lamp”.17,27,28 Recently, the influence of the specific surface area of the catalyst was reported in the second case, but only in the context of lowering the rate of the reaction using MnO2 with a larger particle size (44 μm) for safety purposes27 without the possibility of systematic quantitative analysis. The approach proposed herein is a blend of the above two methods. The procedure is based on observation of the volume of evolved foam (as in the first case) but catalyzed by a solid that is insoluble in water, namely, cobalt spinel (Co3O4).
Cobalt-spinel-based catalysts are very practical materials that have attracted great interest especially in environmental and electrocatalytic applications. The ease of bulk and surface promotion of Co3O4 allows preparation of various catalysts with tailored properties. A very efficient N2O decomposition catalyst can be obtained by surface or bulk promotion or both combined.6,29,30 Total oxidation of an increasingly popular fuel for mobile engines, methane, which reduces CH4 slip to the atmosphere, can be achieved with cobalt spinel used as a reactive support for palladium nanocrystals.31,32 In electrocatalytic applications, bulk-promoted cobalt spinel is one of the most active phases for the oxygen evolution reaction, a half-reaction in water electrolysis.33,34 We previously reported the possibility of using cobalt spinel material to study modification of the electronic properties of a catalyst during students’ hands-on laboratory exercises.35 Moreover, the activity of this catalyst in hydrogen peroxide decomposition is known36 and can be easily adopted for the proposed demonstration. It should be noted that nanocrystalline Fe2O3 was also tested for demonstration purposes, but its activity was not satisfactory.
Demonstration
A detergent solution is prepared with about 10 mL of the detergent per 100 mL of water, It should be stirred well but without intense foaming. Four 250 mL graduated cylinders are charged with 10 mL of the detergent solution. Effort should be made to avoid wetting the cylinders’ walls (e.g., by using a pipet to add the liquid), but high precision is not required in this step. By the use of a funnel with a long stem, 0.25 g samples of Co3O4 spinel calcined at various temperatures are placed into the cylinders (for comparison, results obtained using 0.5 g are given in the Supporting Information). The mixtures are stirred gently to get uniform suspensions, and the cylinders are arranged according to increasing spinel calcination temperature, as shown in Figure 1. Four automatic pipettes with 10 mL tips are filled with 5 mL of 10% w/w hydrogen peroxide (33.3 mL of 30% hydrogen peroxide diluted to 100 mL), and the hydrogen peroxide is added to all four cylinders simultaneously. This part requires two persons using both hands, and it may require some practice to add the contents at the same time and at similar rates. The process should be recorded, as the video can be useful for later data analysis. After the presentation, the contents of the cylinders should be transferred to an appropriate container for disposal.
Figure 1.
Scheme of the experiment and screenshot from the demonstration video after 20 s of reaction.
Experimental Section
Cobalt Spinel Preparation
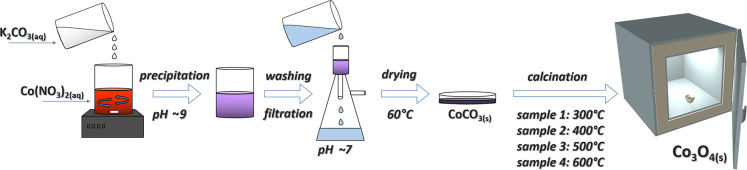
Co3O4 is usually obtained via calcination of cobalt(II) carbonate or hydroxide at various temperatures. Cobalt carbonate can be purchased from a commercial supplier, but it can be easily synthesized from the more common cobalt nitrate hexahydrate (Figure 2). An additional benefit of in-lab synthesis of cobalt carbonate is the certainty that the product is in the nanocrystalline form.
Figure 2.
Schematic of the cobalt spinel synthesis.
A 0.5–2 mol/dm3 solution of cobalt(II) nitrate is used to precipitate the spinel precursor, cobalt(II) carbonate. A solution of potassium or sodium carbonate can be used as the precipitating agent. The amount of carbonate anions can be set as the stoichiometric amount with an extra 20%, or it can be added as a ca. 15 wt % solution until the pH of the solution with the precipitate reaches 9. The slurry is then washed with distilled water on a Büchner funnel. The dry powder precursor is obtained from the wet precipitate after drying at ca. 60 °C for 2 h or more. Cobalt spinel samples are obtained by heat treatment of the precursor at temperatures of 300, 400, 500, and 600 °C for 2 h. Prior to the demonstration, the black Co3O4 materials are thoroughly ground to obtain fine powders.
Video Recording and Data Digitalization
A video-recorded experiment can be analyzed, and on its basis reaction rates can be calculated. Digitalization of the data can be done with video tracking software. For this purpose we used CMA Coach 6,37 the program dedicated for school use. Video analysis requires high contrast between the front of the created foam and the background. The foam is gray, so either an intense bright or deep dark background can be used. However, good lighting in the room is required. Video analysis delivers volume versus time datasets with the selected sampling rate. The results presented below were obtained from video recorded with a frame rate of 29.97 frames/s, and every fourth frame was used for data sampling (the video is available in the Supporting Information). The obtained data are shown in Figure 3 and can be further analyzed in CMA Coach software or transferred to a spreadsheet.
Figure 3.
Screenshot from video analysis of the recorded demonstration.
Hazards
General laboratory safety procedures should be followed throughout this experiment including the use of standard personal protective equipment with mandatory goggles, gloves, and a lab coat. Co3O4 should be handled with care, as it may irritate the skin, eyes, and respiratory tract. It is harmful if swallowed. Grinding of spinels should be done in a fume hood or using respiratory protection. Calcination of cobalt carbonate should be done in a well-ventilated room. The 30% H2O2 is a strong oxidizer; contact with any type of combustibles may cause a fire. Contact of H2O2 with the skin and eyes could cause severe burns. H2O2 should be kept away from small organic molecules such as acetone, which can result in the formation of unstable, highly explosive organic peroxides.38 All chemicals and the after-reaction mixture should be transferred to appropriate containers for disposal.
Discussion
Cobalt spinel samples obtained by calcination of cobalt carbonate at several temperatures are characterized by different values of specific surface areas. The SSA decreases as the heat treatment temperature increases because of recrystallization and sintering (see suggestions for further reading in the Supporting Information). The values of SSA for the obtained Co3O4 samples, measured by nitrogen adsorption at liquid nitrogen temperature, are presented in Figure 4.
Figure 4.

Specific surface areas of Co3O4 samples calcinated at various temperatures.
The differences in catalytic activity of the studied spinels can clearly be seen with the naked eye, but the presentation of the influence of specific surface area on reaction rate in heterogeneous catalysis requires determination of the reaction rate for each case. Video analysis of the recorded demonstration delivers the required data—the changes of foam volume over time. The results for 0.25 g loadings of Co3O4 catalysts calcined at various temperatures are presented in Figure 5, and analogous results for 0.5 g loadings of the catalysts are presented in the Supporting Information. The analysis of the numerical data presented in Figure 5 allows determination of Vt and Vmax, which denote the volume of released oxygen at a particular time of the reaction and the total volume of released oxygen, respectively.
Figure 5.

Video-based analysis of changes of O2 (foam) volume evolved during H2O2 decomposition over time from a demonstration using 0.25 g loadings of spinel catalysts calcined at (a) 300, (b) 400, (c) 500, and (d) 600 °C.
The overall hydrogen peroxide decomposition reaction is presented in eq 1,
| 1 |
and it can be described with the first-order kinetics. The detailed derivation of the mathematical formula is presented in the Supporting Information. Plotting the ln(Vmax – Vt) values against time should yield a straight line with the reaction rate constant equal to the negative value of the slope, as presented in the initial parts of the plots in Figure 6a. For clarity of the demonstration, an open vessel was chosen as the H2O2 decomposition reactor. This setup affects the accuracy of the kinetic analysis of the data to some extent because of losses of the released oxygen, causing deviation of the ln(Vmax - Vt) plot from linearity over time and an erroneous value of Vmax. However, for the initial reaction times, the linear correlation holds and Vmax is similar for all of the samples, allowing the determination of approximate reaction rate constants.
Figure 6.
(a) Natural logarithm of O2 (foam) volume changes over time from a demonstration using 0.25 g loadings of spinel catalysts calcined at (a) 300, (b) 400, (c) 500, and (d) 600 °C. (b) Reaction rate constants determined per unit mass (orange) and per unit surface area (blue).
The reaction rate constants per unit mass of the catalyst, presented in Figure 6b as orange bars, follow the trend of the SSA changes presented in Figure 4, which shows a clear dependence of reactivity on the extent of exposed area of the catalyst and thus on the number of the exposed active sites. Normalization of the reaction rate constants by dividing by SSA of the catalysts gives the rate constant values per unit surface area (blue bars in Figure 6b). Thus, the obtained values represent intrinsic reaction rate constants and show that the specific activities of the catalysts are similar. Using 0.5 g of the catalyst for demonstration makes the differences between reaction rates easily noticeable during visual observation, but the calculated final results are more variable (compare data in the Supporting Information).
Conclusion
The described demonstration allows a clear presentation of the influence of the specific surface area of a catalyst on the reaction rate in heterogeneous catalysis based on hydrogen peroxide decomposition over cobalt spinel materials. Not only can students visually assess the reaction rate, but a simple kinetic analysis can be performed and numerical values of the rate constants can be compared. The results confirm that the catalytic activity of Co3O4 in H2O2 decomposition depends linearly on the specific surface area of the material. The explanation of this observation is that the number of active sites for H2O2 decomposition also scales in a linear way with the SSA.
Acknowledgments
The activity was developed within the Including Responsible Research and Innovation in Cutting Edge Science and Inquiry-Based Science Education to Improve Teacher’s Ability of Bridging Learning Environments (IRRESISTIBLE) Project, which was funded by the European Commission Seventh Framework Programme [FP7/2007-2013] under Grant Agreement 612367.
Supporting Information Available
The Supporting Information is available at https://pubs.acs.org/doi/10.1021/acs.jchemed.0c01101.
The authors declare no competing financial interest.
Supplementary Material
References
- White J. M.; Campbell C. T. Surface Chemistry in Heterogeneous Catalysis: An Emerging Discipline. J. Chem. Educ. 1980, 57 (7), 471. 10.1021/ed057p471. [DOI] [Google Scholar]
- Oyama S. T.; Somorjai G. A. Homogeneous, Heterogeneous, and Enzymatic Catalysis. J. Chem. Educ. 1988, 65 (9), 765. 10.1021/ed065p765. [DOI] [Google Scholar]
- Cusumano J. A. Environmentally Sustainable Growth in the 21st Century: The Role of Catalytic Science in Technology. J. Chem. Educ. 1995, 72 (11), 959. 10.1021/ed072p959. [DOI] [Google Scholar]
- Rothenberg G.Catalysis: Concepts and Green Applications; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Jakubek T.; Kaspera W.; Legutko P.; Stelmachowski P.; Kotarba A. Surface versus Bulk Alkali Promotion of Cobalt-Oxide Catalyst in Soot Oxidation. Catal. Commun. 2015, 71, 37–41. 10.1016/j.catcom.2015.08.014. [DOI] [Google Scholar]
- Stelmachowski P.; Ciura K.; Grzybek G. Morphology-Dependent Reactivity of Cobalt Oxide Nanoparticles in N2O Decomposition. Catal. Sci. Technol. 2016, 6 (14), 5554–5560. 10.1039/C6CY00365F. [DOI] [Google Scholar]
- Mitchell J. A. Heterogeneous Catalysis. J. Chem. Educ. 1932, 9 (2), 261. 10.1021/ed009p261. [DOI] [Google Scholar]
- Ertl G.Reactions at Solid Surfaces; John Wiley & Sons: Hoboken, NJ, 2009. [Google Scholar]
- Somorjai G. A.; Li Y.. Introduction to Surface Chemistry and Catalysis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, 2010. [Google Scholar]
- Battino R.; Letcher T. M.; Rivett D. E. A. The Repeating “Exploding” Flask: A Demonstration of Heterogeneous Catalysis. J. Chem. Educ. 1993, 70 (12), 1029. 10.1021/ed070p1029. [DOI] [Google Scholar]
- Volkovich V. A.; Griffiths T. R.; Smith P. E. Catalytic Oxidation of Ammonia: A Sparkling Experiment. J. Chem. Educ. 2000, 77 (2), 177. 10.1021/ed077p177. [DOI] [Google Scholar]
- Perkins R.; Mattson B.; Fujita J.; Catahan R.; Cheng W.; Greimann J.; Hoette T.; Khandhar P.; Mattson A.; Rajani A.; Sullivan P.; Gonella T. P. Demonstrating Heterogeneous Gas-Phase Catalysis with the Gas Reaction Catalyst Tube. J. Chem. Educ. 2003, 80 (7), 768. 10.1021/ed080p768. [DOI] [Google Scholar]
- Eduard-Job-Foundation for Thermo- and Matterdynamics. Experiment 19.5: Catalysis of Acetone Oxidation by Copper. https://job-stiftung.de/index.php?catalysis-of-acetone (accessed 2020-12-21).
- Raymundo-Piñero E.; Cazorla-Amorós D.; Morallón E. Catalytic Oxidation of Sulfur Dioxide by Activated Carbon: A Physical Chemistry Experiment. J. Chem. Educ. 1999, 76 (7), 958. 10.1021/ed076p958. [DOI] [Google Scholar]
- Mears D. E.; Benson J. E. Liquid Phase Dehydrogenation of Isopropanol: Heterogeneous Catalysis Experiment. J. Chem. Educ. 1966, 43 (6), 325. 10.1021/ed043p325. [DOI] [Google Scholar]
- Menzel P.; Mattson B.; Hulce M.; Cheng W.; Greimann J.; Hoette T. Propanol to Propane: An Advanced Laboratory Experiment Using Heterogeneous Catalysts for Two Successive Gas-Phase Reactions. J. Chem. Educ. 2006, 83 (3), 421. 10.1021/ed083p421. [DOI] [Google Scholar]
- Alyea H. N. MnO2 as a Catalyst for H2O2 Decomposition. Dem. 646. J. Chem. Educ. 1969, 46 (7), A496. 10.1021/ed046pA496.2. [DOI] [Google Scholar]
- Laursen A. B.; Man I. C.; Trinhammer O. L.; Rossmeisl J.; Dahl S. The Sabatier Principle Illustrated by Catalytic H2O2 Decomposition on Metal Surfaces. J. Chem. Educ. 2011, 88 (12), 1711–1715. 10.1021/ed101010x. [DOI] [Google Scholar]
- Bussi J.; Correa C.; Coch Frugoni J. A. An Experiment on Heterogeneous Catalysis. J. Chem. Educ. 1991, 68 (2), 170. 10.1021/ed068p170. [DOI] [Google Scholar]
- Conklin A. R.; Kessinger A. Demonstration of the Catalytic Decomposition of Hydrogen Peroxide. J. Chem. Educ. 1996, 73 (9), 838. 10.1021/ed073p838. [DOI] [Google Scholar]
- Carter G. E. The Feasibility of Using Hydrogen Peroxide Decomposition Studies for High School Chemistry. J. Chem. Educ. 1986, 63 (2), 159. 10.1021/ed063p159. [DOI] [Google Scholar]
- Trujillo C. A.; Senkbeil E.; Krause P. A Modified Demonstration of the Catalytic Decomposition of Hydrogen Peroxide. J. Chem. Educ. 2005, 82 (6), 855. 10.1021/ed082p855. [DOI] [Google Scholar]
- Eldridge D. S. Using Elephant’s Toothpaste as an Engaging and Flexible Curriculum Alignment Project. J. Chem. Educ. 2015, 92 (8), 1406–1408. 10.1021/acs.jchemed.5b00037. [DOI] [Google Scholar]
- Sattsangi P. D. A Microscale Approach to Chemical Kinetics in the General Chemistry Laboratory: The Potassium Iodide Hydrogen Peroxide Iodine-Clock Reaction. J. Chem. Educ. 2011, 88 (2), 184–188. 10.1021/ed100140w. [DOI] [Google Scholar]
- Ragsdale R. O.; Vanderhooft J. C.; Zipp A. P. Small-Scale Kinetic Study of the Catalyzed Decomposition of Hydrogen Peroxide. J. Chem. Educ. 1998, 75 (2), 215. 10.1021/ed075p215. [DOI] [Google Scholar]
- Hernando F.; Laperuta S.; Kuijl J. V.; Laurin N.; Sacks F.; Ciolino A. Another Twist of the Foam: An Effective Test Considering a Quantitative Approach to “Elephant’s Toothpaste.. J. Chem. Educ. 2017, 94 (7), 907–910. 10.1021/acs.jchemed.7b00040. [DOI] [Google Scholar]
- Dolhun J. J. Observations on Manganese Dioxide as a Catalyst in the Decomposition of Hydrogen Peroxide: A Safer Demonstration. J. Chem. Educ. 2014, 91 (5), 760–762. 10.1021/ed4006826. [DOI] [Google Scholar]
- Pratt G.; Curtright R. D.; Hill L.; Clarke S. A Dramatic Demo. J. Chem. Educ. 1988, 65 (10), 896–897. 10.1021/ed065p896. [DOI] [Google Scholar]
- Wójcik S.; Grzybek G.; Stelmachowski P.; Sojka Z.; Kotarba A. Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis. Catalysts 2020, 10 (1), 41. 10.3390/catal10010041. [DOI] [Google Scholar]
- Wójcik S.; Thersleff T.; Gębska K.; Grzybek G.; Kotarba A. Atomic-Level Dispersion of Bismuth over Co3O4 Nanocrystals—Outstanding Promotional Effect in Catalytic DeN2O. Catalysts 2020, 10 (3), 351. 10.3390/catal10030351. [DOI] [Google Scholar]
- Ercolino G.; Stelmachowski P.; Specchia S. Catalytic Performance of Pd/Co3O4 on SiC and ZrO2 Open Cell Foams for Process Intensification of Methane Combustion in Lean Conditions. Ind. Eng. Chem. Res. 2017, 56 (23), 6625–6636. 10.1021/acs.iecr.7b01087. [DOI] [Google Scholar]
- Ercolino G.; Stelmachowski P.; Grzybek G.; Kotarba A.; Specchia S. Optimization of Pd Catalysts Supported on Co3O4 for Low-Temperature Lean Combustion of Residual Methane. Appl. Catal., B 2017, 206, 712–725. 10.1016/j.apcatb.2017.01.055. [DOI] [Google Scholar]
- Monteverde Videla A. H. A.; Stelmachowski P.; Ercolino G.; Specchia S. Benchmark Comparison of Co3O4 Spinel-Structured Oxides with Different Morphologies for Oxygen Evolution Reaction under Alkaline Conditions. J. Appl. Electrochem. 2017, 47 (3), 295–304. 10.1007/s10800-016-1040-3. [DOI] [Google Scholar]
- Stelmachowski P.; Monteverde Videla A. H. A.; Ciura K.; Specchia S. Oxygen Evolution Catalysis in Alkaline Conditions over Hard Templated Nickel-Cobalt Based Spinel Oxides. Int. J. Hydrogen Energy 2017, 42 (46), 27910–27918. 10.1016/j.ijhydene.2017.06.034. [DOI] [Google Scholar]
- Bernard P.; Grzybek G.; Stelmachowski P. Tuning the Electronic Properties of Heterogeneous Catalysts: An Authentic Research-Based Laboratory Course. Chemistry 2014, 23 (3), 392–408. [Google Scholar]
- Makhlouf M. Th; Abu-Zied B. M.; Mansoure T. H. Effect of Calcination Temperature on the H2O2 Decomposition Activity of Nano-Crystalline Co3O4 Prepared by Combustion Method. Appl. Surf. Sci. 2013, 274, 45–52. 10.1016/j.apsusc.2013.02.075. [DOI] [Google Scholar]
- CMA—Centre for Microcomputer Applications . https://cma-science.nl (accessed 2020-12-21).
- Wolffenstein R. Ueber Die Einwirkung von Wasserstoffsuperoxyd Auf Aceton Und Mesityloxyd. Ber. Dtsch. Chem. Ges. 1895, 28 (2), 2265–2269. 10.1002/cber.189502802208. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.