Abstract
The laboratory fruit fly Drosophila melanogaster is one of the leading models for the study of aging. Whereas several behavioral and physiological biomarkers of aging have been identified for Drosophila, life span remains the most robust measure of aging rate. Aging and life span can be modulated by genetic alterations, as well as by drugs and dietary components, to reveal basic and conserved mechanisms of aging. Here methods are presented for Drosophila life span assay, including media preparation, supplementation of media with various drugs, culturing of the flies, passaging flies and recording deaths, and the analysis of life span data.
Keywords: Drosophila, life span, aging, mortality rate, Drosophila media, Gompertz
1. Introduction
Drosophila has a long and storied history of contributions to research in biology and aging [1,2]. In recent years, several biomarkers of aging and remaining life span have been identified for Drosophila, including locomotor activity, decreased egg laying, and the expression of transgenic reporters for immune genes and heat shock protein genes [3–7]. Despite these successes, life span remains the most robust measure of aging rate for Drosophila. Life span data can be fitted to the Gompertz-Makeham equation to reveal if an intervention alters life span by altering aging rate (mortality rate acceleration with age, Gompertz parameter b) or if it does so by altering animal health (initial mortality rate, Gompertz parameter a) [8]. In our hands, the most critical component of the life span assay is the quality of the media, especially the surface of the media in the food vials. The food surface must be moist enough to support optimal feeding/drinking by the fly, but not too wet as to be sticky and create a trap for the flies, and/or promote growth of sticky bacterial species. Here we describe methods for making Drosophila media, including pumping hot media into vials and bottles, cooling, and storage. Many experiments on aging in Drosophila utilize conditional transgene expression systems, where transgene expression is triggered by feeding the flies a drug, such as doxycycline or mifepristone [9,10]. The most precise way to administer a defined concentration of drug to the fly in the media is to add concentrated drug stock solution to the hot liquid media and mix thoroughly before the media cools and hardens. However, this approach has the limitation that some drugs might be sensitive to the heat of the liquid media. Another limitation is that the preparation of media with titrations of drug, and/or various combinations of two or more drugs, becomes prohibitive in terms of time and effort. We describe our simplified method for supplementation of media with various drugs and drug combinations, by applying concentrated drug stock solutions directly to the surface of cooled and solidified media vials [11–13]; this approach has since been adopted by numerous labs.
2. Materials
Drosophila fly vials (Narrow, polystyrene, Genesee Scientific #32-116)
Drosophila fly bottles (6 ounce, Genesee Scientific #32-130)
Fly food pump (Automatic pippeting machine, Brewer Cat#60480 Model#40)
5-headed nozzle (Brass, custom-made by machine shop).
Steam-jacketed kettle
Dextrose (LabScientific #FLY8012-10)
Agar (Gelidium 700, Mooragar Inc. #4004)
Yeast (miniflakes, Red Star #74561)
Cornmeal (ground yellow, MP Biomedical #901411)
Tegosept (Apex Tegosept, Genesee Scientific #20-258, stock solution of 194g/L in ethanol)
Propionic acid (99%, Mallinckrodt Baker)
95% Ethanol
Large Rayon balls (TIDI #969162, Genesee Scientific #51-100)
Flugs for plastic fly bottles (Genesee Scientific #49-100)
Plastic bags (VWR #AA4046SH 40X46X1.1 Mil Clear)
Cheesecloth
Gas dispersion tube (Chemglass #CG-203-02)
Side-arm flask
3. Methods
Use personal protective equipment including lab coat, gloves and splash goggles.
3.1. Making Fly food
1. Prepare each of the components of the fly food ahead of time according to the recipe as follows. Per liter of H2O: 105 g dextrose, 7.5 g agar, 26 g yeast, 50 g cornmeal, 8.5 ml Tegosept stock solution, and 1.9 ml propionic acid. Depending on the setting of the pump (Fig. 1b), vials contain ~6ml media and bottles contain ~30ml media.
Figure 1.

Equipment. (a) Steam-jacketed kettle. (b) Pump. (c) 5-headed nozzle. (d) Bubbler.
2. Add 4/5 of the required amount of water to the kettle (Fig. 1a), keep 1/5 amount nearby in a 20L bucket for use later. Start to stir the water and start the steam to heat.
3. Add each of dextrose, yeast, cornmeal, and agar slowly to the water.
4. When the mixture starts to boil, reduce the steam to maintain a very low boil, and cook for 30 minutes.
5. In case of over-boil, add some water from the 1/5 amount of water in the bucket and turn down the steam.
6. After boiling for 30 min, turn off the steam. Add the remaining water to the kettle (this helps cool the media), and then add the Tegosept solution and propionic acid. Keep stirring.
7. Pump the hot mixture into bottles using plastic tubing.
8. Pump the hot mixture in vials, 5 at a time, using the 5-headed nozzle (Fig. 1c).
9. Move the boxes of hot food onto benchtops or racks. Do not stack any box on top of another – this helps prevent condensation from collecting in the vials and bottles. Cover the food with cheesecloth to prevent any stray flies from accessing the food and laying eggs on the food. Let cool for 24–48 hours.
10. Stuff the vials with Rayon balls. Stuff bottles with bottle flugs. Stack 5 boxes of food into a plastic bag and seal the bag with rubber band. Store the food in 4°C cold room.
3.2. Making drug vials
Here we present methods for making vials supplemented with mifepristone (RU486) to a final concentration of ~200μg/ml (“+drug” vials), and control vials supplemented with ethanol vehicle alone (“−drug” vials). Similar procedures can be used to make vials supplemented with other drugs, including drugs dissolved in water (see Note 1).
1. Prepare 4mg/ml mifepristone stock solution. Place 200mg of RU486 in a 50ml centrifuge tube, add 50ml 100% ethyl alcohol. Mix to dissolve all RU486. Store at −20°C.
2. Pipette 50μl of the 4mg/ml RU486 stock solution onto the surface of each “+drug” vial needed. Pipette 50μl of 100% ethyl alcohol onto the surface of each “−drug” vial needed. Shake the box of vials to distribute the solutions evenly over the top of the food in each vial. Stripe the “+drug” vials with a red marker, and stripe the “−drug” vials with a blue marker. Let dry on benchtop under cheesecloth for 2 days. The 50μl of ethanol solution absorbs into the top ~1ml of media (Fig. 2b), yielding a 1:200 dilution of the stock solution and a final concentration of ~200μg/ml mifepristone.
Figure 2.
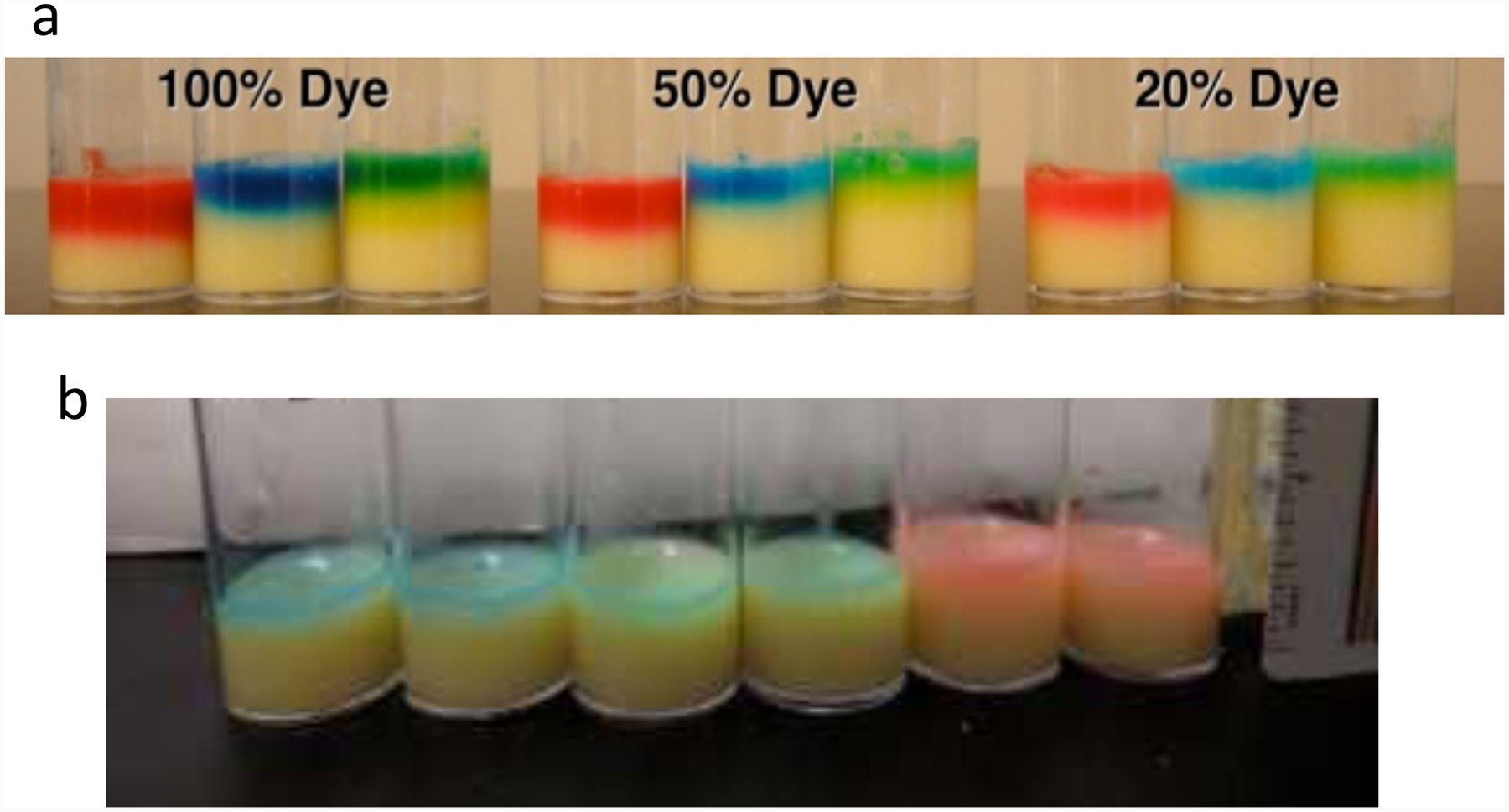
Estimating drug dilution in media using dyes. (a) Each of red, blue and green commercial food colorings were used, directly (100% dye), or were diluted with water to 50% concentration or 20% concentration. 100μl of each solution was added to the surface of a vial with fly food media, and allowed to absorb overnight. (b) Blue, green and red commercial food colorings were diluted into ethanol at 20% dye concentration, and 50μl of each solution was added to the surface of a vial with fly food media (two vials for each color of dye), and allowed to absorb overnight. (a, b) Measurement of the colored area of the food indicates that the solutions absorb into the top ~1ml of media.
3.3. Life span assay
1. The GAL4/UAS binary transgenic system typically involves crossing a GAL4 “driver” strain to a UAS “target” strain to generate over-expression or RNAi flies, and crossing the “driver” to a control strain (often the w[1118] strain) to generate control flies [14]. The conditional systems, for example the Gene-Switch system [15,10,16], are activated by feeding the flies a drug. These experiments typically involve crossing a Gene-Switch “driver” strain to a UAS “target” strain and assay of the flies in presence/absence of drug, plus crossing the “driver” to a control strain (often the w[1118] strain) to generate control flies, which are assayed in presence/absence of drug to identify any potential background effects of the drug [16].
2. Set up bottles of the driver and target strains. Put 20 females and at least 10 males of each strain into fly food bottles. Using more than 20 females may make the bottles overcrowded. To determine the number of bottles required, calculate that you should get ~100 virgins or males from each bottle from a healthy stock.
4. Collect virgins and males for the life span cross. From the bottles set up above in step 1, collect virgins into fresh vials at 20 virgins/vial, and males into fresh vials at 20 males/vial. Label the vials with the date and genotype. If virgins are suspect, wait four days before using to see if there are larvae present. If so, discard that vial.
5. Calculate the number of flies and bottles of each cross required for the experiment. Each sample will require at least 100 flies. Assume you will get ~100 virgins and ~100 males from each cross bottle. For example, if your experiment has 6 treatments, say males +/−drug, virgin females +/−drug and mated females +/−drug, then you would need a total of 600 flies from 6 bottles. However, to ensure all flies come out of the bottles on the same day, set up 3 times that number of bottles. If the target or driver strains are over a balancer, then further double the number of bottles.
6. Set up the crosses to generate progeny for the life span assay. Use 40 virgins and 20 males per bottle (see Notes 2, 3).
7. After 2 days, toss the flies in the bottles into new bottles, these will be used for the second cohort. After 2 days, discard the flies from the second cohort bottles (see Note 4).
8. Collect progeny for life span assay. When pupae turn dark the flies will come out the next day (see Note 5). Collect 20 virgin females per vial, and collect 20 virgin males per vial (see Note 6).
9. Generate mated females. Some experiments will involve analysis of mated females. To generate mated females, add 20 3–5 day old w[1118] males to each vial of 20 virgin females, allow to mate for 48 hours, and then remove the males.
10. Collect flies onto +/−drug vials for the life span assay. If mating is not needed, collect flies directly into +/− drug vials. Make sure to alternate putting flies into −drug and +drug vials: i.e., transfer the first vial of flies you collect into a −drug vial, then transfer next vial of flies you collect into a +drug vial, repeat this alternation for all the flies. This way any potential differences in flies due to handling during collection (time on gas, etc.), will be evenly distributed between the +/− drug groups. For mated flies, also be sure to alternate putting flies into −drug and +drug vials: i.e., transfer first vial of mated flies into a −drug vial, then transfer next vial of mated flies into a +drug vial, repeat this alternation for all the flies. For purposes of life span calculation, the day of collection is considered day 0 of the life span assay.
11. Labeling and handling the life span vials. To make tags for the life span vials, take a piece of colored tape about 3” long, and fold over halfway. This will make a sticky tag that can be transferred from one vial to another every other day. Use blue tape for −drug treatment and red tape for +drug treatment; in this way, the tape tags are color-coordinated with the marker stripes on the vials. Write the vial number on the tape tag with a marker. Make sure tags are placed low on the vials to prevent them from sticking to the rayon. Transfer the flies every other day into fresh +/−drug vials, and transfer the tape tag to the new vial as you toss each vial (see Notes 7, 8, 9).
12. How to score and record deaths. At each transfer record the number of dead flies in each of the numbered vials. Record the data on the raw data sheet (Fig. 3a), which is kept in the box with the cohort. If a fly sticks to the food or the wall of the vial and won’t shake off, even with a vigorous bang of the old vial onto the top of the new vial, then count that fly as dead, even if it is still moving (see Note 10). If there is one or more dead flies in a vial, record the vial number and the number of dead flies, separated by a dash, on the raw data sheet (Fig. 3a). If a fly is dead, but transfers into the new vial, put the number 1 followed by an asterisk on the data sheet (if two dead flies transfer, then put the number 2 with an asterisk, etc.). On the next toss, subtract the number of dead with asterisks on the previous toss from the total number dead in that vial for the current toss (see Note 11). Once all flies in a vial have died, record “C” for “complete” on the raw data sheet next to the last number dead.
Figure 3.
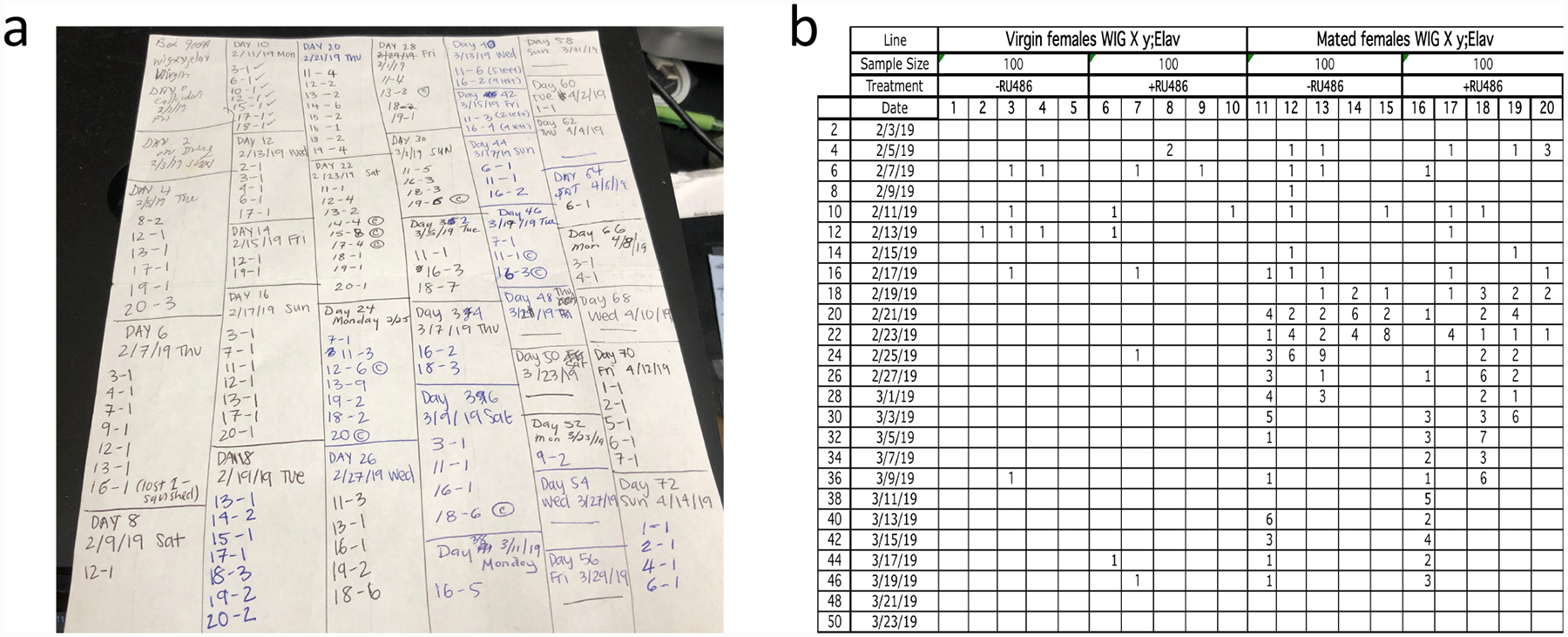
Data recording. (a) Raw data sheet. (b) Excel spreadsheet.
Example:
Day 46
7/26/10 Tue
4–2
14–1
6–2, 1*
85–4 C
On Day 46, 7/26/10, Tuesday, 2 flies died in vial 4, 1 fly died in vial 14, 2 flies died in vial 6 and one dead fly was transferred from vial 6 into a new vial, 4 flies died in vial 85 and that was completed the flies in vial 85.
13. How to organize and handle the data. After all the flies in a cohort have died, all the data should be recorded on the raw data sheet (Fig. 3a). Transfer the data to an Excel spread sheet (Fig. 3b), and retain the raw data sheets for reference. Organize the data in the Excel spread sheet in the order −drug first, +drug second. Don’t enter 0’s. We use a custom script written in R to calculate median life span for each group, to compare groups using log-rank test, and to plot the survival curves. The total number of deaths recorded for the experiment is used as the starting number of flies; this omits any flies lost due to escape. Finally, fitting the life span data to the Gompertz-Makeham equation [8] reveals if an intervention alters life span by altering aging rate (mortality rate acceleration with age, Gompertz parameter b) or if it does so by altering animal health (initial mortality rate, Gompertz parameter a).
4. Notes
1. For drugs dissolved in ethanol, use 50μl per vial. For drugs dissolved in water, use 100μl per vial. In each case the solution will absorb into the top ~1ml of media (Fig. 2).
2. If possible, use virgins for the driver strain, and males for the target strains; this way virgins only need be collected from one strain, and this facilitates crosses to multiple target strains. This will not work if the target is on the X chromosome, and in that case use target strain virgins and driver strain males to set up the cross.
3. Using a relatively large number of parent flies for only two days results in a more synchronous eclosion of the progeny. This in turn facilitates collection of the virgins over the span of one day, resulting in a more age-synchronous cohort.
4. If the bottles start to dry out, add water. Dry bottles will have many small spaces between the food and the side of the vial. Add water slowly using a water squirt bottle carefully inserted between the bottle openin and the flug, so that no flies escape. Add only enough water to fill the spaces, do not leave any water on the surface of the food, or flies will become trapped. If you add too much water, invert the bottle and the excess water will flow into the flug and be absorbed.
5. When pupae start to turn dark in the bottles, put a single layer of Kimwipe on top of the food in the bottles. This provides a dry place for the emerging flies to land without getting trapped in the sticky food surface, or on the sticky side of the bottle. This increases the yield of flies, especially with wet or sticky bottles.
6. Make sure to use a bubbler (Fig. 1d), in which the CO2 gas moves through a sintered glass tube (gas dispersion tube), under the water level, inside a stoppered beaker. In this way, the CO2 is humidified before it reaches the flies. Without a bubbler, the dry CO2 gas will dehydrate the flies, and this can result in male infertility and shortened life span in both males and females. Reduce CO2 flow to lowest amount possible (before the flies start to walk) to limit total CO2 stress on the flies.
7. It is recommended that tossing is practiced several times before the experiment is started, to prevent unnecessary loss of flies during transfers.
8. Make sure that when you toss, the rayon ball is place securely in the new vial. Don’t leave “holes” or gaps for flies to escape through. Don’t toss flies into a foodless vial - this quickly kills the flies.
9. Don’t toss flies into vials where the food is separated from the wall of the vial, as the flies will tend to become trapped in that space. Also, in vials where the food is separated from the wall of the vial, the food will tend to come loose during the toss, fall onto the flies in the new vial, and kill the flies. Do not use vials where there are cracks in the plastic as these will tend to dry out.
10. In our experience flies trapped in this way will be dead within ~12 hours.
11. Usually, the dead flies will stick to the surface of the food, and will not transfer to the new vial.
Acknowledgements
This work was supported by a grant to JT from the Department of Health and Human Services, National Institute on Aging (AG057741). Conflicts of interest: none.
References
- 1.Tower J (2019) Drosophila flies in the face of aging. J Gerontol A Biol Sci Med Sci:In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge L, Tower J (2008) Yeast, a Feast: The Fruit Fly Drosophila as a Model Organism for Research into Aging. In: Guarente LP, Partridge L, Wallace DC (eds) Molecular Biology of Aging. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 267–308 [Google Scholar]
- 3.Carey JR, Papadopoulos N, Kouloussis N, Katsoyannos B, Muller HG, Wang JL, Tseng YK (2006) Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp Gerontol 41 (1):93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller LD, Shahrestani P, Rauser CL (2009) Predicting death in female Drosophila. Exp Gerontol 44 (12):766–772 [DOI] [PubMed] [Google Scholar]
- 5.Rogina B, Wolverton T, Bross TG, Chen K, Muller HG, Carey JR (2007) Distinct biological epochs in the reproductive life of female Drosophila melanogaster. Mech Ageing Dev 128 (9):477–485. doi: 10.1016/j.mad.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, Tavare S, Tower J (2004) Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A 101 (20):7663–7668. doi: 10.1073/pnas.0307605101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Tower J (2009) Expression of hsp22 and hsp70 transgenes is partially predictive of drosophila survival under normal and stress conditions. J Gerontol A Biol Sci Med Sci 64 (8):828–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen J, Landis GN, Tower J (2017) Multiple Metazoan Life-span Interventions Exhibit a Sex-specific Strehler-Mildvan Inverse Relationship Between Initial Mortality Rate and Age-dependent Mortality Rate Acceleration. J Gerontol A Biol Sci Med Sci 72 (1):44–53. doi: 10.1093/gerona/glw005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieschke ET, Wheeler JC, Tower J (1998) Doxycycline-induced transgene expression during Drosophila development and aging. Molecular & general genetics : MGG 258 (6):571–579 [DOI] [PubMed] [Google Scholar]
- 10.Ford D, Hoe N, Landis GN, Tozer K, Luu A, Bhole D, Badrinath A, Tower J (2007) Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp Gerontol 42 (6):483–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren C, Webster P, Finkel SE, Tower J (2007) Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab 6 (2):144–152 [DOI] [PubMed] [Google Scholar]
- 12.Ren C, Finkel SE, Tower J (2009) Conditional inhibition of autophagy genes in adult Drosophila impairs immunity without compromising longevity. Exp Gerontol 44 (3):228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Curtis C, Tavare S, Tower J (2009) A screen of apoptosis and senescence regulatory genes for life span effects when over-expressed in Drosophila. Aging (Albany NY) 1 (2):191211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 (2):401–415 [DOI] [PubMed] [Google Scholar]
- 15.Roman G, Davis RL (2002) Conditional expression of UAS-transgenes in the adult eye with a new gene-switch vector system. Genesis 34 (1–2):127–131 [DOI] [PubMed] [Google Scholar]
- 16.Landis GN, Salomon MP, Keroles D, Brookes N, Sekimura T, Tower J (2015) The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila. Aging (Albany NY) 7 (1):53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]


