Abstract
Coronavirus 2019 disease (COVID-19) continues to challenge healthcare systems globally as many countries are currently experiencing an increase in the morbidity and mortality. Compare baseline characteristics, clinical presentation, treatments, and clinical outcomes of patients admitted during the second peak to those admitted during the first peak. Retrospective analysis of 258 COVID-19 patients consecutively admitted to the Tel Aviv Medical Center, of which, 131 during the first peak (March 21–May 30, 2020) and 127 during the second peak (May 31–July 16, 2020). First and second peak patients did not differ in baseline characteristics and clinical presentation at admission. Treatment with dexamethasone, full-dose anticoagulation, tocilizumab, remdesivir, and convalescent plasma transfusion were significantly more frequent during the second peak, as well as regimens combining 3–4 COVID-19-directed drugs. Compared to the first peak, 30-day mortality and invasive mechanical ventilation rates as well as adjusted risk were significantly lower during the second peak (10.2%, vs 19.8% vs p = 0.028, adjusted HR 0.39, 95% CI 0.19–0.79, p = 0.009 and 8.8% vs 19.3%, p = 0.002, adjusted HR 0.29, 95% CI 0.13–0.64, p = 0.002; respectively). Rates of 30-day mortality and invasive mechanical ventilation, as well as adjusted risks, were lower in the second peak of the COVID-19 pandemic among hospitalized patients. The change in treatment strategy and the experienced gained during the first peak may have contributed to the improved outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-021-02711-1.
Keywords: COVID-19, Treatments, Mortality, Mechanical ventilation
Introduction
The Coronavirus Disease 2019 (COVID-19) epidemic emerged in Wuhan in late December 2019 [1] and spread globally within weeks. By January 30, 2020, the World Health Organization (WHO) declared that COVID-19 is "a public-health emergency of international concern" [2], and in March, with up to 143 countries reporting cases of COVID-19, the WHO declared the disease a pandemic [3].
In Israel, the first COVID-19 patient was diagnosed on February 21, 2020, and was soon followed by an exponential growth rate, leading to a peak of daily confirmed cases of 932 by March 25, 2020. Due to a variety of preliminary measures—isolation and quarantine, population testing, epidemiological investigations, social distancing, and public education—by early May, the rate of new daily confirmed infections plunged to 20 cases per day. However, following the withdrawal of the restrictive measures, a second peak commenced, soon surpassing the infection rate of the first peak, reaching approximately 2000 confirmed cases daily [4] (Figure S1).
The aim of the study is to compare baseline characteristics, disease severity, treatments, and clinical outcomes of patients admitted during the second peak to those admitted during the first peak.
Methods
We retrospectively studied 258 patients with COVID-19 infection consecutively admitted to the Tel Aviv Medical Center. Patients admitted between March 21 and May 30, 2020 were considered first peak patients, while those admitted between May 31 and July 16, 2020 were considered second peak patients. The start of the second peak was defined by the first increase in the rolling 7-day average daily admissions rate, which was followed by further increase. All patients had a positive reverse-transcriptase-polymerase-chain-reaction (PCR) assay for SARS-CoV-2 obtained from respiratory tract sample and were admitted to designated Internal Medicine wards or Intensive Care Units. Demographic status, comorbidities, chronic medical therapy, vital signs, physical examination findings, laboratory results, and chest X-ray findings were recorded from all patients. Upon admission, risk of clinical deterioration was evaluated using the COVID-19 modified early warning score (COVID-19 MEWS) [5] and the Sequential Organ Failure Assessment (SOFA) score [6]. Disease severity was determined in accordance with the National Institutes of Health (NIH) guidelines [7]. Components of the COVID-19 MEWS and the NIH criteria of disease severity are summarized in Supplementary Tables S1, S2, and S3. The study was approved by the Tel Aviv medical center ethics committee (IRB number 0196-20-TLV).
Echocardiography and lung ultrasound
Since the beginning of the COVID-19 pandemic, we have performed echocardiography and lung ultrasound on admission for all patients presenting with respiratory illness due to COVID-19 infection, using a pre-defined step-by-step protocol, as part of a routine patient care protocol. All patients underwent comprehensive lung ultrasound combined with bedside echocardiography within 24 h of admission. Echocardiographic and lung ultrasound examinations were performed by cardiologists with expertise in echocardiographic and lung ultrasound recording and interpretation. Necessary precautions were taken to minimize the risk of infection and disease transmission, in accordance with current guidelines [8]. Measurements of left-ventricular diameters, volumes, and ejection fraction (LVEF) were taken as recommended [9]. Left atrial (LA) volume was calculated using the biplane area length method at end systole. Forward stroke volume was calculated from the LV outflow tract followed by calculation of cardiac output (CO) and CO index. Right ventricular evaluation consisted of end-systolic and end-diastolic areas. Additionally, right-ventricular function was assessed by tricuspid annular plane systolic excursion (TAPSE), systolic tricuspid lateral annular velocity (RV S'), and fractional area change [9, 10].
Lung ultrasound examination included visualization of 6 "windows" on each side: superior and inferior anterior, superior and inferior antero-lateral, and superior and inferior postero-lateral. We used the following scoring system to rate the degree of lung aeration: A-lines, (normal repetitive reverberation of the pleura accompanied by lung sliding) received a score of 0. B-lines (long bands of hyperechoic artifacts representing edematous interlobular septa and/or alveoli) received one or two points, depending on weather the B-lines were separated or coalescent, respectively. Lung consolidation received three points. Ultimately, the lung ultrasound score ranged from 0 (indicating normal lung) to 36 (indicating diffused bilateral lung consolidations) [11].
Outcomes
The primary outcomes were 30-day mortality and 30-day invasive mechanical ventilation. Secondary outcomes included non-invasive high flow oxygen ventilation, time to invasive mechanical ventilation, major bleeding (defined as a fall in hemoglobin ≥ 2 g/dL or transfusion of ≥ 2 packed red blood cells units), and time to discharge.
Statistics
Continuous variables were tested for normality using histograms, quantile–quantile plots, and the Shapiro–Wilk test. Normally distributed continuous variables were compared using the Student's t test and are presented as mean ± standard deviation (SD). Non-normally distributed continuous variables were compared using the Mann–Whitney test, and are expressed as median and interquartile range. Categorial variables were compared using the Chi-square test and Fisher's exact test, and are presented as numbers and percentiles. In addition to the analysis described above, baseline characteristics were also compared using the standardized mean difference test to evaluate the magnitude of the between-group differences, with values < 0.2, 0.3–0.5, and > 0.8 corresponding to a small, medium, and large effect size, respectively. Comparison of mean and median echocardiographic parameters to reference values was done using the one-sample t test and the Wilcoxon rank-sum test, respectively. Clinical outcomes of the two peaks were illustrated using the Kaplan–Meier model and compared using the log-rank test. Adjustment for potential undetected confounders was performed using a multivariate Cox regression model that included age, gender, disease severity, and presence of three or more comorbidities. For all calculations, a p value lower than 0.05 was considered significant. All statistical analysis was performed using SPSS v25.
Results
Patient population
Between March 21 and July 16, 2020, 324 COVID-19 patients were admitted to the Tel Aviv Medical Center. Thirty patients were excluded as they were asymptomatic carriers admitted for either unrelated medical reasons or social arrangements (were unable to self-quarantine). Twenty-seven patients were excluded as they were discharged within less than 24 h from admission. Nine patients were excluded, since they were designated to palliative care only. Hence, the final study cohort included 258 patients, of which 131 admitted during the first peak and 127 during the second. Mean age was 63 ± 18 years and 60.6% were males. Forty-seven percent of patients had at least three comorbidities, hypertension being the most frequent (50%), followed by diabetes mellitus (27%) and obesity (20%). Mean LVEF was 58 ± 7% and 94% had LVEF ≥ 50%. Right ventricle was dilated in approximately 40% of patients. Echocardiographic characteristics and reference values are summarized in Table S4. The median lung ultrasound score was 14 (7–19). The median SOFA score was similar in both peaks. Compared to patients that survived, patients that died had a higher median SOFA score in both the first and second peaks (first peak: 1 [0–2] vs 3.5 [2–8.25], p < 0.001; second peak: 1 [0–2] vs 5 [3–7], p < 0.001), whereas no between-peaks difference was found in the median SOFA score of patients that died (first peak: 3.5 [2–8.25], second peak: 5 [3–7]; p = 0.4) and in those who survived (first peak: 1 [0–2], second peak: 1 [0–2], p = 0.66). Median COVID-19 MEWS was 3 (1–6). According to the NIH definition of disease severity, 47% presented with a mild disease, 19% with a moderate disease, 17% with a severe disease, and 17% with a critical disease. Overall, no major differences were noted between patients of the first and second peaks. Patients’ baseline characteristics are presented in Table 1.
Table 1.
COVID-19 modified early waring score ranges from 0 to 23; Lung ultrasound score ranges from 0 (normal lung) to 36 (diffuse bilateral lung consolidations); Sequential organ failure assessment score ranges from 0 to 24; SMD: standardized mean difference, values < 0.2, 0.3–0.5, and > 0.8 correspond to a small, medium, and large effect size, respectively
| Parameter | All N = 258 | 1st wave N = 131 | 2nd wave N = 127 | SMD | p |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean ± SD (years) | 63.5 ± 18.3 | 65.2 ± 17.8 | 62.1 ± 18.5 | 0.17 | 0.162 |
| Male, n (%) | 157 (60.5) | 80 (61.1) | 76 (59.8) | 0.02 | 0.899 |
| Body mass index, mean ± SD (kg/m2) | 27.2 ± 5.7 | 26.76 ± 5.8 | 27.7 ± 5.6 | 0.16 | 0.23 |
| Medical history | |||||
| Ischemic heart disease, n (%) | 40 (15.4) | 22 (16.8) | 18 (14.2) | 0.05 | 0.608 |
| Congestive heart failure, n (%) | 27 (10.4) | 13 (9.9) | 14 (11) | 0.02 | 0.84 |
| S/P coronary artery bypass graft, n (%) | 11 (4.2) | 8 (6.1) | 3 (2.4) | 0.14 | 0.217 |
| Atrial fibrillation/flutter, n (%) | 43 (16.6) | 23 (17.6) | 20 (15.7) | 0.04 | 0.74 |
| Transient ischemic attack/stroke, n (%) | 26 (10) | 14 (10.7) | 12 (9.5) | 0.03 | 0.837 |
| Peripheral artery disease, n (%) | 8 (3.1) | 4 (3.1) | 4 (3.1) | 0 | 1 |
| Chronic obstructive pulmonary disease, n (%) | 14 (5.4) | 7 (5.3) | 7 (5.5) | < 0.01 | 1 |
| Asthma, n (%) | 14 (5.4) | 7 (5.3) | 7 (5.5) | < 0.01 | 1 |
| Chronic kidney disease, n (%) | 29 (11.2) | 13 (9.9) | 16 (12.6) | 0.07 | 0.557 |
| Diabetes mellitus, n (%) | 69 (26.6) | 39 (29.8) | 30 (23.6) | 0.11 | 0.325 |
| Smoking, n (%) | 27 (10.4) | 17 (13) | 10 (7.9) | 0.13 | 0.224 |
| Hypertension, n (%) | 129 (49.8) | 74 (56.5) | 55 (43.3) | 0.21 | 0.046 |
| Obesity, n (%) | 53 (20.5) | 27 (20.9) | 26 (27.1) | 0.16 | 0.341 |
| Malignancy, n (%) | 13 (5) | 6 (4.6) | 7 (5.5) | 0.03 | 0.782 |
| Comorbidities ≥ 3, n (%) | 122 (47.1) | 62 (47.3) | 60 (47.2) | < 0.01 | 1 |
| Concurrent medical therapy | |||||
| Aspirin, n (%) | 57 (22.1) | 30 (22.9) | 27 (21.3) | 0.03 | 0.766 |
| P2Y12 inhibitor, n (%) | 14 (5.4) | 6 (4.6) | 8 (6.3) | 0.06 | 0.592 |
| Direct oral anticoagulant, n (%) | 31 (12) | 21 (16) | 10 (7.9) | 0.19 | 0.055 |
| Angiotensin-converting enzyme, n (%) | 42 (16.3) | 22 (16.8) | 20 (15.7) | 0.02 | 0.867 |
| Angiotensin receptor blocker, n (%) | 35 (13.6) | 20 (15.3) | 15 (11.8) | 0.08 | 0.47 |
| Diuretics, n (%) | 35 (13.6) | 21 (16) | 14 (11) | 0.11 | 0.277 |
| β-Blocker, n (%) | 62 (24) | 34 (26) | 28 (22) | 0.07 | 0.471 |
| Systemic corticosteroids, n (%) | 10 (3.9) | 4 (3.1) | 6 (4.7) | 0.07 | 0.535 |
| Other anti-inflammatories, n (%) | 7 (2.7) | 5 (3.8) | 2 (1.6) | 0.1 | 0.447 |
| Laboratory | |||||
| Hemoglobin, mean ± SD (g/dL) | 13.1 ± 2 | 13.1 ± 2.1 | 13.2 ± 1.8 | 0.05 | 0.472 |
| White blood cells, median (IQR) (103/μL) | 6.6 (5.1–9.4) | 7.1 (5.3–10) | 6.3 (4.7–8.7) | 0.36 | 0.012 |
| Platelets, median (IQR) (103/μL) | 192 (145–244) | 200 (146–265) | 182 (145–225) | 0.26 | 0.062 |
| Creatinine, median (IQR) (mg/dL) | 0.88 (0.73–1.13) | 0.89 (0.73–1.17) | 0.86 (0.73–1.06) | 0.04 | 0.597 |
| Estimated glomerular filtration rate, mean ± SD (mL/min/1.73 m2) | 77.9 ± 29.2 | 76.37 ± 28.83 | 79.48 ± 29.62 | 0.11 | 0.393 |
| Estimated glomerular filtration rate < 60 mL/min/1.73 m2, n (%) | 65 (25.2) | 35 (26.7) | 30 (23.6) | 0.05 | 0.569 |
| Blood urea nitrogen, median (IQR) (mg/dL) | 16 (11–24) | 18 (14–27) | 14 (10–22) | 0.11 | < 0.001 |
| C-reactive protein, median (IQR) (mg/L) | 51 (17–126) | 57 (18–138) | 36 (16–107) | 0.08 | 0.677 |
| C-reactive protein > 5 mg/L, n (%) | 233 (90) | 115 (87.8) | 118 (92.2) | 0.11 | 0.208 |
| Troponin-I, median (IQR) (ng/L) | 7 (3–20) | 9 (4–23) | 5 (3–13) | 0.06 | 0.005 |
| Troponin-I > 28 ng/L, n (%) | 46 (17.8) | 27 (20.9) | 19 (15) | 0.12 | 0.255 |
| BNP, median (IQR) (pg/mL) | 40 (14–116) | 40 (17–141) | 37 (13–101) | 0.05 | 0.351 |
| BNP > 80 pg/mL, n (%) | 68 (26.3) | 40 (32.8) | 28 (27.7) | 0.09 | 0.466 |
| D-dimer, median (IQR) (mg/L) | 0.81 (0.47–1.54) | 0.9 (0.45–1.56) | 0.77 (0.5–1.43) | 0.19 | 0.954 |
| D-dimer > 0.5 mg/L, n (%) | 180 (69.5) | 87 (69.6) | 93 (77.5) | 0.14 | 0.193 |
| Fibrinogen, mean ± SD (mg/dL) | 520 ± 157 | 531 ± 157 | 510 ± 158 | 0.13 | 0.356 |
| Fibrinogen > 470 mg/dL, n (%) | 125 (48.3) | 62 (67.4) | 63 (57.3) | 0.17 | 0.149 |
| Ferritin, median (IQR) (ng/mL) | 391 (181–945) | 450 (176–1260) | 365 (197–780) | 0.29 | 0.193 |
| Ferritin > 163 ng/mL, n (%) | 174 (67.2) | 85 (78) | 89 (78.8) | 0.01 | 1 |
| Lung imaging | |||||
| Lobar infiltration, n (%) | 38 (14.7%) | 22 (17.1) | 16 (12.8) | 0.09 | 0.382 |
| Bilateral infiltration, n (%) | 101 (39) | 53 (41.1) | 48 (38.4) | 0.04 | 0.701 |
| Pleural effusion, n (%) | 27 (10.4) | 22 (17.1) | 5 (4) | 0.08 | 0.001 |
| Hilar congestion, n (%) | 20 (7.7) | 12 (9.3) | 8 (6.4) | 0.08 | 0.487 |
| Lung ultrasound score, median (IQR) | 14 (7–19) | 15 (7–20) | 12 (9–18) | 0.15 | 0.314 |
| Clinical evaluation of disease severity | |||||
| Sequential Organ Failure Assessment score, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.11 | 0.289 |
| COVID-19 modified early warning score, median (IQR) | 3 (1–6) | 4 (2–7) | 3 (0–6) | 0.26 | 0.075 |
| National institute of health COVID-19 severity | |||||
| Mild disease, n (%) | 121 (46.9) | 59 (45) | 62 (48.8) | 0.06 | 0.618 |
| Moderate disease, n (%) | 49 (19) | 25 (19.1) | 24 (18.9) | < 0.01 | 1 |
| Severe disease, n (%) | 45 (17.4) | 21 (16) | 24 (18.9) | 0.06 | 0.623 |
| Critical disease, n (%) | 43 (16.7) | 26 (19.8) | 17 (13.4) | 0.13 | 0.185 |
COVID-19-directed treatment
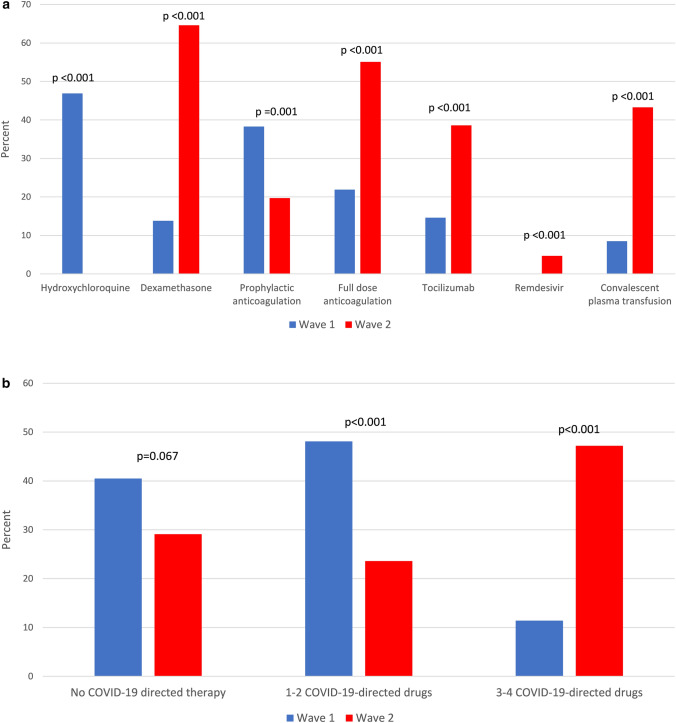
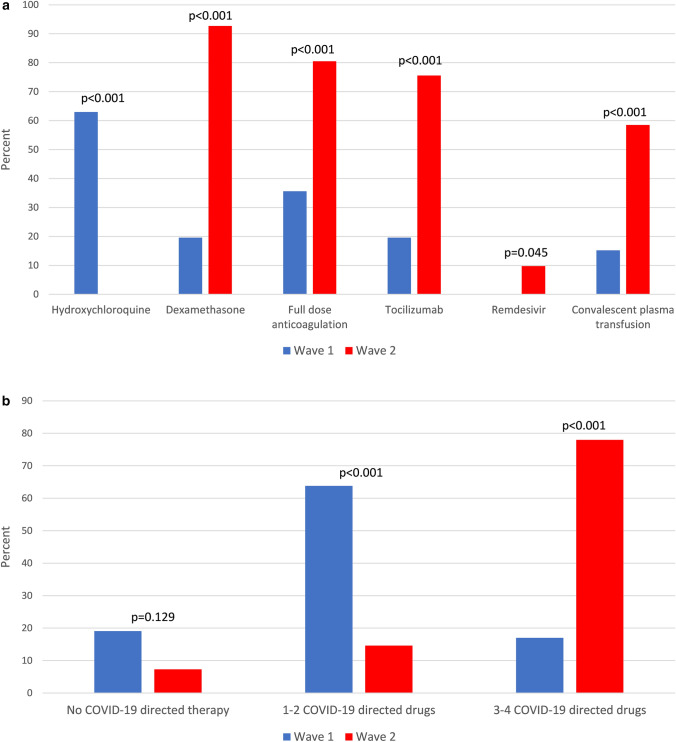
COVID-19-directed therapies of patients in the first and second peaks differed considerably. Hydroxychloroquine was the most frequently used drug in the first peak, administrated to 47% of patients, but the least used drug in the second peak, given to none. Dexamethasone, tocilizumab, remdesivir, and convalescent plasma therapy (CPT) were used significantly more in the second peak (65% vs 14%, p < 0.001; 39% vs 15%, p < 0.001; 4.7% vs 0%, p = 0.014 and 43% vs 8%, p < 0.001 respectively). Treatment with full-dose anticoagulation, mostly in the form of low-molecular-weight heparin, was more frequent during the second peak (55% vs 22%; p < 0.001), whereas prophylactic anticoagulation was more common during the first peak (20% vs 38%; p = 0.001) (Fig. 1a). While no difference was noted in the rate of untreated patients during the second peak, the frequency of treatment with 1–2 COVID-19-directed drugs decreased (48% vs 23%; p < 0.001), whereas the frequency of treatment with a combination of 3–4 COVID-19-directed drugs increased significantly (11% vs 47%; p < 0.001) (Fig. 1b). Among patients with severe-to-critical disease, treatment with dexamethasone, full-dose anticoagulation, tocilizumab, remdesivir, and CPT was significantly more frequent in second peak than in those admitted during the first peak (93% vs 20%, p < 0.001; 80% vs 36%, p < 0.001; 76% vs 20%, p < 0.001; 9.8% vs 0%, p = 0.045 and 58% vs 15%, p < 0.001) (Fig. 2a). In the same group of patients, treatment regimens combining 3–4 COVID-19-directed drugs were more frequent in the second peak (78% vs 17%, p < 0.001) (Fig. 2b). In patients with mild-to-moderate disease, apart from tocilizumab and remdesivir, all other COVID-19-directed drugs, as well as regimens of 3–4 drugs, were more common during the second peak (Figures S2 and S3).
Fig. 1.
a COVID-19-directed drugs in the first and second peaks. b COVID-19-directed combination therapies in the first and second peaks
Fig. 2.
a First vs second peak COVID-19-directed drugs in patients with severe-to-critical disease. b First vs second peak COVID-19-directed combination therapies in patients with severe-to-critical disease
Clinical outcomes
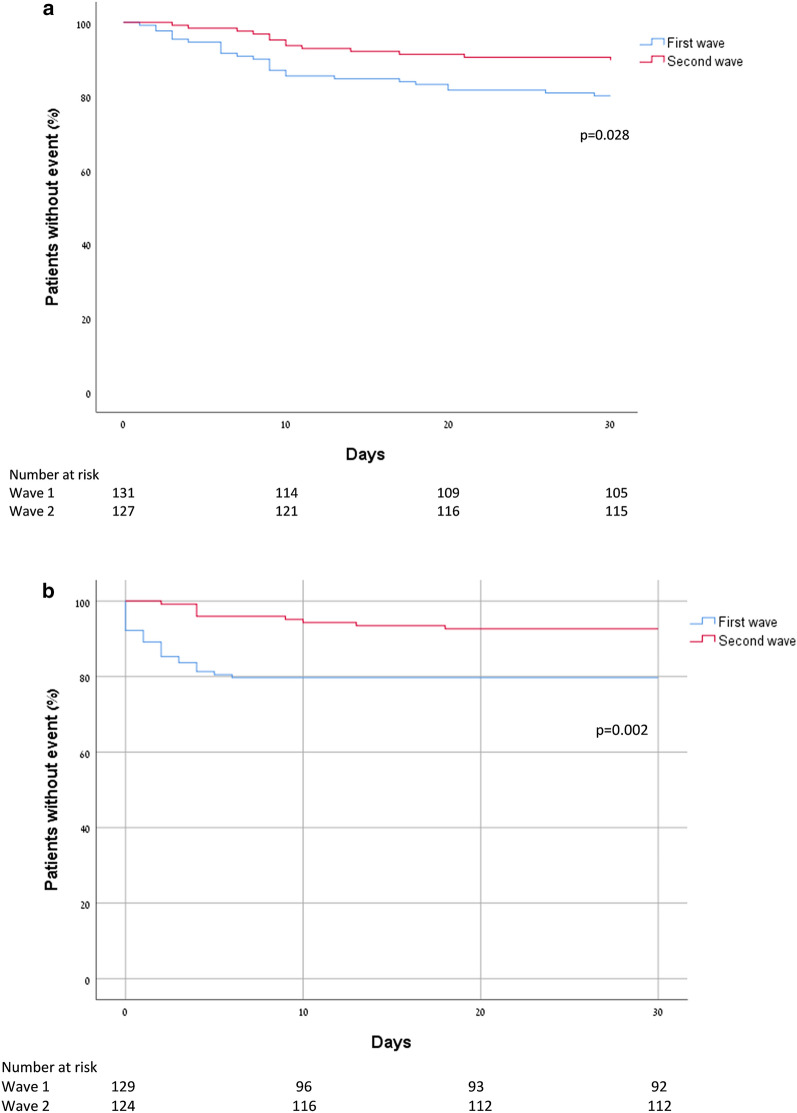
Compared to patients admitted during the first peak, second peak patients had a significantly lower 30-day mortality rate (10.2% vs 19.8%, p = 0.028) and lower 30-day invasive mechanical ventilation rate (8.8% vs 19.3%, p = 0.015) (Fig. 3a,b, respectively). Multivariate Cox regression model adjusting for age, gender, disease severity, and multiple comorbidities showed second peak patients had lower risk for both 30-day mortality and 30-day invasive mechanical ventilation (HR 0.39, 95% CI 0.19–0.79; p = 0.009 and HR 0.29, 95% CI 0.13–0.64; p = 0.002, respectively) (Table 2). While no significant difference was noted in the rate of non-invasive ventilation between the first and second peaks (10.8% vs 15.3%; p = 0.337), the adjusted risk for non-invasive ventilation was significantly higher for patients admitted during the second peak (HR 2.61, 95% CI 1.24–5.5; p = 0.012), and the median time to intubation was longer in the second peak (4 days [4–11.5] vs. 1 day [0–3]; p < 0.001). No difference was noted in the rate of major bleeding between the first and second peaks (2.3% vs 1.6%, p = 1). Finally, time to discharge was significantly shorter for patients admitted during the second peak (4 days [3–8] vs. 6 days [3–13]; p = 0.002).
Fig. 3.
a Kaplan–Meier curve for 30-day mortality of first and second peaks. b Kaplan–Meier curve for 30-day invasive mechanical ventilation of first and second peaks
Table 2.
Multivariate Cox regression for the primary outcomes
| 30-Day mortality | 30-Day mechanical ventilation | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Second wave | 0.398 | 0.199 | 0.797 | 0.009 | 0.296 | 0.136 | 0.642 | 0.002 |
| Age | 1.061 | 1.028 | 1.095 | < 0.001 | 1.007 | 0.982 | 1.033 | 0.563 |
| Gender | 0.608 | 0.304 | 1.216 | 0.159 | 0.549 | 0.257 | 1.170 | 0.12 |
| Mild disease | Reference | Reference | ||||||
| Moderate disease | 0.918 | 0.241 | 3.492 | 0.9 | 1.132 | 0.206 | 6.231 | 0.886 |
| Severe disease | 1.838 | 0.693 | 4.878 | 0.221 | 6.215 | 1.904 | 20.284 | 0.002 |
| Critical disease | 7.489 | 3.187 | 15.599 | < 0.001 | 20.855 | 6.892 | 63.106 | < 0.001 |
| Comorbidities ≥ 3 | 3.639 | 1.304 | 10.156 | 0.014 | 2.03 | 0.881 | 4.678 | 0.096 |
HR hazard ratio, CI confidence interval
Discussion
The main findings of our study are that compared to the first peak of hospitalized COVID-19 patients in our institution, the 30-day mortality and invasive mechanical ventilation rates were significantly lower during the second peak. Importantly, while no major differences were noted between the two groups of patients in baseline characteristics, laboratory parameters, echocardiographic evaluation, lung ultrasound, and disease severity at presentation, the treatment of the two groups differed considerably. The second peak patients were treated with dexamethasone, full-dose anticoagulation, tocilizumab, remdesivir, CPT, as well as a combination of 3–4 drugs at a considerable higher rate compared to patients admitted during the first peak.
Clinical outcomes
The reduced 30-day mortality rate of second peak patients seen in our cohort could be attributed to several factors. It could be argued that patients of the second peak are younger or have a milder clinical course of illness. However, this seems unlikely as we show that the two groups did not differ in baseline characteristics, laboratory tests results, as well as cardiac and pulmonary imaging. Additionally, disease severity was evaluated using three different well-standardized scores, none of which showed significant difference between patients of the two peaks. Moreover, multivariate analysis adjusting for possible undetected confounding effects of disease severity, age, gender, and multiple comorbidities showed a 60.2% risk reduction for 30-day mortality. Similar observations of reduced mortality rate and reduced adjusted mortality risk of hospitalized COVID-19 over time have been reported by Horwitz et al. [12] and Dennis et al. [13]. The findings presented here are novel as they include treatment regimens of all patients. Importantly, as we further corroborate prior findings that survival of hospitalized COVID-19 patients is improving over time, our observations suggest that COVID-19-directed therapies and therapy combination may play a role.
The reduced rate and adjusted risk reduction for invasive mechanical ventilation during the second peak should be interpreted cautiously, as it is a "softer" endpoint that was subjected to physician's discretion. Importantly, during the first peak, experts recommended early invasive ventilation [14], whereas, toward the second peak, as local and global experience was gained, the benefits of early intubation were questioned [15, 16], shifting the pendulum toward a practice favoring late intubation and prolonging non-invasive ventilation. Considering that the adjusted risk for non-invasive ventilation was significantly higher during the second peak, it is reasonable that the decrease in invasive mechanical ventilation was, to certain extent, due to physicians' predilection to prolong non-invasive ventilation and avoid early invasive mechanical ventilation. While it is likely that the reduction in both invasive mechanical ventilation and mortality observed in the second peak is a result of changes in medical treatment, we cannot exclude the possibility that avoiding mechanical ventilation, as a clinical approach, is at least partially accountable for the decrease in mortality during the second peak. The lack of randomization as well as the confounding by the difference in therapy regimens do not allow us to determine a causal relationship between these observations.
Inspecting the SOFA score within each peak reveals that, in both peaks, not surprisingly, the patients who died were sicker (i.e., had higher SOFA score) compared to those who survived. There was no significant difference in the SOFA score of survivors between the two peaks. There was a non-significant trend toward higher SOFA score of patients who died in the second peak compared to those who died in the first peak. Taken together, these results strengthen the possibility that the change in treatment was the key for the favorable outcomes during the second peak.
COVID-19-directed therapies
Of all the therapies evaluated to date, including those reported in this study, dexamethasone is the only agent that proved to decrease mortality in a large randomized-controlled trial [17]. Although early in the course of the pandemic, the WHO recommended against the routine use of steroids for patients with COVID-19 infection, the preliminary results from the randomized-controlled RECOVERY trial [18] published on June 16, 2020, established dexamethasone role as the corner-stone in the treatment of hospitalized COVID-19 patients. In our study, dexamethasone use was scarce during the first peak, whereas during the second peak, its use became routine, especially in patients with severe and critical illness, and could account for the favorable outcome observed during the second peak.
To date, data from large randomized-controlled trials concerning the efficacy of anticoagulation in patients with COVID-19 are not available. However, pulmonary post-mortem findings of platelet–fibrin thrombi in small arterial vessels [19, 20], as well as the characteristic laboratory coagulation abnormalities of COVID-19 patients [21], suggest that coagulopathy plays a key role in the pathomechanism of the disease and in the respiratory failure associated with severe illness. In our previous study [22] that included the first 100 hospitalized patients of the first peak, we reported that all patients that developed popliteal and femoral deep vein thrombosis, did so while receiving prophylactic doses of low-molecular-weight heparin. Consequently, a more liberal approach favoring treatment with full-dose anticoagulation was adopted during the second peak. Notably, this change in anticoagulation strategy was not associated with increased major bleeding, suggesting that patient selection for full-dose anticoagulation was well balanced. Whether anticoagulation contributed to the decreased mortality and invasive ventilation rates observed during the second peak could not be determined from our study, and randomized-controlled trials evaluating its direct effect are ongoing.
Remdesivir, an inhibitor of viral RNA polymerase, was regarded as a potential therapeutic agent as it showed to inhibit SARS-Cov-2 in vitro [23]. However, the largest randomized-controlled trial held to date [24] reported that although treatment with remdesivir was associated with a shorter time to recovery, it did not decrease mortality compared to placebo. In our study, remdesivir became available shortly prior to the end of the study period, and only six patients admitted during the second peak received it. Thus, the impact of remdesivir on the outcomes reported in our study and its use in combination with other drugs should be considered with caution.
Hydroxychloroquine, initially regarded as a promising therapeutic agent [25], was authorized for emergency use by the Food and Drug and Administration (FDA) [26] only to be revoked shortly thereafter [27], as data raising safety concerns and questioning its efficacy became available [28, 29]. In our cohort, hydroxychloroquine was withdrawn as of April 2, 2020, almost 2 months prior to its revocation by the FDA. Whether the decreased mortality observed in patients of the second peak is, at least partially, due to the exclusion of hydroxychloroquine, remains unknown.
Tocilizumab, a recombinant humanized anti IL-6 monoclonal antibody approved for the treatment of cytokine release syndrome [1] was evaluated in a randomized-controlled trial [30] and was not found to be effective in preventing intubation or death. Importantly, none of the patients in the tocilizumab group received concomitant treatment with dexamethasone, whereas in our study, 49 patients admitted during the second peak were treated with tocilizumab; of those, 98% received concomitant treatment with dexamethasone.
The therapeutic benefits of CPT have been known for over a century [31], and open-label, non-randomized trials have found it to be efficacious in previous epidemics [32–34]. However, two recently published randomized-controlled trials that evaluated CPT in COVID-19 patient reported conflicting results [35, 36], and the role of CPT remains unclear.
The COVID-19 pandemic has far-reaching consequences on health care systems and their ability to contain the morbidity burden while at the same time treating non-COVID-19 patients. While the availability of a vaccine in such a short time frame is unprecedented and nothing short of a monumental achievement for the scientific community [37], it is probable that COVID-19 will continue to challenge health care systems globally, and it is imperative to continue the search for the optimal treatment for those who will be infected in the future.
Our study has several limitations. It is a single center, retrospective observational study, and a selection bias cannot be excluded. Due to the observational nature of our study and lack of randomization into different treatment groups conclusions regarding individual COVID-19-directed therapies cannot be made, as most patients, especially those with a severe and critical disease, received combination therapy. The reduced 30-day mortality and need for ventilation during the second peak could potentially be due to factors not considered in this study.
Conclusions
Compared to the first peak of hospitalized COVID-19 patients, the 30-day mortality and invasive mechanical ventilation rates as well as the adjusted risks were significantly lower during the second peak. Change in treatment strategies and gain of clinical experience may be responsible for the favorable outcomes. A classic Hebrew phrase says: "All peaks rise in succession and break in their turn, as does man" [38] and Shakespeare wrote: "Like as the waves make toward the pebbled shore, So do our minutes hasten to their end; Each changing place with that which goes before, In sequent toil all forwards do contend." The unique and humbling experience of practicing medicine during a large-scale pandemic, while implementing new treatment strategies in an ever-changing environment, has taught us to never break down, and always pursue better outcomes for our patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
None.
Declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Human and animal rights statement
The study was approved by the Tel Aviv medical center ethics committee (IRB number 0196-20-TLV).
Informed consent
For this retrospective study- formal consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ariel Banai and Philippe Taieb contributed equally to this work.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Wang W, Zhao X, et al. Transmission dynamics and evolutionary history of 2019-nCoV. J Med Virol. 2020 doi: 10.1002/jmv.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucinotta D, Vanelli M (2020) WHO declares COVID-19 a Pandemic. Acta Biomed 91(1):157–160. 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed]
- 4.European Centre for Disease Prevention and Control An agency of the European Union (2020). https://www.ecdc.europa.eu/en/publications-data/download-todays-data-geographic-distribution-covid-19-cases-worldwide. Accessed 17 Aug 2020
- 5.Liao X, Wang B, Kang Y. Novel coronavirus infection during the 2019–2020 epidemic: preparing intensive care units—the experience in Sichuan Province, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambden S, Laterre PF, Levy MM, Francois B (2019) The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 23(1):374. 10.1186/s13054-019-2663-7 [DOI] [PMC free article] [PubMed]
- 7.(2020) COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed 1 Aug 2020 [PubMed]
- 8.Kirkpatrick JN, Mitchell C, Taub C, et al. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Coll Cardiol. 2020;75:3078–3084. doi: 10.1016/j.jacc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Topilsky Y, Khanna AD, Oh JK, et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929–1939. doi: 10.1161/CIRCULATIONAHA.110.991018. [DOI] [PubMed] [Google Scholar]
- 11.Lichter Y, Topilsky Y, Taieb P, et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020;46:1873–1883. doi: 10.1007/s00134-020-06212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020 doi: 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]
- 13.Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 15.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duca A, Memaj I, Zanardi F, et al. Severity of respiratory failure and outcome of patients needing a ventilatory support in the Emergency Department during Italian novel coronavirus SARS-CoV2 outbreak: preliminary data on the role of Helmet CPAP and Non-Invasive Positive Pressure Ventilation. EClinicalMedicine. 2020;24:100419. doi: 10.1016/j.eclinm.2020.100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on dexamethasone (2020) Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19. In: 16 June. https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalized-patients-with-severe-respiratory-complications-of-covid-19. Accessed 14 Nov 2020
- 19.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-center descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikdeli B, Madhavan M, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac manifestations in COVID-19. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Hinton Denise M (2020) Request for emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 coronavirus disease. https://www.fda.gov/media/136534/download. Accessed 1 Aug 2020
- 27.Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. In: 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and. Accessed 1 Aug 2020
- 28.Hraiech S, Bourenne J, Kuteifan K, et al. Lack of viral clearance by the combination of hydroxychloroquine and azithromycin or lopinavir and ritonavir in SARS-CoV-2-related acute respiratory distress syndrome. Ann Intensive Care. 2020;10:63. doi: 10.1186/s13613-020-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szekely Y, Lichter Y, Shrkihe BA, et al. Chloroquine-induced torsade de pointes in a COVID-19 patient. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 33.Hung IF, To KK, Lee C-K, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonovich VA, Pratx LDB, Scibona P, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libster R, Marc GP, Wappner D, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021 doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shitrit M (2016) Sometimes. https://www.youtube.com/watch?v=TxeYFbC1HWM. Accessed 18 Aug 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.