Abstract
Optimal vancomycin exposure is important to minimize treatment failure of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. We aimed to analyze the impact of initial vancomycin pharmacokinetic/pharmacodynamic (PK/PD) parameters, including the initial vancomycin C trough and the area under the curve (AUC)/minimal inhibitory concentration (MIC) on the outcomes of pediatric MRSA bacteremia. The study population consisted of hospitalized children aged between 2 months and 18 years with MRSA bacteremia, in whom C trough was measured at least one time within the time period of January 2010 to March 2018. Demographic profiles, underlying diseases, and clinical/microbiological outcomes were abstracted retrospectively. During the study period, 73 cases of MRSA bacteremia occurred in children with a median age of 12.4 months. Severe clinical outcomes leading to intensive care unit stay and/or use of mechanical ventilation occurred in 47.5% (35/73); all-cause 30-day mortality was 9.7% (7/72). The median dosage of vancomycin was 40.0 mg/kg/day. There was a weak linear relationship between C trough and the corresponding AUC/MIC (r = 0.235). ROC curves for achieving an AUC/MIC of 300 suggested that the initial C trough at 10 μg/mL could be used as a cut-off value with a sensitivity of 90.5% and a specificity of 44%. Although persistent bacteremia at 48–72 hours after vancomycin administration was observed more frequently when the initial C trough was < 10 μg/mL and initial AUC/MIC was < 300, initial AUC/MIC < 300 was the only risk factor associated with persistent bacteremia at 48–72 hours (adjusted OR 3.05; 95% CI, 1.07–8.68). Initial C trough and AUC/MIC were not associated with 30-day mortality. Although there was a weak relationship between C trough and AUC/MIC, initial AUC/MIC < 300 could be used as a predictor of persistent MRSA bacteremia at 48–72 hours. Further prospective data on optimal vancomycin dosing are necessary to improve clinical and microbiological outcomes in pediatric MRSA bacteremia.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the major health concerns in both healthcare-associated and community-acquired infections in children, and clinical outcomes of MRSA bacteremia are considered to be worse than cases caused by methicillin-susceptible S. aureus (MSSA) [1, 2]. Vancomycin is an important treatment option for invasive MRSA infections [1]. To achieve favorable clinical outcomes while avoiding vancomycin toxicity associated with an overdose, the methods of monitoring of vancomycin administration have been studied. The results of these studies suggested that a ratio of the area under the curve to the minimum inhibitory concentration (AUC/MIC) has been correlated with efficacy in experiments conducted in vitro [3]. Although there is an assumption that a vancomycin C trough of > 15 μg/mL when the MIC is ≤ 1 μg/mL can be used as a good surrogate marker for AUC/MIC ratio of ≥ 400 with the MIC determined by broth microdilution (BMD) for achieving clinical effectiveness in adults [4], vancomycin dosing information to ensure optimal drug exposure in the pediatric population remains limited [5]. However, recent studies have suggested that high concentrations of vancomycin may not be necessary for pediatric populations; vancomycin C trough 7–10 μg/mL could predict the achievement of AUC/MIC > 400 at a dose of 15 mg/kg/dose every 6 hours in the case of patients with normal renal function, if the MIC ≤ 1 μg/mL [6], and the risk of vancomycin nephrotoxicity may increase if C trough exceeds the predetermined range [7–9]. In addition, C trough is not highly correlated with AUC/MIC and is insufficient as a surrogate marker for clinical outcomes, not only in pediatric populations but also in adults [10, 11].
According to our previous study, initial vancomycin C trough of < 10 μg/mL was associated with microbiologic failure at 48 hours after vancomycin administration for pediatric MRSA bacteremia [12]. Another study on the impact of vancomycin C trough on the duration of MRSA bacteremia in children also suggested a C trough of > 10 μg/mL within the first 72 hours was the most predictive factor for persistent MRSA bacteremia lasting for > 3 days [13]. However, both studies focused only on the parameter of C trough without any evaluation of the impact of AUC/MIC on the clinical and microbiological outcomes.
Herein, we conducted a study elucidating the pharmacokinetics/pharmacodynamics (PK/PD) parameters, including both initial C trough and AUC/MIC, for optimal vancomycin dosing to achieve favorable clinical and microbiological outcomes in pediatric MRSA bacteremia. In addition, we aimed to determine whether C trough could be used as an optimal surrogate marker replacing AUC/MIC in clinical practice.
Materials and methods
Study design
This retrospective study was performed at a single center, the Asan Medical Center Children’s Hospital, which is a 253-bed academic-tertiary medical center located in Seoul, South Korea. Pediatric patients aged between 2 months and 18 years who were admitted during the study period from January 2010 to March 2018 with culture-confirmed MRSA bacteremia were eligible for inclusion. Patients were included if they received vancomycin for at least 48 hours, and their initial C trough had been sampled before the 4th or 5th dose within the 30 minutes before the next dose of vancomycin. The following MRSA bacteremia cases were excluded from this study: patients with chronic renal diseases requiring renal replacement therapy such as hemodialysis or peritoneal dialysis, and cases treated in the neonatal intensive care unit.
The analysis only included the first MRSA isolates detected during a single clinical episode occurring within a 4-week period, and duplicates from the same patient were excluded. Demographic profile and clinical data including primary sites of infection, underlying diseases, duration of hospital stay, and blood laboratory results, including serum creatinine, and initial vancomycin dose were extracted from the electronic medical records. The antimicrobial susceptibilities of the S. aureus isolates were decided using a MicroScan Walk-Away 96-Combo Pos 28 panel (Siemens, West Sacramento, CA, USA).
The primary outcome of this study is to clarify the relationship between the initial C trough and AUC/MIC, and the potential impact of the variables on clinical and microbiological outcomes. This study was approved by the Institutional Review Board of Asan Medical Center with a waiver of informed consent due to the study being retrospective and using de-identified data collection and analysis (IRB No. 2019–1638).
Definitions
Most of the definitions used in this study, including fever, the primary focus of infection, and persistent bacteremia, were the same as those described in a previous study [12]. Recurrent MRSA bacteremia was defined as another MRSA bacteremia event 1 month after MRSA bacteremia resolution. Co-infection was defined as the case in which clinically significant bacteria were identified from the blood culture at the same time as the diagnosis of MRSA bacteremia. Clinically severe cases included those who required an intensive care unit stay, mechanical ventilation, and/or fatal cases. Acute kidney injury (AKI) was defined as an increase in serum creatinine levels by 0.5 mg/dL [14]. The clinically significant renal toxicity of vancomycin was defined as an additional need for renal replacement therapy during vancomycin treatment or the need to replace vancomycin with an alternate antibiotic because of serum creatinine elevation.
Clinical and microbiological outcomes
The clinical and microbiological outcomes were analyzed according to the initial vancomycin C trough, vancomycin MIC of MRSA isolates, and initial AUC/MIC, respectively. As measures of clinical outcomes, recurrence of MRSA bacteremia and 30-day all-cause mortality were evaluated. Microbiological outcomes were determined using the time-to-negative conversion of blood culture and failure of clearance of bacteremia at 48–72 hours after the initiation of vancomycin administration.
Pharmacokinetic data
PK parameters including AUC and vancomycin clearance were calculated as follows:
Statistical analysis
Categorical data were analyzed with X2 tests and Fisher’s exact test, and continuous variables with independent t-tests or one-way ANOVA. Spearman`s correlation coefficient was used to evaluate relationships between C trough and AUC/MIC. A receiver operating characteristic (ROC) curve was used to determine the sensitivity and specificity of each PK/PD parameter. A multivariate logistic regression analysis was used to assess the potential impact of the variables on clinical and microbiological outcomes. A P value of < 0.05 was considered statistically significant for the comparisons. Statistical Program for Social Science release 21 (SPSS Inc., Chicago, IL, USA) was used for all statistical calculations.
Results
Patient characteristics
During the study period from January 2010 to March 2018, a total of 98 patients aged between 2 months and 18 years experienced MRSA bacteremia in our institute. Excluding 25 patients because of hemodialysis (n = 17), unavailable data of C trough (n = 3), and use of an alternative antibiotic instead of vancomycin (n = 5), a total of 73 patients who fulfilled the inclusion criteria were analyzed in this study. The patients’ median age was 12.4 months (range, 2 months– 17.3 years), and 97.3% (71 out of 73) had underlying medical comorbidities, of which congenital heart disease was the most common underlying disease (28.8%; 21/73) (Table 1). With the exception of one case, all other cases of MRSA bacteremia were healthcare-associated infections. Bacterial co-infection occurred in a total of 5 patients, all of which were bacteremia due to Enterococcus faecalis (n = 2), Klebsiella aerogenes (n = 1), K. pneumoniae (n = 1), and Pseudomonas aeruginosa (n = 1).
Table 1. Demographic and clinical characteristics of 73 pediatric patients with MRSA bacteremia.
| Characteristics | Number of cases (%) or median values (interquartile range) |
|---|---|
| Median age, months | 12.4 (5.3–36.1) |
| Sex (number; % of male) | 43 (58.9%) |
| Hospital stay before the onset of the MRSA bacteremia, days | 13.0 (4.0–33.0) |
| Presence of bacterial co-infectiona | 5 (6.8%) |
| Presence of invasive device b | 58 (79.5%) |
| Presence of fever ≥ 38°C at the onset of bacteremia | 55 (75.3%) |
| Presence of underlying diseases | 71 (97.3%) |
| Congenital heart disease | 21 (28.8%) |
| Malignancy | 12 (16.4%) |
| Chronic lung disease | 4 (5.5%) |
| Neurologic diseases | 5 (6.8%) |
| Others c | 15 (20.5%) |
| Primary focus of MRSA bacteremia | |
| None | 31 (42.5%) |
| Central vascular catheter | 25 (34.2%) |
| Lung | 8 (11.0%) |
| Others d | 9 (12.3%) |
| Initial laboratory findings | |
| Serum WBC (/uL) | 13,500 (8,600–19,300) |
| CRP (mg/dL) | 3.1 (0.8–6.7) |
| Serum Creatinine (mg/dL) | 0.3 (0.2–0.4) |
| Parameters related to vancomycin regimen | |
| Initial vancomycin dose (mg/kg/day) | 40.0 (40.0–55.0) |
| Initial vancomycin C trough concentration (mg/L) | 6.4 (4.3–11.6) |
| Initial AUC/MIC | 336.4 (271.4–422.6) |
| Simultaneous use of other antibiotics (%) | 63 (86.3%) |
| Recurrent bacteremia e | 14 (19.4%) |
| Persistent bacteremia at 48–72 hours | 29 (39.7%) |
| Acute kidney injury | 3 (4.1%) |
| All-cause 30 day-mortality e | 7 (9.7%) |
a Bacterial co-infection occurred in a total of 5 patients, all of which were bacteremia due to Enterococcus faecalis (n = 2), Klebsiella aerogenes (n = 1), K. pneumoniae (n = 1), and Pseudomonas aeruginosa (n = 1).
b Invasive devices included central vascular catheter (n = 46), gastrostomy (n = 5), tracheostomy (n = 5), ventriculo-peritoneal shunt (n = 2).
c Others included congenital diaphragmatic hernia (n = 2), congenital megacolon (n = 2), short bowel syndrome (n = 3), acute respiratory distress syndrome (n = 3), omphalocele (n = 1), trachea-esophagus fistula (n = 1), jejunal atresia (n = 1), pseudohypoaldosteronism (n = 1), VACTERL (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities association) (n = 1).
d Others included skin and soft tissue infection (n = 7), ventriculo-peritoneal shunt infection (n = 2).
e One out of 73 patients with MRSA bacteremia was transferred to another hospital during treatment, allowing analysis of mortality and recurrence rates in a total of 72 patients excluding it.
A primary focus of MRSA bacteremia was found in 57.5% (42 out of 73), which consisted of central vascular catheters (59.5%, 25 out of 42), pneumonia (19.0%, 8 out of 42), skin and soft tissue infections (16.7%, 7 out of 42), and ventriculoperitoneal shunt (4.8%, 2 out of 42); the remaining 42.5% (31 out of 73) were primary bacteremia. Among the 25 cases of central line-associated bloodstream infection, 12 (48%) retained the central vascular catheter as salvage therapy. The simultaneous use of other antibiotics with vancomycin was observed in 86.3% (63 out of 73).
Fever was the most common initial symptom (75.3%, 55 out of 73). One out of 73 patients with MRSA bacteremia was transferred to another hospital during treatment, allowing analysis of mortality and recurrence rates in a total of 72 patients: all-cause 30-day mortality was 9.7% (7 out of 72). Recurrent MRSA bacteremia and persistent bacteremia at 48–72 hours occurred in 19.4% (14/72) and 39.7% (29/73) patients, respectively. Although AKI occurred in 3 out of 73 in this study, there was no clinically significant renal toxicity associated with vancomycin use.
Vancomycin MIC of MRSA isolates
All of the 73 MRSA isolates were susceptible to vancomycin with MIC ≤ 2.0 μg/mL. Most of the MRSA isolates belonged to the group with vancomycin MIC 1.0 μg/mL (n = 53; 72.6%), followed by the group with vancomycin MIC ≤ 0.5 μg/mL (n = 10; 13.7%), and 2.0 μg/mL (n = 10; 13.7%), respectively (Table 2). During the study period, the proportion of MRSA isolates with vancomycin MIC ≤ 0.5 μg/mL showed a decreasing trend; 30.8% (2010–2012), 3.3% (2013–2015), and 5.9% (2016–2018) (P for trend = 0.246).
Table 2. Annual trend of vancomycin MIC of MRSA isolates.
| Year | Vancomycin MIC range determined by Microscan | ||
|---|---|---|---|
| < 0.5 μg/mL | 1.0 μg/mL | 2.0 μg/mL | |
| 2010–2012 | 30.8% (8/26) | 53.8% (14/26) | 15.4% (4/26) |
| 2013–2015 | 3.3% (1/30) | 86.7% (26/30) | 10.0% (3/30) |
| 2016–2018 | 5.9% (1/17) | 76.5% (13/17) | 17.6% (3/17) |
PK/PD of vancomycin use
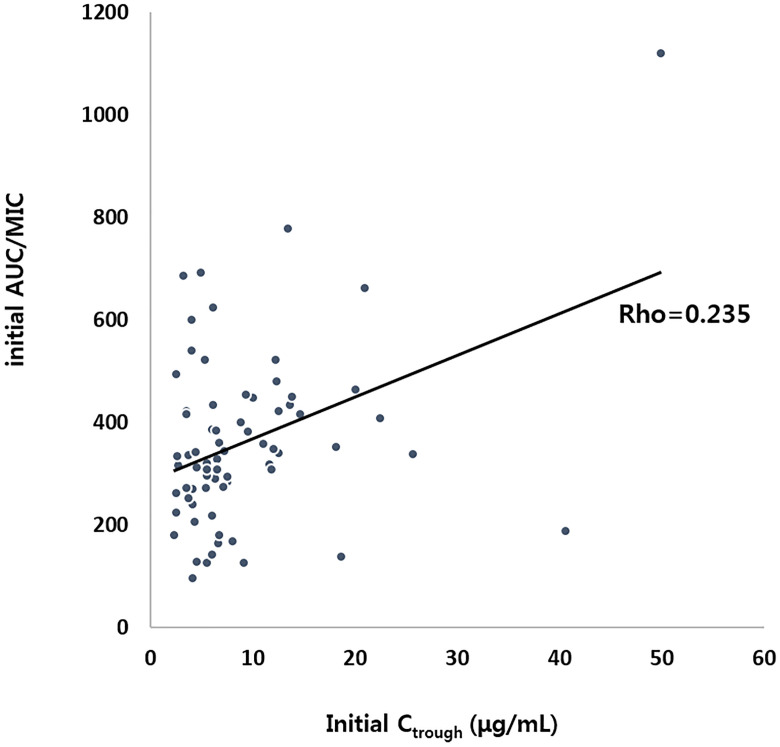
The initial median dose of vancomycin was 40.0 mg/kg/day [interquartile range (IQR), 40.0–55.0 mg/kg/day], and an initial vancomycin dose of ≥ 60 mg/kg/day was used in 28.3% of cases (17 out of 73). Initial median C trough was 6.4 μg/mL (IQR, 4.3–11.6 μg/mL); 71.2% (52 out of 73) had an initial C trough ≤ 10 μg/mL and an initial C trough > 15 μg/mL was achieved in only 8 patients. The median initial AUC/MIC was 336.4 (IQR, 271.4–422.6), and an initial AUC/MIC ≥ 300 and ≥ 400 was achieved in 64.4% and 32.9%, respectively. There was a weak positive linear relationship between C trough and AUC/MIC (r = 0.235) (Fig 1).
Fig 1. Correlation between initial C trough and AUC/MIC.
ROC curves for predicting the achievement of AUC/MIC ≥ 300 suggested that the initial C trough of 10 μg/mL could be used as a cut-off value with a sensitivity of 90.5% and a specificity of 44.0%; an AUC of 0.721 [95% confidence interval (CI), 0.593–0.849]. An achievement of AUC/MIC ≥ 400 suggested sensitivity of 57.1% and specificity of 78.8% at the cut-off value of initial C trough of 10 μg/mL.
Impact on clinical and microbiological outcomes
Compared to the group with initial C trough ≥ 10 μg/mL, persistent bacteremia at 48–72 hours was observed more frequently in those with an initial C trough < 10 μg/mL (44.2% vs 19.0%; P = 0.044) (Table 3). The mean time for negative conversion of MRSA bacteremia in those with an initial C trough < 10 μg/mL was 4.1 ± 4.0 days, but it was 2.5 ± 1.5 days in those with a C trough ≥ 10 μg/mL (P = 0.018). However, there was no difference in the percentage of persistent bacteremia or duration of MRSA bacteremia with a cut off value of C trough of 15 μg/mL. In addition, there was no statistical significance between the initial vancomycin C trough and the 30-day mortality rate or recurrence of MRSA bacteremia.
Table 3. Clinical and microbiological outcomes of children with MRSA bacteremia according to the pharmacokinetic parameters of vancomycin and the MIC of MRSA isolates.
| Microbiological outcome | Clinical outcome a | |||
|---|---|---|---|---|
| Time to negative conversion (days) | Persistent bacteremia at 48–72 hours | Recurrent MRSA bacteremia b (n = 14) | All-cause 30-day mortality (n = 7) | |
| Initial vancomycin Ctrough (μg/mL) | ||||
| < 10 (n = 52) | 4.1 ± 4.0 | 23 (44.2%) | 9/52 (17.3%) | 6/52 (11.5%) |
| ≥ 10 (n = 21) | 2.5 ± 1.5 | 4 (19.0%) | 5/20 (25.0%) | 1/20 (5.0%) |
| P-value c | 0.018 | 0.044 | 0.460 | 0.402 |
| < 15 (n = 65) | 3.7 ± 3.7 | 24 (36.9%) | 12/64 (18.8%) | 7/64 (10.9%) |
| ≥ 15 (n = 8) | 2.9 ± 1.9 | 3 (37.5%) | 2/8 (25.0%) | 0/8 (0%) |
| P-value c | 0.546 | 0.975 | 0.674 | Not applicable |
| Initial vancomycin AUC/MIC | ||||
| < 300 (n = 25) | 3.9 ± 3.0 | 14 (53.8%) | 6/25 (24.0%) | 3/25 (12.0%) |
| ≥ 300 (n = 48) | 3.4 ± 3.8 | 13 (27.7%) | 8/47 (17.0%) | 4/47 (8.5%) |
| P-valuec | 0.632 | 0.026 | 0.476 | 0.634 |
| < 400 (n = 49) | 3.2 ± 2.6 | 19 (38.8%) | 10/48 (20.8%) | 4/48 (8.3%) |
| ≥ 400 (n = 24) | 4.4 ± 4.9 | 8 (33.3%) | 4/24 (16.7%) | 3/24 (12.5%) |
| P-valuec | 0.184 | 0.651 | 0.674 | 0.574 |
| Vancomycin MIC (μg/mL) | ||||
| ≤ 1.0 (n = 63) | 6.5 ± 17.4 | 21 (33.3%) | 10/62 (16.1%) | 7/62 (11.3%) |
| 2.0 (n = 10) | 4.0 ± 3.5 | 6 (60.0%) | 4/10 (40.0%) | 0/10 (0%) |
| P-valuec | 0.659 | 0.105 | 0.077 | Not applicable |
| Initial vancomycin dose | ||||
| ≥ 60 mg/kg/day (n = 17) | 8.2 ± 23.5 | 3 (17.6%) | 5/17 (29.4%) | 1/17 (5.9%) |
| < 60 mg/kg/day (n = 56) | 5.6 ± 13.3 | 24 (42.9%) | 9/55 (16.4%) | 6/55 (10.9%) |
| P-valuec | 0.154 | 0.059 | 0.235 | 0.541 |
a One out of 73 patients with MRSA bacteremia was transferred to another hospital during treatment, allowing analysis of mortality and recurrence rates in a total of 72 patients excluding it.
b Recurrent MRSA bacteremia defined as another MRSA bacteremia event 1 month after MRSA bacteremia resolution.
c Categorical data were analyzed with X2 tests.
Persistent bacteremia at 48–72 hours was observed more frequently in the group with initial AUC/MIC < 300 than those with AUC/MIC ≥ 300 (53.8% vs 27.7%; P = 0.026). However, in terms of initial microbiological outcomes, there was no statistically significant difference between the two groups with an initial AUC/MIC < 400 and ≥ 400. Furthermore, the time to negative conversion of MRSA bacteremia, 30-days mortality, or recurrent MRSA infection were indifferent to the initial AUC/MIC.
Compared to the group with vancomycin MIC ≤ 1.0 μg/mL, persistent bacteremia at 48–72 hours and recurrent MRSA bacteremia were more frequently observed in those with vancomycin MIC 2.0 μg/mL although there was no statistical significance (33.3% vs 60.0%; P = 0.105, and 16.1% vs 40.0%; P = 0.077, respectively).
Compared to the initial vancomycin dose ≥ 60 mg/kg/day group, the <60 mg/kg/day group tended to have more cases of persistent bacteremia at 48–72 hours, but there was no statistical significance (17.6% vs 42.9%; P = 0.059). Clinical outcomes, including recurrent MRSA infection and 30-day all-cause mortality, were not influenced by the initial vancomycin dose.
Risk factors for persistence of MRSA bacteremia and 30-day all-cause mortality
Unadjusted logistic regression analysis revealed initial C trough < 10 μg/mL and initial AUC/MIC < 300 were risk factors associated with persistent bacteremia at 48–72 hours [odds ratio (OR), 3.37; 95% confidence interval (CI), 1.00–11.41, and 3.43; 95% CI, 1.24–9.45, respectively] (Table 4).
Table 4. Risk factors for persistence of MRSA bacteremia at 48–72 hours and 30-day all-cause mortality.
| Variable | Persistent bacteremia at 48–72 hours | 30-day all-cause mortality | ||
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Age | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.01 (0.99–1.02) | 1.00 (0.99–1.02) |
| Bacterial co-infection a | 1.15 (0.18–7.34) | 1.37 (0.20–9.49) | 0.00 | 0.00 |
| Presence of invasive device b | 1.81 (0.51–6.37) | 1.60 (0.42–6.13) | 2.57 (0.30–22.07) | 2.42 (0.27–21.76) |
| Presence of primary focus | 2.38 (0.87–6.51) | 1.92 (0.66–5.56) | 1.87 (0.44–7.89) | 1.85 (0.41–8.28) |
| Initial Ctrough < 10 μg /mL | 3.37 (1.00–11.41) | 3.14 (0.86–11.41) | 1.73 (0.34–8.91) | 1.42 (0.26–7.78) |
| Initial AUC/MIC < 300 | 3.43 (1.24–9.45) | 3.05 (1.07–8.68) | 0.80 (0.19–3.40) | 0.60 (0.13–2.73) |
a Bacterial co-infection occurred in a total of 5 patients, all of which were bacteremia due to E. faecalis (n = 2), K. aerogenes (n = 1), K. pneumoniae (n = 1), and P. aeruginosa (n = 1).
b Invasive devices included central vascular catheter (n = 46), gastrostomy (n = 5), tracheostomy (n = 5), ventriculo-peritoneal shunt (n = 2).
Although initial C trough < 10 μg/mL tended to increase the risk of persistent bacteremia at 48–72 hours (adjusted OR, 3.14; 95% CI, 0.86–11.41), in multivariate analysis adjusting for age, co-infection, indwelling medical device, and presence of primary focus, initial AUC/MIC < 300 was the only statistically significant risk factor associated with persistent bacteremia at 48–72 hours (adjusted OR, 3.05; 95% CI, 1.07–8.68). Co-infection, indwelling medical device, ICU stay, concurrent antibiotics use, and vancomycin MIC were not statistically significant risk factors for persistent bacteremia in unadjusted and adjusted logistic regression analysis.
Furthermore, 30-day all-cause mortality was not influenced by initial C trough nor initial AUC/MIC by adjusted or unadjusted logistic regression analysis. Age, co-infection, indwelling medical device, and presence of primary focus of bacteremia were not risk factors associated with 30-day all-cause mortality under adjusted nor unadjusted logistic regression analysis.
Discussion
In this study, we analyzed the relationship between initial vancomycin C trough and AUC/MIC, and the impact of these PK/PD parameters on clinical/microbiological outcomes of MRSA bacteremia in a pediatric population. Initial C trough < 10 μg/mL and AUC/MIC < 300 were associated with microbiological failure at 48–72 hours of vancomycin use, and initial AUC/MIC < 300 nearly tripled the risk for persistent MRSA bacteremia in children. However, these initial PK/PD parameters were not correlated with clinical outcomes in terms of recurrent MRSA infection and all-cause mortality within 30 days.
Our previous study analyzed the impact of initial C trough on the clinical and microbiological outcomes in pediatric MRSA bacteremia without data regarding AUC/MIC [12]. Herein, we extended the previous study, including more cases with MRSA bacteremia and estimated the AUC/MIC using vancomycin dose, vancomycin clearance, patient`s weight, and serum creatinine in a calculation proposed by Le et al. [5]. This study revealed a weak linear relationship between C trough and estimated AUC/MIC, but it is still inconclusive as to whether there is a positive correlation between C trough and AUC [16, 17]. A recent pediatric study suggested a positive correlation between C trough and AUC; a C trough of 10 μg/mL, 15 μg/mL, and 20 μg/mL can predict AUC of 413, 548, and 714, respectively [18]. However, Chhim et al. reported that the correlation between C trough and estimated AUC/MIC was poor (R2 = 0.14–0.20) [19]. Among the various methods for calculating AUC, Le’s equation tended to present the highest AUC value [17]. Extrapolation of AUC from serum C trough might underestimate AUC by up to 25%, and the AUC value varied between patients with similar results by up to 30-fold [8]. Accordingly, simultaneous monitoring of C trough and C peak has been suggested for accurate calculation of AUC/MIC. In a comparison of methods for calculating AUC, the trapezoidal method using both C trough and C peak could estimate AUC/MIC more accurately than the other methods [5, 16, 17]. Considering the trough-only concentration monitoring method might not reflect the actual AUC value in clinical settings and it is not always feasible to draw blood from young children at least two times per day, the Bayesian computer software programs could be used to estimate the appropriate vancomycin AUC value with minimal sampling [14, 20].
Although a recent study in adult patients showed that AUC/MIC of > 300 could be used as a surrogate marker for favorable clinical outcomes [10], a value of AUC/MIC 300–400 might be subtherapeutic, because AUC/MIC > 400 has been used as a standard surrogate marker for predicting the effectiveness of vancomycin use [21]. In this study, initial vancomycin AUC/MIC < 300 could be used as a predictor of persistent MRSA bacteremia at 48–72 hours, and AUC/MIC was a more reliable pharmacokinetic parameter predicting favorable microbiological outcomes compared to C trough. However, given that the initial AUC/MIC > 400 was only achieved in 33% in this study, the role of MIC should be addressed in correlating AUC with MIC, dose, C trough, and bacteremia clearance.
There is a controversy about a limited activity of vancomycin against MRSA isolates with a MIC at the high end of the susceptibility range [22, 23]; vancomycin MIC ≥ 1.5 μg/mL is associated with treatment failure in MRSA bacteremia [24]. In other study, however, the clinical outcomes of MRSA bacteremia were not different between vancomycin MIC < 2.0 μg/mL or 2.0 μg/mL [25]. Our study suggested that persistent or recurrent bacteremia tended to occur more often in the group with vancomycin MIC 2.0 μg/mL compared to those with MIC ≤ 1.0 μg/mL although there was no statistical significance. Even though it is difficult to say clearly that vancomycin “MIC creep” was observed during this study, which was defined as a gradual increase in the central tendency of the vancomycin MIC above 1 or 1.5 μg/mL for the dominant wild-type population [26], there was no increased trend of the proportion of MRSA isolates with vancomycin MIC > 1.0 μg/mL. However, it is important to clearly distinguish MIC creep from an increased occurrence of specified epidemic clones for which vancomycin MIC are elevated. So, it is necessary to monitor long-term changes in vancomycin MIC and the increase of specific clones with higher MIC. Additionally, there is considerable variability in MIC results between the susceptibility testing methods [27, 28], and the current critical PK/PD index was accepted with the MIC determined by BMD [14]. Because our institution perform MIC testing using automated systems, MicroScan WalkAway, it may be helpful to consider performing BMD in future studies to minimize method-dependent differences in MIC results.
Recent studies of pediatric MRSA bacteremia suggested that a higher C trough does not correlate with clinical outcomes [11], and the therapeutic discordance between AUC and C trough may lead to suboptimal outcomes among patients with infections due to pathogens with higher MIC values [18, 20]. In addition, targeting vancomycin trough levels > 15 μg/mL in pediatrics could expose them to adverse effects such as nephrotoxicity or ototoxicity [18]. It is difficult to give clear meaning due to the small number, but in a total of 3 patients who experienced transient AKI in this study, the initial C trough were all < 10 μg/mL.
The recurrence rate was relatively high in this study, although it was not possible to clearly distinguish between recurrence or reinfection of MRSA bacteremia because genotyping analysis using pulsed field gel electrophoresis or multilocus sequence typing was not performed. This might be explained by a high proportion of patients with severe underlying disease and long-term hospitalization, and clinician`s preference for salvage therapy for catheter associated infection.
This study had some limitations. Because of the small study population from a single center, its generalizability may be poor, and the low event rate of clinical outcome including mortality makes multivariable analysis challenging. We conducted this study retrospectively, and checked only C trough during vancomycin treatment as the standard of care. So, the estimated AUC without C peak might not reflect the actual AUC in pediatric patients. However, there have been few clinical studies to determine the impact of the PK/PD parameters of vancomycin on clinical and microbiological outcomes in MRSA bacteremia especially among children population. We adopted methods for calculating AUCs that could be applicable for children under 2 years old, and determined the impact of the vancomycin PK/PD parameters of C trough, AUC, and MIC on clinical and microbiological outcomes in MRSA bacteremia which occurred in young children. Finally, this is a study of initial C trough or AUC/MIC, rather than a study of PK/PD parameters over time during a treatment course. Given the clinical significance of subsequent C trough or AUC/MIC, a delicate analysis of the relationship between PK/PD parameters and clinical/microbiological outcomes throughout the treatment period will be essential.
In conclusion, initial vancomycin AUC/MIC < 300 increased the risk for early microbiologic failure during treatment for pediatric MRSA bacteremia, although it did not impact 30-day fatalities or recurrence. In addition, C trough and AUC/MIC had only weak relationships in children. Future studies using more accurate PK/PD parameters are needed to determine the optimal vancomycin exposure and to improve the clinical and microbiological outcomes in children.
Supporting information
(XLSX)
Acknowledgments
This manuscript was presented in part at the ASM microbe 2019, in San Francisco, California, USA, June 20–24, 2019.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015; 28:603–661. 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev. 2012; 25:362–386. 10.1128/CMR.05022-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rybak MJ. The Pharmacokinetic and Pharmacodynamic Properties of Vnaocmycin. Clin Infect Dis. 2006; 42:35–39. 10.1086/491712 [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011; 52:e18–55. 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 5.Le J, Bradley JS, Murray W, Romanowski GL, Tran TT, Nguyen N, et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J. 2013; 32:e155–163. 10.1097/INF.0b013e318286378e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frymoyer A, Guglielmo BJ, Hersh AL. Desired Vancomycin Trough Serum Concentration for Treating Invasive Methicillin-resistant Staphylococcal Infections. Pediatr Infect Dis J. 2013; 32:1077–1079. 10.1097/INF.0b013e318299f75c [DOI] [PubMed] [Google Scholar]
- 7.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009; 49:507–514. 10.1086/600884 [DOI] [PubMed] [Google Scholar]
- 8.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014; 58:309–316. 10.1128/AAC.01653-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ragab AR, Al-Mazroua MK, Al-Harony MA. Incidence and predisposing factors of vancomycin-induced nephrotoxicity in children. Infect Dis Ther. 2013; 2:37–46. 10.1007/s40121-013-0004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mogle BT, Steele JM, Seabury RW, Dang UJ, Kufel WD. Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2018; 52:805–810. 10.1016/j.ijantimicag.2018.08.024 [DOI] [PubMed] [Google Scholar]
- 11.McNeil JC, Kok EY, Forbes AR, Lamberth L, Hulten KG, Vallejo JG, et al. Healthcare-associated Staphylococcus aureus Bacteremia in Children: Evidence for Reverse Vancomycin Creep and Impact of Vancomycin Trough Values on Outcome. Pediatr Infect Dis J. 2016; 35:263–268. 10.1097/INF.0000000000000991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo RN, Kim SH, Lee J. Impact of Initial Vancomycin Trough Concentration on Clinical and Microbiological Outcomes of Methicillin-Resistant Staphylococcus aureus Bacteremia in Children. J Korean Med Sci. 2017; 32:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu AJ, Hamdy RF, Huang Y, Olson JA, Ghobrial S, Gerber JS, et al. Association between vancomycin trough concentrations and duration of Methicillin-Resistant Staphylococcus aureus bacteremia in children. J Pediatric Infect Dis Soc. 2018; 7:338–341. 10.1093/jpids/pix068 [DOI] [PubMed] [Google Scholar]
- 14.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillinresistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists,the Infectious Diseases Society of America, the Pediatric Infectious DiseasesSociety, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020; 77:835–864. 10.1093/ajhp/zxaa036 [DOI] [PubMed] [Google Scholar]
- 15.Frymoyer A, Hersh AL, Coralic Z, Benet LZ, Joseph Guglielmo B. Prediction of vancomycin pharmacodynamics in children with invasive methicillin-resistant Staphylococcus aureus infections: a Monte Carlo simulation. Clin Ther. 2010; 32:534–542. 10.1016/j.clinthera.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang D. Influence of malignancy on the phramacokinetics of vancomycin in infants and children. Pediatr Infect Dis J. 1995; 14:667–673. 10.1097/00006454-199508000-00004 [DOI] [PubMed] [Google Scholar]
- 17.Kishk OA, Lardieri AB, Heil EL, Morgan JA. Vancomycin AUC/MIC and Corresponding Troughs in a Pediatric Population. J Pediatr Pharmacol Ther. 2017; 22:41–47. 10.5863/1551-6776-22.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alsultan A, Abouelkheir M, Alqahtani S, Aljabri A, Somily AM, Alsubaie S, et al. Optimizing Vancomycin Monitoring in Pediatric Patients. Pediatr Infect Dis J. 2018; 37:880–885. 10.1097/INF.0000000000001943 [DOI] [PubMed] [Google Scholar]
- 19.Chhim RF, Arnold SR, Lee KR. Vancomycin Dosing Practices, Trough Concentrations, and Predicted Area Under the Curve in Children With Suspected Invasive Staphylococcal Infections. J Pediatric Infect Dis Soc. 2013; 2:259–262. 10.1093/jpids/pis083 [DOI] [PubMed] [Google Scholar]
- 20.Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014; 77:50–57. 10.1016/j.addr.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 21.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of Vancomycin and Other Antimicrobials in Patients with Staphylococcus aureus Lower Respiratory Tract Infections. Clinical Pharmacokinetics. 2004; 43:925–942. 10.2165/00003088-200443130-00005 [DOI] [PubMed] [Google Scholar]
- 22.Chang CN, Lo WT, Chan MC, Wang CC. A retrospective study to estimate serum vancomycin trough concentrations in pediatric patients with current recommended dosing regimen. JMed Sci. 2018; 38:275–279. 10.4103/jmedsci.jmedsci_103_18 [DOI] [Google Scholar]
- 23.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009; 66:82–98. 10.2146/ajhp080434 [DOI] [PubMed] [Google Scholar]
- 24.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008; 52:3315–3320. 10.1128/AAC.00113-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adani S, Bhowmick T, Weinstein MP, Narayanan N. Impact of Vancomycin MIC on Clinical Outcomes of Patients with Methicillin-Resistant Staphylococcus aureus Bacteremia Treated with Vancomycin at an Institution with Suppressed MIC Reporting. Antimicrob Agents Chemother. 2018; 62:e02512–17. 10.1128/AAC.02512-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sader HS, Fey PD, Limaye AP, Madinger N, Pankey G, Rahal J, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother. 2009; 53:4127–4132. 10.1128/AAC.00616-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu DI, Hidayat LK, Quist R, Hindler J, Karlsson A, Yusof A, et al. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of meticillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents. 2008; 32:578–585. 10.1016/j.ijantimicag.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 28.Kruzel MC, Lewis CT, Welsh KJ, Lewis EM, Dundas NE, Mohr JF, et al. Determination of vancomycin and daptomycin MICs by different testing methods for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2011; 49:2272–2273. 10.1128/JCM.02215-10 [DOI] [PMC free article] [PubMed] [Google Scholar]