Abstract
BACKGROUND
White tea (Camellia sinensis) has anti-inflammatory and antioxidant properties and a protective effect against wrinkles, sunburn and UV damages on the skin. Thus, we aimed to evaluate the effect of white tea extract on the healing process of skin wounds in rats.
METHODS
This study was done in the Research Center of Shahrekord University of Medical Sciences, Shahrekord, Iran in 2019. Excisional skin wounds were created on five groups of healthy male Wistar rats (200-250 g, n=21) including control group, Eucerin-treated group, white tea 5% ointment (Eucerin) treated group, gel-treated group, white tea 5% gel treated group. Treatment was begun on day 1 and repeated every day at the same time until day 15. Pathologic samples were taken on days 4, 7 and 15 for histopathological examinations. Kruskal-Wallis test was used to analyze data by SPSS. Statistical significance was defined as P<0.05.
RESULTS
Wound closure rate of control group was more than other groups on day 4 (P<0.05). On day 7, reepithelisation and granulation tissue of control group were more than white tea 5% ointment-treated and its inflammation was less than others (P<0.05). Neo-vascularization of white tea 5% ointment-treated group was more than control group on days 4 and 15 (P<0.05). On day 4, intact mast cells of control group were more than white tea treated groups (P<0.05). Degranulated mast cells of white tea 5% gel treated group was significantly (P<0.05) more than control group on days 4 and 15.
CONCLUSION
Five percent white tea extract could not help the skin wound healing process.
Key Words: Antioxidant, Camellia sinensis, Cutaneous wound, Healing process, White tea
INTRODUCTION
Skin protects our bodies against dehydration and foreign pathogens influx and maintains body homeostasis and temperature 1, 2. Therefore, wounds by causing a disturbance in skin anatomy and function may lead to complications such as infection and even shock 3, 4. Because of the importance of healthy skin, wounds prevalence and the costs of skin wound treatment, it is essential to find a low-cost and effective treatment for skin wounds 2,5,6.
Chemical drugs are expensive and have side effects, while, medicinal plants are safe and cheap 7-9. Thus, the wound-healing effects of some medicinal plants have been studied and proven 4.
The extracts of some medicinal plants such as Lemon, Aloe vera, Jojoba, and Ginseng were used for wound healing and their common feature was the production of flavonoid compounds with phenolic structures 10. Flavonoids and polyphenols have anti-oxidative effect 11.
In a normal physiological state, production and neutralization of reactive species are in balance 12. Overproduction of ROS is cytotoxic and delays wound healing process. Therefore, one way to improve wound healing is reducing ROS 5.
The genus Camellia is a member of the Theaceae family 13. Tea (C. sinensis L) has various beneficial effects including antioxidant, anti-diabetic, neuroprotective, hypocholesterolemic, antimicrobial and antifungal activities 14,15. It also prevents cancers and heart disease and protects our body from Cd and Pb accumulation 16. C. sinensis water extract has anti-wrinkle effects 17.
Different types of tea are classified based on their formation process. White and green tea are non-fermented, red tea is semi-fermented and black tea is fermented 14,15,18. There are polyphenol oxidase and other oxidative enzymes in the leaves of tea; thus, during the fermentation process from white tea to black tea, the polyphenols of C. sinensis’ leaves become more and more oxidized 14,18. Therefore, white tea has the most antioxidants among all types of teas 15. The antioxidant property of tea seems to be due to tea flavonoids and polyphenols 17. Some of the features of polyphenols are antioxidant, antineoplastic and anti-inflammatory effects 15, 19. Moreover, they are good for skin health 19.
The catechins of white tea are catechin (C), epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epigallocatechin (EGC) and epicatechin (EC) 20. They are anti-oxidants that improve wound healing by increasing collagen volume and keratinocytes reproduction 21.
Considering similarities between green tea and white tea, and benefits of green tea ethanolic extract on the surgical and burn wounds 1,21, more anti-oxidants in white tea in comparison with green tea and the positive effects of antioxidants and antimicrobial agents on the wound healing process and all the properties of white tea, we aimed to evaluate the effect of white tea extract on the healing process of excisional skin wounds in rats 2,14,15,18.
MATERIALS AND METHODS
After obtaining approval from Ethics Committee of Shahrekord University of Medical Sciences, this experiment was carried out in the Research Center of Shahrekord University of Medical Sciences in 2019.
Preparation of white tea extract
The Maceration method was used for preparing the extract. Dried leaves of white tea (Lahijan Refah tea, TEAMAN CO., Iran. Herbarium No. 1020, Medical Plants Research Center, SKUMS) were pulverized by a grinder machine. A mixture of 100 gr of white tea powder and 1 L of ethanol 70% (Hamoon Teb Markazi Pharmaceutical Chemical Industrial, Iran.) was made in an Erlenmeyer and kept for 48 h in the laboratory temperature. Subsequently, all the materials were filtered by filter paper, evaporated by a rotary and dried by a heater 21.
The powder of extract was uniformly mixed with Eucerin (Orand Eucerin, Abi Darya Co., Iran) to prepare Eucerin-based 5% ointment. A gel containing 5% extract of white tea was produced, too.
Animals
Healthy male Wistar rats (105 rats, 200-250 g) were housed in the same situation for light, food, and water. A standard glass cage was allocated to each rat. After acclimatization to the environment of the laboratory, 105 rats were randomly divided into five groups containing 21 rats each: control group, vehicle (Eucerin)-treated group, white tea 5% ointment (Eucerin)-treated group, vehicle (gel)-treated group and white tea 5% gel-treated group.
Surgical wounds
On day 0, all the rats were anesthetized with an intramuscular injection of 20 mg/kg ketamine 10% (Merck, Germany) and 2 mg/kg xylazine 2% (Merck, Germany). Then, after the disinfection of the skin of dorsal surface of their neck by betadine 10% (Behvazan pharmaceutical Co. Iran) and shaving the hair, a full-thickness circular excisional skin wound was created with 1 cm diameter on the dorsal surface of the neck by a sterile scissor 21, 22.
Treatments
All the rats were treated every day for 15 d and the treatment began on day 1 which means one day after the surgery. Every day, all the wounds of all rats were washed by normal saline serum (Daru-Pakhsh, Iran) and after that, all of them, except the control group, was given a layer of their local treatment which covered the wound completely. Therefore, the wounds of vehicle (Eucerin)-treated group were treated with the Eucerin vehicle, the wounds of white tea 5% ointment (Eucerin)-treated group with white tea 5% ointment, the wounds of vehicle (gel)-treated group with vehicle gel and wounds of white tea 5% gel treated group with gel 5% of white tea.
Data collecting
Seven rats from each group were randomly selected on days 4, 7 and 15. The surface of the remaining wound of each rat was drawn on a transparent paper and with the aid of AutoCAD software, the area of the remaining wound was measured. The following equation was used for wound closure rate calculation.
Wound closure rate %= [(area at day 0 – area at each day of measurement)/area at day 0] ×100
For the histological study, the rats were euthanized and then full-thickness remaining wounds with the surrounding healthy skin were sampled and fixed in tubes containing formalin buffer 10% 21, 22. Subsequently, tissues were dehydrated and processed for paraffin and with the means of microtome, 5 µm thick incisions were made and then stained by Toluidine Blue, and Hematoxylin and Eosin (H&E) 21, 23.
The evaluation of re-epithelialization, neovascularization, granulation tissue, inflammation and collagen alignment of the samples were accomplished by a pathologist who was blinded in grouping by evaluating H&E stained tissues and the given scores were based on Table 1.
Table 1.
System of histological scoring of samples
| Histological features | Score | ||||
|---|---|---|---|---|---|
| Granulation tissue | _ | Thin granular layer | Moderate granular layer | Thick granular layer | Very thick granular layer |
| Re-epithelialization | No epithelial organization |
Just basal layer formed | Moderate epithelial organization | Keratinization is done | _ |
| Neo-vascularization | zero formed capillary vessels |
Newly formed capillary vessels<3 | 3-6 formed capillary vessels | formed capillary vessels>6 | _ |
| Collagen alignment | _ | 25% of collagen tissue is parallel to wound surface | 25-50% of collagen tissue is parallel to wound surface | 50-75% of collagen tissue is parallel to wound surface | More than 75% of collagen tissue is parallel to wound surface |
| Inflammation | No inflammation | Mild | Moderate | Sever | _ |
A pathologist who was blinded in grouping quantified the Mast cells of tissues with the use of Toluidine Blue stained samples.
Data analysis
The normal distribution of the variables was evaluated using the one-sample Kolmogorov-Smirnov test. Because the data were not distributed normally, the medians with interquartile range (IQR) were used for presenting the data. Kruskal-Wallis test followed by Dunn’s test was used to compare variables between the groups. Statistical significance was defined as P<0.05 and analysis was performed by SPSS version 23 (Chicago, IL, USA).
Ethics
The experiment was approved by the Ethical Committee of Shahrekord University of Medical Sciences (ethical code: IR.SKUMS.REC.1396.22). We followed the ethics principles of working on animals.
RESULTS
Measurement of phenolic and flavonoid compounds
The phenolic compounds were totally 226.45 mg/g in Gallic acid equivalent.
The flavonoid compounds were totally 9.95 mg Rutin equivalent/g.
Wound closure rate
Median with Interquartile ranges (IQR) of wound closure rates in groups are shown in Table 2.
Table 2.
Median with Interquartile range (IQR) of wound closure rates in groups
| Group | Day4 | Day7 | Day15 |
|---|---|---|---|
| Control | 69.5(66.6-73.6)* | 61.6(58-86.4) | 100(97.8-100) |
| Eucerin-treated | 51.1(48.1-61.8)* | 77.3(75-81.9) | 100(99-100) |
| White tea 5% ointment-treated | 46.1(41.4_58.9)* | 65(55.4-70.2) | 100(100-100) |
| Gel-treated | 60.9(48.4-65) | 76(67.3-83.7) | 99.5(98-100) |
| White tea 5% gel-treated | 50.8(36.8-57)* | 82(81-85.8) | 100(100-100) |
On day 4, the wound closure rate of control group was significantly (P<0.05) more than Eucerin-treated group, white tea 5% ointment (Eucerin)-treated group and white tea 5% gel treated group.
Histological findings
Median with Interquartile ranges (IQR) of histological findings in groups are shown in Table 3. There was no significant difference in Collagen alignments between groups. Histological differences among groups with H&E staining, on day 4, are shown in Figure 1. Histological differences among groups with H&E staining, on day 7, are shown in Figure 2.
Table 3.
Median with Interquartile range (IQR) of histological findings in groups
| Group | Granulation tissue | Re-epithelialization | Neo-vascularization | Collagen alignment | Inflammation | |
|---|---|---|---|---|---|---|
| Control | Day 4 Day 7 Day 15 |
2(2-2) 3(3-4)❶ 3(3-3)❷ |
0(0-0) 1(1-2)❸ 3(2/5-3) |
2(2-2)❺ 2(2-3) 0(0-1)❻ |
1(1-1) 2(2-3) 3(2/5-3) |
2(1-2) 0(0_0)❼ 0(0_0) |
| Eucerin-treated | Day 4 Day 7 Day 15 |
2(1-2) 3(2-3) 3(2-3) |
0(0-2) 0(0-2) 3(2-3) |
2(2-2) 2(2-3) 1(1-2) |
1(1-1) 2(1-2) 2(1-3) |
1(1-1) 1(0-1)❼ 0(0_0) |
| White tea 5% ointment-treated | Day 4 Day 7 Day 15 |
2(2-2) 2(2-3)❶ 3(3-3) |
0(0-0) 0(0-0/25)❸ 3(3-3) |
3(2-3)❺ 3(2-3) 1(1-2)❻ |
1(1-1) 2(1-2) 3(3-3) |
2(1-2) 1(1-1)❼ 0(0_0) |
| Gel-treated | Day 4 Day 7 Day 15 |
2(1/5-3) 3(3-4) 4(3/75-4)❷ |
0(0-0) 3(2-3)❹ 3(2/25-3) |
2(1/5-3) 2(1/5-2/5) 1(0/75-2) |
1(0/5-2) 3(2/5-3) 4(2/75-4) |
1(0/5-1/5) 0(0-1) 0(0_0/25) |
| White tea 5% gel-treated | Day 4 Day 7 Day 15 |
2(2-2) 3(3-4) * |
0(0-0) 0(0-0)❹ 3(3-3) |
1(1-2) 2(1-3) * |
1(1-2) 2(2-2) * |
2(1-2) 1(0-2)❼ 0(0_0) |
*: IQR was not calculable due to the lack of enough data.
On day 7, the granulation tissue of control group was significantly (P<0.05) more than white tea 5% ointment (Eucerin)-treated group (❶).
On day 15, the granulation tissue of vehicle (gel)-treated group was significantly (P<0.05) more than control group (❷).
On day 7, the re-epithelialization of control group was significantly (P<0.05) more than white tea 5% ointment (Eucerin)-treated group (❸).
On day 7, the re-epithelialization of vehicle (gel)-treated-group was significantly (P<0.05) more than white tea 5% gel-treated group (❹).
On days 4 and 15, the neovascularization of white tea 5% ointment (Eucerin)-treated group was significantly (P<0.05) more than control group (❺, ❻).
On day 7, the inflammation of control group was significantly (P<0.05) less than vehicle (Eucerin)-treated group, white tea 5% ointment (Eucerin)-treated group and white tea 5% gel-treated group (❼).
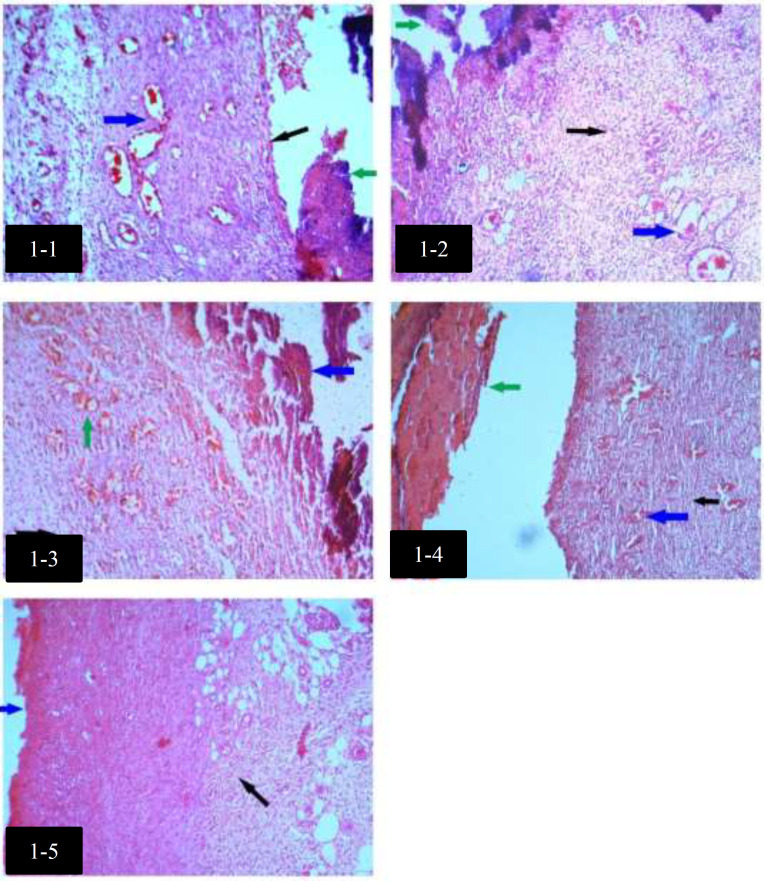
Fig. 1.
Histological differences among groups with H&E staining, on day 4
1-1: Granulation tissue formation with abundant blood vessels (blue arrow), no epidermis (black arrow) and presence of superficial clot (green arrow) in control group on day 4 (H&E, X10).
1-2: Granulation tissue formation with irregular collagen fibers (black arrow), abundant blood vessels (blue arrow), no epidermis and presence of superficial clot (green arrow) in Eucerin-treated group on day 4 (H&E, X10).
1-3: Filling of the wound with granulation tissue and irregular collagen fibers (black arrow), abundant blood vessels (green arrow), no epidermis and presence of superficial clot (blue arrow) in white tea 5% ointment (Eucerin)-treated group on day 4 (H&E, X10).
1-4: Filling of the wound with granulation tissue and regular collagen fibers (black arrow), abundant blood vessels (blue arrow), no epidermis and presence of superficial clot (green arrow) in gel-treated group on day 4 (H&E, X10).
1-5: Filling of the wound with granulation tissue and partially regular collagen fibers (black arrow), no superficial epidermis (blue arrow) in white tea 5% gel-treated group on day 4 (H&E, X10).
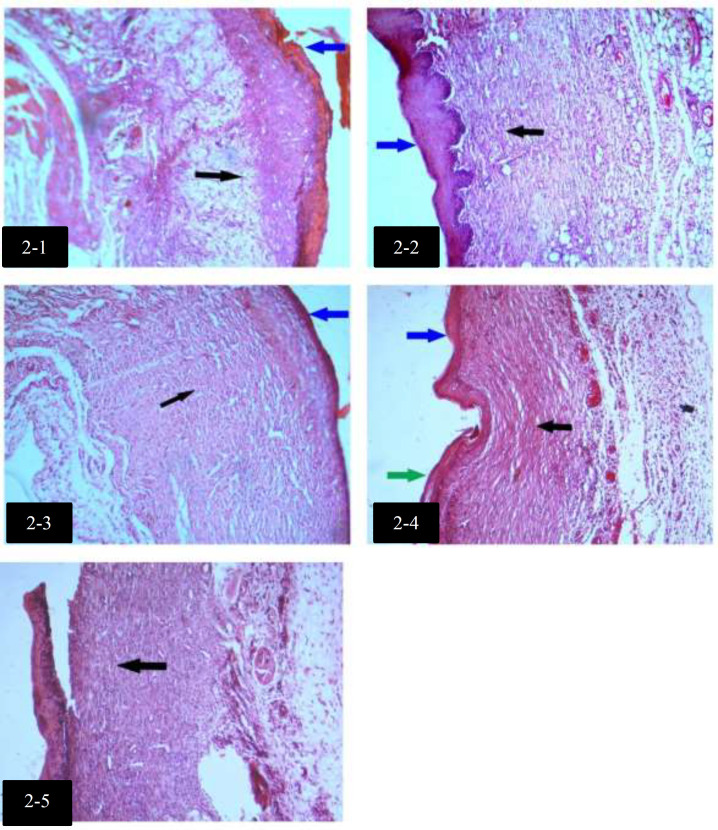
Fig. 2.
Histological differences among groups with H&E staining, on day 7
2-1: Filling of the wound with granulation tissue and irregular collagen fibers (black arrow), no epidermis and presence of superficial clot (blue arrow) in control group on day 7 (H&E, X10).
2-2: Filling of the wound with granulation tissue and regular collagen fibers (black arrow), epidermis formation (blue arrow) in Eucerin-treated group on day 7 (H&E, X10).
2-3: Filling of the wound with granulation tissue and regular collagen fibers (black arrow), epidermis formation (blue arrow) in white tea 5% ointment (Eucerin)-treated group on day 7 (H&E, X10).
2-4: Filling of the wound with granulation tissue and regular collagen fibers (black arrow), Keratinized (green arrow) superficial epidermis formation (blue arrow) in gel-treated group on day 7 (H&E, X10).
2-5: Filling of the wound with granulation tissue and dense regular collagen fibers (black arrow) in white tea 5% gel-treated group on day 7 (H&E, X10).
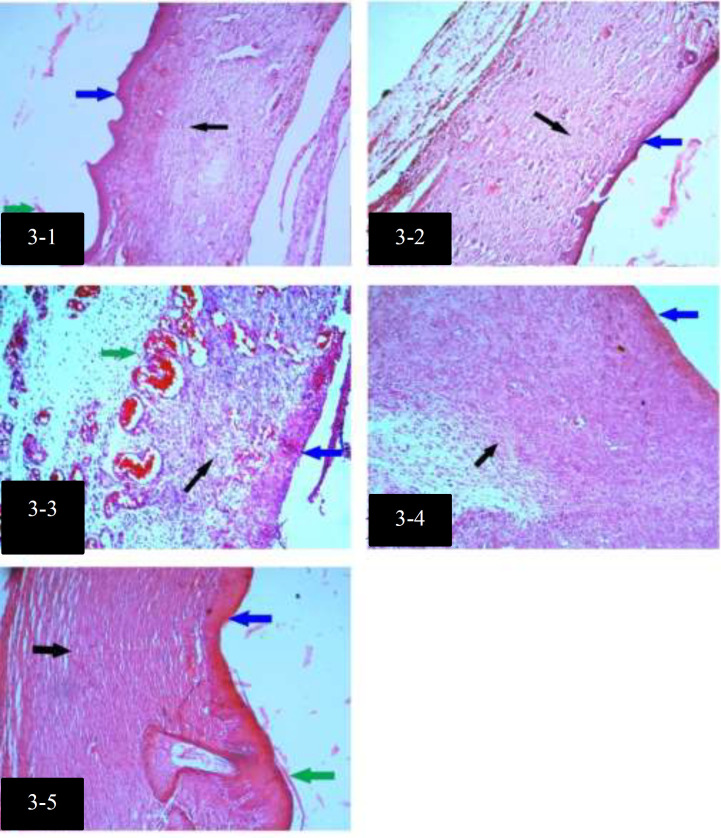
Histological differences among groups with H&E staining, on day 15, are shown in Figure 3. Median with Interquartile ranges (IQR) of intact and degranulated Mast cells in groups are shown in Table 4.
Fig. 3.
Histological differences among groups with H&E staining, on day 15
3-1: Filling of the wound with granulation tissue and regular collagen fibers (black arrow), epidermis formation (blue arrow) along with superficial keratinization (green arrow) in control group on day 15 (H&E, X10).
3-2: Filling of the wound with granulation tissue and regular collagen fibers (black arrow), superficial epidermis formation (blue arrow) in Eucerin-treated group on day 15 (H&E, X10).
3-3: Filling of the wound with granulation tissue and sparse irregular collagen fibers (black arrow), abundant blood vessels (green arrow), no superficial epidermis formation (blue arrow) in white tea 5% ointment (Eucerin)-treated group on day 15 (H&E, X10).
3-4: Filling of the wound with granulation tissue and partially regular collagen fibers (black arrow), superficial epidermis formation (blue arrow) in gel-treated group on day 15 (H&E, X10).
3-5: Filling of the wound with granulation tissue and dense regular collagen fibers (black arrow), Keratinized (green arrow) superficial epidermis formation (blue arrow) in white tea 5% gel-treated group on day 15 (H&E, X10).
Table 4.
Median with Interquartile range (IQR) of intact and degranulated Mast cells in groups
| Group | Intact Mast cells | Degranulated Mast cells | |
|---|---|---|---|
| Control | Day 4 Day 7 Day 15 |
14(7-18)① 3(2-4) 3(1-3) |
4(0-6)③ 4(2-6) 0(0-1)④ |
| Eucerin-treated | Day 4 Day 7 Day 15 |
6(5-12) 2(1-3) 2(2-3) |
8(2-9) 3(2-6) 1(0-2) |
| White tea 5% ointment-treated | Day 4 Day 7 Day 15 |
3(2-4.5)① 2(1.5-3.5) 2(2-3) |
8(5-9.5) 5(3-6) 0(0-1) |
| Gel-treated | Day 4 Day 7 Day 15 |
4(4-4) 4(4-4) 2(1-2)② |
6(3-7) * 0(0-1)④ |
| White tea 5% gel-treated | Day 4 Day 7 Day 15 |
2(2-3)① 3(3-7) 3.5(3-4.75)② |
9(7-16)③ 5(5-9) 2.5(1.25-3)④ |
On day 4, intact Mast cells of control group were significantly (p<0.05) more than intact Mast cells of white tea 5% ointment-treated group and white tea 5% gel treated group (①).
On day 15, intact Mast cells of white tea 5% gel treated group were significantly (p<0.05) more than intact Mast cells of gel-treated group (②).
On day 4, degranulated Mast cells of white tea 5% gel treated group were significantly (p<0.05) more than degranulated Mast cells of control group (③).
On day 15, degranulated Mast cells of white tea 5% gel treated group were significantly (p<0.05) more than degranulated Mast cells of control group and gel-treated group (④).
*: IQR was not calculable due to the lack of enough data.
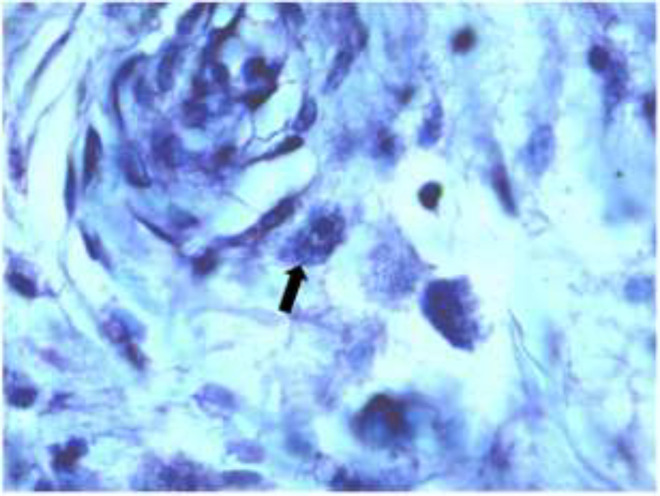
Mast cell in the wound with Toluidine Blue staining is shown in Figure 4.
Fig. 4.
Mast cell in the wound (black arrow) (X100, Toluidine Blue)
DISCUSSION
This study aimed to evaluate the effect of white tea extract on the wound healing process in male Wistar rats (105 rats, 200-250 g) showed that despite flavonoids and antioxidant properties of white tea, it did not improve wound healing.
After occurrence of a wound (which is an interruption of the continuity of cells of the skin), the wound healing process begins; in which the four overlapping phases including hemostasis, inflammation, cellular proliferation, and remodeling should be done in proper sequence and time frame 8, 24, 25. Some factors have effects on the wound healing process; such as age, sex, stress, alcohol, smoking, nutritional condition, and diabetes 4.
Plant-based medicines are used by 80% of world’s population 26. Various plants have been studied to evaluate their effects on the wound healing process. A blend of Alchemilla vulgaris and Mimosa had positive effects on re-epithelialization, collagen synthesis and angiogenesis 27. Ethyl acetate extract of Gmelina arborea has anti-inflammatory effects and could promote keratinocytes’ wound healing 28. Green tea extract could help wound healing of the surgical wound of rats 21. Iris forentina caused higher wound closure and density of fibroblast and collagen bundles in rats 22. The positive effects of these plants have been attributed to their phenolic compounds, especially the flavonoid components.
Different studies showed the burn wound healing activity of polyphenols and antioxidants 29-31. Besides, polyphenols and white tea have a protective effect against wrinkles, sunburn and UV damages on the skin17, 19.
Therefore, the lack of positive effects in the present study is somewhat unexpected.
In another study, the effect of henna, pomegranate, myrrh, the mixture of these three and Gentamycin on wound healing was compared. The most wound contracture and the least epithelialization duration were reported in the group treated with the mixture of plants 24.
Some other plants used for cutaneous wound treatment have flavonoid compounds 10. Several properties have been attributed to flavonoids, such as anti-inflammatory properties 19. Inflammation is part of normal wound healing. Neutrophils, macrophages and T lymphocytes are the main cells of this phase in which neutrophils arrive at the wound location in 24-36 h, while, macrophages and T lymphocytes in 72 hours 32.
Results of inflammation among the groups of this study (Table 3) showed that on day 7, inflammation in control group was significantly (P<0.05) less than Eucerin-treated, white tea 5% ointment (Eucerin)-treated and white tea 5% gel-treated groups; while, we do not expect inflammation until this day 32. Impaired wound healing has some complication such as displeasing scar formation and infection 21.
There are a lot of factors that can increase the risk of impaired wound healing and chronic wounds including genetic disorders like down-syndrome and ataxia-telangiectasia, aging, psychological stress by harming the immune system, malnutrition including zinc, vitamin B12, vitamin C and vitamin D deficiencies, the excess activity of macrophages through the release of pro-inflammatory cytokines, infection, diabetes through various means such as hyperglycemia, hypoxia and impaired immunity, coagulation defects, inadequate angiogenesis in patients with DM, and venous stasis and elderly 6, 25, 32-38
Other influential factors are foreign bodies, some diseases such as jaundice and uremia, obesity, and medications like glucocorticoids 25.
CONCLUSION
White tea 5% extract did not improve the wound healing process except neovascularization, attributed to the observed inflammation. The only factor ameliorated by white tea extract was neovascularization which may be due to polyphenols and antioxidant effects of white tea. There might be additional influential factors for impaired wound healing; therefore, additional studies are needed.
ACKNOWLEDGMENTS
This work was supported by grant No. 2409 by Deputy of Research of SKUMS, Shahrekord, Iran. We also thank Medical Plants Research Center staff, SKUMS, and all people who helped us in this experiment.
CONFLICT OF INTEREST
There is not any conflict of interest in this manuscript.
References
- 1.Karimi M, Parsaei P, Asadi SY, Ezzati S, Khadivi Boroujeni R, Zamiri A, et al. Effects of Camellia sinensis ethanolic extract on histometric and histopathological healing process of burn wound in rat. Middle-East J Sci Res. 2013;13(1):14–19. [Google Scholar]
- 2.Khodaii Z, Afrasiabi S, Hashemi SA, Ardeshirylajimi A, Natanzi MM. Accelerated wound healing process in rat by probiotic Lactobacillus reuteri derived ointment. J Basic Clin Physiol Pharmacol. 2019;30:3. doi: 10.1515/jbcpp-2018-0150. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Wu C, Zhang X, Bian W, Liu N, Yin S, et al. A short peptide potentially promotes the healing of skin wound. Biosci Rep. 2019;39(3) doi: 10.1042/BSR20181734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derakhshanfar A, Moayedi J, Derakhshanfar G, Fard AP. The role of Iranian medicinal plants in experimental surgical skin wound healing: An integrative review. Iran J Basic Med Sci. 2019;22(6):590–600. doi: 10.22038/ijbms.2019.32963.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pazyar N, Houshmand GH, Yaghoobi R, Hemmati AA, Zeineli Z, Ghorbanzadeh B. Wound healing effects of topical Vitamin K: A randomized controlled trial. Indian J Pharmacol. 2019;51(2):88–92. doi: 10.4103/ijp.IJP_183_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017;8(1):23–33. doi: 10.1007/s13167-017-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daemi A, Farahpour MR, Oryan A, Karimzadeh S, Tajer E. Topical administration of hydroethanolic extract of Lawsonia inermis (henna) accelerates excisional wound healing process by reducing tissue inflammation and amplifying glucose uptake. Kaohsiung J Med Sci. 2019;35(1):24–32. doi: 10.1002/kjm2.12005. [DOI] [PubMed] [Google Scholar]
- 8.Güzel S, Özay Y, Kumaş M, Uzun C, Özkorkmaz EG, Yıldırım Z, et al. Wound healing properties, antimicrobial and antioxidant activities of Salvia kronenburgii Rech and Salvia euphratica Montbret, Aucher & Rech var euphratica on excision and incision wound models in diabetic rats. Biomed Pharmacother. 2019;111:1260–1276. doi: 10.1016/j.biopha.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Karimi A, Majlesi M, Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol. 2015;4(1):27–30. [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu S. Green tea and the skin. J Am Acad Dermatol. 2005;52(6):1049–59. doi: 10.1016/j.jaad.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 11.Dehghan Shahreza F. Oxidative stress, free radicals, kidney disease and plant antioxidants. Immunopathol Persa. 2017;3(2):e11. [Google Scholar]
- 12.Dehghan Shahreza F. From oxidative stress to endothelial cell dysfunction. J Prev Epidemiol. 2016;1(1):e04. [Google Scholar]
- 13.Zeng W, Endo Y. Lipid Characteristics of Camellia Seed Oil. J Oleo Sci. 2019;68(7):649–658. doi: 10.5650/jos.ess18234. [DOI] [PubMed] [Google Scholar]
- 14.Camargo L, Pedroso L, Vendrame S, Mainardes R, Khalil N. Antioxidant and antifungal activities of Camellia sinensis Kuntze leaves obtained by different forms of production. Braz J Biol. 2016;76(2):428–34. doi: 10.1590/1519-6984.18814. [DOI] [PubMed] [Google Scholar]
- 15.Nunes AR, Alves MG, Tomás GD, Conde VR, Cristóvao AC, Moreira PI, et al. Daily consumption of white tea (Camellia sinensis ( ) improves the cerebral cortex metabolic and oxidative profile in prediabetic Wistar rats. Br J Nutr. 2015;113(5):832–42. doi: 10.1017/S0007114514004395. [DOI] [PubMed] [Google Scholar]
- 16.Winiarska-Mieczan A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure–A rat model study. Regul Toxicol Pharmacol. 2015;73(2):521–9. doi: 10.1016/j.yrtph.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Lee KO, Kim SN, Kim YC. Anti-wrinkle effects of water extracts of teas in hairless mouse. Toxicol Res. 2014;30(4):283–9. doi: 10.5487/TR.2014.30.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, Dashwood WM, Löhr CV, Fischer KA, Pereira CB, Louderback M, et al. Protective versus promotional effects of white tea and caffeine on PhIP-induced tumorigenesis and β-catenin expression in the rat. Carcinogenesis. 2008 Apr;29(4):834–9. doi: 10.1093/carcin/bgn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saric S, Sivamani R. Polyphenols and sunburn. Int J Mol Sci. 2016;17(9):1521. doi: 10.3390/ijms17091521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinosa C, López‐Jiménez JA, Pérez‐Llamas F, Guardiola FA, Esteban MA, Arnao MB, et al. Long‐term intake of white tea prevents oxidative damage caused by adriamycin in kidney of rats. J Sci Food Agric. 2016;96(9):3079–87. doi: 10.1002/jsfa.7483. [DOI] [PubMed] [Google Scholar]
- 21.Asadi SY, Parsaei P, Karimi M, Ezzati S, Zamiri A, Mohammadizadeh F, et al. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int J Surg. 2013;11(4):332–7. doi: 10.1016/j.ijsu.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Mirmalek SA, Parsa T, Parsa Y, Yadollah-Damavandi S, Salimi-Tabatabaee SA, Jangholi E, et al. The wound healing effect of Iris forentina on full thickness excisional skin wounds: A histomorphometrical study. Bangladesh J Pharmacol. 2016;11(1):86–90. [Google Scholar]
- 23.Castro Souza Junior Neto Jd, Estevão LRdM, Baratella-Evêncio L, Vieira MGF, Simões RS, Florencio-Silva R, et al. Mast cell concentration and skin wound contraction in rats treated with Ximenia americana L. Acta Cir Bras. 2017;32(2):148–156. doi: 10.1590/s0102-865020170207. [DOI] [PubMed] [Google Scholar]
- 24.Elzayat EM, Auda SH, Alanazi FK, Al-Agamy MH. Evaluation of wound healing activity of henna, pomegranate and myrrh herbal ointment blend. Saudi Pharm J. 2018;26(5):733–738. doi: 10.1016/j.jsps.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Sa, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upadhyay A, Chattopadhyay P, Goyary D, Mitra Mazumder P, Veer V. Ixora coccinea enhances cutaneous wound healing by upregulating the expression of collagen and basic fibroblast growth factor. ISRN Pharmacol. 2014:2014:751824. doi: 10.1155/2014/751824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J, Park Y-G, Yun M-S, Seol J-W. Effect of herbal mixture composed of Alchemillavulgaris and Mimosa on wound healing process. Biomed Pharmacother. 2018;106:326–332. doi: 10.1016/j.biopha.2018.06.141. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann-Klemd AM, Konradi V, Steinborn C, Ücker A, Falanga CM, Woelfle U, et al. Influence of traditionally used Nepalese plants on wound healing and immunological properties using primary human cells in vitro. J Ethnopharmacol. 2019;235:415–423. doi: 10.1016/j.jep.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Działo M, Mierziak J, Korzun U, Preisner M, Szopa J, Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int J Mol Sci. 2016;17(2):160. doi: 10.3390/ijms17020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghlissi Z, Kallel R, Sila A, Harrabi B, Atheymen R, Zeghal K, et al. Globularia alypum methanolic extract improves burn wound healing process and inflammation in rats and possesses antibacterial and antioxidant activities. Biomed Pharmacother. 2016;84:1488–1495. doi: 10.1016/j.biopha.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Wang K, Li W, Soteyome T, Xiao H, Hu Z. Protective effects of polyphenolic extracts from longan seeds promote healing of deep second-degree burn in mice. Food Funct. 2019;10(3):1433–1443. doi: 10.1039/c8fo02330a. [DOI] [PubMed] [Google Scholar]
- 32.Stolzenburg-Veeser L, Golubnitschaja O. Mini-encyclopaedia of the wound healing-Opportunities for integrating multi-omic approaches into medical practice. J Proteomics. 2018;188:71–84. doi: 10.1016/j.jprot.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Gould L, Abadir P, Brem H, Carter M, Conner‐Kerr T, Davidson J, et al. Chronic wound repair and healing in older adults: current status and future research. Wound Repair Regen. 2015;23(1):1–13. doi: 10.1111/wrr.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiecolt-Glaser JK, Marucha PT, Mercado A, Malarkey WB, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346(8984):1194–6. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 35.Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998;60(3):362–5. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monroe DM, Hoffman M. The clotting system–a major player in wound healing. Haemophilia. 2012:18 Suppl 5:11–6. doi: 10.1111/j.1365-2516.2012.02889.x. [DOI] [PubMed] [Google Scholar]
- 38.DiPietro LA. Angiogenesis and scar formation in healing wounds. Curr Opin Rheumatol. 2013;25(1):87–91. doi: 10.1097/BOR.0b013e32835b13b6. [DOI] [PubMed] [Google Scholar]