On Dec 20, 2020, the Israeli Ministry of Health launched a national COVID-19 vaccination campaign that aimed to rapidly vaccinate all high-risk individuals by the end of January, 2021, using the Pfizer BNT162b2 mRNA vaccine.1, 2 Vaccines were readily available and free of charge. Patients with cancer who have been treated with systemic anticancer therapy are at a significantly increased risk of mortality from COVID-19,3, 4, 5, 6 and therefore should be considered as a high-priority group for COVID-19 vaccination.
Because the pivotal vaccination study for BNT162b2 included only healthy individuals or those with stable chronic medical conditions,1 a major obstacle faced by the Ministry of Health and by the National Council for the Prevention Diagnosis and Treatment of Malignant Disease was an absence of data regarding the safety and efficacy of the vaccine in patients with cancer who have been or are being treated. Based on available knowledge regarding other routinely used vaccines (eg, the influenza vaccine) the Ministry of Health recommended vaccination of all patients with cancer.7 However, some experts at the National Council raised concerns regarding the ability of the vaccine to provoke or enhance immune-related side-effects in patients who are being treated with immune checkpoint inhibitors. Thus, the Ministry of Health left the decision about vaccinating individuals treated with immune checkpoint inhibitors to the discretion of their treating physician.
The institutional policy of the Oncology Division at the Tel Aviv Sourasky Medical Center (TLVMC) and at the Oncology Unit at Bnei-Zion Medical Center was to allow and encourage vaccination for all patients with cancer being actively treated, regardless of treatment type. Because COVID-19 is particularly dangerous to these susceptible patients, vaccination was encouraged regardless of disease stage, performance status, or life expectancy. Only patients previously infected with SARS-CoV-2 or those with acute conditions (ie, active infection or active, uncontrolled immune-related side-effects) were excluded from the vaccination campaign. The vaccine was administered at the standard recommended dose on days 1 and 21. The second dose was omitted if the patient contracted SARS-CoV-2 infection after the first dose.
Due to the aforementioned safety concerns, side-effects among patients being treated with immune checkpoint inhibitors were monitored via detailed telephone questionnaires done 17–21 days after the first vaccine dose and at a median of 19 days (IQR 12–31) after the second vaccine dose. Here we report on the safety of the BNT162b2 mRNA vaccine in a cohort of patients treated with immune checkpoint inhibitors at the two participating institutions. We compared side-effects in the patients treated with immune checkpoint inhibitors with a control group. At the beginning of the national campaign, all staff, including volunteers, at TLVMC were encouraged to receive the BNT162b2 mRNA vaccine and 2241 vaccinated staff members also participated in the side-effect survey. Each patient with cancer who had received the second vaccine dose was matched by sex and year of birth to a healthy individual from among the TLVSMC cohort. The only patient who was not matched by year of birth was aged 93 years, and they were matched with a TLVMC volunteer aged 89 years.
Between Jan 11 and Feb 25, 2021, 170 patients who were being treated with immune checkpoint inhibitors were offered the vaccine and surveyed. 33 (19%) refused the vaccination, mostly due to fear of side-effects. 137 (81%) patients received the first vaccination dose, of whom 134 (98%) received the second dose. Three patients died after the first dose, one due to COVID-19, and two due to disease progression. Characteristics of the remaining 134 patients are presented in the appendix p 1. The most common side-effects after the first dose were local, with 28 (21%) of 134 patients reporting pain at the site of injection. Systemic side-effects included fatigue (five [4%]), headache (three [2%]), muscle pain (three [2%]), and chills (one [1%]).
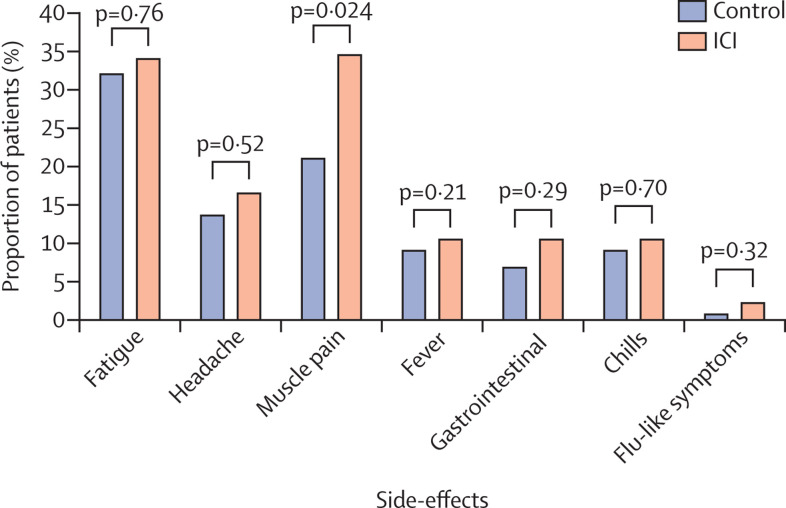
During the observation period after the second dose, four (3%) of 134 patients were admitted to hospital, three due to cancer-related complications and one due to fever, and all were discharged after treatment. As previously reported,1 more systemic and local side-effects were observed after the second dose of vaccine than after the first dose. The most common local side-effects were pain at the injection site (85 [63%] of 134), local rash (three [2%]), and local swelling (12 [9%]), whereas the most common systemic side-effects were muscle pain (46 [34%]), fatigue (45 [34%]), headache (22 [16%]), fever (14 [10%]), chills (14 [10%]), gastrointestinal complications (14 [10%]), and flu-like symptoms (three [2%]; figure ; appendix p 2). None of the reported side-effects required admission to hospital or any other special intervention.
Figure.
Systemic side-effects after the second dose of the BNT162b2 mRNA vaccine among patients with cancer treated with immune checkpoint inhibitors and matched controls
ICI=patients with cancer treated with immune checkpoint inhibitors. Bars show the proportion of participants reporting on each side-effect. Differences between the groups were calculated using the χ2 test.
Most patients (97 [72%] of 134) were treated with immune checkpoint inhibitors alone and 37 (28%) were treated with a combination of immune checkpoint inhibitors and chemotherapy. A similar rate of side-effects was observed in both treatment groups (31 [32%] of 97 vs 16 [43%] of 37; χ2 test p=0·22).
Most importantly, no new immune-related side-effects or exacerbation of existing immune-related side-effects were observed.
The side-effect profile was similar in the healthy controls and the patients with cancer, apart from muscle pain, which was significantly more common among patients with cancer (figure). However, no immune-related myositis was diagnosed after the vaccine in either patients or controls. This observation further supports the safety of the BNT162b2 mRNA vaccine in patients with cancer being treated with immune checkpoint inhibitors.
The potential association between previous immune-related side-effects and the vaccine side-effects was also examined. 47 (35%) of 134 patients had previously had grade 2 or worse immune checkpoint inhibitor-related side-effects before they had the vaccine. There was no significant difference in the number of patients who reported systemic side-effects after the second dose of vaccine between those who had reported previous immune-related side-effects and those who had not (16 [34%] of 47 vs 30 [34%] of 87; p=0·96). Importantly, even in patients with previous immune-related side-effects, the vaccine-related side-effects were mild and did not lead to admission to hospital or cessation of cancer treatment.
Little data are available regarding the safety of various vaccines in the context of treatment with immune checkpoint inhibitors, but vaccination had generally been suggested to be safe in this population.8 For example, the influenza vaccine was found to be safe in 370 patients with cancer actively treated with immune checkpoint inhibitors.9 Our data support the short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer being treated with immune checkpoint inhibitors. Because no vaccine-related or immune checkpoint inhibitor-related severe adverse events were observed in this cohort of 134 patients who received two doses of the vaccine, it is unlikely that any common side-effects were missed. However, rare side-effects might be identified when larger cohorts are investigated. Considering the high mortality of patients with cancer who contract COVID-19, which can be as high as 40% in some patient populations,3 the benefits of vaccination seem to outweigh the potential harms. Although further studies are needed to determine if these data are also applicable to the other COVID-19 vaccines, our findings might provide some reassurance for their use in patients being treated with immune checkpoint inhibitors.
Obviously, larger studies over longer periods of follow-up are required to fully assess the benefits and harms of vaccines against COVID-19. However, the decision to vaccinate patients in regions severely affected by the pandemic cannot wait for the accumulating data from well designed, prospective clinical trials. Thus, despite the absence of evidence, the March 5, 2021, update of the American Society of Clinical Oncology guidelines state that “At this time, patients undergoing treatment may be offered vaccination against COVID-19 as long as any components of the vaccine are not contraindicated”.
The data we present here provide, for the first time, a reassuring safety signal regarding the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Considering the high mortality due to COVID-19 in patients with cancer who are being treated, our data support current guidelines and call for vaccination of patients being treated with immune checkpoint inhibitors, especially during pandemic surges.
This online publication has been corrected. The corrected version first appeared at thelancet.com/oncology on April 30, 2021
IW and BW contributed to conception and design of the study. BW, AA, ES, and HP contributed to collection and analysis of data. BW and IW contributed to data analysis and interpretation. All authors contributed to writing of the Comment and approved the final version before submission. This study had no funding. BW reports speaker fees and fees for consultancy from MSD. AA reports speaker fees and fees for consultancy from MSD, Roche, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Pfizer, Bristol Meyers Squibb, Novartis, Merck Serono, Sanofi, Oncotest-Teva, Medison, AbbVie, and Takeda; grants from Altman Health; and is a member of the board for the Israeli Lung Cancer Advocacy Group. IW reports speaker fees and fees for consultancy from Roche, Bristol Myers Squibb, MSD, and Novartis, and research funding from Roche and MSD. ES and HP declare no competing interests. No individual participant-level data will be shared.
Supplementary Material
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harel A. Israel's ambitious vaccine drive is set for success as Netanyahu seeks election campaign boost. Haaretz. Dec 29, 2020. https://www.haaretz.com/israel-news/.premium-israel-s-ambitious-vaccine-drive-is-set-for-success-as-netanyahu-seeks-election-gain-1.9406513
- 3.Venkatesulu BP, Chandrasekar VT, Girdhar P, et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. medRxiv. 2020 doi: 10.1101/2020.05.27.20115303. published online May 29. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee LYW, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidelines for COVID-19 vaccination Ministry of Health, Epidemiology division. Jan 9, 2021. https://www.health.gov.il/UnitsOffice/HD/PH/epidemiology/td/docs/365_Corona.pdf (in Hebrew).
- 8.Desage AL, Bouleftour W, Rivoirard R, et al. Vaccination and immune checkpoint inhibitors: does vaccination increase the risk of immune-related adverse events? A systematic review of literature. Am J Clin Oncol. 2021;44:109–113. doi: 10.1097/COC.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 9.Chong CR, Park VJ, Cohen B, Postow MA, Wolchok JD, Kamboj M. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;70:193–199. doi: 10.1093/cid/ciz202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.