Abstract
Ciguatera fish poisoning (CFP) is a syndrome caused by the bioaccumulation of lipophilic ciguatoxins in coral reef fish and their subsequent consumption by humans. These phycotoxins are produced by Gambierdiscus spp., tropical epiphytic dinoflagellates that live on a variety of macrophytes, as well as on dead corals and sand. Recent taxonomic studies have identified novel diversity within the Gambierdiscus genus, with at least 18 species and several sub-groups now identified, many of which co-occur and differ significantly in toxicity. The ability to accurately and quickly distinguish Gambierdiscus species in field samples and determine community composition and abundance is central to assessing CFP risk, yet most Gambierdiscus species are indistinguishable using light microscopy and other enumeration methods are semi-quantitative. In order to investigate the spatial and temporal dynamics of Gambierdiscus species and community toxicity, new tools for species identification and enumeration in field samples are needed. Here, fluorescence in situ hybridization (FISH) probes were designed for seven species commonly found in the Caribbean Sea and Pacific Ocean, permitting their enumeration in field samples using epifluorescence microscopy. This technique enables the assessment of community composition and accurate determination of cell abundances of individual species. Molecular probes detecting G. australes, G. belizeanus, G. caribaeus, G. carolinianus, G. carpenteri, and the G. silvae/G. polynesiensis clade were designed using alignments of large subunit ribosomal RNA (rRNA) sequences. These probes were tested for specificity and cross-reactivity using Gambierdiscus cultures, and a series of experiments were performed in which field samples spiked with known concentrations of Gambierdiscus cultures were analyzed to confirm that Gambierdiscus can be successfully detected and enumerated by FISH in the presence of detritus and other organisms. These probes were then used to characterize Gambierdiscus community structure of field samples collected from the Florida Keys and Hawai’i, USA. The probes revealed the co-occurrence of multiple species at each location. Time-series FISH analyses of samples collected from the Florida Keys quantified seasonal shifts in community composition as well as fluctuations in overall Gambierdiscus cell abundance. Application of species-specific FISH probes provides a powerful new tool to those seeking to target individual Gambierdiscus species, including significant toxin-producers, in field populations. Moving forward, analysis of Gambierdiscus community composition across multiple environments and over time will also allow species dynamics to be linked to environmental parameters, improving our ability to understand and manage the current and changing risks of CFP worldwide.
Keywords: Ciguatera, Gambierdiscus, ciguatoxin, fluorescence in situ hybridization, harmful algal bloom
Graphical Abstract
1. Introduction
Ciguatera fish poisoning (CFP) is a syndrome caused by the bioaccumulation of lipophilic ciguatoxins in coral reef fish and subsequent consumption by humans (Scheuer et al., 1967; Lehane and Lewis, 2000). These phycotoxins are produced by members of the genus Gambierdiscus, a tropical epiphytic dinoflagellate genus that lives on many varieties of macroalgae but also may occur on dead corals and sand (reviewed in Parsons et al., 2012). Different species of Gambierdiscus have been shown to exhibit algal host preferences, and high densities of cells have been found on diverse algal taxa including calcified species (Jania), brown algae (Dictyota), green algae (Chaetomorpha) and turf algae (Rains and Parsons, 2015; Cruz-Rivera and Villareal, 2006). Species of Gambierdiscus have also been shown to have different growth characteristics and toxin-producing capabilities, topics that continue to be actively researched (Xu et al., 2016; Kibler et al., 2012; Litaker et al., 2017). Given the substantial physiological and toxicological diversity within the genus, risk of CFP may be largely determined by Gambierdiscus species community composition, their distribution and abundance across available benthic habitat, and the prevalence of certain toxic species or strains.
As Gambierdiscus species are almost indistinguishable under the light microscope, current techniques to identify cells to the species level include scanning electron microscopy, sequencing of large subunit ribosomal DNA (LSU rDNA) sequences, and recently developed restriction fragment length polymorphism (RFLP) typing methods (Lyu et al., 2017; Lozano-Duque et al., 2018). Semi-quantitative qPCR has also been developed for large scale analysis of field samples (Vandersea et al., 2012), but it does not have the same quantitative strength as microscope counts due to varying levels of rDNA within Gambierdiscus cells as well as possible matrix effects (currently under evaluation; D. Anderson, unpublished data). Species-specific fluorescence in situ hybridization (FISH) probes offer an alternative approach, allowing for the determination of Gambierdiscus community composition and abundance in field samples while preserving the accuracy of microscopic cell counts. This differentiation of Gambierdiscus species allows the direct elaboration of community dynamics and shifting patterns of community toxicity, and thus provides a valuable tool for monitoring toxic species.
Molecular FISH probes targeting LSU rRNA have long been used in monitoring bloom populations of Alexandrium species associated with paralytic shellfish poisoning (PSP) in the Gulf of Maine region, as well as in the detection and enumeration of other harmful algal bloom species (e.g., Anderson et al., 2005; Chen et al. 2008; Mikulski et al., 2005; Parsons et al., 1999; Scholin et al., 1996). In cases where the target taxon is only a minor member of a phytoplankton assemblage or where there may be detritus in field samples, as is true in both cases for Gambierdiscus, FISH probes can enable accurate identification of cells (Anderson et al., 2005). In this study, probes detecting G. australes, G. belizeanus, G. caribaeus, G. carolinianus, G. carpenteri, and the G. polynesiensis clade (G. polynesiensis and G. silvae) were designed using alignments of large subunit ribosomal RNA (rRNA) sequences in the D1-D3 and D8-D10 regions of the LSU rRNA gene. These species were selected based on their common occurrence in the Caribbean Sea and/or Pacific Ocean, and in the case of G. polynesiensis and G. silvae, their high toxicity (Chinain et al. 1999, 2010; Longo et al., 2019; Robertson et al,. 2018). Candidate probes were tested for specificity and cross-reactivity using cultured isolates that included both target and non-target species, and a series of experiments were performed in which field samples from St. Thomas, USVI were spiked with known concentrations of Gambierdiscus cultures were analyzed to confirm that Gambierdiscus can be successfully detected and enumerated by FISH in the presence of detritus and other organisms. These probes were then used to characterize Gambierdiscus community structure of field samples collected from the Florida Keys and Hawai’i, USA.
The genus Gambierdiscus has a circumtropical distribution, but individual species can have more limited ranges (Lewis 2001; Litaker et al., 2010). Florida lies on the northern edge of ciguatera incidence although Gambierdiscus species have been isolated as far north as off the coast of North Carolina (Litaker et al,. 2009; Radke et al., 2015). Hawai’i also has a relatively low incidence of ciguatera compared to other Pacific islands even though multiple species of Gambierdiscus have been reported (Skinner et al., 2011; Litaker et al., 2010). Conversely, St, Thomas, USVI, historically has had high incidences of ciguatera poisoning and much work has gone into forecasting ciguatera risk for the region (e.g., Radke et al. 2013). In this study we take advantage of field samples and cultured isolates collected from all three locations to test Gambierdiscus probes against various species assemblages and substrate types. Molecular probes were applied both individually and in combination using different fluorophores, permitting multiplexing in subsequent analyses of field samples.
Given the potential for variable toxicity among co-occurring Gambierdiscus, species, it is now commonly accepted that species composition can have a greater influence on risk of CFP than total genus abundance, as high local abundance of Gambierdiscus cells has not always led to a CFP events (discussed in Litaker et al., 2010). The hypothesized link between ciguatera outbreaks and the toxicity of individual species was first proposed by Holmes et al. (1991) and Legrand (1998), suggesting that certain “super-producing” strains of Gambierdiscus were responsible for high ciguatoxin levels leading to outbreaks. The discovery of highly toxic species such as G. polynesiensis, G. silvae, and G. excentricus lend support to these early hypotheses. The approach outlined herein allows for accurate, quantitative determination of community composition, as well as the selective enumeration of species known to exhibit high toxicity. Species abundance measurements can be integrated into routine monitoring efforts and used to investigate community structure over spatiotemporal gradients, allowing establishment of seasonal patterns, or elucidation of community response to disturbance events such as storms or coral bleaching.
2. Materials and Methods
2.1. Probe Design and Testing Overview
Fluorescent probes were designed to target the large subunit (LSU) region of rRNA of seven species of Gambierdiscus: G. australes, G. belizeanus, G. caribaeus, G. carolinianus, G. carpenteri, G. polynesiensis and G. silvae. DNA sequences from the D1-D3 and D8-D10 domains of the LSU were obtained from GenBank (www.ncbi.nlm.nih.gov/genbank/) and aligned. Regions of conservation within species were identified, and candidate probes were designed that contained at least two nucleotides of difference between species (Table 1). Every effort was made to design probes with similar melt temperatures (Tms) to aid in multiplexing. Candidate probes were conjugated to a fluorophore for initial testing (Cy3 or FITC) using cultures of Gambierdiscus. Based on their availability and viability in culture, eight Gambierdiscus species and one ribotype were included in this testing (Table 2): G. australes, G. belizeanus, G. caribaeus, G. carolinianus, G. carpenteri, G. pacificus, G. polynesiensis, G. silvae, and Gambierdiscus sp. ribotype 2.
Table 1.
Probe combinations for the analysis of samples from the Caribbean and Gulf of Mexico, and Pacific Ocean. Table shows probe target species, sequence, melting temperature (Tm), microscope filter, and fluorophore used for illumination. LP=long pass; BP=band pass.
| CARIBBEAN SEA REGION PROBE COMBINATIONS | ||||||
|---|---|---|---|---|---|---|
| Group | Probe | Target Species | Sequence | Tm | Filter | Fluorophore |
| 1 | Gcarib_D1D3 | G. caribaeus | TGAGACCCACATGTGGAGATTC | 53 | FITC (LP, Zeiss 09) | AF488 |
| Gbeliz_D8D10 | G. belizeanus | AGATCAGTACGCCAGAGTGACTA | Cy3 (LP, Chroma 49016) | AF532 | ||
| 2 | Gcarpent_D1D3 | G. carpenteri | TGATGTAACGCAAGACGCACAG | 55 | FITC (LP, Zeiss 09) | AF488 |
| Gribo1_D8D10 | G. polynesiensis clade | CGATCAGAGACATACTTTGGCGC | Cy3 (LP, Chroma 49016) | AF532 or Cy3 | ||
| Gcarol_D8D10 | G. carolinianus | AGCAAGCCACAGATCCACTGAG | TxRd (LP, Chroma 19006 | AF594 | ||
| PACIFIC OCEAN PROBE COMBINATIONS | ||||||
| Group | Probe | Target Species | Sequence | Tm | Filter | Fluorophore |
| 1 | Gcarib_D1D3 | G. caribaeus | TGAGACCCACATGTGGAGATTC | 52 | FITC (LP, Zeiss 09) | AF488 |
| Gaust_D1D3 | G. australes | TGCCAATCCAGTTGTGTATCTC | Cy3 (LP, Chroma 49016) | AF532 | ||
| 2 | Gcarpent_D1D3 | G. carpenteri | TGATGTAACGCAAGACGCACAG | 53 | FITC (LP, Zeiss 09) | AF488 |
| Gbeliz_D8D10 | G. belizeanus | AGATCAGTACGCCAGAGTGACTA | Cy3 (LP, Chroma 49016) | AF532 or Cy3 | ||
| Gpoly_D8D10 | G. polynesiensis clade | CTCCGCCAGTGACGTTAAGTAG | TxRd (LP, Chroma 19006) | AF594 | ||
Table 2.
Cultured isolates of Gambierdiscus spp. used for candidate probe testing. Conditions for culture maintenance are described in Xu et al. (2014).
| Isolates | Geographic Origin | Species |
|---|---|---|
| CCMP 1653 | Hawai’i, USA | G. australes |
| CCMP 399 | St. Barthélemy Island, French West Indies | G. belizeanus |
| GTBNAC1 | Florida Keys, FL, USA | G. caribaeus |
| BP Aug08 | St. Thomas, USVI, USA | G. caribaeus |
| BB Apr10_6 | St. Thomas, USVI, USA | G. carolinianus |
| BP Mar10_1 | St. Thomas, USVI, USA | G. carolinianus |
| HGB6 | Florida Keys, FL, USA | G. carpenteri |
| 350509-27 | Marakei, Republic of Kiribati | G. pacificus |
| D50511-08 | Marakei, Republic of Kiribati | G. pacificus |
| RIK-8 | Mangareva, Gambier, French Polynesia | G. polynesiensis |
| RAI-1 | Raivavae, Australes, French Polynesia | G. polynesiensis |
| RG-92 | Rangiroa, Tuamotu, French Polynesia | G. polynesiensis |
| SH Apr11-1 | St. Thomas, USVI, USA | G. silvae |
| TRL23 | Florida Keys, FL, USA | G. silvae |
| FC May10_9 | St. Thomas, USVI, USA | G. silvae |
| BP Mar10_5 | St. Thomas, USVI, USA | Gambierdiscus sp. ribotype 2 |
During initial testing, cultures were hybridized in separate reactions within a hybridization manifold with individual candidate probes labeled with either a Cy3 or FITC fluorophore, which were cost-effective for candidate probe screening. These experiments included a separate reaction with a universal probe targeting small subunit (SSU) rRNA (Univ-1390, Zheng et al., 1996), which served as a positive control. Each test also included a negative control reaction (no probe). These positive and negative control reactions were used to confirm that the culture cells’ rRNA complement was compatible with FISH probe labeling, and that probe signal could successfully distinguish a labeled cell from unlabeled cells. Specific preservation and hybridization steps are detailed in Sections 2.3-2.5, and involved concentrating formalin/methanol preserved samples on cyclopore filters, followed by examination using epifluorescence microscopy. Subsequent to candidate probe testing, probe labeling was also carried out using Alexa Fluor® dyes (see Section 2.2), which provided a brighter signal in field samples. All samples in this study were analyzed using a Zeiss Axio Vert.A1 inverted microscope (Carl Zeiss AG, Oberkochen, Germany) at 100×.
Subsequent to initial cross-reactivity testing described in Section 2.4, experiments were performed in which preserved aliquots of cell cultures with a known concentration were added to field samples known to be devoid of Gambierdiscus cells (see Section 2.5). These samples were hybridized and enumerated, and compared to cell culture concentrations to verify that the method provides a quantitative assessment of Gambierdiscus species abundance.
2.2. Overview of multiplexing approach for detecting multiple species in field samples
To enable enumeration of multiple species within a sample, probes that were successfully tested for cross-reactivity were combined into groups based on the region of their target species (Caribbean or Pacific), as well as their Tms, which ranged from 52 to 55°C (Table 1). In order to best combine probes into regional groups for field sample analysis, probes with most similar Tms were combined. Probes that were grouped in two different combinations at different Tms (G. carpenteri and G. caribaeus, both present in Caribbean and Pacific) were tested at both Tms to ensure they still maintained specificity at a slightly different Tm. Available laSpecifics regarding procedures for sample preservationboratory microscope filters (FITC long pass, Zeiss 09, Cy3 long pass, Chroma 49016, Tx Red long pass, Chroma 19006) enabled three different fluorophores to be used simultaneously in the same reaction. Given the availability of three flours and the requirement to screen five species within both Pacific and Caribbean regions, each sample was split and screened with either the Group I or Group II species-specific probes (Table 1). Calcafluor White M2R cellulose stain (Sigma-Aldrich, MO, USA), which renders phytoplankton cells visible under the DAPI filter set (Fritz and Triemer, 1985), was applied in the final step of the hybridization process (see Section 2.4) to facilitate initial identification of Gambierdiscus cells to the genus level as well as enumeration of any unlabeled Gambierdiscus in field samples.
When analyzing multiplexed samples, each slide was initially scanned under the DAPI filter set. Whenever a Gambierdiscus cell was encountered, the Cy3, TxRd, and FITC filters were applied in sequence in order to determine whether the cell was labeled with a fluorophore. The filter set was then returned to DAPI, and scanning continued until another Gambierdiscus cell was located. Depending on density of sample contents and quantity of Gambierdiscus, time required for a fully trained personnel to analyze 1 mL in a Sedgwick-rafter slide averaged around 20 minutes.
Initially Cy3 was used for groups of two probes, but was replaced by Alexa Fluor® 532 in the groups of three probes, as the Cy3 fluorescence caused some overlap within the Texas Red excitation filter, and Cy3 labeled cells exhibited higher autofluorescence under the Texas Red filter compared with Alexa Fluor® 532. However, since Cy3 is less expensive and still effective in combination with Alexa Fluor® 488, it was kept for the two-member group. Both options are listed in Table 1. Alexa Fluor® 488 was used in place of FITC, and Alexa Fluor® 594 in place of Texas Red fluorophores that were used in initial culture testing as they produced a brighter signal that was easier to detect over detritus that can have varying levels of autofluorescence under different filters. This is a common issue with FISH labeling of field samples, which was also combated through the addition of Calcafluor White stain during sample processing to facilitate initial identification of Gambierdiscus cells to the genus level. This two-group multiplexing approach enabled visualization of multiple Gambierdiscus species at the same time, and was used for subsequent analysis of field samples (Section 2.6). Specifics regarding procedures for sample preservation, processing, and analysis are detailed below.
2.3. Sample Preservation
Sample preservation procedures were identical for both cultured isolates and field samples, and followed those described in Anderson et al. (2005). Each 14 mL sample was preserved with 750 μl of formalin and stored for at least five minutes, but no more than 24 hours. The sample was then centrifuged at 3000 g for 10 minutes, the supernatant removed via aspiration, and each sample was then resuspended in ice-cold methanol (volume was adjusted to accommodate sample density). Samples were stored at −20°C for at least 24 hours prior to whole cell hybridization. Prompt and proper preservation of samples is extremely important, otherwise cells may exhibit high levels of autofluorescence which can interfere with probe visualization.
2.4. Cross-reactivity testing using cultures and field samples
The Gambierdiscus cultures used in cross-reactivity testing were labeled with FISH probes following a whole-cell hybridization method adapted from Anderson et al. (2005), which involved collecting samples on a cyclopore membrane using a filtration manifold, and mounting filters on a microscope slide for enumeration. These procedures are detailed below; note that a centrifugation based method (described in Section 2.5) was subsequently developed to enable processing of larger sample quantities.
Each candidate probe was tested with the cultures listed in Table 2 to verify specificity and efficacy. For this initial cross-reactivity testing, 300 μl of formalin-methanol preserved culture was loaded into each chamber of a hybridization manifold. Methanol was removed via vacuum filtration, and particulates were collected on a Cyclopore membrane (5 μm pore size, 25mm diameter; Whatman, NJ, USA). Samples were treated with prehybridization buffer (5X SET, 0.1% IGEPAL CA-630, Poly A 10 mg mL−1, 10% formamide) and incubated at room temperature for five minutes. Prehybridization buffer was removed via vacuum filtration and replaced with hybridization buffer containing FISH probes at a concentration of 2 ng μl−1. During all protocol steps, exposure of probes to light was minimized in order to prevent fluorophore quenching. Samples were incubated in a hybridization manifold at 52, 53, or 55 °C for an hour, depending on the Tm of the probe mixture. Hybridization buffer was removed via vacuum filtration and 0.2X SET wash solution was added and incubated for five minutes at room temperature. The wash solution was removed via vacuum filtration, and hybridization filters were placed on glass slides, mounted with 5–10 μl glycerol (depending on sample density), and a glass cover slip applied. Slides were stored in the dark at 4°C until microscopic enumeration using fluorescence microscopy, and all analyses were completed within 72 hours of hybridization to avoid loss of signal.
Following the initial culture-based testing for specificity, probes were then tested using field samples from the Florida Keys (Heine Grass Bed, HGB: 24.859667; −80.73816) and St. Thomas, USVI (Flat Cay, FC: 18.31822083; −64.99103593; Black Point, BP: 18.34417968; −64.98543862) that were routinely collected as part of an ongoing study to monitor and model Gambierdiscus abundance and toxicity (CiguaHAB). Two turf algae samples collected from the Florida Keys in March 2018 and Feb 2019 (West Washer Woman: WWW: 24.5475; −81.5866; Tennessee Reef Lighthouse, TRF: 24.745; −80.7812) were used in spiking experiments using cultures of known to assess recovery and to confirm that Gambierdiscus can be successfully quantified by FISH in the presence of detritus and other organisms. These samples had been previously enumerated and were known to be devoid of Gambierdiscus. A description of sample collection and processing procedures can be found in Parsons et al. (2017).
Macroalgal samples from St. Thomas were first used in qualitative testing. These samples were characterized by high amounts of detritus, allowing testing of probe efficacy in the presence of potentially confounding environmental particulates. For this analysis, samples were spiked with known Gambierdiscus species from culture (~0.5 culture per 14 mL sample); these spiked samples were used to determine whether probe concentrations were sufficient to label cells when they were a minor fraction of a larger benthic community (as determined from preliminary data collected over several years at these locations). Hybridizations were performed as described above using 1 mL of sample. Probes were tested at 2 ng μl−1, 3 ng μl−1, and 4 ng μl−1 in each hybridization reaction using the Cy3 fluorophore, and 1.3 μl mL−1 of a 10 mg mL−1 working stock solution of Calcofluor White M2R was added to the SET wash solution. As cells were easily visualized at a probe concentration of 2 ng μl−1, this was selected as the working concentration for further analysis.
2.5. Quantitative assessment of FISH approach for Gambierdiscus cell enumeration
In order to confirm that Gambierdiscus can be successfully quantified by FISH in the presence of detritus and other organisms, a series of experiments were performed in which field samples were spiked with known concentrations of Gambierdiscus cultures, and these samples were enumerated using FISH. For these experiments, cultures of G. silvae, G. carolinianus, and G. caribaeus were preserved as described in Section 2.3. Cell concentrations of each were first determined by staining 1 mL of sample with Calcafluor White (n=6), and these samples were enumerated at 100× using a DAPI filter.
Two turf algae samples from sites in the Florida Keys (WWW & TRF) which had been previously identified as being devoid of Gambierdiscus spp. based on prior sample enumeration were pooled and used for these experiments. Each methanol-preserved field sample was spiked with 1 mL of culture (n=3), hybridized with the corresponding species-specific probe as described below, and enumerated.
For this experiment, a modified centrifugation method was used for hybridization rather than the filtration manifold approach described previously. In this modified protocol, samples were centrifuged (5 min x 10000 g) to pellet contents and overlying methanol was aspirated, taking care to leave the pellet undisturbed. The pellet was resuspended in 1 mL hybridization buffer (5X SET, 0.1% IGEPAL CA-630, Poly A 10 mg mL−1, 10% formamide) and incubated at room temperature for five minutes. Probe was added at a final concentration of 1.6 ng μl−1, and samples were incubated in the dark at 53 or 55°C (Tm-dependent) for one hour. Following the incubation, samples were pelleted via centrifugation, and hybridization buffer was aspirated and replaced with 1 mL wash buffer (0.2X SET solution). Samples were incubated at room temperature for five minutes and centrifuged. Wash buffer was aspirated and pellet was resuspended in 1 mL of 5X SET solution containing 10 μl of a working stock solution of Calcafluor White (10 mg mL−1), and 1 mL of sample was loaded into a Sedgewick-Rafter slide for identification and enumeration under fluorescence. This modified centrifugation method has proven effective for processing large numbers of samples, and quality of labeling is comparable to the manifold filtration method.
As described in Section 2.1, each slide was initially scanned under the DAPI filter set. Whenever a Gambierdiscus cell was identified (to genus), the Cy3, Texas Red, and FITC filters were applied in sequence in order to determine whether the cell was labeled with a fluorophore. The filter set was then returned to DAPI, and scanning continued until another Gambierdiscus cell was located. Differences in cell concentrations determined by microscopic counts versus FISH enumeration were compared using a non-parametric Wilcoxon test performed using JMP 11 (SAS Corporation, NC, USA).
2.6. Field Sample Testing
In the Caribbean, field samples collected from the HGB site within the Florida Keys (Parsons et al., 2017) were selected for a seasonal comparison of Gambierdiscus community diversity. These samples were collected from two macroalgal hosts (Thalassia testudinum and Halimeda incrassata) from June to December in 2013. As these samples were analyzed prior to development of the multiplexing approach, individual probes detecting G. belizeanus, G. caribaeus, G. carolinianus, G. carpenteri, and the G. silvae/G. polynesiensis clade were used, each attached to the Cy3 fluorophore. Multiple hybridizations carried out for each sample as described in Section 2.4, using a probe concentration of 2 ng μl-1. In the Pacific, field samples were collected from sites within the Wai’Ōpae Tide Pools on the southeastern shore of the Island of Hawai’i (19.487994, −154.821981) in July 2015 to determine probe efficacy in this region. Macroalgae were scarce in this environment, so artificial substrates (e.g., window screens) were used in sampling to provide representative sampling of the benthic dinoflagellate community. Artificial substrates were deployed for 24 hours at several locations in this system over the course of one week and processed following established methods (Tester et al., 2014). As in previous studies, screen surface area for Gambierdiscus abundance measurements was calculated by considering the screen to be composed of cylindrical filaments resulting in a total surface area of 156.74 cm2 (Tester et al., 2014). These samples were analyzed with the Pacific assay probes (Table 1), following the multiplexing approach. This testing illustrated the efficacy of the Pacific probes as well as multiplexing with combinations of three and two probes.
3. Results
3.1. Probe design
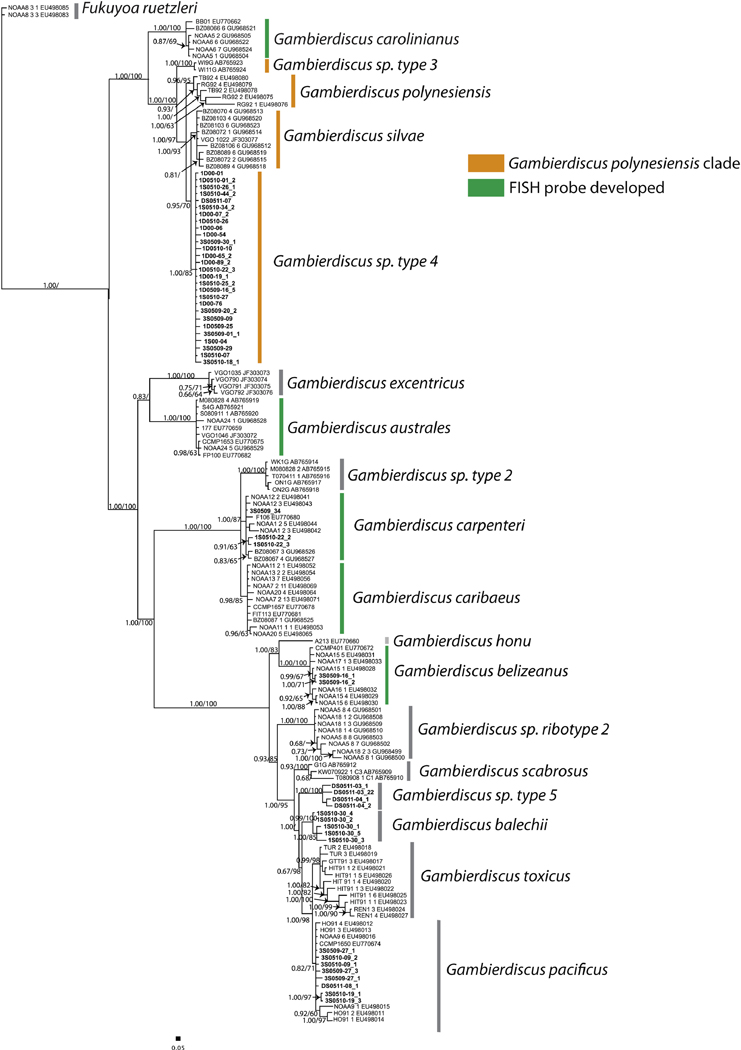
Molecular probes were designed for a range of Gambierdiscus species and clades as shown in Figure 1, based on their common occurrence at our sampling sites in St. Thomas, USVI and the Florida Keys, as documented in the scientific literature (Litaker et al., 2010; LozanoDuque et al., 2018), and depending on the availability of cultured isolates that could be used in testing. Therefore, not all species were addressed in this effort, including G. scabrosus, G. balechii, G. toxicus, G. excentricus and G. pacificus. Molecular probe sequences are shown in Table 1.
Figure 1.
Phylogeny based on analysis of the D8-D10 region of LSU rRNA gene of Gambierdiscus species, adapted from Xu et al. (2014). Scale bar = 0.05 substitutions per site. Support values are Bayesian posterior probability and bootstrap support values from maximum likelihood analysis. In orange is highlighted the ‘Gambierdiscus polynesiensis clade’. In green, species for which this study has developed species-specific probes are highlighted.
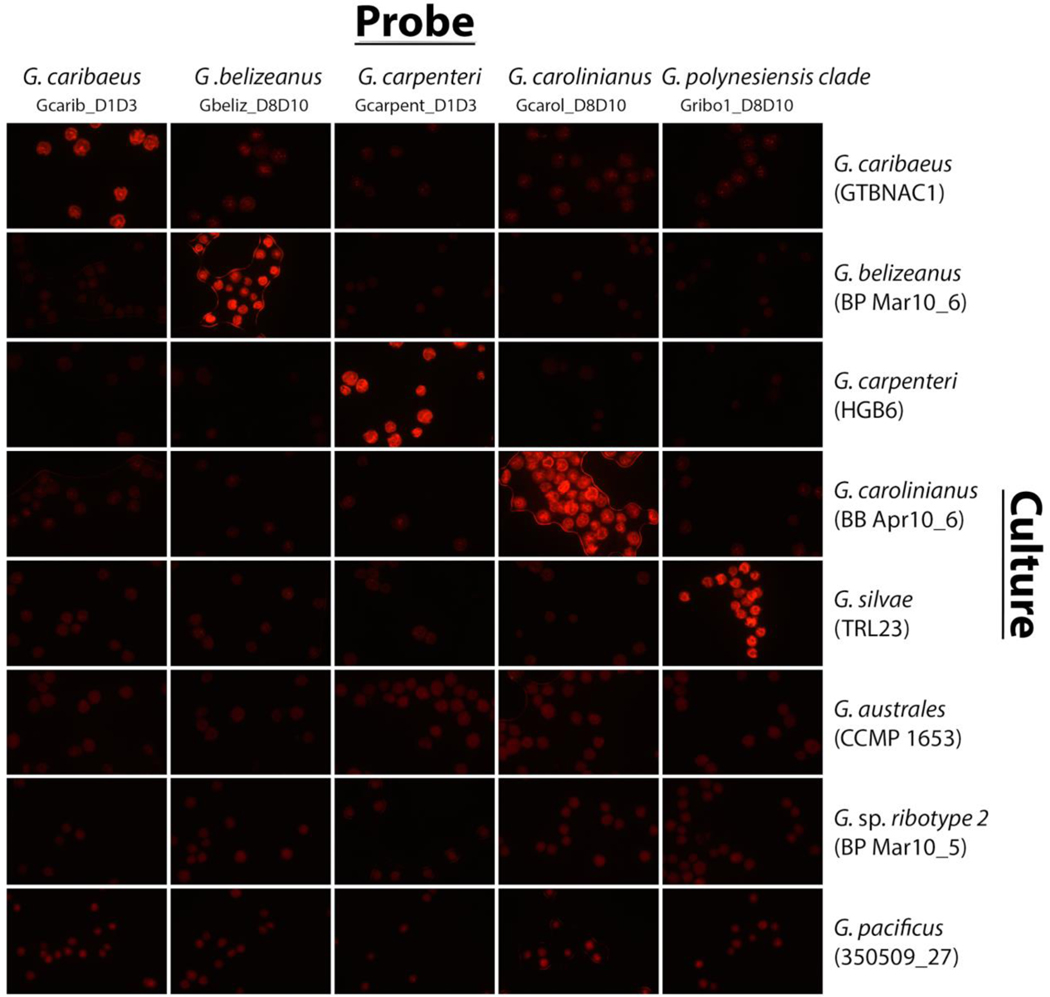
3.2. Cross-reactivity testing using Gambierdiscus cultures
Species-specific candidate probes labeled with Cy3 fluorophores were tested using cultured isolates of Gambierdiscus (Tables 2 and 3) to establish specificity and identify cross-reactivity (Table 3, Fig. 2). Determination of labeling specificity was based on visual intensity of fluorescence in comparison with non-target cells (Fig. 2).
Table 3.
Results of cross-reactivity using cultured isolates of Gambierdiscus. Cultures were preserved in formalin and methanol, hybridized with a Cy3-labeled probe, and viewed under Cy3 filter using fluorescence microscopy. Probes that successfully hybridized with an isolate and were highly visible under the fluorescent microscope are denoted positive “+”‘Pos’. Probes that failed to detect a culture are denoted negative “-”. Probes were tested at all Tms listed in Table 1.
| Probe/Target Species | ||||||||
|---|---|---|---|---|---|---|---|---|
| Culture ID | Species | Gaust_D1D3/ G. australes | Gcarib_D1D3/ G. caribaeus | Gbeliz_D8D10/ G. belizeanus | Gpoly_D8D10/ G. polynesiensis clade | Gcarpent_D1D/ G. carpenteri | Gcarol_D8D10/ G. carolinianus | Gribo1_D8D10/ G. polynesiensis clade |
| CCMP 1653 | G. australes | + | - | - | - | - | - | - |
| CCMP 399 | G. belizeanus | - | - | + | - | - | - | - |
| BP Apr 11_7 | G. belizeanus | - | - | + | - | - | - | - |
| BP Mar 10_6 | G. belizeanus | - | - | + | - | - | - | - |
| GTBNAC1 | G. caribaeus | - | + | - | - | - | - | - |
| BP Aug08 | G. caribaeus | - | + | - | - | - | - | - |
| BB Apr10_6 | G. carolinianus | - | - | - | - | - | + | - |
| BP May10_1 | G. carolinianus | - | - | - | - | - | + | - |
| HGB6 | G. carpenteri | - | - | - | - | + | - | - |
| RIK-8 | G. carpenteri | - | - | - | - | + | - | - |
| RAI-1 | G. polynesiensis | - | - | - | + | - | - | + |
| RG-92 | G. polynesiensis | - | - | - | + | - | - | + |
| 350509_271 | G. pacificus | - | - | - | - | - | - | - |
| D50511–08 | G. pacificus | - | - | - | - | - | - | - |
| SH Apr11–1 | G. silvae | - | - | - | + | - | - | + |
| TRL23 | G. silvae | - | - | - | + | - | - | + |
| FC May 10_9 | G. silvae | - | - | - | + | - | - | + |
| BP Mar 10_5 | G. ribotype 2 | - | - | - | - | - | - | - |
Figure 2.
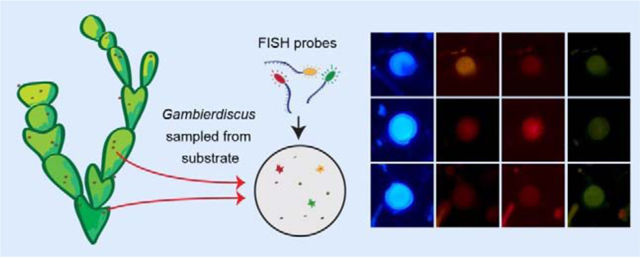
Fluorophore labels targeted to five species (G. caribaeus, G. belizeanus, G. carpenteri, G. carolinianus, G. silvae) hybridized across seven different cultures. Strain-specific information for each culture is indicated to the right. During initial testing, cultures were hybridized in separate reactions within a hybridization manifold with individual candidate probes labeled with either a Cy3 or FITC fluorophore, which were cost-effective for candidate probe screening.
Probes Gaust_D1D3, Gcarib_D1D3, Gbeliz_D8D10, Gcarpent_D1D3, and Gcarol_D8D10 only detected their target species (Fig. 2) but probes Gpoly_D8D10 and Gribo1_D8D10 detected both G. polynesiensis and G. silvae. For all known ribosomal large subunit sequences both Gpoly_D8D10 and Gribo1_D8D10 have at least two base pairs of difference between the species; these species are very closely related, however (Fig. 1). The clade comprising G. polynesiensis and G. silvae can potentially be labeled by both probes Gpoly_D8D10 and Gribo1_D8D10 (the species G. carolinianus was not labeled by these probes); therefore, for this and future analyses, probes Gpoly_D8D10 and Gribo1_D8D10 have been redefined to detect species within the highly toxic “G. polynesiensis clade”.
3.3. Multiplexing fluorophores to detect multiple species
Multiplexed probe assays for each geographic region were created based on optimal Tm for probe hybridizations (Tables 1 and 3). Up to three probes were multiplexed via differential labeling by multiple fluorophores (See Table 1, Fig. 3). These fluorophores were chosen based on labeling efficacy and their ability to be combined due to their emission of light in separate spectral regions, and allows multiple species in a single sample to be distinguished and enumerated.
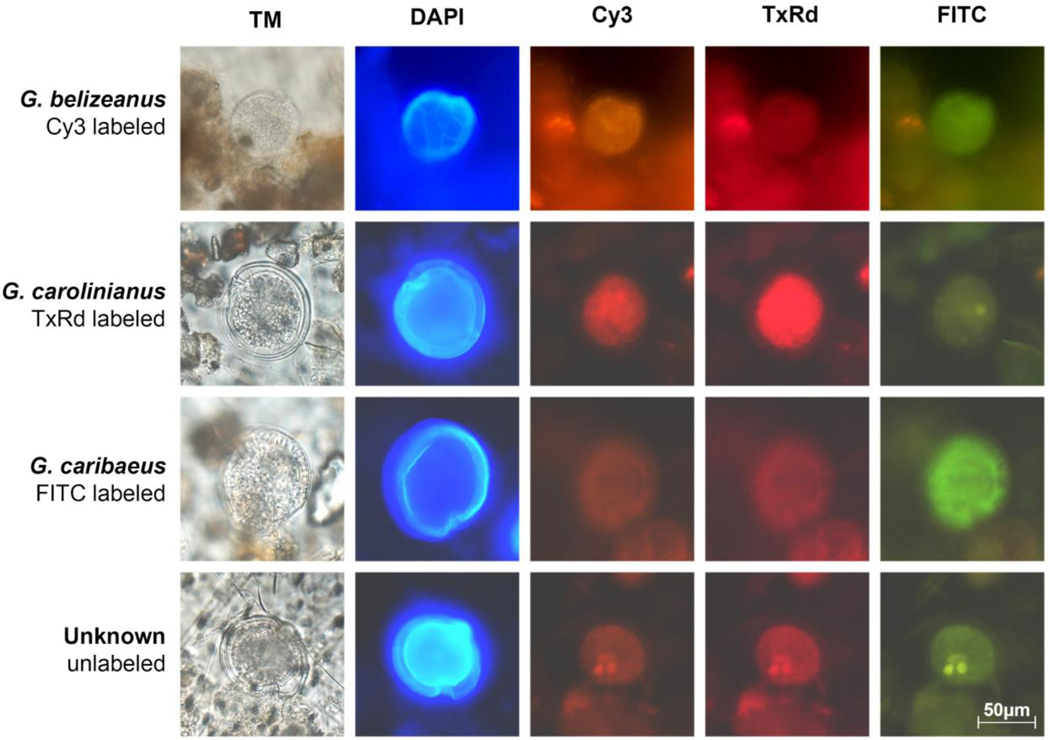
Figure 3.
Fluorophore labeling and identification of Gambierdiscus cells hybridized to species-specific probes using multiplexed fluorophores. Each row of micrographs displays a single Gambierdiscus cell under transmitted light followed by DAPI, Cy3, Texas Red and FITC filters. The species and labeling of each cell are indicated on the left-hand side of the figure. The final row of micrographs displays an unlabeled cell. The use of Calcofluor White allows visualization of thecal plates under DAPI filter, and also aids in distinguishing Gambierdiscus cells from surrounding detritus.
The use of Calcafluor White to stain cells allows for initial identification of Gambierdiscus at the genus level when field samples are scanned under the DAPI filter set. While other members of the algal community, such as Ostreopsis and Prorocentrum, are also stained by Calcafluor White, their morphology is readily distinguishable from Gambierdiscus based on thecal plate structure and other cellular characteristics. The Calcafluor stained cells are also readily distinguished from background detritus. Once cells were identified at the genus level, species-level identification was possibly based on determined by overall brightness, relative intensity, and color of the cell when visualized under the three filter sets (Fig. 3). For example, cells labeled with Cy3 (or Alexa Fluor® 532) were brightly labeled and orange in appearance under the Cy3 filter set; these cells are visible but dark and faded under the FITC and Texas red filters. Cells labeled with the Texas Red fluorophore (or Alexa Fluor® 594) appeared brightly lit and vibrantly red under the Texas Red filter, but appeared as faded red under Cy3 filter, and barely visible under the FITC filter. Cells labeled with FITC ( or Alexa Fluor® 488) were a bright and chalky green under the FITC filter, and barely visible under the other two filters. Unlabeled cells are only faintly visible under all three filter sets. Labeling can be thus be distinguished by a combination of color intensity and brightness, and for the multiplexing approach to be effective, it is important to carefully visualize each cell under all three filter sets.
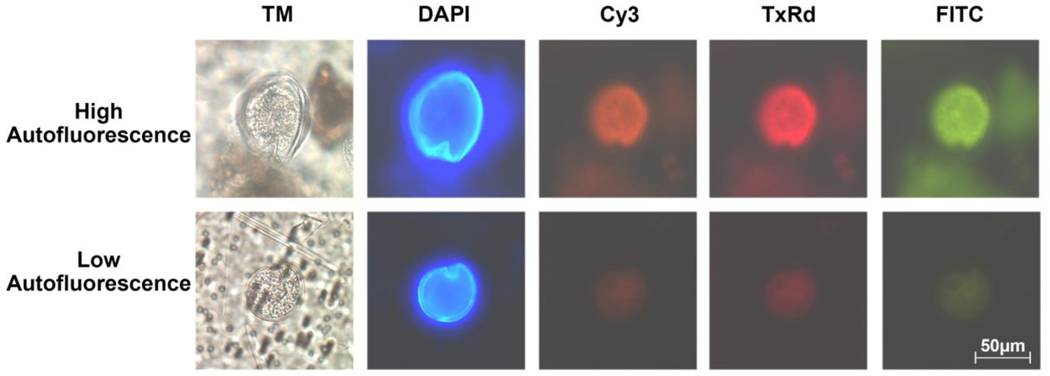
A note regarding autofluorescence: this can introduce interference if field samples are not properly preserved (Section 2.3). High levels of autofluorescence (Fig. 4) will manifest as a bright appearance under all three filter sets, complicating cell identification. In our experience autofluorescence is minimized by following preserving samples promptly, and that delays in preservation of over 24 hours introduced by occasional logistical disruptions to field operations produced samples with higher autofluorescence.
Figure 4.
Demonstration of autofluorescence variability in field samples caused by improper sample preservation. Each row of micrographs displays a single unlabeled Gambierdiscus cell viewed under transmitted light followed by DAPI, Cy3, Texas Red and FITC filters. The top row displays a highly autofluorescent cell from a sample that was stored for over 24 hours before formalin-methanol fixation, and the bottom row shows a minimally autofluorescent cell from a field sample that was preserved properly (see Section 2.3 for preservation protocol).
3.4. Quantitative Assessment of Gambierdiscus species enumeration using FISH
Experiments were performed to confirm that FISH allows quantitative enumeration of Gambierdiscus species in the community matrix typically present in field samples (e.g., detritus and other organisms). Field samples collected from the Florida Keys spiked with Gambierdiscus cultures yielded reliable labeling and detection of cells (Table 4). No significant differences in cell density were found between cultures and spiked field samples for any of the three species tested (G. caribaeus, p=0.52; G. carolinianus, p=1; G. silvae, p=0.25). These results indicate that the FISH method results in robust quantification of Gambierdiscus cells in field samples, and that the processing steps involved do not result in significant cell loss. Low variability among replicate samples also demonstrates that the centrifugation method is a viable alternative to the manifold filtration method. This latter approach also allows for processing of larger sample volumes; this is highly advantageous when enumerating Gambierdiscus spp., which are often present at low concentrations and are frequently a minor component of the benthic dinoflagellate assemblage (e.g., Richlen and Lobel, 2007).
Table 4.
Quantitative assessment of FISH approach for Gambierdiscus cell enumeration. Cultures of G. silvae, G. carolinianus, and G. caribaeus were enumerated using microscopic cell counts, and known concentrations added to field samples. These samples were analyzed using FISH with corresponding species-specific probes.
| Species | Cell abundance microscopic cell counts (1 mL, n=6) | Cell abundance FISH (1 mL, n=3) |
|---|---|---|
| G. carolinianus | 66±4.5 | 67±9.3 |
| G. silvae | 54±8.9 | 72±2.7 |
| G. caribaeus | 42±6.2 | 51±8.7 |
3.5. Field Sample Analysis
To determine the efficacy of Gambierdiscus cell detection in field samples, benthic samples collected from the HGB site in the Florida Keys were labeled with individual probes detecting G. belizeanus, G. caribaeus, G. carolinianus, G. carpenteri, and the G. polynesiensis clade. These analyses showed that the Gambierdiscus community primarily consisted of the G. polynesiensis clade, G. caribaeus, and G. carpenteri; G. carolinianus was a minor component of the Gambierdiscus community, and G. belizeanus was absent (Fig. 5). The cells in the G. polynesiensis clade were likely G silvae, as G. polynesiensis has not been reported in the Caribbean (Litaker et al., 2010; Lozano-Duque et al., 2018).
Figure 5.
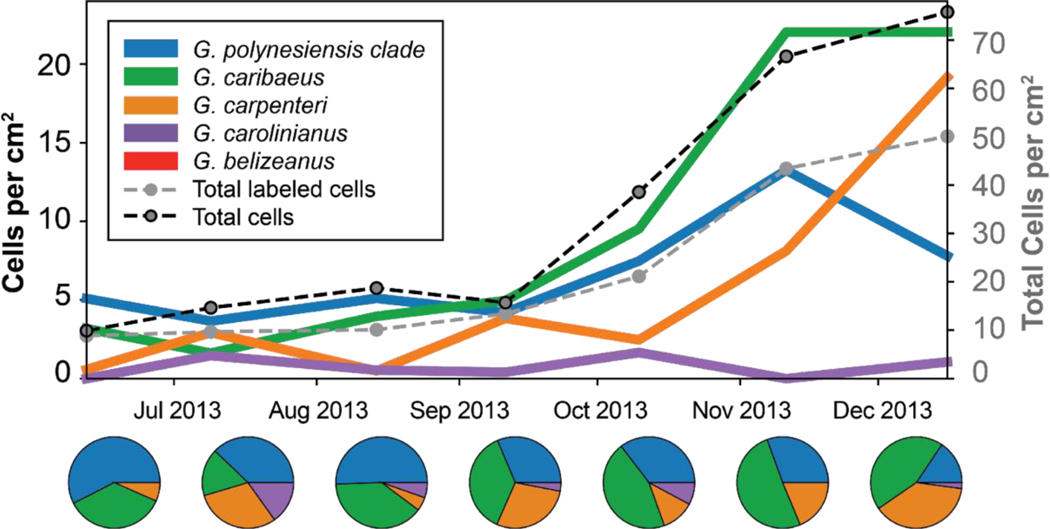
Community dynamics of Gambierdiscus species on Halimeda macroalgae at the Heine Grassbed (HGB) site over a six-month period. Species composition is similar to that observed in other studies in the Caribbean. Total abundance of Gambierdiscus spp. (genus level count) is shown by the dashed black line and summed abundance of enumerated species is shown by the dashed grey line, illustrating the fraction of unidentified cells. Gambierdiscus species detections shown by colored lines. Pie charts show relative species composition at each sampling point.
The Gambierdiscus community composition shifted over time as total Gambierdiscus spp. abundance increased, predominantly during November and December. All species detected were at low concentrations (<5 cells cm−2) from the outset of the sampling in June until November when three of the four species present increased (Fig. 5). Gambierdiscus caribaeus and G silvae become the dominant taxa in November, whereas in December, the two dominant species were G. caribaeus and G. carpenteri. Overall Gambierdiscus genus abundance also increased in the fall and early winter portion of the sampling period.
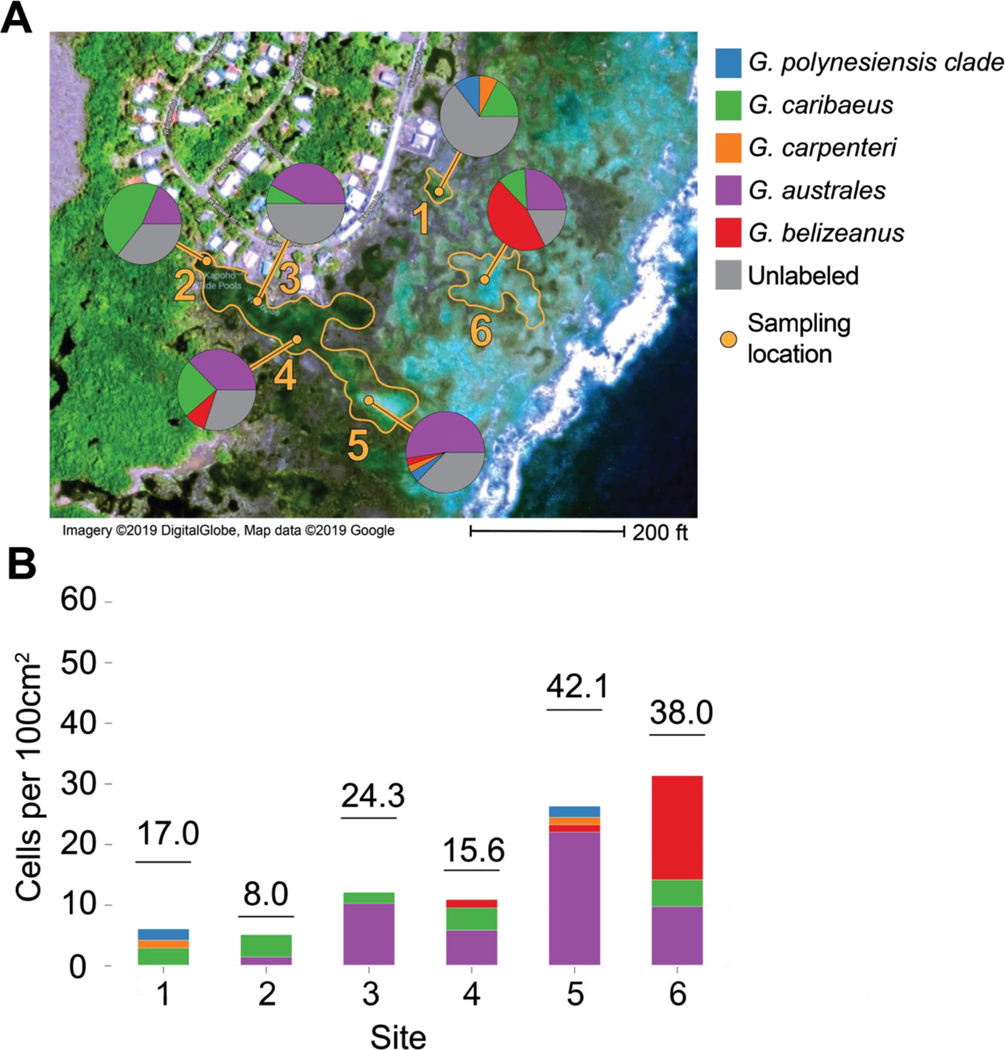
To test probes specifically designed for Pacific Gambierdiscus species and to determine efficacy of probe multiplexing (using multiple fluorophores), field samples collected in 2015 from Wai’Ōpae Tide Pools, Hawai’i were labeled with probes detecting G. australes, G. belizeanus, G. caribaeus, G. carpenteri, and the G. polynesiensis clade (Table 1). All species were readily detected at the site with these specific probes and fluorophores. Operationally, using combinations of 2–3 fluorophores per sample permitted visualization of up to three species at once using the three filter sets. Variation in species composition was observed between sites and pools across Wai’Opae during a single week-long sampling period (Fig. 6). There was a large proportion of G. australes present overall with G. caribaeus and G. belizeanus also proportionally the most abundant in sites 2 and 6, respectively. The highest concentration of Gambierdiscus cells was found in the sites furthest from shore.
Figure 6.
Gambierdiscus community at Wai’Ōpae Tide Pools on the southeastern shore of the Island of Hawai’i during July 2015 as taken from deployed artificial substrates. (A) Tide pool boundaries are outlined in orange with sampling location and sampling site number indicated. Pie charts show relative Gambierdiscus species abundance at each site. (B) Gambierdiscus cells per 100cm2 of artificial substrate for each site. Colored portions of bars show abundance of each species per site as shown in (A), and horizontal line and number indicates total abundance including unlabeled cells at each site.
4. Discussion
Recent advances in research on the taxonomy and toxicity of Gambierdiscus spp. have demonstrated that multiple species with widely differing toxicities co-occur within a particular locale and even on the same host alga (e.g., Vandersea et al., 2012; Nishimura et al., 2016), yet the cryptic diversity of this genus precludes identification beyond the genus level when analyzing samples using traditional light microscopy. Some dinoflagellate and diatom HAB genera exhibit variability in toxin content among species and strains (e.g., Alexandrium, Pseudonitzschia; Maranda et al., 1985; Cembella et al., 1987; Bates et al., 1998), a trait that has also been documented in Gambierdiscus (Chinain et al. 1999; 2010, Litaker et al., 2017; Robertson et al. 2018, Longo et al. 2019). It is therefore likely that Gambierdiscus communities at a given location are comprised of both highly toxic and non-toxic (or low toxicity) species, making species-specific enumeration a critical component in determining risk of ciguatera fish poisoning. The development of species-specific fluorescence in situ hybridization (FISH) probes allows for accurate enumeration of Gambierdiscus species in field samples, and offers a quantitative method for determining community composition in field samples, and particularly for monitoring the toxic species G. silvae and G. polynesiensis. As additional species-specific PCR primers and probes become available, the approach outlined herein can also be adapted for additional species and clades, including other toxic species of interest (e.g., G. excentricus).
Fluorescence in situ hybridization has been adapted for enumeration of several harmful algal bloom (HAB) organisms worldwide, including toxin-producing Alexandrium catenella (Anderson et al., 2005; John et al., 2005), Prorocentrum micans (Chen et al., 2013), Pseudo-nitzschia spp. (Greenfield et al., 2006; Parsons et al., 1999; Scholin et al., 1996), and the fish killing raphidophyte Heterosigma akashiwo (Chen et al., 2008). These species are sometimes present in relatively low proportions compared to the rest of the planktonic community, and identification may be further confounded by morphological similarities to other more innocuous organisms. The FISH method enables quick and high-confidence visual identification of toxic taxa. Given the potential for variable toxicity among Gambierdiscus species and strains, species composition may have a greater impact on risk of CFP than total genus abundance. The latter is, however, the parameter typically reported in field studies related to CFP. As a result of this study, probes can be targeted towards specific Gambierdiscus species or groups, allowing selective enumeration of species known to exhibit high toxicity. An example of the magnitude of this effect is seen in Figure 5. In mid-November, G. caribaeus was the dominant taxa, with roughly twice the abundance of the G. polynesiensis clade (presumably G. silvae), yet the latter is several orders of magnitude more toxic (Robertson et al., 2018), and is the most likely source of ciguatoxins in fish at this location based on our prior characterization of Gambierdiscus community composition and structure in St. Thomas and the Florida Keys (Lozano-Duque et al., 2018). Even when G. silvae concentrations decreased in December as G. caribaeus and G. carpenteri increased, the vast majority of the ciguatoxin at that location would still be attributable to G. silvae.
Using FISH, species abundance measurements could be integrated into routine monitoring efforts and analyzed over spatiotemporal gradients, allowing establishment of species-specific seasonal patterns and elucidation of community responses to disturbances, such as storms or coral bleaching. Preference for substrates or other environmental niche parameters can also be determined via experimental manipulations in the field or in the laboratory, followed by species-specific FISH probe analyses. In this context, note that in the field samples analyzed for this study, discrepancies were observed between the total abundance of Gambierdiscus cells and the summed abundance of all species detected using the FISH probes (Figs. 5, 6). One possible explanation is the presence of unlabeled cells or incomplete labeling, but this could also reflect the presence of one or more known or unknown species for which there is currently no FISH probe developed, and is a fertile area for further investigation. As knowledge of Gambierdiscus species taxonomy expands (e.g., Fraga and Rodríguez, 2014) the discovery of new species will force re-evaluation of detection techniques. The FISH method can be readily be adapted to target new species of interest. An additional benefit of this method is that it can be used to interrogate archived field samples preserved in methanol, allowing retrospective analysis of samples that had previously been used for genus or species enumeration.
Current methods for species-level Gambierdiscus detection such as electron micrograph imaging or Sanger DNA sequencing of cultures require extensive time and effort, which can be prohibitive for routine monitoring. Another approach that has shown promise is the use of a semi-quantitative qPCR assay to detect species presence in field samples (Vandersea et al., 2012). Through DNA extraction, the qPCR assay can examine a greater volume of sample more rapidly than FISH analysis. However, this technique may be subject to matrix effects associated with algal host substrate, which can impact limits of detection. The FISH approach described in this study allows for quantitative enumeration of all types of field samples in a time-efficient framework. Integration of multiple methods is likely to be an effective approach, as suggested in Kibler et al. (2015). For example, qPCR could be used to determine presence-absence or the relative abundance of an array of species (e.g., Nishimura et al., 2016; Vandersea et al., 2012) and then FISH probes could be used to the abundance of certain select taxa.
In this regard, accurate identification is essential to establishing patterns of abundance in field samples, and ultimately predicting future blooms. To date, predictive modeling efforts for Gambierdiscus have been limited to the genus level (Parsons et al., 2010) and are not yet able to forecast CFP risks. Field studies have been inconsistent in establishing a positive relationship between toxicity and cell abundance, or with environmental factors such as ocean warming (Chateau-Degat et al., 2005, Radke et al., 2013). To resolve these questions, spatiotemporal dynamics of individual species, quantitatively measured by FISH probe analysis, can be integrated with environmental data to update and refine models and analyses of trends. Measured abundances can be correlated with parameters such as temperature, nutrient levels, light, salinity, wave action, and season. Species-specific growth and toxicity data under various treatments (Xu et al., 2012, Kibler et al., 2014) can be used to inform new models, with the ultimate goal of improving CFP predictive abilities.
5. Conclusions
Species-specific FISH probes are a powerful new tool enabling those working with field populations of Gambierdiscus species to investigate community structure, and monitor the abundances of the most toxic, and thus the most important taxa. Due to Gambierdiscus species’ cryptic diversity and variance in toxicity, species-specific enumeration is a critical component in the determination of regional risk of ciguatera fish poisoning. This novel approach was successfully applied to samples from both the Caribbean Sea and Pacific Ocean, demonstrating high specificity in field samples and cultures from both regions. Moving forward, this method will facilitate the creation of datasets with high resolution at the species level. Analysis of Gambierdiscus community composition across multiple environments and over time will allow species abundance to be linked to environmental parameters, improving our ability to understand and manage the current and changing risks of CFP worldwide.
Highlights.
FISH probes developed to detect Caribbean and Pacific species of Gambierdiscus in field samples.
Multiple Gambierdiscus species shown to co-occur in natural populations in the Caribbean and the Pacific.
FISH probes detected temporal changes in Gambierdiscus community composition in the Florida Keys.
Acknowledgements
This work was supported by NOAA NOS (CiguaHAB program; NA11NOS4780060 and NA11NOS4780028; CiguaTOX program; N1A17NOS4780181), NSF PIRE (OISE Award # 1743802), and the Greater Caribbean Center for Ciguatera Research (NIH 1P01ES028949-01 and NSF 1841811), and the WHOI Ocean Ventures Fund. We thank Mireille Chinain at the Louis Malardé Institute (Tahiti, French Polynesia) for samples of preserved G. polynesiensis cultures used in testing. In addition, we thank Dave Kulis, Taylor Sehein, Kelsey Furman, and Finn Morrison for laboratory assistance, and sample analysis. The authors also thank Jason Adolf at the University of Hawai’i at Hilo for lab space, as well as Louise Economy for assistance collecting field samples. This is ECOHAB publication number 939.
Abbreviations
- CFP
ciguatera fish poisoning
- FISH
fluorescence in situ hybridization
- qPCR
quantitative polymerase chain reaction
- LSU rDNA
large subunit ribosomal DNA
- LSU rRNA
large subunit ribosomal RNA
- USVI
US Virgin Islands
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, and Scholin CA, 2005. Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep Sea Research Part II: Topical Studies in Oceanography, 52(19–21), 2467–2490. doi: 10.1016/j.dsr2.2005.06.015 [DOI] [Google Scholar]
- Bates SS, Garrison DL and Horner RA, 1998. Bloom dynamics and physiology of domoic-acid-producing Pseudo-nitzschia species. NATO ASI series G ecological sciences, 41, pp.267–292. [Google Scholar]
- Cembella AD, Sullivan JJ, Boyer GL, Taylor FJR and Andersen RJ, 1987. Variation in paralytic shellfish toxin composition within the Protogonyaulax tamaronsis/catenella species complex; red tide dinoflagellates. Biochemical systematics and ecology, 15(2), pp.171–186. [Google Scholar]
- Chateau-Degat ML, Chinain M, Cerf N, Gingras S, Hubert B and Dewailly E, 2005. Seawater temperature, Gambierdiscus spp. variability and incidence of ciguatera poisoning in French Polynesia. Harmful Algae, 4(6), pp.1053–1062. [Google Scholar]
- Chen GF, Wang GC, Zhang CY, Zhang BY, Wang XK and Zhou BC, 2008. Development of rRNA and rDNA-targeted probes for fluorescence in situ hybridization to detect Heterosigma akashiwo (Raphidophyceae). Journal of Experimental Marine Biology and Ecology, 355(1), pp.66–75. [Google Scholar]
- Chen GF, Liu Y, Zhang CY, Ma CS, Zhang BY, Wang GC, XU Z, Lu DD, 2013. Development of rRNA-targeted probes for detection of Prorocentrum micans (Dinophyceae) using whole cell in situ hybridization. J Appl Phycol, 25(4), pp1077–1089. 10.1007/s10811-012-9920-3 [DOI] [Google Scholar]
- Chinain M, Faust MA and Pauillac S, 1999. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. Journal of Phycology, 35(6), pp.1282–1296. [Google Scholar]
- Chinain M, Darius HT, Ung A, Cruchet P, Wang Z, Ponton D, Laurent D and Pauillac S, 2010. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon, 56(5), pp.739–750. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Baden DG, Bean JA, Weisman R, and Blythe DG, 1998. Marine Seafood Toxin Diseases: Issues In Epidemiology and Community Outreach. In Reguera B, Blanco J, Fernandez ML, and Wyatt T (Eds.), Harmful Algae (pp. 245–248). Paris: Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO. Retrieved from http://yyy.rsmas.miami.edu/groups/niehs/mfbsc/science/pdf/SeafoodToxinDiseasesIssues.pdf [Google Scholar]
- Fraga S, and Rodríguez F, 2014. Genus Gambierdiscus in the Canary Islands (NE Atlantic Ocean) with Description of Gambierdiscus silvae sp. nov., a new potentially toxic epiphytic benthic dinoflagellate. Annals of Anatomy, 165(6), 839–853. doi: 10.1016/j.protis.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Fritz L and Triemer RE, 1985. A rapid simple technique utilizing calcofluor white M2R for the visualization of dinoflagellate thecal plates 1. Journal of phycology, 21(4), pp.662–664. [Google Scholar]
- Greenfield DI, Marin III R, Jensen S, Massion E, Roman B, Feldman J and Scholin CA, 2006. Application of environmental sample processor (ESP) methodology for quantifying Pseudo‐nitzschia australis using ribosomal RNA‐targeted probes in sandwich and fluorescent in situ hybridization formats. Limnology and Oceanography: Methods, 4(11), pp.426–435. [Google Scholar]
- Holland WC, Litaker RW, Tomas CR, Kibler SR, Place AR, Davenport ED, and Tester PA, 2013. Differences in the toxicity of six Gambierdiscus (Dinophyceae) species measured using an in vitro human erythrocyte lysis assay. Toxicon, 65, 15–33. doi: 10.1016/j.toxicon.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Medlin LK and Groben R, 2005. Development of specific rRNA probes to distinguish between geographic clades of the Alexandrium tamarense species complex. Journal of plankton research, 27(2), pp.199–204. [Google Scholar]
- Kibler SR, Litaker WC, Vandersea MW, and Tester PA, 2015. A practical approach for Gambierdiscus species monitoring in the Caribbean. In: Lincoln MacKenzie A (Ed.), Marine and Freshwater Harmful Algae. Proceedings of the 16 International Conference on Harmful Algae, Wellington, New Zealand 27th-31st October 2014. Cawthron Institute, Nelson, New Zealand and International Society for the Study of Harmful Algae. [Google Scholar]
- Kibler SR, Litaker WR, Holland WC, Vandersea MW, and Tester PA, 2012. Growth of eight Gambierdiscus (Dinophyceae) species: Effects of temperature, salinity and irradiance. Harmful Algae, 19, 1–14. doi: 10.1016/j.hal.2012.04.007 [DOI] [Google Scholar]
- Kohler ST, and Kohler C, 1992. Dead bleached coral provides new surfaces for dinoflagellates implicated in ciguatera fish poisonings. Environmental Biology of Fishes, 35, 413–416. [Google Scholar]
- Lehane L, and Lewis RJ, 2000. Ciguatera: recent advances but the risk remains. International Journal of Food Microbiology, 61(2–3), 91–125. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11078162 [DOI] [PubMed] [Google Scholar]
- Litaker RW, Vandersea MW, Faust MA, Kibler SR, Nau AW, Holland WC, Chinain M, Holmes MJ and Tester PA, 2010. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon, 56(5), pp.711–730. [DOI] [PubMed] [Google Scholar]
- Litaker RW, Holland WC, Hardison DR, Pisapia F, Hess P, Kibler SR and Tester PA, 2017. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PloS one, 12(10), p.e0185776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo S, Sibat M, Viallon J, Darius HT, Hess P and Chinain M, 2019. Intraspecific variability in the toxin production and toxin profiles of in vitro cultures of Gambierdiscus polynesiensis (Dinophyceae) from French Polynesia. Toxins, 11(12), p.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Duque Y, Richlen ML, Smith TB, Anderson DM and Erdner DL, 2018. Development and validation of PCR-RFLP assay for identification of Gambierdiscus species in the Greater Caribbean Region. Journal of Applied Phycology, pp.1–12. [PMC free article] [PubMed] [Google Scholar]
- Lyu Y, Richlen ML, Sehein TR, Chinain M, Adachi M, Nishimura T, Xu Y, Parsons ML, Smith TB, Zheng T and Anderson DM, 2017. LSU rDNA based RFLP assays for the routine identification of Gambierdiscus species. Harmful algae, 66, pp.20–28. [DOI] [PubMed] [Google Scholar]
- Maranda L, Anderson DM and Shimizu Y, 1985. Comparison of toxicity between populations of Gonyaulax tamarensis of eastern North American waters. Estuarine, Coastal and Shelf Science, 21(3), pp.401–410. [Google Scholar]
- Mikulski CM, Morton SL and Doucette GJ, 2005. Development and application of LSU rRNA probes for Karenia brevis in the Gulf of Mexico, USA. Harmful Algae, 4(1), pp.49–60. [Google Scholar]
- Nishimura T, Sato S, Tawong W, Sakanari H, Uehara K, Shah MMR, Suda S, Yasumoto T, Taira Y, Yamaguchi H and Adachi M, 2013. Genetic diversity and distribution of the ciguatera-causing dinoflagellate Gambierdiscus spp. (Dinophyceae) in coastal areas of Japan. PLoS One, 8(4), p.e60882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Hariganeya N, Tawong W, Sakanari H,. Yamaguchi H Adachi M, 2016. Quantitative PCR assay for detection and enumeration of ciguatera-causing dinoflagellate Gambierdiscus spp. (Gonyaulacales) in coastal areas of Japan. Harmful Algae 52:11–22 [DOI] [PubMed] [Google Scholar]
- Parsons ML, Scholin CA, Miller PE, Doucette GJ, Powell CL, Fryxell GA, Dortch Q, Soniat TM, 1999. Pseudo-nitzschia in Louisiana coastal waters: molecular probe field trials, genetic variability, and domoic acid analyses. Journal of Phycology 35: 13681378. [Google Scholar]
- Parsons ML, Settlemier CJ, Bienfang PK, 2010. A simple model capable of simulating population dynamics of Gambierdiscus, the benthic dinoflagellate responsible for ciguatera fish poisoning. Harmful Algae, 10: 71–80 [Google Scholar]
- Parsons ML, Brandt AL, Ellsworth A, Leynse AK, Rains LK and Anderson DM, 2017. Assessing the use of artificial substrates to monitor Gambierdiscus populations in the Florida Keys. Harmful Algae, 68, pp. 52–66. [DOI] [PubMed] [Google Scholar]
- Pisapia F, Holland WC, Hardison DR, Litaker RW, Fraga S, Nishimura T, Adachi M, Nguyen-Ngoc L, Séchet V, Amzil Z and Herrenknecht C, 2017. Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae, 63, pp.173–183. [DOI] [PubMed] [Google Scholar]
- Radke EG, Grattan LM, Cook RL, Smith TB, Anderson DM and Morris JG Jr, 2013. Ciguatera incidence in the US Virgin Islands has not increased over a 30-year time period despite rising seawater temperatures. The American journal of tropical medicine and hygiene, 88(5), pp. 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke EG, Reich A and Morris JG Jr, 2015. Epidemiology of ciguatera in Florida. The American journal of tropical medicine and hygiene, 93(2), pp.425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlen ML and Lobel PS, 2011. Effects of depth, habitat, and water motion on the abundance and distribution of ciguatera dinoflagellates at Johnston Atoll, Pacific Ocean. Marine ecology progress series, 421, pp.51–66. [Google Scholar]
- Robertson A, Richlen ML, Erdner D, Smith TB, Anderson DM, Liefer J, Xu Y, McCarron P, Miles C, Parsons M, 2018. Toxicity, chemistry, and implications of Gamberdiscus silvae: A ciguatoxin superbug in the Greater Caribbean Region. International Conference on Harmful Algae (ICHA), 21–26 October, Nantes, France. [Google Scholar]
- Ruff TA, 1989. Ciguatera in the Pacific: A Link with Military Activities. The Lancet, 201–205. [DOI] [PubMed] [Google Scholar]
- Scheuer PJ, Takahashi W, Tsutsumi J, and Yoshida T, 1967. Ciguatoxin: isolation and chemical nature. Science (New York, N.Y.), 155(3767), 1267–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6018649 [DOI] [PubMed] [Google Scholar]
- Scholin CA, and Anderson DM, 1993. Population analysis of toxic and nontoxic Alexandrium species using ribosomal RNA signature sequences. In: Toxic Phytoplankton Blooms in the Sea, Smayda TJ and Shimizu Y, Eds. Amsterdam: Elsevier. [Google Scholar]
- Scholin CA, Buck KR, Britschgi T, Cangelosi G, and Chavez FP 1996. Identification of Pseudo-nitzschia australis (Bacillariophyceae) using rRNA-targeted probes in whole cell and sandwich hybridization formats. Phycologia: May 1996, Vol. 35, No. 3, pp. 190–197. [Google Scholar]
- Sparrow L, Momigliano P, Russ GR and Heimann K, 2017. Effects of temperature, salinity and composition of the dinoflagellate assemblage on the growth of Gambierdiscus carpenteri isolated from the Great Barrier Reef. Harmful algae, 65, pp. 52–60. [DOI] [PubMed] [Google Scholar]
- Vandersea MW, Kibler SR, Holland WC, Tester PA, Schultz TF, Faust MA, Holmes MJ, Chinain M and Litaker RW, 2012. Development of semi-quantitative PCR assays for the detection and enumeration of Gambierdiscus species (Gonyaulacales, Dinophyceae). Journal of Phycology, 48(4), no–no. doi: 10.1111/j.1529-8817.2012.01146.x [DOI] [PubMed] [Google Scholar]
- Xu Y, Richlen ML, Morton SL, Mak YL, Chan LL, Tekiau A, and Anderson DM, 2014. Distribution, abundance and diversity of Gambierdiscus spp. from a ciguatera-endemic area in Marakei, Republic of Kiribati. Harmful Algae, 34, 56–68. doi: 10.1016/j.hal.2014.02.007 [DOI] [Google Scholar]
- Zheng D, Alm EW, Stahl DA, and Raskin L, 1996. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Applied and Environmental Microbiology, 62(12), 4504–4513. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8953722 [DOI] [PMC free article] [PubMed] [Google Scholar]