Abstract
Background
Mechanical ventilation with variable tidal volumes (VT) may improve lung function and reduce ventilator-induced lung injury in experimental acute respiratory distress syndrome (ARDS). However, previous investigations were limited to less than 6 h, and control groups did not follow clinical standards. We hypothesised that 24 h of mechanical ventilation with variable VT reduces pulmonary inflammation (as reflected by neutrophil infiltration), compared with standard protective, nonvariable ventilation.
Methods
Experimental ARDS was induced in 14 anaesthetised pigs with saline lung lavage followed by injurious mechanical ventilation. Pigs (n=7 per group) were randomly assigned to using variable VT or nonvariable VT modes of mechanical ventilation for 24 h. In both groups, ventilator settings including positive end-expiratory pressure and oxygen inspiratory fraction were adjusted according to the ARDS Network protocol. Pulmonary inflammation (primary endpoint) and perfusion were assessed by positron emission tomography using 2-deoxy-2-[18F]fluoro-d-glucose and 68Gallium (68Ga)-labelled microspheres, respectively. Gas exchange, respiratory mechanics, and haemodynamics were quantified. Lung aeration was determined using CT.
Results
The specific global uptake rate of 18F-FDG increased to a similar extent regardless of mode of mechanical ventilation (median uptake for variable VT=0.016 min−1 [inter-quartile range, 0.012–0.029] compared with median uptake for nonvariable VT=0.037 min−1 [0.008–0.053]; P=0.406). Gas exchange, respiratory mechanics, haemodynamics, and lung aeration and perfusion were similar in both variable and nonvariable VT ventilatory modes.
Conclusion
In a porcine model of ARDS, 24 h of mechanical ventilation with variable VT did not attenuate pulmonary inflammation compared with standard protective mechanical ventilation with nonvariable VT.
Keywords: ARDS, mechanical ventilation, positron emission tomography, pulmonary neutrophilic inflammation, variable ventilation
Editor's key points.
-
•
Mechanical ventilation with variable tidal volumes (VT) may reduce ventilator-induced lung injury in experimental acute respiratory distress syndrome (ARDS).
-
•
Previous experimental investigations have been short and did not adhere to gold standard ventilation for ARDS.
-
•
In an experimental porcine model of ARDS, the authors examined whether prolonged (24 h) variable VT ventilation reduced pulmonary inflammation, compared with protective nonvariable VT ventilation.
-
•
Variable VT ventilation failed to alter either pulmonary inflammation or perfusion, as assessed by positron emission tomography.
Patients with acute respiratory distress syndrome (ARDS) usually require mechanical ventilation. Despite being lifesaving, mechanical ventilation may itself damage the lungs (ventilator-induced lung injury [VILI]).1 The ARDS Network2 proposed a mechanical ventilation protocol that avoids excessive mechanical stress on the lungs through the use of low tidal volumes (VT, 4–8 ml kg−1), inspiratory plateau pressure (Pplat) <30 cm H2O, and combinations of positive end-expiratory pressure/fraction of inspired oxygen (PEEP/F io 2) which take into account the severity of gas exchange impairment.
The use of low VT, which is rather constant (nonvariable) breath to breath, has been considered one of the most important elements of the ARDS Network ventilation protocol. Different studies, however, have suggested that variable VT (termed variable ventilation) improved gas exchange and lung mechanics in experimental ARDS3 by reducing lung inflammation.4, 5, 6 However, these studies have been of short duration (<6 h) and have not included protective mechanical ventilation as standard care, as defined by the ARDS Network low PEEP.7
VILI results in infiltration and activation of neutrophils in lung tissue.8 , 9 Activated neutrophils compared with other cells have a higher glucose uptake,10 , 11 which can be quantified in vivo with positron emission tomography (PET/CT) using the glucose analogue 2-deoxy-2-[18F]fluoro-d-glucose (18F-FDG). In this study, we evaluated the effects of 24 h of variable ventilation combined with the ARDS Network protocol on lung inflammation (primary endpoint), gas exchange and respiratory system mechanics in experimental ARDS. We hypothesised that 24 h of mechanical ventilation with variable VT reduces neutrophilic inflammation, as compared with standard protective ventilation with nonvariable VT.
Methods
The study protocol was approved by the Institutional Animal Care and Welfare Committee and the Government of the State of Saxony, Germany (AZ 24-9168.11-1/2013-53). All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the US National Academy of Sciences Guide for the Care and Use of Laboratory Animals and complied with relevant aspects of the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Animals were kept in an environment with controlled temperature (23°C) and light–dark cycles, with free access to water and food.
Animal preparation
Fourteen female pigs (German landrace, weighing 29.5–40 kg; Danish Specific Pathogen Free Certification; www.spf.dk) were pre-medicated with midazolam (1 mg kg−1 i.m.) and ketamine (10 mg kg−1 i.m.); intravenous anaesthesia was induced and maintained with midazolam (bolus 0.5–1 mg kg−1, followed by 1–2 mg kg−1 h−1) and ketamine (bolus 3–4 mg kg−1, followed by 10–18 mg kg−1 h−1). Neuromuscular block was achieved with atracurium (bolus 3–4 mg kg−1, followed by 1–2 mg kg−1 h−1). Adequacy of anaesthesia was assessed continuously by lack of spontaneous movements, absence of reaction to painful stimulation between the front hooves, and absence of cardiovascular signs of sympathetic stimulation (increases in heart rate or arterial blood pressure). The trachea of the animals was intubated transorally with a cuffed single-lumen tracheal tube (8.0 mm internal diameter; Mallinckrodt, Athlone, Ireland), and lungs were ventilated with an EVITA XL (Dräger Medical AG, Lübeck, Germany) ventilator. All skin incisions were preceded by infiltration of 2–5 ml lidocaine 2%. After surgical preparation of the right internal carotid artery, an indwelling catheter was inserted and the mean arterial pressure continuously monitored. A 7.5 Fr pulmonary artery catheter (Opticath; Abbott, Abbott Park, IL, USA) was advanced through an 8.5 Fr sheath, placed in the right external jugular vein until typical pulmonary arterial pressure waveforms could be observed. Urine was collected with a bladder catheter inserted through a median mini-laparotomy. Parameters of gas exchange, respiratory signals, and haemodynamics were recorded before ARDS induction (time point BASELINE1).
Experimental ARDS
Lung injury was induced with a double hit model consisting of isotonic saline lung lavage alternating prone and supine position (four times each), followed by injurious ventilation with VT of 20 ml kg−1 and zero PEEP until the Pao 2/F io 2 is <13.3 kPa for at least 30 minutes. Measurements were performed immediately thereafter (Injury). PET/CT imaging data were acquired (Day 1) and measurements performed (Baseline2). Thereafter, animals were randomly assigned to mechanical ventilation with: (i) variable VT (VV) or (ii) nonvariable VT (NV). In both groups, mechanical ventilation settings followed the recommendations of the ARDS Network protocol using the low F io 2/PEEP table.7 Measurements were performed in intervals of 6 h (Time1 to Time4). An illustration of the experiment's time course is available in the Supplementary material. Intravascular volume was maintained with a crystalloid solution (E153; Serumwerk Bernburg AG, Bernburg, Germany) at a rate of 4 ml kg−1 h−1. Norepinephrine was used to maintain a mean arterial pressure of at least 60 mm Hg throughout the experiments. After 24 h, PET/CT imaging was repeated (Day 2) and animals were killed with an intravenous injection of thiopental (2 g), followed by potassium chloride (1 M, 50 ml). Postmortem lung tissue samples were obtained for histological and molecular biology analyses.
Ventilator settings
Until randomisation, animals were ventilated in volume-controlled mode with VT=6 ml kg−1, F io 2=1.0, inspiratory/expiratory (I/E) ratio=1:1, PEEP=10 cm H2O, constant inspiratory flow of 35 L min−1, and ventilatory frequency (VF) adjusted to achieve an arterial pH between 7.38 and 7.42. During initial PET/CT, PEEP was set to 16 cm H2O to standardise imaging conditions before randomisation. After randomisation, VF was set to reach a pH of >7.3, the lowest tolerable pH being 7.15 with a maximum VF of 35 breaths min−1; otherwise, an increase of VT was allowed. Combinations of F io 2 and PEEP were set according to the low-PEEP table of the ARDS Network.7
Variable VT ventilation
During variable ventilation, VT changed from breath to breath, based on a predefined sequence of 600 random VT values (mean VT=6 ml kg−1, normal distribution) which looped infinitely. The sequence of 600 variable VT cycles achieved the same minute volume as nonvariable ventilation. The targeted variance of the VT values was 30%, which is roughly the variability in healthy spontaneously breathing subjects.12 The flow rate was 35 L min−1, and active inspiratory time was adjusted at each cycle to achieve the target VT. Because the I/E ratio was fixed at 1:1, the inspiratory pause varied.
Lung imaging
A low-dose helical CT scan (Biograph16 Hirez PET/CT; Siemens, Knoxville, TN, USA) of the thorax was obtained at mean airway pressure holds, and used for attenuation correction of the subsequent PET scans (attenuation correction CT [ACCT]). ACCT scans were used for manual segmentation of the lungs, excluding major airways and vessels. Segmented ACCT scans were used to define ventral, mid-ventral, central, mid-dorsal, and dorsal lung regions (region of interest [ROI]) of equal lung tissue mass,13 and to calculate gas fraction (FGAS) from the linear relation between tissue attenuation expressed in Hounsfield units (HU) and lung density (FGAS=HU [–1000]−1).
Primary outcome: pulmonary inflammation
After the ACCT scan, a bolus of 18F-FDG (∼200 MBq) was infused to assess the infiltration of activated neutrophils which have a higher glucose uptake.10 , 11 Sequential PET frames and a series of blood samples were acquired over a period of 75 min. Blood samples were spun down, and the plasma activity was measured in a gamma counter, cross-calibrated with the PET scanner. The field of view (cranio-caudal extension: 15 cm) of the dynamic PET scan was set above the diaphragmatic dome to reduce motion artifacts. The Patlak two-compartment model14 was used to calculate the 18F-FDG uptake rate (K i). To account for differences in lung inflation and blood volume between ROI, animals and time points, K i was normalised to lung tissue fraction (FTISSUE), thus computing the specific K i (K iS) as shown (equation (1)):
| (1) |
where F BLOOD is the fractional blood volume obtained from the three-compartment Sokoloff model.15 K iS was determined for each ROI of comparable lung mass (regional K iS) and for the whole lung (global K iS).
Secondary outcomes
Lung perfusion
After the acquisition of the residual 18F-FDG activity by a PET scan, 68Ga-labelled (ITG Isotope Technologies Garching GmbH, Munich, Germany) microspheres (ROTOP Pharmaka AG, Dresden, Germany) were injected i.v. (∼100 MBq) to quantify lung perfusion, as assessed by a static PET scan.16 Specific regional perfusion was determined for each ROI of comparable lung mass as count rate per ROI relative to that of the whole lung and multiplied by the cardiac output at the time point of the 68Ga PET measurement.
Lung aeration
A static high-resolution helical CT scan of the thorax was acquired under a respiratory hold at mean airway pressure and muscle paralysis. In those high-resolution images, segmentation was performed in every fifth slice to define the lung contour. Segmented CT scans were used to differentiate between hyper-aerated, normally aerated, poorly aerated, and nonaerated compartments, for which the respective mass relative to that of the whole lung was calculated.17 , 18
Markers of lung injury and inflammation
The wet/dry ratio of the right lung was measured. The diffuse alveolar damage score19 was evaluated by an expert blinded to group allocation. Gene expression and protein levels of markers associated with inflammation (interleukin [IL]-6 and IL-8), fibrosis (type III procollagen), and endothelial cell damage (vascular endothelial growth factor [VEGF] and intercellular adhesion molecule 1 [ICAM-1]) were analysed using quantitative real-time polymerase chain reaction (PCR) (iCycler IQ Real-Time PCR System; Bio-Rad Laboratories Inc., Hercules, CA, USA, and PerfeCta SYBR Green FastMix; Quanta Biosciences, Gaithersburg, MD, USA) and enzyme-linked immunosorbent assay (ELISA) (R&D Systems Europa, Abingdon, UK).
Experimental protocol
Gas exchange, respiratory signals (obtained from internal sensors of the ventilator), and haemodynamics were assessed at time points defined as Baseline1, Injury, Baseline2, and at 6 h intervals (Time1 to Time4). At the end of the experiments, the left and right lungs were removed for tissue analysis.
Statistical analysis
At the time of study planning, there was no literature available with suitable data for sample size estimation. Therefore, the number of animals per group was derived from the expertise of the group; thus, the analysis is exploratory in nature. Data are presented as median and 25% and 75% quartiles unless indicated otherwise. Student's t-test, generalised linear model, Mann–Whitney U-tests, and Wilcoxon's test were used as appropriate. Calculations were performed using the SPSS software package (SPSS version 22.0; IBM, Armonk, NY, USA); multiple comparisons were adjusted according to the Bonferroni–Holm procedure. Global statistical significance was accepted at P<0.05.
Results
ARDS model
Respiratory variables were similar between each group, apart from the coefficient of variation of VT, which was close to 30% in the group with variable VT ventilation (Table 1 ). Arterial pH was maintained at >7.15 in both groups. All other parameters, including fluid therapy, anaesthetic requirements, and number of lavages were similar between groups (Supplementary material).
Table 1.
Respiratory mechanics and gas exchange. Values are given as mean and standard deviation. Effects of Injury on variables were tested with paired t-test (Baseline vs Injury, P<0.05). Differences between and within groups (Group effect; Time × Group effect) were tested with general linear model statistics with BASELINE2 as a covariate. Global statistical significance was accepted at P<0.05. VT, tidal volume; CV VT, coefficient of variation of tidal volume; VF, ventilatory frequency; MV, minute ventilation; Pplat, plateau airway pressure averaged over 100 single breaths; Pmean, mean airway pressure averaged over 100 single breaths; Ppeak, peak airway pressure averaged over 100 single breaths; ERS, elastance of the respiratory system; RRS resistance of the respiratory system; VV, variable ventilation; NV, nonvariable ventilation; Pao2/FIo2, arterial partial oxygen pressure divided by inspiratory oxygen fraction; Pao2; arterial partial oxygen pressure; Paco2, arterial partial carbon dioxide pressure; pH, arterial pH value
| Variable | Group | Baseline1 | Injury | Baseline2 | Time1 | Time2 | Time3 | Time4 | Group effect P-value | Time × Group effect P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| VT (ml kg−1) | VV | 6.5 (0) | 6.5 (0.1) | 6.5 (0) | 6.2 (0.5) | 6.2 (0.5) | 6.2 (0.6) | 6.1 (0.5) | 0.931 | 0.20 |
| NV | 6.6 (0.2) | 6.6 (0.2) | 6.9 (1.0) | 6.5 (0.3) | 6.4 (0.5) | 6.5 (0.4) | 6.6 (0.2) | |||
| CV VT (%) | VV | 0.6 (0.1) | 0.6 (0.2) | 0.5 (0.2) | 28 (2.4) | 26.6 (6.8) | 27.6 (4.3) | 27.4 (4) | ≤0.001 | 0.843 |
| NV | 0.7 (0.3) | 0.6 (0.2) | 0.6 (0.4) | 0.5 (0.2) | 0.4 (0.1) | 0.5 (0.2) | 0.5 (0.1) | |||
| VF (min−1) | VV | 33.6 (2.5) | 33.6 (2.5) | 35.1 (0.1) | 28.3 (7.2) | 27.5 (8.1) | 25.3 (8.6) | 26 (9.3) | 0.522 | 0.162 |
| NV | 33.6 (2.5) | 33.6 (2.5) | 35.1 (0.0) | 32.9 (2.7) | 29.3 (6.1) | 27.9 (5.7) | 26.4 (5.6) | |||
| MV (L min−1) | VV | 7.9 (0.6) | 7.9 (0.6) | 8.3 (0.7) | 6.2 (1.4) | 6.0 (1.6) | 5.4 (1.5) | 5.5 (1.5) | 0.333 | 0.349 |
| NV | 7.6 (0.7) | 7.6 (0.6) | 8.2 (0.9) | 7.3 (0.6) | 6.4 (0.8) | 6.1 (1) | 6 (1.4) | |||
| ERS (cm H2O L−1) | VV | 24.1 (2.7) | 81.2 (7) | 69.2 (12) | 74.6 (22) | 74.1 (24) | 71.3 (23) | 70.1 (23) | 0.746 | 0.647 |
| NV | 23.6 (4.3) | 67.7 (9.8) | 69.1 (8.8) | 79.3 (14) | 78 (14) | 74.6 (11) | 71 (10) | |||
| RRS (cm H2O L−1 s) | VV | 7.3 (0.6) | 10.7 (2) | 7.4 (0.3) | 8.2 (0.6) | 9.1 (1.1) | 9.8 (2.1) | 10.7 (4.2) | 0.479 | 0.101 |
| NV | 7.6 (1.1) | 10 (1.6) | 8.5 (1.9) | 7.9 (0.6) | 8.4 (2.1) | 9.4 (2.2) | 9.6 (2.4) | |||
| Ppeak (cm H2O) | VV | 21 (0.7) | 34.4 (2.4) | 27.6 (4.2) | 27.1 (5.2) | 27.6 (6) | 26.8 (5) | 27.2 (4.7) | 0.713 | 0.152 |
| NV | 20.9 (0.7) | 31.1 (2.6) | 30.5 (3.6) | 29.1 (3.9) | 28.1 (2.3) | 28 (2.5) | 26.6 (2.4) | |||
| Pplat (cm H2O) | VV | 17.4 (0.7) | 30.8 (2.3) | 25.6 (4.3) | 24.6 (5.2) | 24.7 (7) | 23.5 (6.1) | 23.4 (6.2) | 0.608 | 0.188 |
| NV | 17.3 (0.6) | 27 (2.3) | 27.8 (4.8) | 27.1 (4.2) | 25.7 (3.4) | 25.2 (3.1) | 23.6 (3) | |||
| Pmean (cm H2O) | VV | 14 (0.2) | 19.2 (0.8) | 15.5 (3.2) | 14.2 (2.7) | 14.4 (3.5) | 13.8 (3.1) | 14 (3.2) | 0.479 | 0.101 |
| NV | 14 (0.3) | 17.9 (0.9) | 17.6 (3.1) | 15.9 (2.8) | 15.3 (2.2) | 15.2 (2.1) | 13.9 (1.7) | |||
| PEEP (cm H2O) | VV | 10 (0.0) | 9.8 (0.2) | 7.7 (2.9) | 6.2 (1.5) | 6.3 (2) | 5.8 (1.9) | 6.2 (2) | 0.519 | 0.088 |
| NV | 10 (0.0) | 9.8 (0.2) | 9.7 (2.8) | 7.6 (2) | 6.9 (1.9) | 6.7 (1.9) | 5.6 (1.5) | |||
| Pao2/Fio2 (kPa) | VV | 80.1 (80.3) | 9.2 (2.1) | 27.1 (10.9) | 28.5 (10.5) | 29.3 (10.3) | 29.7 (9.9) | 30.4 (11.5) | 0.990 | 0.530 |
| NV | 80 (8) | 8.5 (2) | 21.1 (5.6) | 22 (4.3) | 25.2 (5.9) | 26.4 (6.4) | 25.3 (5.6) | |||
| Pao2 (kPa) | VV | 80.1 (80.3) | 9.2 (2.1) | 11.6 (1.7) | 11.1 (2.4) | 11 (2.1) | 11.1 (1.9) | 11.7 (2.4) | 0.349 | 0.483 |
| NV | 80 (8) | 8.5 (2) | 10.8 (0.8) | 10.1 (1.5) | 9.9 (1.3) | 10.3 (1.4) | 9.9 (1.2) | |||
| Paco2 (kPa) | VV | 6.4 (0.8) | 11.9 (1.4) | 11.7 (2.4) | 11.2 (1.5) | 10.9 (1.0) | 12.2 (1.4) | 12.8 (2.1) | 0.237 | 0.587 |
| NV | 6.8 (0.8) | 11.8 (3.7) | 11.9 (2.7) | 10.7 (2.0) | 10.7 (2.1) | 11.6 (2.6) | 11.2 (1.4) | |||
| pH | VV | 7.38 (0.04) | 7.23 (0.06) | 7.26 (0.08) | 7.30 (0.06) | 7.33 (0.06) | 7.32 (0.06) | 7.30 (0.07) | 0.188 | 0.401 |
| NV | 7.38 (0.04) | 7.25 (0.11) | 7.22 (0.08) | 7.30 (0.05) | 7.32 (0.04) | 7.34 (0.05) | 7.35 (0.05) |
Primary outcome: pulmonary inflammation
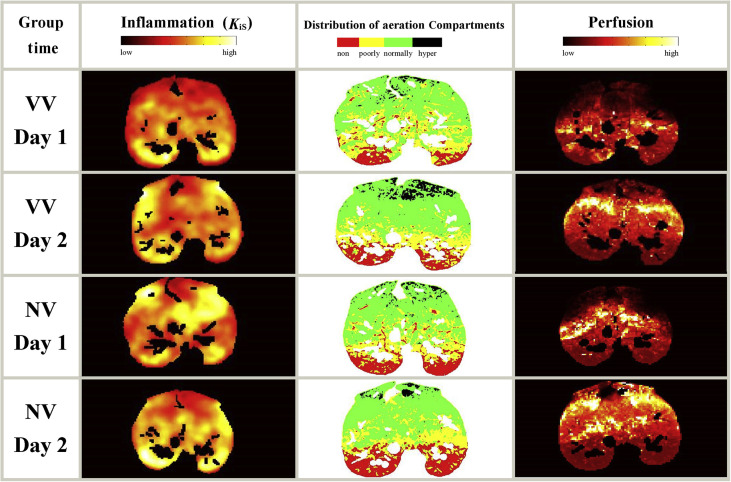
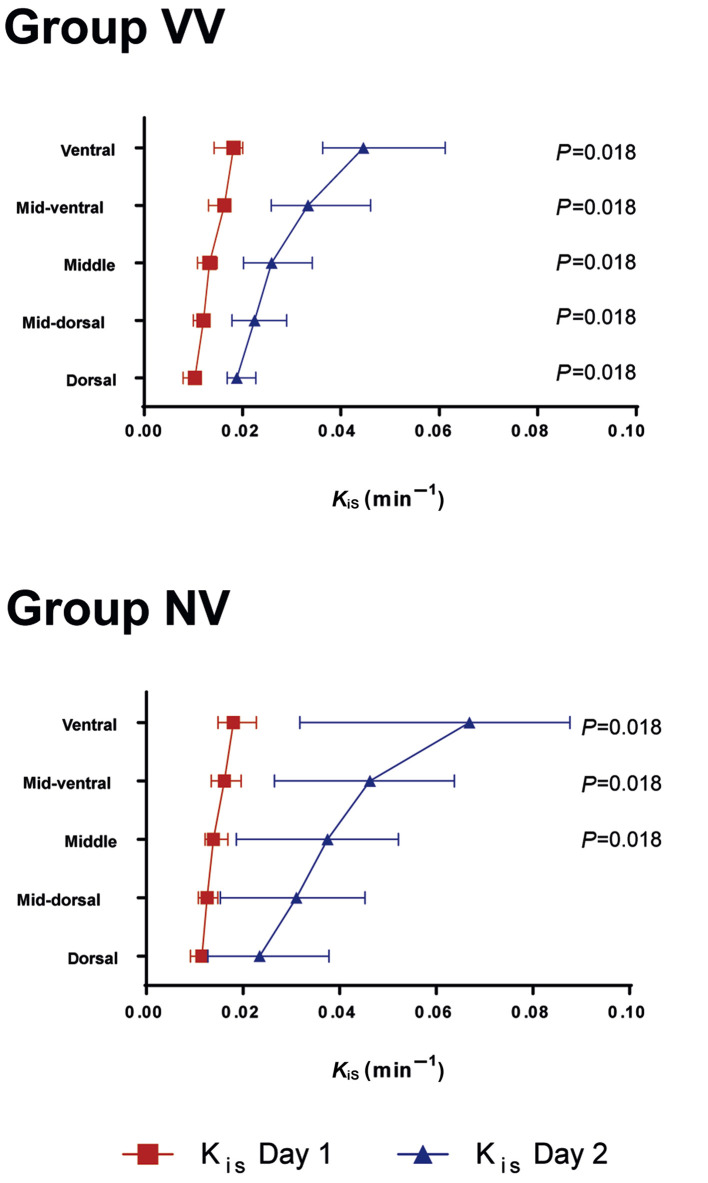
Figure 1 shows the maps of the distribution of lung inflammation, aeration compartments, and perfusion of one representative animal of each group before randomisation at 24 h. At 24 h, global K iS increased significantly in both groups, but values did not differ significantly between groups (VV: 0.016, 0.012–0.029 min−1; NV: 0.037, 0.008–0.053 min−1; P=0.406). Similarly, the increase in regional K iS from before randomisation (Day 1) to 24 h after randomisation (Day 2) did not differ significantly between VV and NV in the investigated subregions. The same global and regional behaviour was observed when inflammation was assessed by non-normalised K i measurements. Within each group, regional K iS values were higher on Day 2 compared with Day 1 in all regions for group VV, and in ventral, mid-ventral, and mid-dorsal regions for group NV (Fig. 2 ).
Fig 1.
Transversal slices of inflammation, aeration, and perfusion of one representative animal per group before and 24 h after randomisation. Left column: transversal slice of the specific uptake rates of 2-deoxy-2-[18F]fluoro-d-glucose (KiS). KiS was determined with positron emission tomography/CT and kinetic modelling according to the Patlak method. The resulting Ki values were normalised to the tissue fraction (KiS=Ki/FTISSUE=Ki/(1 – gas fraction – blood fraction); gas fraction determined from CT; blood fraction determined using the Sokoloff three-compartment model). Middle column: aeration compartments obtained from CT; hyper, hyper-aerated compartment; normally, normally aerated compartment; poorly, poorly aerated compartment; non, nonaerated compartment. Right column: distribution of perfusion obtained with 68Ga-labelled microspheres and positron emission tomography/CT. VV, variable ventilation; NV, nonvariable ventilation; Day 1, before randomisation; Day 2, 24 h after randomisation.
Fig 2.
Regional specific uptake rates of 2-deoxy-2-[18F]fluoro-d-glucose (KiS) before and 24 h after randomisation. KiS were determined by positron emission tomography/CT and kinetic modelling according to the Patlak method. Resulting Ki values were normalised to tissue fraction (KiS=Ki/FTISSUE=Ki/(1–gas fraction–blood fraction); gas fraction was determined from CT; blood fraction determined using the Sokoloff three-compartment model). Symbols and horizontal lines represent the median and inter-quartile range. Global statistical significance was accepted at P<0.05, Bonferroni–Holm adjustment for multiple testing. Differences between Day 1 and Day 2 within the same region and group were tested with Wilcoxon test (depicted P-values). No differences were found between groups VV and NV (Mann–Whitney U-test). n=7 per group. VV, variable ventilation; NV, nonvariable ventilation. Day 1, before randomisation; Day 2, 24 h after randomisation.
Secondary outcomes
Lung aeration
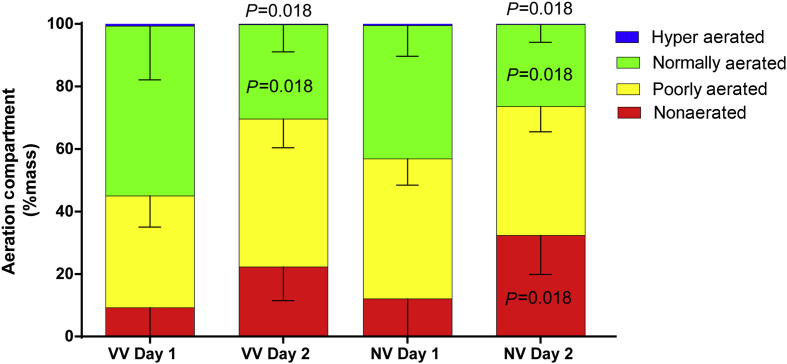
The amount of normally aerated and hyper-aerated lung tissue decreased during the observation period in both groups. The amount of nonaerated lung tissue increased in the NV group after 24 h, but not in the VV group. No significant differences were found between groups VV and NV (Fig. 3 ).
Fig 3.
Size of aeration compartments of the whole lung expressed as % mass of the whole lung. Bars represent the mean values of the hyper aerated (blue), normally aerated (green), poorly aerated (yellow), and nonaerated compartments (red). Vertical lines represent standard deviations. Global statistical significance was accepted at P<0.05, Bonferroni–Holm adjustment for multiple testing. Differences between Day 1 and Day 2 within the same group and same compartment were tested with Wilcoxon tests (depicted P-values). No differences were found between groups VV and NV (Mann–Whitney U-test). n=7 per group. VV, variable ventilation; NV, nonvariable ventilation; Day 1, before randomisation; Day 2, 24 h after randomisation.
Pulmonary perfusion
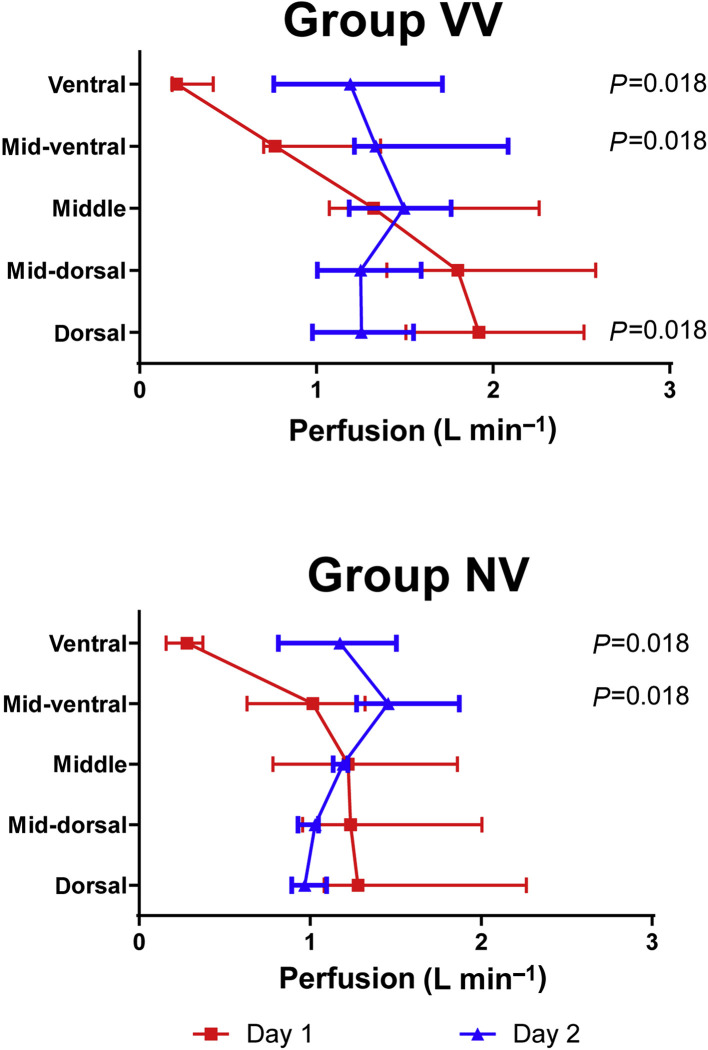
The regional pulmonary perfusion increased in ventral and mid-ventral regions after 24 h in both groups. In the VV group, but not in the NV group, pulmonary perfusion decreased in the dorsal region after 24 h. No significant differences were found between groups VV and NV (Fig. 4 ).
Fig 4.
Specific regional pulmonary perfusion in regions of equal lung mass from ventral to dorsal determined by positron emission tomography/CT and 68Ga-labelled microspheres. Symbols and horizontal lines represent median and inter-quartile ranges. Global statistical significance was accepted at P<0.05, Bonferroni–Holm adjustment for multiple testing. Differences between Day 1 and Day 2 within the same group and same region were tested with Wilcoxon tests (depicted P-values). No differences were found between groups VV and NV (Mann–Whitney U-test). n=7 per group. VV, variable ventilation; NV, nonvariable ventilation; Day 1, before randomisation; Day 2, 24 h after randomisation.
Histologic lung injury score, inflammatory markers, and wet/dry ratio
The cumulative DAD score, the wet/dry ratio, and gene expression of IL-6 and IL-8, VEGF, ICAM-1, and type III procollagen did not differ significantly between groups (Table 2 ).
Table 2.
DAD score, protein levels [pg mg−1], and gene expression of pulmonary markers [relative mRNA expression, fold-change compared with the house-keeping-gen hypoxanthin-phosphoribosyl-transferase-1]. Values are given as median and quartiles. Statistical significance was accepted at P>0.05. Dad score diffuse alveolar damage score; IL-6, interleukin 6; ELISA, enzyme-linked immunosorbent assay, IL-8, interleukin 8; mRNA, messenger ribonucleic acid; VEGF, vascular endothelial growth factor; ICAM-1, intercellular adhesion molecule 1. VV, variable ventilation, NV nonvariable ventilation; mRNA, messenger RNA.
| Group | DAD score | IL-6 ELISA | IL-8 ELISA | IL-6 mRNA | IL-8 mRNA | VEGF mRNA | ICAM-1 mRNA | Type III procollagen mRNA |
|---|---|---|---|---|---|---|---|---|
| VV | 15.1 (12.5–17.1) | 44.2 (38.6–49.3) | 109.3 (99.4–136.3) | 0.6 (0.5–1.1) | 0.8 (0.8–1.2) | 0.9 (0.9–1.2) | 1.0 (0.9–1.2) | 1.2 (1.0–1.3) |
| NV | 10.4 (8.0–23.3) | 47.0 (38.5–60.3) | 165.6 (111.4–272.2) | 0.7 (0.4–1.7) | 1.7 (1.1–4.0) | 1.1 (0.9–1.2) | 1.0 (0.9–1.4) | 1.1 (0.9–1.1) |
| P-value | 0.565 | 0.482 | 0.085 | 0.655 | 0.110 | 0.749 | 0.565 | 0.142 |
Gas exchange and haemodynamics
During the observation period, gas exchange and haemodynamics variables did not differ significantly between groups (Supplementary material).
Discussion
In this experimental model of ARDS in pigs, 24 h of mechanical ventilation according to the ARDS Network protocol with variable compared with nonvariable VT did not attenuate pulmonary inflammation. In addition, gas exchange, respiratory mechanics, lung aeration, pulmonary perfusion, histologic lung injury score, gene expression of inflammatory and endothelial damage markers and the wet/dry ratio did not differ between groups.
To our knowledge, this is the first study to address the effects of 24 h of variable ventilation on the distribution of lung inflammation. Of note, mechanical ventilation settings followed closely the clinical standard recommended by the ARDS Network. We used a double hit model consisting of saline lung lavage and injurious ventilation, as it reproduces several typical features of human ARDS, including alveolar haemorrhage, hyaline membrane formation, neutrophilic infiltration, decreased compliance, and gas-exchange deterioration, and results in a serious lung injury.20 A further strength of our study is that lung inflammation was determined with PET and the radiotracer 18F-FDG, which is an established method to assess lung inflammation in vivo. 15 Our study also quantified the distribution of pulmonary perfusion in vivo using 68Ga-labelled microspheres and PET, which allows quantifying even minimal regional differences21 and assessed the distribution of aeration in lungs with a gold standard technique, namely CT.17 , 18
Previous studies have reported conflicting results with respect to the effects of variable ventilation on lung damage and inflammation. In a porcine oleic acid model22 and a lavage-endotoxin-VILI model of ARDS in rabbits,23 variable ventilation with different PEEP levels did not reduce the level of pro-inflammatory cytokines (IL-8) and cell counts in bronchial alveolar lavage fluid22 and histological lung injury scores.23 In contrast, reduced lung damage has been observed in small animals ventilated with variable ventilation compared with conventional ventilation in hydrochloric acid injured24 and noninjured lungs.5 , 25 In pigs with saline lung lavaged lungs, the histologic damage, but not the level of cytokines, was reduced during variable ventilation compared with nonvariable ventilation when PEEP was ≥12 cm H2O.6 In contrast, variable ventilation worsened epithelial cell damage when combined with higher PEEP levels,24 whereas we opted for the use of the ARDS Network7 low PEEP table, which represents current clinical practice.2 , 26 , 27
The present observation that 24 h of variable ventilation did not improve gas exchange nor respiratory mechanics, differs from recent experimental3 and clinical28 reports. However, in line with the results of our study, variable ventilation compared with conventional ventilation did not improve gas exchange in oleic acid-induce ARDS in pigs29 and dogs.30 Furthermore, variable ventilation did not improve ERS in oleic acid injury in pigs.4 These results might be partly explained by differences in the severity of the lung damage. In fact, in saline lung lavage, variable ventilation resulted in lower mean and peak airway pressures, lower ERS, and higher arterial oxygenation values,31 whereas these beneficial effects could not be seen in the present double hit model of ARDS. Furthermore, PEEP values used in that study31 were higher (12 cm H2O) than those used in clinical practice. These higher PEEP values might have favoured stabilisation of lungs after recruitment with variable ventilation. Also, the observation period was much shorter (6 h)31 than in the present study, and it cannot be ruled out that respiratory mechanics would have deteriorated after that period.
Our finding that variable ventilation did not increase lung aeration compared with nonvariable ventilation differs from similar studies in the field. In a previous study, variable ventilation resulted in a significant recruitment of nonaerated and poorly aerated lung volume and a significant increase of normally aerated lung volume in oleic acid-induced ARDS in pigs.29 Furthermore, redistribution of perfusion towards dependent lung zones was observed after saline lung lavage in pigs treated with variable ventilation,6 suggesting improved lung aeration in dorsal areas. In our study pulmonary perfusion was redistributed from dependent towards nondependent lungs, which is probably attributable to hypoxic pulmonary vasoconstriction. These differences in aeration and perfusion are likely explained by the use of higher PEEP values in other studies.6 , 29 , 32 Another possible explanation is that the time constant for derecruitment could have been lower in our study, leading to increased end-expiratory lung collapse. In fact, in a study with a computer model of lung recruitment33 variable ventilation combined with low PEEP levels was not able to recruit lungs when the time constants of recruitment and derecruitment were the same.
Limitations
Our study has several limitations. First, the double hit model does not fully mimic all features of the human ARDS, even though this model has been considered clinically relevant.20 Second, the metabolic activity as indicated by 18F-FDG uptake was used as a surrogate of VILI, which involves not only metabolism, but also structural damage. Nevertheless, different studies showed that K iS is a reliable marker of lung injury.34 , 35 Third, we set PEEP in both groups according to minimal adequate oxygenation; thus we did not evaluate the effects of variable ventilation in combination with alternative ways of PEEP titration aimed at stabilising lungs. However, in large clinical trials, the low PEEP table of the ARDS network has proved superior to other strategies that aim at an open lung with respect to clinical outcome.2 , 26 , 27 Fourth, we did not determine the pressure vs volume curve of the respiratory system. A previous study suggested that PEEP values according to the low PEEP table most likely centre the ventilation at the lower part of that curve.36 Thus, variable ventilation was likely conducted in a low compliance range, that is, was not optimised for respiratory mechanics. Fifth, we have targeted for a mathematically derived VT variance of 30% during VV. Although we cannot rule out that different levels or distributions of variance would have yielded different results,23 this value corresponds to the value of young healthy volunteers12 and led to most pronounced improvement in lung function in two experimental studies in rats19 and pigs.31 Sixth, the sample size was mainly derived from the expertise of the authors. An increased sample size might have resulted in a statistically significant difference presumably not being clinically relevant.
Conclusion
In this model of ARDS in pigs, 24 h of ventilation according to the ARDS Network protocol with variable VT did not attenuate pulmonary neutrophilic inflammation compared with nonvariable VT. These data reinforce the need to compare emerging ventilatory strategies with established gold-standard therapy.
Authors' contributions
Acquisition of data: JW, MS, AB, RH, TB, MH, AG, LB, IR, MGA, TKi
Data processing: JW, MS, AB, RH, TB, MH, AG, LB, IR, MGA, TKi
Drafting of the manuscript: JW, MS, AB, RH, PRMR, PP, MGA, TKi
All authors contributed to the conception and/or design of this study, analysis and interpretation of data; critically revised the manuscript for important intellectual content; and approved the final version to be submitted.
Acknowledgements
We thank Susanne Henninger Abreu, and the research fellows of the Pulmonary Engineering Group, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany, for their assistance in conducting the experiments. We also thank Gabriele Kotzerke and Kathrin Rosenow, technical radiology assistants, and Liane Oehme, physicist, at the Department of Nuclear Medicine, University Hospital Dresden, Dresden, Germany, for their valuable support.
Handling editor: Gareth Ackland
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.12.040.
Declarations of interest
MGdeA and TK were granted a patent on the variable pressure support ventilation mode of assisted ventilation, which has been licensed to Dräger Medical AG (Lübeck, Germany). All other authors have disclosed that they do not have any conflicts of interest.
Funding
German Research Foundation (Deutsche Forschungsgemeinschaft), Bonn, Germany (grant number GA 1256/6-2). The positron emission tomography/computed tomography device was a gift from the German Federal Ministry of Education and Research (BMBF contract 03ZIK42/OncoRay), Bonn, Germany. National Institutes of Health (NIH; Bethesda, MD, USA) (grant number R01 HL121228 to MFVM).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 2.ARDS-Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Huhle R., Pelosi P., de Abreu M.G. Variable ventilation from bench to bedside. Crit Care. 2016;20:62. doi: 10.1186/s13054-016-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boker A., Graham M.R., Walley K.R. Improved arterial oxygenation with biologically variable or fractal ventilation using low tidal volumes in a porcine model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:456–462. doi: 10.1164/ajrccm.165.4.2108006. [DOI] [PubMed] [Google Scholar]
- 5.Arold S.P., Suki B., Alencar A.M., Lutchen K.R., Ingenito E.P. Variable ventilation induces endogenous surfactant release in normal Guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2003;285:L370–L375. doi: 10.1152/ajplung.00036.2003. [DOI] [PubMed] [Google Scholar]
- 6.Spieth P.M., Carvalho A.R., Pelosi P. Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. Am J Respir Crit Care Med. 2009;179:684–693. doi: 10.1164/rccm.200806-975OC. [DOI] [PubMed] [Google Scholar]
- 7.Brower R.G., Lanken P.N., MacIntyre N. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 8.Kawano T., Mori S., Cybulsky M. Effect of granulocyte depletion in a ventilated surfactant-depleted lung. J Appl Physiol (1985) 1987;62:27–33. doi: 10.1152/jappl.1987.62.1.27. [DOI] [PubMed] [Google Scholar]
- 9.Tsuno K., Miura K., Takeya M., Kolobow T., Morioka T. Histopathologic pulmonary changes from mechanical ventilation at high peak airway pressures. Am Rev Respir Dis. 1991;143:1115–1120. doi: 10.1164/ajrccm/143.5_Pt_1.1115. [DOI] [PubMed] [Google Scholar]
- 10.Musch G. Positron emission tomography: a tool for better understanding of ventilator-induced and acute lung injury. Curr Opin Crit Care. 2011;17:7–12. doi: 10.1097/MCC.0b013e32834272ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiss M., Roos D. Differences in oxygen metabolism of phagocytosing monocytes and neutrophils. J Clin Invest. 1978;61:480–488. doi: 10.1172/JCI108959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobin M.J., Mador M.J., Guenther S.M., Lodato R.F., Sackner M.A. Variability of resting respiratory drive and timing in healthy subjects. J Appl Physiol (1985) 1988;65:309–317. doi: 10.1152/jappl.1988.65.1.309. [DOI] [PubMed] [Google Scholar]
- 13.Gattinoni L., Pesenti A., Avalli L., Rossi F., Bombino M. Pressure–volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis. 1987;136:730–736. doi: 10.1164/ajrccm/136.3.730. [DOI] [PubMed] [Google Scholar]
- 14.Patlak C.S., Blasberg R.G., Fenstermacher J.D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder T., Melo M.F., Venegas J.G. Analysis of 2-[Fluorine-18]-fluoro-2-deoxy-d-glucose uptake kinetics in PET studies of pulmonary inflammation. Acad Radiol. 2011;18:418–423. doi: 10.1016/j.acra.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotzerke J., Andreeff M., Wunderlich G., Wiggermann P., Zophel K. [Ventilation–perfusion-lungs cintigraphy using PET and 68Ga-labeled radiopharmaceuticals] Nuklearmedizin. 2010;49:203–208. doi: 10.3413/nukmed-0348-10-09. [DOI] [PubMed] [Google Scholar]
- 17.Rouby J.J., Puybasset L., Cluzel P., Richecoeur J., Lu Q., Grenier P. Regional distribution of gas and tissue in acute respiratory distress syndrome: II. Physiological correlations and definition of an ARDS Severity Score. CT Scan ARDS Study Group. Intensive Care Med. 2000;26:1046–1056. doi: 10.1007/s001340051317. [DOI] [PubMed] [Google Scholar]
- 18.Reske A.W., Reske A.P., Gast H.A. Extrapolation from ten sections can make CT-based quantification of lung aeration more practicable. Intensive Care Med. 2010;36:1836–1844. doi: 10.1007/s00134-010-2014-2. [DOI] [PubMed] [Google Scholar]
- 19.Kiss T., Silva P.L., Huhle R. Comparison of different degrees of variability in tidal volume to prevent deterioration of respiratory system elastance in experimental acute lung inflammation. Br J Anaesth. 2016;116:708–715. doi: 10.1093/bja/aew093. [DOI] [PubMed] [Google Scholar]
- 20.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oehme L., Zophel K., Golgor E. Quantitative analysis of regional lung ventilation and perfusion PET with (68)Ga-labelled tracers. Nucl Med Commun. 2014;35:501–510. doi: 10.1097/MNM.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 22.Funk D.J., Graham M.R., Girling L.G. A comparison of biologically variable ventilation to recruitment manoeuvres in a porcine model of acute lung injury. Respir Res. 2004;5:22. doi: 10.1186/1465-9921-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fodor G.H., Bayat S., Albu G. Variable ventilation is equally effective as conventional pressure control ventilation for optimizing lung function in a rabbit model of ARDS. Front Physiol. 2019;10:803. doi: 10.3389/fphys.2019.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thammanomai A., Hamakawa H., Bartolak-Suki E., Suki B. Combined effects of ventilation mode and positive end-expiratory pressure on mechanics, gas exchange and the epithelium in mice with acute lung injury. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camilo L.M., Motta-Ribeiro G.C., de Avila M.B. Variable ventilation associated with recruitment maneuver minimizes tissue damage and pulmonary inflammation in anesthetized lung-healthy rats. Anesth Analg. 2018;127:784–791. doi: 10.1213/ANE.0000000000003582. [DOI] [PubMed] [Google Scholar]
- 26.Writing Group for the Alveolar Recruitment for Acute Respiratory. Distress Syndrome Trial I., Cavalcanti A.B., Suzumura E.A. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constantin J.M., Jabaudon M., Lefrant J.Y. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019 doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 28.Candik P., Kolesar A., Nosal M. Use of programmed multilevel vntilation as a superior method for lung recruitment in heart surgery. Int J Crit Care Emerg Med. 2019;5:67. [Google Scholar]
- 29.Graham M.R., Gulati H., Kha L., Girling L.G., Goertzen A., Mutch W.A. Resolution of pulmonary edema with variable mechanical ventilation in a porcine model of acute lung injury. Can J Anaesth. 2011;58:740–750. doi: 10.1007/s12630-011-9517-3. [DOI] [PubMed] [Google Scholar]
- 30.Nam A.J., Brower R.G., Fessler H.E., Simon B.A. Biologic variability in mechanical ventilation rate and tidal volume does not improve oxygenation or lung mechanics in canine oleic acid lung injury. Am J Respir Crit Care Med. 2000;161:1797–1804. [PubMed] [Google Scholar]
- 31.Spieth P.M., Carvalho A.R., Guldner A. Effects of different levels of pressure support variability in experimental lung injury. Anesthesiology. 2009;110:342–350. doi: 10.1097/ALN.0b013e318194d06e. [DOI] [PubMed] [Google Scholar]
- 32.Ruth Graham M., Goertzen A.L., Girling L.G. Quantitative computed tomography in porcine lung injury with variable versus conventional ventilation: recruitment and surfactant replacement. Crit Care Med. 2011;39:1721–1730. doi: 10.1097/CCM.0b013e3182186d09. [DOI] [PubMed] [Google Scholar]
- 33.Ma B., Suki B., Bates J.H. Effects of recruitment/derecruitment dynamics on the efficacy of variable ventilation. J Appl Physiol (1985) 2011;110:1319–1326. doi: 10.1152/japplphysiol.01364.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha D., Takahashi K., de Prost N. Micro-autoradiographic assessment of cell types contributing to 2-deoxy-2-[(18)F]fluoro-d-glucose uptake during ventilator-induced and endotoxemic lung injury. Mol Imaging Biol. 2013;15:19–27. doi: 10.1007/s11307-012-0575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones H.A., Sriskandan S., Peters A.M. Dissociation of neutrophil emigration and metabolic activity in lobar pneumonia and bronchiectasis. Eur Respir J. 1997;10:795–803. [PubMed] [Google Scholar]
- 36.Guldner A., Braune A., Ball L. Comparative effects of volutrauma and atelectrauma on lung inflammation in experimental acute respiratory distress syndrome. Crit Care Med. 2016;44:e854–e865. doi: 10.1097/CCM.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.