Abstract
The catecholamine 3,4-dihydroxyphenethylamine, or dopamine (DA), acts as a neurotransmitter across a broad phylogenetic spectrum. Functions attributed to dopamine in the mammalian brain include regulation of motor circuits, valuation of sensory stimuli, and mediation of reward or reinforcement signals. Considerable evidence also supports a neurotransmitter role for DA in gastropod molluscs and there is growing appreciation for its potential common functions across phylogeny. This article reviews evidence for DA’s transmitter role in the nervous systems of gastropods. The functional properties of identified dopaminergic neurons in well characterized neural circuits suggests a hypothetical incremental sequence by which DA accumulated it’s diverse roles. At each stage of this sequence, it is proposed that existing functions of dopaminergic neurons favored their recruitment to fulfill additional information processing demands. Common functions of DA in other intensively studied groups, ranging from mammals and insects to nematodes, suggests an ancient origin for this progression.
Keywords: central pattern generators, reward, reinforcement, associative conditioning
Introduction
Dopamine (DA) is arguably the most intensively studied neurotransmitter, with a query of PubMed yielding more than 5,000 citations for the year 2019. In the mammalian brain, dopaminergic signaling participates in functions ranging from lactation to higher cognitive operations (Iversen et al., 2010; Di Chiara et al., 2014). However, two archetypal functions of DA, initially reported nearly fifty years ago, have withstood the test of time. Its involvement in motor control originally emerged from clinical observations (Barbeau, 1967, 1974; Hornykiewicz, 1973, 1982), and its pivotal role in reward-related signaling was established in studies on animal learning and memory (Yokel and Wise 1975; Wise and Rompre, 1989; Wise, 2004, 2008; Schultz, 2007, 2010).
Many neurotransmitters, including dopamine, are widely conserved across animal taxa (Kehoe and Marder, 1976; Strausfeld and Hirth, 2013; Fieber, 2017). In his highly influential review of the early literature on invertebrate neurotransmitters, Gerschenfeld (1973) concluded that the evidence for DA as a neurotransmitter in molluscs remained “rather indirect and circumstantial”. Subsequent neuroanatomical, biochemical, pharmacological, and physiological findings resolved this uncertainty, firmly establishing DA as a gastropod neurotransmitter (reviewed by Kerkut, 1973; Walker, 1986). This foundation set the stage for exploring dopaminergic signaling in well characterized neuronal circuits that produce gastropod behavior. This article surveys the diverse contributions of DA signaling to gastropod sensorimotor integration and plasticity, with an emphasis on its functions that are shared by other complex nervous systems (see Barron et al., 2012; Gillette and Brown, 2015). A hypothetical sequence charting the acquisition of functions by dopaminergic neurons suggests that each role favored their recruitment into successive information processing tasks. The presence of common functions of DA across a broad range of taxa implies that this proposed sequence could reflect an ancient prototype that has been implemented to varying degrees in different groups.
Histochemical, Biochemical, and Pharmacological Foundations
The histochemical fluorescence method of Falck and Hillarp (Falck et al., 1962) was initially used to demonstrate the presence of catecholamines in neurons of the cerebral, pedal, and visceral ganglia of Helix pomatia (Dahl et al., 1962, 1966). The green fluorescence indicative of dopamine was also observed in the central nervous systems of Tritonia diomedia (Sakharov et al., 1965, Sakharov and Zs.-Nagy, 1968), Planorbis corneus (Marsden and Kerkut, 1970), Strophocheilus oblongus (Jaeger et al., 1971), Limax maximus (Osborne and Cottrell, 1971), Biomphalaria glabrata (Chiang et al. 1974), and Hermissenda crassicornis (Croll, 1987). These observations were corroborated by fluorometric and chromatographic measurements (Cardot, 1963; Sweeney, 1963; Kerkut et al., 1966, 1967; Osborne and Cottrell, 1970; Juorio and Killick, 1972; Straub and Kuhlman, 1984; Heatherington et al., 1994).
Early pharmacological studies demonstrated excitatory and inhibitory responses to bath application dopamine in neurons of Cornu (Helix) aspersum (Kerkut and Walker, 1961, 1962; Walker et al., 1968) and inhibitory effects in the Argentine land snail Crytomphallus aspersa (Gerschenfeld, 1964). More precise application of drugs using microiontophoresis enabled Ascher (1968, 1972) to show that some neurons of Aplysia possess both excitatory and inhibitory DA receptors. Swann and Carpenter (1975) observed common properties between responses to dopamine and acetylcholine on Aplysia neurons, and proposed that the receptors for both transmitters were associated with common Na+, Cl−, and K+ ionophores (see also Yavari et al., 1979). Several studies tested the efficacy of agonists and antagonists on gastropod DA receptors, with particular attention to their possible utility for screening potential therapeutic compounds, but the pharmacological profiles of the gastropod dopamine receptors were found to differ from their vertebrate counterparts (reviewed by Gospe, 1983; Walker, 1986).
Some actions of DA in gastropod nervous systems are long-lasting and reflect activation of second messenger signaling cascades. Cedar and Schwartz (1972) showed that dopamine and serotonin, but not carbachol, glutamate, or norepinephrine increased levels of 3', 5'-cyclic adenosine monophosphate (cAMP) in Aplysia nervous tissue. In specific neurons of Cornu (Helix) aspersum, DA and serotonin both produced a decreased K+ conductance and had additive stimulatory effects on adenylate cyclase activity, indicating that their signaling converged at the second messenger level (Deterre et al. 1982). Additional second messenger mediated actions of DA were demonstrated on K+ and Ca++ currents that contribute to somatic action potentials in identified Cornu (Helix) aspersum neurons (Paupardin-Tritsch et al., 1985).
Dopamine was also found to regulate slow voltage-dependent currents in Aplysia neurons. In the endogenous bursting cell R15, dopamine eliminated bursting by reducing the regenerative inward current underlying slow oscillations of the membrane potential (Wilson and Wachtel, 1978). In RB neurons of the Aplysia abdominal ganglion, dopamine augmented a voltage-dependent calcium current that was activated at holding potentials more positive than −40 mV and maximal near 0 mV (Pellmar, 1981a). This current was also elicited by serotonin, octopamine, and by injection of cAMP, suggesting convergence at the level of the second messenger (Pellmar and Carpenter, 1980, 1981; but see Pellmar, 1981b). Enhancement of the voltage-dependent calcium current by neurotransmitters was proposed as a mechanism by which heterosynaptic modulation could alter action potentials or slow waves that underlie bursting (Pellmar and Carpenter, 1980).
A role for DA at gastropod central synapses was supported by studies on a prolonged inhibitory postsynaptic potential (inhibition of long duration) produced in specific neurons in response to nerve stimulation. Dopamine produced hyperpolarizing responses in neurons that exhibited inhibition of long duration and ergonovine, a DA antagonist, blocked both the synaptic and DA responses (Kerkut et al., 1969; Walker et al., 1971; Boisson and Gola, 1976). Thus, together with the histological and pharmacological findings reviewed above, there was abundant support for the role of DA as a neurotransmitter in the gastropod CNS prior to the identification dopaminergic neurons.
Dopaminergic Regulation of Peripheral Systems
The involvement of dopamine in peripheral signaling was initially examined in the gill of Aplysia californica, due to its participation in simple forms of learning (Peretz and Estes, 1974). High levels of DA were measured in the gill and paraformaldehyde-induced green fluorescence was observed in neurons along the peripheral nerve trunks (Carpenter et al., 1971; Peretz and Estes, 1974). The observation that DA enhanced contractions produced by non-dopaminergic gill motor neurons appears to be the first instance in which its actions were described as modulatory (Swann et al., 1978). Perfusion of the gill with micromolar concentrations of DA also decreased the habituation of responses to repeated motor neuron stimuli (Ruben and Lukowiak, 1979, 1983).
Tritt and Byrne (1982) examined the potential role of dopamine in control of the opaline gland of Aplysia californica. Infusion of DA through a branch of the anterior aorta produced opaline gland contractions that were blocked by fluphenazine. Dopamine also increased the amplitude of excitatory junction potentials and contractions produced by stimulation of three motor neurons in the pleural ganglion. DA was proposed to act as both a neurotransmitter and as a neuromodulator in the Aplysia opaline gland (Tritt and Byrne, 1982). The source of DA innervation of the opaline gland remained unspecified, however, as the glyoxylic acid technique did not produce fluorescence in the identified central motor neurons (Tritt et al., 1983).
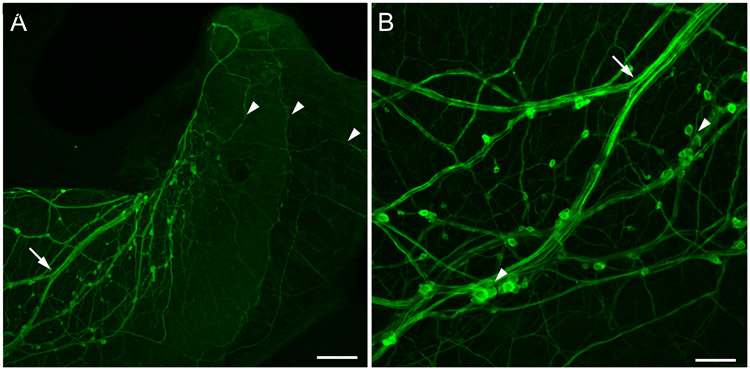
Finally, histochemical fluorescence and microspectrofluorometric observations led to a proposed role for dopamine in regulating the female reproductive system of gastropods (Hartwig et al., 1980). Using formaldehyde histofluorescence, a plexus of intraepithelial cells was detected in the duct of the albumen gland of the pond snail Bulinus truncatus and the carrefour of the land snail Archachatina marginata. Microspectrofluorometry confirmed the presence of dopamine in these tissues (Hartwig et al., 1980). In Helisoma duryii, tyrosine hydroxylase-like immunoreactive (THli) axons were shown to form a nerve tract in the central region of the albumen gland and a uniform network of fibers spreading over the entire gland (Kiehn et al., 2001). Recently, we detected a network of THli neurons in the albumen gland of Bursatella leachii (ragged sea hare, Fig. 1), a marine gastropod (Ramos et al., 1995; Miller, 1997). This observation broadens the potential involvement of DA in female reproductive function to the Euopisthobranchia.
Figure 1.
TH-like immunoreactivity in the albumen gland of the opisthobranch Bursatella leachii. (A) Low power image showing bundles of THli fibers (arrow) entering the albumen gland. Small immunoreactive somata were observed adhering to the fiber tracts. Fine axons (arrowheads) covered the entire surface of the gland. Calibration bar = 200 μm. (B) At higher magnification, some of the small (5 – 10 μm diameter) THli cell bodies were solitary, and others were clustered into small groups (arrowheads). Branch point of the fiber bundle (arrow) corresponds to arrow in panel A. Calibration bar = 50 μm. (Unpublished data; G. Rosado-Mattei and M.W. Miller, Institute of Neurobiology, University of Puerto Rico).
Application of dopamine to the albumen gland of Helisoma duryii and Biomphalaria glabrata stimulated glycoprotein secretion (Saleuddin et al., 2000; Santhanagopalan and Yoshino, 2000; Mukai et al., 2004). Moreover, DA levels were increased in the albumen glands of B. glabrata during the initial stage of egg production, when perivitelline fluid is being secreted around each ovum (Boyle and Yoshino, 2002). The intrinsic dopaminergic network was proposed to coordinate fluxes between the albumen gland and the carrefour, where fertilized eggs are coated (Boyle and Yoshino, 2002; see also Brisson, 1983; de Jong-Brink and Goldschmeding, 1983). Collectively, the functions of DA on the gill, opaline gland, and albumen gland established its role in the regulation of gastropod peripheral systems.
Dopamine as a Sensory Neurotransmitter
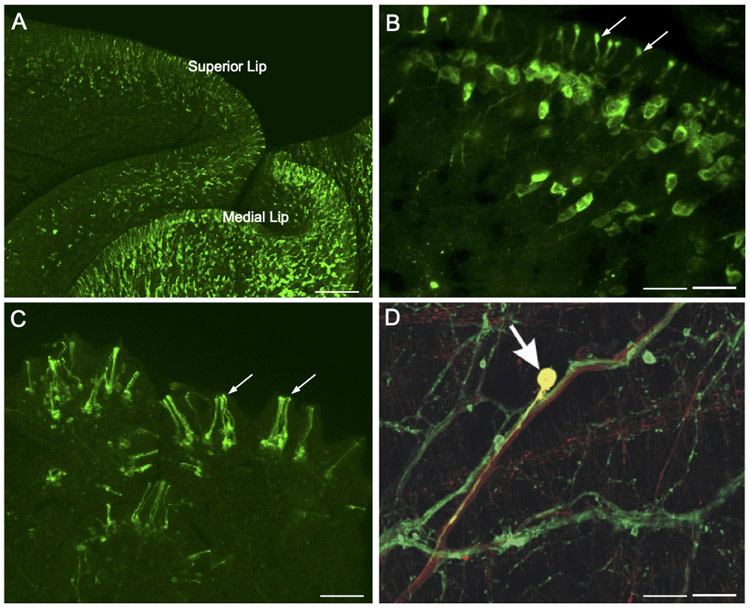
In gastropods that have been studied, the number of peripheral dopaminergic neurons far exceeds the total present in the CNS (Croll, 2001, 2003). Peripheral catecholaminergic innervation of the integument was initially reported in Biomphalaria glabrata (Chiang et al., 1974), Aplysia depilans and Aplysia fasciata (Salimova et al., 1987b), and Cornu (Helix) aspersum (Hernádi and Elekes, 1999). More refined techniques enabled Croll (2001, 2003) to detect thousands of tyrosine hydroxylase-like immunoreactive neurons with subepithelial somata and processes that penetrate the overlying body wall in Aplysia californica. Axons from these cells project centrally via the major nerves and terminate diffusely in all ganglia, supporting their role in transmission of sensory information. Faller et al. (2008) examined THli subepidermal sensory neurons in the cephalic sensory organs of several opisthobranchs and advanced a role for these cells in contact chemoreception or mechanoreception. Similar cells are abundant in the cephalic sensory organs of the pulmonates Lymnaea stagnalis and Biomphalaria glabrata (Wyeth and Croll, 2011; Vallejo et al., 2014; Fig. 2A, B).
Figure 2.
Dopamine as a sensory neurotransmitter in gastropods. (A) Low magnification image shows abundant TH-like immunoreactive innervation of the lips in Biomphalaria glabrata. Scale bar = 100 μm. (B) Higher magnification of THli cells in the mantle integument of Biomphalaria alexandrina. A single process from each cell projects through the epithelium, terminating as a bulbous enlargement at the surface (arrows). Scale bar = 20 μm. A, B: Reprinted with permission from Vallejo et al., J. Comp. Neurol. 522: 2532-2552, 2014. (C) THli cells in the oral veil of Pleurobranchaea californica. Groups of cilia-like terminations (arrows) penetrate the epithelial surface. Scale bar = 20 μm. Reprinted with permission from Brown et al., PLoS ONE 13: e0208891, 2018. (D) Esophageal nerve tracing (biocytin, red) and THli (green) on the surface of the pharynx of Aplysia californica. One double-labeled cell (yellow, arrow) in this merged image is a THli neuron that projects toward the CNS via the esophageal nerve. Scale bar = 50 μm. Reprinted with permission from Martínez-Rubio et al., J. Comp. Neurol. 514: 329-342, 2009.
The potential sensory function of peripheral dopaminergic neurons was recently examined in the nudipleuran Pleurobranchaea californica (Brown et al., 2018). Ciliated THli cells were highly concentrated in the oral veil and tentacle, sensory structures known to be important for food-seeking behavior (Fig. 2C). In freely behaving specimens, application of the D2 antagonist sulpiride to the oral veil-tentacle complex produced a delay to initiate feeding responses. Sulpiride also reduced spiking responses to tactile stimuli in the tentacle nerve. Together, these observations support a role of dopamine in the peripheral processing of food-related stimuli (Brown et al., 2018).
Dopamine is also proposed to function as a proprioceptive signal in gastropod feeding systems. Early histological studies reported intense staining of catecholaminergic fibers in the esophageal nerve (Fekete et al., 1984; Salimova et al., 1987a; Rathousz and Kirk, 1988; Goldstein and Schwartz, 1989; Kabotyanski et al., 1998). Double-labeling (biocytin nerve tracing x THli) revealed that some THli fibers in the esophageal nerve originate from DA neurons on the pharynx (Martínez-Rubio et al., 2009; Fig. 2D). These histological observations prompted studies exploring the role of DA in mediating reinforcement in associative conditioning (see Dopaminergic Reinforcement in Associative Learning).
Giant Dopaminergic Neurons in Aquatic Pulmonates
Although most of the catecholaminergic neurons in gastropods are relatively small (10-40 μm soma diameter), a large (>200 μm) dopaminergic neuron (GDC: giant dopaminergic cell) was detected by Marsden and Kerkut (1970) in the left pedal ganglion of Planorbis corneus (see also Pentreath and Berry, 1975). Electrophysiological experiments demonstrated direct inhibitory and excitatory synaptic potentials from the GDC in neurons of the visceral and left parietal ganglion (Berry and Cottrell, 1973, 1975). These studies supported the role of dopamine as a multi-action neurotransmitter, capable of producing both excitatory and inhibitory actions on distinct synaptic targets upon release from a single neuron (Cottrell, 1967, 1977).
The giant dopaminergic cell of Planorbis corneus was used to monitor dopamine storage and dynamics using capillary electrophoresis with electrochemical detection and microelectrochemistry (reviewed by Anderson and Ewing, 1999). A low concentration of DA (2.2 ± 0.52 μM) was measured in the cytoplasm (Olefirowicz and Ewing, 1990). Amperometric measurements with a carbon fiber electrode detected current transients near the GDC soma in response to a high potassium stimulation pulse (Chen et al., 1995). Histograms constructed from the amplitudes of these current transients exhibited two peaks, which were proposed to represent somatic release of two distinct populations of DA-containing vesicles (Chen et al., 1995).
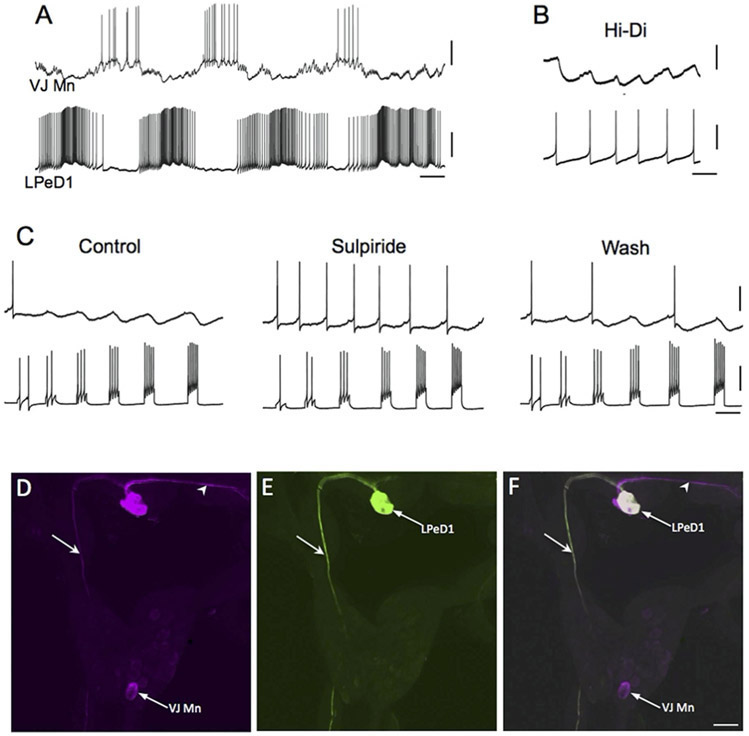
A giant dopamine cell is present in the left pedal ganglion of sinistral aquatic snails that possess a pneumostome breathing apparatus, including the genera Helisoma (H. trivolvis: McCaman et al., 1979: Harris and Cottrell, 1995; H. duryi: Kiehn et al., 2001) and Biomphalaria (B. glabrata, B. alexandrina: Vallejo et al., 2014; Fig. 3). In the dextral pulmonate Lymnaea stagnalis, a corresponding giant dopaminergic cell, designated RPeD1, was identified in the right pedal ganglion (McCaman et al., 1979; Cottrell et al., 1979; Winlow et al., 1981; Haydon et al., 1981; Elekes et al., 1991). Syed and Winlow (1991a,b) showed that RPedD1 participates in the central pattern generator circuit controlling respiration. In the isolated central nervous system, direct intracellular stimulation RPeD1 initiated coordinated rhythmic activity in the respiratory CPG (Syed et al., 1990). In an in vitro reconstruction of the respiratory CPG, firing the RPeD1 neuron or pulsed application of dopamine were both capable of activating repetitive cycling (Syed et al., 1990; Fig. 4A, B). These findings showed that dopaminergic signaling is sufficient for initiating or activating motor programs in the Lymnaea respiratory system.
Figure 3.
The giant dopaminergic pedal neuron of Biomphalaria glabrata. (A) Intracellular recording from LPeD1 (lower trace) of B. glabrata; isolated CNS in normal saline solution. The recording exhibits alternating phases of activity and quiescence. A putative cardio-respiratory motor neuron in the visceral ganglion (VJ Mn, upper trace) was observed to burst out of phase with LPeD1. Calibration bars: 5 s, 20 mV. (B) Changing the bathing medium to a raised divalent saline solution (Hi-Di) eliminated polysynaptic signaling, enabling resolution of direct one-to-one inhibitory postsynaptic potential (IPSPs, upper record). Each IPSP occurred with a brief and constant latency following each LPeD1 impulse (lower record). Calibration bars = 1 s; 5 mV, upper record; 20 mV, lower record. (C) Pharmacological evidence for direct dopaminergic signaling from LPeD1 to VJ Mn. Control: Depolarizing current pulses were passed into LPeD1 producing sequentially greater number of impulses (lower record). The IPSPs produced in the VJ Mn became progressively larger as more impulses were stimulated. When the dopaminergic (D2) antagonist sulpiride was added to the solution (100 uM), the IPSPs were blocked. They returned following approximately 15 min Wash (right panel). Calibration bars = 0.5 s, 20 mV, 20 mV. (D-F) Morphological confirmation of LPeD1 and VJ Mn identity. (D) Neurobiotin was injected into LPeD1, a second neighboring cell in the pedal ganglion, and the VJ Mn. The three injected neurons were visualized with Avidin 546 (false color magenta). The axon of LPeD1 can be seen descending through the left pleural ganglion (arrow), while the neighboring cell projects to the contralateral pedal ganglion (arrowhead). (E) When the preparation was processed for TH-like immunoreactivity and viewed with avidin 488 (green), only the LPeD1 neuron and its descending axon (arrow) were labeled. (F) When the fill (D) and immunohistochemical (E) panels were overlaid, only the LPeD1 neuron and its descending axon (arrow) appear white (colocalization). The other injected cells, VJ Mn and the neighboring pedal neuron (arrowhead), appear magenta. Calibration bar = 50 μm, applies to D-F. Reprinted from Vallejo et al., 2014, J. Comp. Neurol. 522: 2532-2552, with permission from John Wiley & Sons, Inc.
Figure 4.
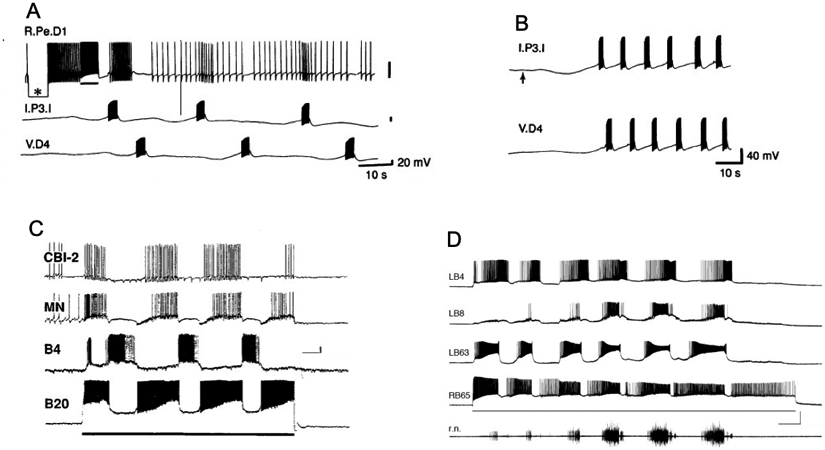
Dopamine activates central pattern generator circuits of gastropods. (A) The dopaminergic RPeD1 interneuron of Lymnaea stagnalis was co-cultured with interneurons Ip3I and VD4 for 24 h, allowing the three cells to extend neurites and form synapses. Passing hyperpolarizing current into RPeD1 (asterisk) established that there was no electrical coupling between the cells. Passing depolarizing current that caused RPeD1 to fire (bar below upper recording) initiated repetitive expression of the respiratory CPG rhythm. Reprinted with permission from Syed et al., Science 1990; 250: 282-285. (B) Two non-dopaminergic interneurons that belong to the respiratory CPG, Ip3I and VD4, were co-cultured for four days, allowing synapses to form between them. Pulsed application of DA (1 s pulses at 0.3 Hz, beginning at upward arrow) from a fire-polished micropipette produced alternating bursts of impulses in the two interneurons. Reprinted with permission from Syed et al., Science 250: 282-285. (C) Stimulation of dopaminergic neuron B20 produces rhythmic motor patterns in the buccal ganglion of Aplysia californica. Depolarizing current (line below B20 recording) was passed from an intracellular microelectrode. The buccal motor program was monitored in the buccal interneuron B4, an unidentified motor neuron (MN) in the ventral cluster of the buccal ganglion, and a cerebral-buccal interneuron (CBI-2). Calibration bars = 10 s, 10 mV. Reprinted with permission from Teyke et al., Brain Res. 1993; 630: 226-237. (D) Stimulation of dopaminergic neuron B65 produces rhythmic motor patterns in the buccal ganglion of Aplysia californica. Depolarizing current (3.3 nA, line below B65 recording). B65 fired in phase with the protraction phase neuron LB63, and out of phase with the retraction phase interneuron LB4. Activity in the radula closer motor neuron LB8 and on the radula nerve (r.n.) increased in successive cycles of the motor program. Calibration bars = 10 s, 40 mV, 100 μV). Reprinted with permission from Kabotyanski et al., J. Neurophysiol. 1998; 79: 605–621.
Direct synaptic signals from the RPeD1 neuron of Lymnaea to its followers provided an opportunity to characterize the central dopaminergic synapses of gastropods. Magoski et al. (1995) showed that exogenous application of DA to identified follower neurons mimicked both excitatory and inhibitory synaptic actions of RPeD1. The D2 antagonist sulpiride blocked both inhibitory synaptic potentials evoked by RPeD1 and the inhibitory effects of exogenous DA (IC50 = 47 μM; see Fig. 3C). Excitatory postsynaptic potentials were blocked by picrotoxin (Magoski et al., 1998). Injection of postsynaptic neurons with GDP-β-S or incubation with pertussis toxin antagonized synaptic transmission, suggesting involvement of G-proteins in rapid (100 – 200 ms) dopaminergic signaling (Magoski et al., 1995).
Feeding Motor Systems of Pulmonates
Involvement of dopamine in the regulation of gastropod feeding was initially demonstrated in the terrestrial slug Limax maximus (Wieland and Gelperin, 1983). High levels of DA were measured in the buccal and cerebral ganglion using high performance liquid chromatography with electrochemical detection (buccal: 19.4 ± 2.4 pmol; cerebral: 124 ± 33 pmol). No norepinephrine, epinephrine, or other metabolites of dopamine were detected. Application of exogenous dopamine (> 1 x 10−6 M) to the isolated cerebral-buccal system produced coordinated feeding motor programs that resembled programs elicited by stimulation of the chemosensory lips with apple juice. Moreover, feeding motor programs produced by lip stimulation were eliminated in the presence of the DA antagonist ergonovine.
In Helisoma trivolvis, high levels of 3H-dopamine were synthesized from 3H-tyrosine in the pedal, cerebral, and buccal ganglia (Trimble et al., 1984). The Falck-Hillarp and glyoxylic acid protocols both labeled small (15 – 35 μm) cell bodies in the buccal ganglion and bath application of dopamine (10−8 to 10−4 M) to the isolated buccal ganglion initiated the patterned motor output characteristic of feeding (Trimble and Barker, 1984). In preparations that were producing spontaneous bouts of patterned motor output, addition of dopamine increased the impulses per burst and burst duration of buccal motor neurons.
Stimulation of one labeled neuron in each buccal hemiganglion of Helisoma also initiated feeding motor patterns (Quinlan and Murphy, 1997). The motor programs produced by this cell, designated N1a, and those elicited by application of dopamine, were both blocked by sulpiride. As N1a was excited by feeding stimulants applied to the buccal cavity, and programs could be attenuated when it was hyperpolarized, dopaminergic signaling from N1a was proposed to serve as a key determinant in the initiation of feeding behavior (Quinlan and Murphy, 1997).
In Lymnaea stagnalis, superfusion of dopamine to the isolated CNS also activated feeding motor programs (Kyriakides and McCrohan, 1989). In spontaneously active preparations, DA increased the frequency of motor programs. Dopamine also increased the firing rate of the cerebral giant cell, a higher-order serotonergic neuron that projects to the buccal ganglion (Kyriakides and McCrohan, 1989).
Collectively, studies on feeding networks of the pulmonates Limax, Helisoma, and Lymnaea demonstrated that dopamine can activate coordinated motor programs upon global application to complex CPG circuits. These actions tend to occur at concentrations (micromolar) that are lower than those reached within the synaptic cleft (millimolar) and last as long as DA is present, i.e. they do not desensitize (see Matsumoto et al., 1987). In common with the respiratory central pattern generator circuit of pulmonates described above, individual dopaminergic neurons that are intrinsic to the feeding circuits can activate coordinated motor programs.
Multi-level Dopaminergic Control of a Multifunctional CPG
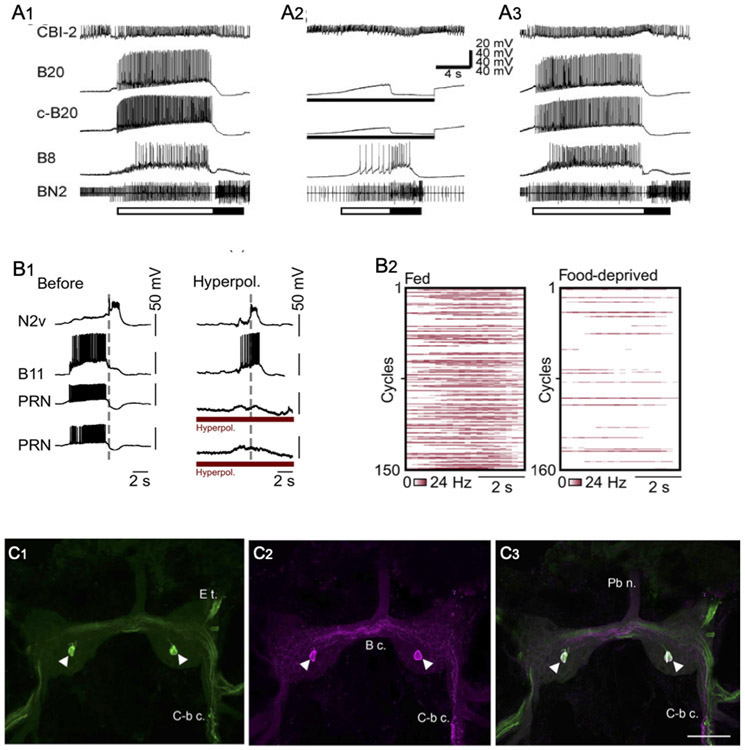
Aplysia feeding is a complex motivated behavior that can be elicited and modified via diverse sensory pathways (Kupfermann, 1974; Cropper et al. 2016). The central pattern generator network that controls Aplysia feeding exhibits multifunctionality, producing distinct behaviors via the activation or suppression of specific interneurons (Kupfermann and Weiss, 2001; Jing and Weiss, 2001). The presence of identified DA interneurons within the Aplysia feeding CPG provides opportunities to examine the contribution of dopaminergic synaptic signaling to the generation and selection of behavior by a multifunctional neural network (Fig. 4C, D; 5A). Modifications of the paraformaldehyde and glutaradehyde technique revealed five catechol aminergic neurons in the buccal ganglion of Aplysia californica (Rathouz and Kirk, 1988; Goldstein and Schwartz, 1989; Croll, 2001). Teyke et al. (1993) observed staining in a pair of small (20 – 30 μm) bipolar buccal interneurons, designated B20, that could evoke coordinated buccal motor programs when stimulated (Fig. 4C). The B20 neurons were activated during programs produced by sensory input (pharyngeal stretch) and by higher cerebral-buccal order interneurons. They were proposed to act as intrinsic modulators of the buccal feeding CPG circuit (Teyke et al., 1993).
Figure 5.
Motor program selection and stimulus valuation by identified gastropod interneurons. (A) Preventing firing of dopaminergic interneuron B20 converts egestive buccal motor patterns to ingestive programs in Aplysia californica. Programs were elicited by tonic firing of cerebral-buccal interneuron 2 (CBI-2, top record). Bar below bottom record shows the protraction (open bar) and retraction (filled bar) phases of the motor programs, as determined from the extracellular recording of buccal nerve 2 (BN2). When no current was passed into the two B20 neurons (A1 and A3), they fired intensively during the protraction phase. The B8 radula closer motor neuron was also highly active during the protraction phase, indicative of egestive motor programs. When both B20 neurons were prevented from firing by passing intracellular current (A2), the strongest B8 firing occurred during the retraction phase, characteristic of ingestive motor programs. Reprinted with permission from Jing and Weiss, J. Neurosci. 2001; 21: 7349-7362. (B) The PRNs of Lymnaea stagnalis specify egestive motor programs and are active according to hunger state. (B1) Preventing the PRNs of Lymnaea from participating in feeding motor programs converts them from their egestive to their ingestive form. In a preparation dissected from a well-fed specimen (left panel), a tactile stimulus to the esophagus produced an egestive motor pattern, in which the motor neuron B11 fired during the protraction phase (left of the vertical dashed line; out of phase with the retraction interneuron N2V). When the two PRN neurons were hyperpolarized by intracellular current injection (right panel, red bars below PRN recordings), the esophageal stimulus produced an ingestive motor program, evidenced by predominant B11 firing during the retraction phase (right of vertical dashed line; in phase with retraction interneuron N2v). (B2) Heat plots of PRN firing rates during in vitro feeding cycles from fed (n = 150 cycles, 15 preparations), versus food-deprived (160 cycles, 16 preparations). Reprinted with permission from Crossley et al., Sci. Adv. 2018; 4: eaau9180. (C) Colocalization of TH-like immunoreactivity and GABA-like reactivity in presumptive PRN of Lymnaea stagnalis. (C1) As reported by Crossley et al., 2018, THli was detected in a single cell on the ventral surface of the buccal ganglion of Lymnaea. (C2) GABA-like immunoreactivity was present in the same cell (magenta pseudo-color). (C3) Merge of panels C1 and C2 confirms colocalization of THli and GABAli (cell appears white). Calibration bar = 100 μm applies to all C panels. Reprinted with permission from Vaasjo et al., 2018; J. Comp. Neurol. 526: 1790–1805.
In addition to evoking coordinated buccal motor programs, B20 can specify the type of program elicited by higher order interneurons (Jing and Weiss, 2001; Fig. 4A) or by peripheral nerve stimulation (Proekt et al., 2004, 2007). B20 fires during the protraction phase of motor programs, providing strong excitatory signals to motor neurons that cause radula closure and hence egestive motor patterns (Fig. 5A, left panel). When the two B20 neurons were hyperpolarized and prevented from firing via intracellular current injection, motor programs exhibited their ingestive configuration (Fig. 5A; Jing and Weiss, 2001). Rapid dopaminergic signaling was shown to be responsible for such motor program specification, as EPSPs from B20 to decisive buccal motor neurons were occluded by exogenous dopamine and blocked by sulpiride (Díaz-Ríos and Miller, 2002).
Recently, a bilateral pair of dopaminergic cells, termed pattern reversing neurons (PRNs) was identified in the buccal ganglion of Lymnaea stagnalis (Crossley et al., 2018). Firing an individual PRN produced egestive motor patterns, i.e. programs that would cause the snail to reject a potential food stimulus. The PRN was highly active during spontaneous egestive motor programs recorded from nervous systems dissected from satiated specimens (Fig. 5B2, left panel). PRN activity was low in ingestive feeding cycles produced by ganglia dissected from food-deprived specimens (Fig. 5B2, right panel). As shown for B20 in Aplysia, when the two PRNs were prevented from firing, egestive programs were converted to their ingestive form (Fig. 5B1). These observations led to the proposal that dopaminergic signaling defines the perceived value of a stimulus and biases the feeding motor circuit to reflect the motivational state of the snail (Crossley et al. 2018). In view of their morphology, neurotransmitter phenotype, and ability to bias the buccal network toward its egestive mode, the PRNs of Lymnaea (Crossley et al., 2018) are likely to be homologous to the B20 neurons of Aplysia californica (see also Murphy, 2001; Vaasjo et al., 2018).
A second pair of dopaminergic interneurons, termed B65, was localized to the lateral region of the caudal surface of the Aplysia buccal ganglion (Kabotyanski et al., 1998). In contrast to B20, but like neuron N1a of Helisoma (see Murphy, 2001), B65 is a monopolar neuron that does not project to the cerebral ganglion. Each B65 produces excitatory and inhibitory PSPs in both ipsilateral and contralateral buccal motor neurons and can initiate repeated bouts of buccal motor programs (Fig. 4D). Dopaminergic signaling by B65 was proposed to contribute to specification of motor patterns by the polymorphic buccal CPG (Kabotyanski et al., 1998). This hypothesis was supported by experiments in which both B65 neurons were prevented from firing during BMPs produced by stimulation of the esophageal nerve (Due et al., 2004; see also Dacks and Weiss, 2013). Hyperpolarizing both B65 neurons, or application of the DA antagonist sulpiride, resulted in motor programs that were biased toward their ingestive conformation.
Thus, while firing dopaminergic interneurons B20 and B65 in Aplysia, or PRM in Lymnaea, can elicit coordinated buccal programs, their activity is not required for program expression. When motor patterns are generated via other pathways, dopaminergic signaling can qualitatively specify the output from the multifunctional feeding central pattern generator network. Interestingly, the excitatory B65-B20-motor neuron pathway constitutes a disynaptic dopaminergic feedforward network motif that promotes a specific response, i.e. egestive movements (Jing and Weiss, 2001; Díaz-Ríos and Miller, 2004, 2006; Proekt et al., 2007).
While the specification of egestive motor programs by interneurons B20 and B65 is largely achieved via rapid dopaminergic synaptic signals, DA can also exert modulatory actions that modify the intrinsic properties of motor neurons in the feeding system of Aplysia. Application of DA or firing B65 converts sporadic and asynchronous burst activity into rhythmic and synchronized bursting in the bilaterally paired pharyngeal B67 motor neurons (Serrano and Miller, 2006). The increased synchrony was proposed to reflect dopaminergic enhancement of an intrinsic burst-forming ‘driver potential’ and increased electrical coupling between the B67 pair (Serrano and Miller, 2006; see also Martínez-Rubio et al., 2010). As the capacity for a neuromodulator to elicit burst activity from otherwise quiescent neurons was previously designated “conditional bursting” (Marder and Eisen, 1984; Harris-Warrick and Marder, 1991), the rhythmicity and synchrony produced by dopamine in the paired B67 cells were referred to as “conditional rhythmicity” and “conditional synchrony”, respectively (Serrano and Miller, 2006; Nargeot et al., 2009).
Finally, the feeding motor circuits of Aplysia are also influenced by dopaminergic signaling originating from extrinsic sources, including higher order neurons projecting from the cerebral ganglion (Rosen et al., 1991). A bilateral pair of cerebral-buccal interneurons, termed CBI-1, exhibits catecholamine histofluroescence when treated with the paraformaldehyde-glutaraldehyde technique. Firing this higher order neuron elicits a single cycle of buccal motor program activity, that incorporates dopaminergic buccal interneuron B20 (Rosen et al., 1991). In semi-intact preparations of Aplysia kurodai, a pair of dopaminergic cerebral neurons (CBm1) that are thought to be homologous to CBI-1 of A. californica biases the feeding motor circuitry toward rejection of nonpreferred foods (Narusuye and Nagahama, 2002). As noted previously for B65-B20-motor neuron signaling that is intrinsic to the buccal CPG, the extrinsic CBI-1 - B20 - motor neuron pathway also utilizes a feedforward dopaminergic configuration that promotes rejection of unwanted material.
Dopamine Colocalization with GABA
TH-like immunoreactivity and CA histofluorescence was reported to be colocalized with GABA-like immunoreactivity in five neurons in the buccal ganglion of Aplysia californica (Díaz-Ríos et al., 2002). DA-GABA colocalization was not detected elsewhere in the central or peripheral nervous systems. Physiological experiments showed that four of the DA-GABA cells correspond to the paired B20 and paired B65 neurons (see previous text). The fifth DA-GABA neuron is an unpaired cell on the caudal surface of the left buccal ganglion that has not yet been identified. Rapid synaptic signaling from both B20 and B65 to identified follower motor neurons was occluded by DA and blocked by sulpiride, supporting their dopaminergic phenotype (Due et al., 2004; Díaz-Ríos and Miller, 2005).
GABA antagonists did not block signals from B20 or B65, but GABA and the GABAB agonist baclofen did increase the amplitude of their dopaminergic EPSPs (Díaz-Ríos and Miller, 2005). When B20 was fired repetitively to mimic its activity during motor programs, GABA and baclofen also enhanced two forms of synaptic plasticity, facilitation and summation (Díaz-Ríos and Miller, 2006). In view of the DA-GABA colocalization in B20, this modulation was designated “homosynaptic modulatory metaplasticity” (Díaz-Ríos and Miller, 2006). GABAergic modulation of dopaminergic signaling from B20 was shown to be mediated via postsynaptic activation of G protein-dependent protein kinase C (PKC; Svensson et al., 2014).
Recently, colocalization of TH-like and GABA-like immunoreactivity was observed in five neurons in the buccal ganglia of the pulmonates Biomphalaria glabrata, Helisoma trivolvis, and Lymnaea stagnalis (Fig. 5C; Vaasjo et al., 2018). These observations support the hypothesis that the central pattern generator for gastropod feeding exhibits a common plan across the panpulmonate and euopisthobranch clades (Murphy, 2001; Wentzell et al., 2009). They also support the proposal that the pattern reversing neuron of Lymnaea corresponds to B20 in Aplysia californica (see previous text).
Dopaminergic Reinforcement in Associative Learning
Accumulating evidence supports a role for dopamine in both classical and operant conditioning of the Aplysia californica feeding system (see Baxter and Byrne, 2006; Mozzachiodi et al., 2013; Nargeot and Simmers, 2010, 2012; Nargeot and Bédécarrats, 2017). Pavlovian appetitive conditioning was demonstrated in this system by pairing a neutral tactile stimulus (conditioned stimulus: CS) with food presentation (unconditioned stimulus: US; Colwill et al., 1997; Lechner et al., 2000). Appetitive operant conditioning was shown by stimulating a branch of the esophageal nerve (En.2, see following text; Brembs et al., 2002) or presenting a food reward (Nargeot et al., 2007) contingent upon expression of consummatory biting behavior. Neurophysiological sequelae of both forms of conditioning could be detected in nervous systems dissected from trained specimens (Lechner et al., 2000b; Brembs et al., 2002). Moreover, in vitro analogs for both paradigms exhibit modifications of fictive feeding motor patterns that recapitulate in vivo conditioning (classical conditioning: Mozzachiodi et al., 2003; Reyes et al., 2005; operant conditioning: Nargeot et al., 1997; 1999a).
The anterior branch of the esophageal nerve (En.2) was identified as a pathway that was necessary and sufficient for transmission of the US in the Pavlovian paradigm and reinforcement in operant conditioning (Nargeot et al., 1997; Lechner et al., 2000a; Brembs et al., 2002; Mozzachiodi et al., 2003). As described above, En.2 is rich in catecholaminergic fibers, some of which originate from THli cells on the wall of the pharynx (Martinez-Rubio et al., 2009; see Dopamine as a Sensory Neurotransmitter; Fig. 2D).
Low concentrations (1 nM) of the dopamine antagonist methylergonovine blocked conditioned enhancement of ingestive motor programs produced by stimulation of the esophageal nerve in analogs of operant and classical conditioning (Nargeot et al., 1999c; Reyes et al., 2005). Investigation therefore focused on a specific identified neuron, B51, that acts as a pivotal decision-making element for the specification of ingestive buccal motor programs (Nargeot et al., 1999a,b). Brembs et al. (2002) tested a single cell analog for operant conditioning using pulsed application of dopamine onto B51 isolated from the buccal ganglion of naïve specimens. When DA application was contingent upon expression of plateau potentials by B51, subsequent tests revealed a significant increase in the input resistance and a decrease in burst threshold (Brembs et al., 2002). These biophysical changes were shown to reflect activation of second messenger pathways acting through protein kinase A and protein kinase C via a D1-like dopamine receptor (Barbas et al., 2006; Lorenzetti et al., 2008; Mozzachiodi et al., 2008).
Using a modified version of the operant conditioning paradigm in behaving Aplysia, Nargeot and coworkers (2007, 2009) observed a significant increase in the frequency and rhythmicity of biting. Several changes in the buccal network, including regularization of the burst discharge and increased electrical coupling of pattern-initiating cells, were detected following dissection from contingent-reward trained specimens (Nargeot et al., 2009). These modifications were replicated in an in vitro paradigm that used stimulation of the dopamine-rich En.2 as a reinforcing stimulus (Bédécarrats et al., 2013). When the D1 receptor antagonist flupenthixol was present during training, rhythmic programs were not observed in trained preparations and physiological changes did not occur in the pattern-initiating cells. The regularly repeating, compulsive-like expression of feeding behavior was proposed to require dopaminergic contingent reward signals from En.2 (Bédécarrats et al., 2013).
Discussion
Evolution of Dopamine as a Multifunctional Neurotransmitter
The findings reviewed here support the role of dopamine as a multifunctional neurotransmitter in gastropods and suggest a hypothetical progression by which DA acquired its diverse roles. This sequence can be readily examined in the networks that control feeding-related behaviors (Fig. 6). At each stage of this progression, the function of DA neurons is proposed to favor their recruitment to their subsequent role, as the circuits gained increasing complexity and adaptive control. Common functions of DA across phylogeny imply the ancient origin of this sequence, possibly preceding the divergence of deuterostomes and protostomes (see Hills, 2006; Barron et al., 2010). There is presently some debate regarding the body plan and nervous system organization of the last common bilaterian ancestor (see Moroz, 2009; Hirth, 2010; Northcutt, 2012; Strausfeld and Hirth, 2013). However, paleontological evidence suggests that the oldest well-documented bilaterian, Kimberella (555 Mya), had a slug-like form and fed by scratching the microbial surface on which it lived (Fedonkin and Waggoner, 1997). Such an organism would, in all likelihood, possess a nervous system that could sense potential nutrients and activate a scraping apparatus.
Figure 6.
Sequential acquisition of dopamine functions in nervous systems. The proposed sequence is shown on the left (shaded boxes), using the feeding system of Aplysia as a prototype. Mechanisms or levels of nervous complexity that enabled DA to assimilate each function are shown at right. According to this hypothesis: (A) Initially, DA mediated sensory-motor synaptic signals could influence food searching networks. (B) With the advent of central pattern generator circuits, the role of DA as a sensory neurotransmitter favored its utilization as an activator of motor programs (E: egestive motor program). (C) With the elaboration of more complex multifunctional CPG circuits, the ability of DA to activate motor programs facilitated its implementation in the specification of one pattern versus another. Here, DA is shown selecting an egestive program (E, dark shading) from a network that can also produce ingestive (I, light shading) programs. (D) The ability of DA neurons to select a specific program from a multifunctional CPG led to their utility for factoring the value of a stimulus into such decisions. Here, state sensitivity causes dopaminergic signaling to be down-regulated (potentiometer, lighter blue shading) as the hunger level of the organism is increased, decreasing activation of egestive motor programs (lighter shading) and increasing the tendency toward ingestive programs (dark shading). (E) The ability of DA to activate motor networks via modulatory second messenger cascades facilitated its deployment as a phasic reinforcing signal. Here, a DA pulse originating from pharyngeal sensory neurons is shown following a spontaneous ingestive motor pattern (I). Through activity-dependent modulation of rhythm and burst-generating currents in key interneurons, the dopaminergic reinforcing signal causes the feeding CPG to produce repetitive ingestive programs. (F) The ability of dopaminergic sensory neurons to inform the feeding CPG about the consequences of its activity also led to their participation in stimulus coincidence detection. Here, modulatory DA signals enable a conditioned stimulus (CS) to evoke a specific behavior (ingestive motor pattern shown) following pairing with an unconditioned stimulus (US). Strengthening of synaptic input from the CS pathway to the feeding CPG is proposed to reflect enhancement of an intrinsic plasticity, such as long-term potentiation, In this paradigm, the actions of DA constitute a form of modulatory metaplasticity.
Sensation.
The presence of subepithelial dopaminergic neurons lining the cephalic sensory organs of several gastropod groups (Fig. 2A-C) indicates that DA signaling participates in the transduction of stimuli associated with foraging or food-seeking behavior (Fig. 6, panel A: Sensation). To date, the modality of the peripheral dopaminergic neurons remains unresolved, but their projections through the body wall suggest either a tactile or chemosensory function (see Croll, 2001; Faller et al., 2008; Wyeth and Croll, 2011; Vallejo et al., 2014). The participation of sensory DA signaling in food-seeking behavior is also supported by pharmacological observations in Pleurobranchaea (Brown et al., 2018).
Sensory signaling in foraging behaviors appears to be an ancient function of dopamine, at least predating divergence of the Ecdysozoa and Lophotrochozoa superphyla (581 – 531 Mya; Hedges and Kumar, 2009). In the simpler nervous system of the nematode Caenorhabditis elegans, consisting of only 302 neurons, all known dopaminergic neurons (8 in the hermaphroditic form and 14 in the male) have a sensory function (Chase and Koelle, 2007). Stimulation of dopaminergic mechanosensory neurons by bacterial food reduces crawling speed and increases turning behavior (Sawin et al., 2000; Hills et al., 2004; Rivard et al., 2010). Both of these changes in reward-seeking behavior promote an area-restricted search, increasing the likelihood that C. elegans will remain in a location where food is encountered (Hills, 2006).
Activation.
When repetitive stereotyped movements came under the control of central pattern generator circuits, dopaminergic sensory neurons may have been recruited to regulate their activation (Fig. 6, panel B: Activation). In the gastropods, a limited number of neurons could have migrated to the CNS and become integrated into certain CPGs (see McCallister et al., 1983; Gillette and Brown, 2015). In the aquatic pulmonates, the respiratory system CPG contains a dopaminergic neuron that is capable of activating the quiescent central circuit (Fig. 3, 4A, B). In the snails that have been studied, Helisoma trivolvis, and Lymnaea stagnalis, the giant dopaminergic cell participates in each cycle of the respiratory motor pattern, acting as an intrinsic constituent of the respiratory CPG (see Fig. 4A, B).
Activation of the Aplysia feeding motor system can also be achieved by dopaminergic signaling from a neuron that is extrinsic to the feeding CPG itself (CBI-1 in Aplysia californica and CBm1 in Aplysia kurodai). In contrast to the giant neurons in the pulmonate respiratory CPGs, the cerebral-buccal interneurons typically serve a command-like function and are not sensu stricto participants in each cycle of the feeding motor pattern. Such extrinsic activation of motor systems by dopamine occurs in multiple taxa, including the cardiac and stomatogastric CPGs of crustaceans (Anderson and Barker, 1981; Miller et al., 1984; Fort et al., 2004), the crawling CPG of the medicinal leech (Puhl and Mesce, 2008), and the spinal locomotor CPGs of fish and mammals (Svennson et al., 2003; Barrière et al., 2004; Sharples et al., 2014). Recently, two-photon calcium imaging and optogenetics were used to demonstrate that dopaminergic nigrostriatal projections signal the initiation of locomotion bouts in mice (Howe and Dombeck, 2016; see also Jin and Costa, 2010).
Selection.
When CPGs gained a level of complexity that supported multifunctionality (see Getting, 1989; Briggman and Kristan, 2008), the ability of dopaminergic neurons to activate motor systems could have facilitated their implementation for specification of motor programs (Fig. 6, panel C: Selection). This function is illustrated by the B20 and B65 interneurons in the buccal feeding network of Aplysia and the PRM neuron of Lymnaea. Activity of these dopaminergic neurons causes the buccal circuit to select one motor pattern, egestion, over another, ingestion (Fig. 5A, B). Interestingly, B20, B65, and the PRM all possess the ability to activate buccal motor patterns when stimulated (Kabotyanski et al., 1998; Jing and Weiss, 2001; Crossley et al., 2018). However, all are selectively recruited by stimuli that generate one class of motor program, egestion, from the multifunctional feeding circuit. When their participation in motor program generation is prevented, the circuit produces ingestive patterns (Fig. 5A, B). Thus, dopaminergic synaptic signaling from B20, B65 of Aplysia and the PRM neuron of Lymnaea specifies or selects the performance of egestive programs (Jing and Weiss, 2001; Due et al., 2004; Crossley et al. 2018).
In vertebrates, resolution of the “selection problem”, i.e. execution of a specific behavior while inhibiting competing actions, is commonly attributed to the basal ganglia (Graybiel, 1995; Mink, 1996; Redgrave et al., 1999). Participation of nigrostriatal dopaminergic neurons in action selection was suggested by early pharmacological studies (Cools, 1980). Recently, fast-scan cyclic voltammetry was used to measure sub-second changes in dopamine levels in the dorsal striatum of mice that were trained to select between two alternative actions. Higher dopamine levels and firing rates of nigrostriatal DA neurons were both specifically associated with behavioral choice (Howard et al., 2017).
Valuation.
The capacity for dopaminergic neurons to specify behavior produced by multifunctional motor networks could have favored their implementation by systems that factor an assessment of stimulus value or utility into such selection (Fig. 6, panel C: Valuation). Such valuation could be implemented by adjusting the strength of DA signaling according to the motivational state of the organism (denoted by the potentiometer in Fig. 6C).
Dopaminergic signaling by the pattern reversing neurons of Lymnaea stagnalis causes the feeding circuit to produce egestive motor programs in satiated specimens in response to tactile or chemical stimuli (Crossley et al., 2018). The PRNs are not active in food-deprived snails, which produce ingestive behaviors in response to the same stimuli. It was proposed that these higher order interneurons encode hunger state (satiated versus hungry) and select corresponding motor patterns (egestive versus ingestive) from the multifunctional feeding circuit (Crossley et al., 2018). Suppression of PRN activity thus enables hungry snails to increase their valuation of food stimuli.
A similar neural architecture is present in Drosophila, where six dopaminergic interneurons in the mushroom bodies prevent expression of conditioned feeding in satiated flies (Krashes et al., 2009). Inhibition of these interneurons, termed MB-MP cells, by an ortholog of the mammalian neuropeptide Y (neuropeptide F), mimics food deprivation and promotes memory performance in satiated flies. Interestingly, the Aplysia ortholog of neuropeptide Y was shown to mediate satiety signals from the esophagus, at least in part by increasing the activity of the DA-GABA interneuron B20 (Jing et al., 2007), proposed here to correspond to the PRN of Lymnaea.
In vertebrates, motivational state can assign value to sensory stimuli by modulating or filtering afferent signaling. Dopamine is a major neurotransmitter in the olfactory bulb of mice, where it is colocalized with GABA in juxtaglomerular cells that have extensive interglomerular projections (Kosaka et al., 1985; Kosaka and Kosaka, 2008; Kiyokage et al., 2010). In feeding systems, signaling in the olfactory bulb is modified by nutritional status (Aimé et al., 2007; Prud’homme et al., 2009). The extensive dopaminergic network in the cephalic sensory organs of gastropods has been likened to the vertebrate juxtaglomerular cells, and it has been proposed that they could participate in the peripheral computation of stimulus value reflecting hunger or satiety (Gillette and Brown, 2015; Brown et al., 2018).
Association.
The ability to identify a temporal association between internal or external events is required for animals to form adaptive predictions (Pavlov, 1927; Skinner, 1938; Gallistel, 1990). To date, stimulation of the esophageal nerve has been used as the unconditioned stimulus for in vitro analogs of classical conditioning and as the reinforcing stimulus for two forms of operant conditioning in the Aplysia feeding system (Nargeot et al., 1999a, b; Mozzachiodi et al., 2003; Brembs et al., 2002, 2004; Bédécarrats et al., 2013). In all cases, it has been proposed that associations result from the release of DA within the feeding CPG (Brembs et al., 2002; Reyes et al., 2005; Bédécarrats et al., 2013). This hypothesis was supported by the demonstration of dopaminergic afferent neurons on the wall of the pharynx (Fig. 1D; Martínez-Rubio et al., 2009). Such cells could provide rapid preabsorptive feedback to the buccal CPG concerning the consequences of feeding movements.
In some CPGs, slow currents in key interneurons can produce spontaneous behavior that is not elicited by a specific stimulus (see Nargeot and Simmers, 2012; Hawkins and Byrne, 2015). The ability of dopaminergic neurons to activate and select motor programs based upon an assessment of value (Fig. 6A-D) could have favored the participation of dopamine in providing proprioceptive feedback to feeding CPGs (Fig. 6, panel E: Association). For example, if a food-searching bite motor program was followed by a DA pulse signifying successful ingestion, the production of subsequent biting programs could be increased. As discussed above (Activation), tonic application of DA can initiate repetitive rhythmic motor activity in gastropod CPGs (e.g. Fig. 4B; see also Wieland and Gelperin, 1983; Trimble and Barker, 1984; Serrano and Miller, 2006). Its implementation as a phasic reinforcing signal that can increase the occurrence of an operant is likely to reflect similar mechanistic actions, i.e. modulation of rhythm- and burst-generating currents in key CPG elements (Nargeot et al., 2009; Nargeot and Simmers, 2012). The ability of such phasic reinforcing DA signals to associate the occurrence of a motor program with its consequences could reflect long-lasting activity-dependent modulation of these currents.
Coincidence Detection.
The ability of dopaminergic neurons to influence feeding CPGs based upon the consequences of their activity could have set the stage for their use in detecting temporal contiguity between a sensory stimulus and its consequences. In this scenario, a neutral stimulus that precedes successful passage of food through the pharynx would take on a subsequent predictive function (Fig. 6, panel F: Coincidence detection).
Although both instrumental and Pavlovian conditioning involve modulatory actions of DA originating from the esophageal nerve, the mechanisms for establishing associations differ for the two types of learning. While operant conditioning affects the intrinsic rhythm- and burst-generating currents in decision making neurons (see previous text), classical conditioning tends to reflect regulation of synaptic signals that reach those neurons through sensory pathways (Lechner et al., 2000b; Mozzachiodi et al., 2003, 2013; Mozzachiodi and Byrne, 2010). Such modification of synaptic signals could be achieved via dopaminergic enhancement of an intrinsic coincidence detection mechanism, such as Hebbian long-term potentiation, in the sensory input to the feeding CPG.
In vertebrate nervous systems, midbrain dopaminergic neurons involved in appetitive conditioning are thought to receive sensory information, e.g. taste or odor, that conveys the potential utility of a food stimulant (see Glimcher, 2011). Present models posit that these DA neurons encode a reward prediction error that uses such sensory information, or a proxy cue, to calculate whether the expectation of future reward requires revision (Montague et al., 1996; Schultz et al., 1997). Midbrain DA neurons project to motor control circuits of the basal ganglia and frontal cortex where DA release reflects the reward prediction error magnitude (Williams and Goldman-Rakic, 1998; Björkland and Dunnett, 2007). In a leading mechanism for associative conditioning, DA modulation can produce a pairing specific strengthening of sensorimotor pathways by enhancing long-term potentiation of synaptic connections (Levine et al., 1996; Pawlak and Kerr, 2008; Lutzu and Castillo, 2020). Thus, DA may make parallel contributions to Pavlovian conditioning in the gastropod and vertebrate nervous systems, i.e. modulation of long-lasting coincidence-detecting synaptic plasticity.
Future Directions and Perspective
Emerging research should continue to reveal how dopamine acquired and executes its various functions in gastropods. Promising areas of investigation include: 1) use of voltage sensitive dyes to explore global effects of DA on neural circuits (Neveu, 2017), 2) omics data that will lead to the identification, characterization, and localization of dopamine receptors involved in motor control and learning (Barbas et al., 2006; Tamvacakis et al., 2015; Mansour et al., 2017), and the use of identified DA-GABA neurons to learn how brains encode stimulus value (Jing et al., 2007; Crossley et al., 2018).
Surveying the literature on dopamine as a multifunctional neurotransmitter in gastropod molluscs, it is striking how many of its functions are shared with other taxa, including vertebrates (Table I). Here, a sequence is proposed that traces the incorporation of DA into various functions as nervous systems became more complex and proficient, from its sensory role in appetitive food finding to its reinforcing role in appetitive conditioning. The presence of common dopaminergic functions across phylogeny implies the ancient origin of this sequence, possibly predating the split of the protostome and deuterostome lineages. Moving forward, advances emerging from the simpler gastropod systems should continue to inform, and be informed by, exploration of dopamine’s multifunctional contributions to sensorimotor integration and behavioral plasticity across phylogeny.
Table I.
Anatomic substrates of DA functions in gastropod and mammalian nervous systems.
| Function | Gastropod nervous system | Mammalian nervous system |
|---|---|---|
| Sensation |
Cephalic sensory organs (Wyeth and Croll, 2011, Faller et al., 2017; Brown et al., 2018) |
Olfactory bulb (Kosaka et al., 1985; Kosaka and Kosaka, 2008; Kiyokage et al., 2010) |
| Activation |
Cerebral-buccal command-like interneurons (Rosen et al., 1991; Narusuye and Nagahama, 2002) |
Nigrostriatal projections Howe and Dombeck, 2016; see also Jin and Costa, 2010) |
| Selection |
Aplysia buccal interneurons (B20 & B65) (Teyke et al., 1993; Kabotyanski et al, 1998; Jing and Weiss, 2001) |
Nigrostriatal projections (Graybiel, 1995; Mink, 1996; Redgrave et al., 1999; Howard et al., 2017) |
| Valuation |
Aplysia B20; Lymnaea PRN (Jing et al., 2007; Crossley et al., 2018) |
VTA projections to ventral striatum (Kroemer et al., 2016) |
| Association |
Pharyngeal DA afferents (Martínez-Rubio et al., 2009; Bédécarrats et al., 2013) |
VTA-striatal projections (Graybiel, 2005, 2008) |
| Coincidence Detection |
Pharyngeal DA afferents (Brembs et al., 2002; Reyes et al., 2005; Martínez-Rubio et al., 2009) |
VTA-striatal and cortical projections (Levine et al., 1996; Williams and Goldman-Rakic, 1998; Schultz, 2010) |
Selected references shown for each function.
Acknowledgements.
The author’s research is supported by the National Institutes of Health: RCMI MD007600, MBRS GM087200; National Science Foundation: DBI-1337284, HRD-1137725. OISE 1545803; National Academy of Sciences (NAS; USA): U.S.-Egypt Science and Technology (S&T) Joint Fund 2000007152; Science and Technology Development Fund (STDF, Egypt): USC17-188.
This article is derived from the Subject Data funded in whole or part by NAS and USAID. Any opinions, findings, conclusions, or recommendations expressed are those of the authors alone, and do not necessarily reflect the views of USAID or NAS.
Abbreviations:
- CBI
cerebral-buccal interneuron
- DA
dopamine
- cAMP
3', 5'-cyclic adenosine monophosphate
- Mya
million years ago
- THli
tyrosine hydroxylase-like immunoreactive
- CPG
central pattern generator
- BMP
buccal motor program
- PRN
pattern reversing neuron
Literature Cited
- Aimé P, Duchamp-Viret P, Chaput MA, Savigner A, Mahfouz M, and Julliard AK. 2007. Fasting increases and satiation decreases olfactory detection for a neutral odor in rats. Behav. Brain Res 179: 258–264. doi: 10.1016/j.bbr.2007.02.012 [DOI] [PubMed] [Google Scholar]
- Anderson WW, and Barker DL. 1981. Synaptic mechanisms that generate network oscillations in the absence of discrete synaptic potentials. J. Exp. Zool 216:187–191. [DOI] [PubMed] [Google Scholar]
- Anderson BB, and Ewing AG. 1999. Chemical profiles and monitoring dynamics at an individual nerve cell in Planorbis corneus with electrochemical detection. J. Pharm. Biomed. Anal 19: 15–32. doi: 10.1016/s0731-7085(98)00088-0 [DOI] [PubMed] [Google Scholar]
- Aonuma H, Kaneda M, Hatakeyama D, Watanabe T, Lukowiak K, and Ito E. 2016. Relationship between the grades of a learned aversive-feeding response and the dopamine contents in Lymnaea. Biol. Open 5: 1869–1873. doi: 10.1242/bio.021634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P 1968. Electrophoretic injections of dopamine on Aplysia neurones. J. Physiol 198: 48–49 P. [Google Scholar]
- Ascher P 1972. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J. Physiol 225: 173–209. doi: 10.1113/jphysiol.1972.sp009933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, and Chesnoy-Marchais D. 1982. Interactions between three slow potassium responses controlled by three distinct receptors in Aplysia neurones. J. Physiol 324: 67–92. doi: 10.1113/jphysiol.1982.sp014101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas D, Zappulla JP, Angers S, Bouvier M, Mohamed HA, Byrne JH, Castellucci VF, and DesGroseillers L. 2006. An aplysia dopamine 1-like receptor: molecular and functional characterization. J. Neurochem 96: 414–427. doi: 10.1111/j.1471-4159.2005.03561.x [DOI] [PubMed] [Google Scholar]
- Barbeau A 1967. The “pink spot”, 3,4-dimethoxyphenylethylamine and dopamine. Relationship to Parkinson’s disease and to schizophrenia. Rev. Can. Biol 26: 55–79. [PubMed] [Google Scholar]
- Barbeau A 1974. Drugs affecting movement disorders. Ann. Rev. Pharmacol 14: 91–113. doi: 10.1146/annurev.pa.14.040174.000515 [DOI] [PubMed] [Google Scholar]
- Barrière G, Mellen N, and Cazalets J-R. 2004. Neuromodulation of the locomotor network by dopamine in the isolated spinal cord of newborn rat. Eur J. Neurosci 19: 1325–1335. doi: 10.1111/j.1460-9568.2004.03210.x [DOI] [PubMed] [Google Scholar]
- Barron AB, Sovik E, and Cornish JL. 2010. The roles of dopamine and related compounds in reward-seeking behavior across animal phyla. Front. in Behav. Neurosci 4: doi: 10.3389/fnbeh.2010.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter DA, and Byrne JH. 2006. Feeding behavior of Aplysia: A model system for comparing cellular mechanisms of classical and operant conditioning. Learn. & Mem 13: 669–680. doi: 10.1101/lm.339206 [DOI] [PubMed] [Google Scholar]
- Bédécarrats A, Cornet C, Simmers J, and Nargeot R. 2013. Implication of dopaminergic modulation in operant reward learning and the induction of compulsive-like feeding behavior in Aplysia. Learn. & Mem 20: 318–327. doi: 10.1101/lm.029140.11 [DOI] [PubMed] [Google Scholar]
- Berridge KC, and Robinson TE. 1998. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev 28: 309–369. [DOI] [PubMed] [Google Scholar]
- Berry MS, and Cottrell GA. 1973. Dopamine: Excitatory and inhibitory transmission from a giant dopamine neurone. Nature New Biol. 242: 250–253. doi: 10.1038/newbio242250a0 [DOI] [PubMed] [Google Scholar]
- Berry MS, and Cottrell GA. 1975. Excitatory, inhibitory and biphasic synaptic potentials mediated by an identified dopamine-containing neurone. J. Physiol 244: 589–612. doi: 10.1113/jphysiol.1975.sp010814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, and Dunnett SB. 2007. Dopamine neuron systems in the brain: an update. Trends in Neurosci 30: 194–202. doi: 10.1016/j.tins.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Boisson M, and Gola M. 1976. Current-voltage relations in ILD- or dopamine-stabilized bursting neurone in Aplysia. Comp. Biochem. Physiol 54C: 109–113. [DOI] [PubMed] [Google Scholar]
- Boyle JP, and Yoshino TP. 2002. Monoamines in the albumen gland, plasma, and central nervous system of the snail Biomphalaria glabrata during egg-laying. Comp. Biochem. Physiol. A: Mol. & Integ. Physiol 132: 411–422. doi: 10.1016/s1095-6433(02)00091-0 [DOI] [PubMed] [Google Scholar]
- Brembs B, Lorenzetti FD, Reyes FD, Baxter DA, and Byrne JH. 2002. Operant reward learning in Aplysia: Neuronal correlates and mechanisms. Science 296: 1706–1709. doi: 10.1126/science.1069434 [DOI] [PubMed] [Google Scholar]
- Brembs B, Baxter DA, and Byrne JH. 2004. Extending in vitro conditioning in Aplysia to analyze operant and classical processes in the same preparation. Learn. Mem 11: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, and Kristan WB Jr. 2008. Multifunctional pattern-generating circuits. Ann. Rev. Neurosci 31: 271–294. doi: 10.1146/annurev.neuro.31.060407.125552 [DOI] [PubMed] [Google Scholar]
- Brisson P 1983. Aminergic structures in the genital tract of pulmonate gastropods and their possible role in the reproductive system. In: Molluscan Neuroendocrinology (Edited by Lever J, and Boer HH). Amsterdam, North Holland Publishing: 120–125. [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, and Hikosaka O. 2010. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 68: 815–834. doi: 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardot J 1963. Sur la présence de dopamine dans le systeme nerveux et ses relations avec la décarboxylation de la dioxyphénylalanine chez le Mollusque Helix pomatia. C. r. Acad. Sci. Paris 257: 1364–1366. [PubMed] [Google Scholar]
- Carpenter DO, Breese G, Schanberg S, and Kopin I. 1971. Serotonin and dopamine: distribution and accumulation in Aplysia nervous and non-nervous tissues. Intern. J. Neurosci 2: 49–56. [DOI] [PubMed] [Google Scholar]
- Cedar H, and Schwartz JH. 1972. Cyclic adenosine monophosphate in the nervous system of Aplysia californica. II. Effect of serotonin and dopamine. J. Gen. Physiol 60: 570–587. doi: 10.1085/jgp.60.5.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Koelle MR. 2007. Biogenic amine neurotransmitters in C. elegans (February 20, 2007), WormBook, ed. The C. elegans Research Community, WormBook, 10.1895/wormbook.1.132.1, http://www.wormbook.org. Accessed: 8/4/2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gavin P, Luo G, and Ewing A. 1995. Observation and quantitation of exocytosis from the cell body of a fully developed neuron in Planorbis corneus. J. Neurosci 15: 7747–7755. doi: 10.1523/jneurosci.15-11-07747.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang PK, Bourgeois JG, and Bueding E. 1974. 5-Hydroxytryptamine and dopamine in Biomphalaria glabrata. J. Parasitol 60: 264–271. [PubMed] [Google Scholar]
- Chien JB, Wallingford RA, and Ewing AG. 1990. Estimation of free dopamine in the cytoplasm of the giant dopamine cell of Planorbis corneus by voltammetry and capillary electrophoresis. J. Neurochem, 54: 633–638. doi: 10.1111/j.1471-4159.1990.tb01918.x [DOI] [PubMed] [Google Scholar]
- Colwill RM, Goodrum K, and Martin A. 1997. Pavlovian appetitive discriminative conditioning in Aplysia californica. Animal Learn. Behav 25: 268–276. doi: 10.3758/bf03199084 [DOI] [Google Scholar]
- Cools AR 1980. Role of the neostriatal dopaminergic activity in sequencing and selecting behavioural strategies: Facilitation of processes involved in selecting the best strategy in a stressful situation. Behav. Brain Res 1: 361–378. doi: 10.1016/0166-4328(80)90035-2 [DOI] [PubMed] [Google Scholar]
- Cottrell GA 1977. Identified amine-containing neurones and their synaptic connexions. Neurosci. 2: 1–18. doi: 10.1016/0306-4522(77)90064-1 [DOI] [PubMed] [Google Scholar]
- Cottrell GA, Abernethy KB, and Barrand MA. 1979. Large amine-containing neurones in the central ganglia of Lymnaea stagnalis. Neuroscience 4: 685–689. doi: 10.1016/0306-4522(79)90145-3 [DOI] [PubMed] [Google Scholar]
- Cottrell GA 1967. Amines in the molluscan nervous tissue and their subcellular localization. In: Symposium on Neurobiology of Invertebrates. Salánki J, Ed. Budapest: Akadémiai Kiadó. p. 353–364. [Google Scholar]
- Croll RP 1987. Distribution of monoamines in the central nervous system of the nudibranch gastropod, Hermissenda crassicornis. Brain Res. 405: 337–347. doi: 10.1016/0006-8993(87)90303-9 [DOI] [PubMed] [Google Scholar]
- Croll RP 2001. Catecholamine-containing cells in the central nervous system and periphery of Aplysia californica. J. Comp. Neurol 441: 91–105. doi: 10.1002/cne.1399 [DOI] [PubMed] [Google Scholar]
- Crossley M, Staras K, and Kemenes G. 2018. A central control circuit for encoding perceived food value. Sci. Adv 4: eaau9180. doi: 10.1126/sciadv.aau9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks AM, and Weiss KR. 2013. Latent modulation: A basis for non-disruptive promotion of two incompatible behaviors by a single network state. J. Neurosci 33: 3786–3798. doi: 10.1523/jneurosci.5371-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl E, Falck B, Lindqvist M, and von Mecklenburg C. 1962. Monoamines in mollusk neurons. Kgl. fysiogr. Sällsk. Lund Förh 32: 89–92. [Google Scholar]
- Dahl E, Falck B, von Mecklemburg C, Myhrberg H, and Rosengren E. 1966. Neuronal localization of dopamine and 5-hydroxytryptamine in some molluscs. Z. Zellforsch 71: 489–498. [DOI] [PubMed] [Google Scholar]
- de Jong-Brink M, and Goldschmeding JT. 1983. Endocrine and nervous regulation of female reproductive activity in the gonad and the albumen gland of Lymnaea stagnalis. In: Molluscan Neuroendocrinology (Edited by Lever J, and Boer HH). Amsterdam, North Holland Publishing: 126–131. [Google Scholar]
- Diana M, Di Chiara G, and Spano P (Eds.) 2014. Dopamine. Prog. Brain Res 211. Elsevier. Amsterdam. PMID 24968786 DOI: 10.1016/B978-0-444-63425-2.10000-5. [DOI] [PubMed] [Google Scholar]
- Deterre P, Paupardin-Tritsch D, Bockaert J, and Gerschenfeld HM. 1982. c-AMP mediated decrease in K+ conductance evoked by serotonin and dopamine in the same neuron: A biochemical and physiological single-cell study. Proc. Natl. Acad. Sci. USA 79: 7934–7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Ríos M, and Miller MW. 2005. Rapid dopaminergic signaling by interneurons that contain markers for catecholamines and GABA in the feeding circuitry of Aplysia. J. Neurophysiol 93: 2142–2156. doi: 10.1152/jn.00003.2004 [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, and Miller MW. 2006. Target-specific regulation of synaptic efficacy in the feeding central pattern generator of Aplysia: Potential substrates for behavioral plasticity? Biol. Bull 210: 215–229. doi: 10.2307/4134559 [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, Oyola E, and Miller MW. 2002. Colocalization of γ-aminobutyric acid-like immunoreactivity and catecholamines in the feeding network of Aplysia californica. J. Comp. Neurol 445: 29–46. doi: 10.1002/cne.10152 [DOI] [PubMed] [Google Scholar]
- Drummond AH, Bucher F, and Levitan IB. 1978. LSD labels a novel dopamine receptor in molluscan nervous system. Nature, 272: 368–370. doi: 10.1038/272368a0 [DOI] [PubMed] [Google Scholar]
- Drummond AH, Bucher F, and Levitan IB. 1980. Distribution of serotonin and dopamine receptors in Aplysia tissues: Analysis by [3H]LSD binding and adenylate cyclase stimulation. Brain Res. 184: 163–177. doi: 10.1016/0006-8993(80)90595-8 [DOI] [PubMed] [Google Scholar]
- Due MR, Jing J, and Weiss KR. 2004. Dopaminergic contributions to modulatory functions of a dual-transmitter interneuron in Aplysia. Neurosci. Lett 358: 53–57. doi: 10.1016/j.neulet.2003.12.058 [DOI] [PubMed] [Google Scholar]
- Elekes K, Kemenes G, Hiripi L, Geffard M, and Benjamin PR. 1991. Dopamine-immunoreactive neurones in the central nervous system of the pond snail Lymnaea stagnalis. J. Comp. Neurol 307, 214–224. doi: 10.1002/cne.903070205 [DOI] [PubMed] [Google Scholar]
- Emaduddin M, Liu GJ, and Takeuchi HH, 1995. Effects of dopamine on snail neurones. Eur. J. Pharmacol 283: 113–124. doi: 10.1016/0014-2999(95)00301-z [DOI] [PubMed] [Google Scholar]
- Falck B, Hillarp N-A, Thieme G, and Torp A. 1962. Flourescence of catecholamines and related compunds condensed with formadehyde. J. Histochem. Cytochem 10: 348–354. [Google Scholar]
- Faller S, Staubach S, and Klussmann-Kolb A. 2008. Comparative immunohistochemistry of the cephalic sensory organs in Opisthobranchia (Mollusca, Gastropoda). Zoomorphology 127: 227–239. [Google Scholar]
- Fedonkin MA, and Waggoner BM. 1997. The Late Precambrian fossil Kimberella is a mollusc-like bilaterian organism. Nature 388: 868–871. doi: 10.1038/42242 [DOI] [Google Scholar]
- Fekete E 1984. Distribution of fluorogenic monoamines in the gastrointestinal musculature of Helix pomatia. Histochem. 81: 311–312. [DOI] [PubMed] [Google Scholar]
- Fieber LA 2017. Neurotransmitters and neuropeptides of invertebrates. Oxford Handbooks Online. https://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780190456757.001.0001/oxfordhb-9780190456757-e-10. Accessed 8/4/2020. [Google Scholar]
- Fort TJ, Brezina V, and Miller MW. 2004. Modulation of an integrated central pattern generator-effector system: Dopaminergic regulation of cardiac activity in the blue crab Callinectes sapidus. J. Neurophysiol 92: 3455–3470. [DOI] [PubMed] [Google Scholar]
- Gallistel CR 1990. The organization of learning. Bradford Books/MIT Press. Cambridge, MA. [Google Scholar]
- Gerschenfeld HM 1964. A non-cholinergic synaptic inhibition in the central nervous system of a mollusc. Nature 203: 415–416. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld HM 1973. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol. Rev 53: 11–19. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld HM, and Tauc L. 1961. Pharmacological properties of neurones in an elementary central nervous system. Nature 189: 924–925. [DOI] [PubMed] [Google Scholar]
- Getting PA 1989. Emerging principles governing the operation of neural networks. Ann. Rev. Neurosci, 12: 185–204. doi: 10.1146/annurev.ne.12.030189.001153 [DOI] [PubMed] [Google Scholar]
- Ghosh DD, Sanders T, Hong S, McCurdy LY, Chase DL, Cohen N, Koelle MR, and Nitabach MN. 2016. Neural architecture of hunger-dependent multisensory decision making in C. elegans. Neuron 92: 1049–1062. doi: 10.1016/j.neuron.2016.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, and Brown JW. 2015. The sea slug, Pleurobranchaea californica: A signpost species in the evolution of complex nervous systems and behavior. Integ. Comp. Biol 1–12 doi: 10.1093/icb/icv081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW 2011. Foundations of Neuroeconomic Analysis. Oxford University Press, New York. [Google Scholar]
- Goldstein RS, and Schwartz JH. 1989. Catecholamine neurons in Aplysia: Improved light-microscopic resolution and ultrastructural study using paraformaldehyde and glutaraldehyde (FaGlu) cytochemistry. J. Neurobiol 20: 203–218. doi: 10.1002/neu.480200404 [DOI] [PubMed] [Google Scholar]
- Gospe SM 1983. Studies of dopamine pharmacology in molluscs. Life Sci. 33: 1945–1957. doi: 10.1016/0024-3205(83)90732-4 [DOI] [PubMed] [Google Scholar]
- Graybiel AM 1995. Building action repertoires: memory and learning functions of the basal ganglia. Curr. Opin. Neurobiol 5: 733–741. doi: 10.1016/0959-4388(95)80100-6 [DOI] [PubMed] [Google Scholar]
- Graybiel AM 2005. The basal ganglia: learning new tricks and loving it. Current Opinion in Neurobiol. 15: 638–644. doi: 10.1016/j.conb.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Haber SN 2014. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 282: 248–257. doi: 10.1016/j.neuroscience.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SJ, and Cottrell GA. 1995. Properties of an identified dopamine containing neuron in culture from the snail Helisoma. Exp. Physiol 80: 37–51. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, and Marder E. 1991. Modulation of neural networks for behavior. Annu. Rev. Neurosci 14: 39–57. [DOI] [PubMed] [Google Scholar]
- Hartwig HG, Brisson P, Lyncker I, and Collin JP. 1980. Aminergic systems in pulmonated gastropod molluscs. III. Microspectrofluorometric characterization of the monoamines in the reproductive system. Cell Tiss. Res 210: 223–234. [DOI] [PubMed] [Google Scholar]
- Hedges SB, and Kumar S. 2009. The Timetree of Life. Oxford University Press. New York. [Google Scholar]
- Hernádi L, and Elekes KK, 1999. Topographic organization of serotonergic and dopaminergic neurons in the cerebral ganglia and their peripheral projection patterns in the head areas of the snail Helix pomatia. J. Comp. Neurol 411: 274–287. doi: [DOI] [PubMed] [Google Scholar]
- Hetherington MS, McKenzie JD, Dean HG, and Winlow W. 1994. A quantitative analysis of the biogenic amines in the central ganglia of the pond snail, Lymnaea stagnalis (L.). Comp. Biochem. Physiol. C 107: 83–93. [Google Scholar]
- Hills TT 2006. Animal foraging and the evolution of goal-directed cognition. Cog. Sci 30: 3–41. [DOI] [PubMed] [Google Scholar]
- Hirth F 2010. On the origin and evolution of the tripartite brain. Brain, Behav. Evol 76: 3–10. doi: 10.1159/000320218 [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O 1982. Imbalance of brain monoamines and clinical disorders. Prog. in Brain Res 419–429.doi: 10.1016/s0079-6123(08)64212-0 [DOI] [PubMed] [Google Scholar]
- Howard CD, Li H, Geddes CE, and Jin X. 2017. Dynamic nigrostriatal dopamine biases action selection. Neuron 93: 1436–1450.e8. doi: 10.1016/j.neuron.2017.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe MW, and Dombeck DA. 2016. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature,535: 505–510. doi: 10.1038/nature18942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen LL, Iversen SD, Dunnett SB, and Björkland A, Eds. 2010. Dopamine Handbook. Oxford University Press. New York, NY. [Google Scholar]
- Jaeger CP, Jaeger EC, and Welsh JH. 1971. Localization of monoamine-containing neurones in the nervous system of Strophocheilus oblongus (Gastropoda). Z. Zellforsch 112: 54–68. doi: 10.1007/bf00665621 [DOI] [PubMed] [Google Scholar]
- Jing J, and Weiss KR. 2001. Neural mechanisms of motor program switching in Aplysia. J. Neurosci 21: 7349–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, and Weiss KR. 2007. From hunger to satiety: Reconfiguration of a feeding network by Aplysia neuropeptide Y. J. Neurosci 27: 3490–3502. doi: 10.1523/jneurosci.0334-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C 1981. Presynaptic modulation of synaptic transmission in Helix pomatia: The effects of serotonin and dopamine antagonists. Neuropharmacol., 20: 323–326. doi: 10.1016/0028-3908(81)90003-4 [DOI] [PubMed] [Google Scholar]
- Juel C 1982. Enkephalin increases the efficacy of dopaminergic transmission in Helix pomatia. Neuropharmacol., 21: 1301–1303. doi: 10.1016/0028-3908(82)90137-x [DOI] [PubMed] [Google Scholar]
- Juorio AV, and Killick SW. 1972. Monoamines and their metabolism in some molluscs. Comp. gen. Pharmacol 3: 283–295. [DOI] [PubMed] [Google Scholar]
- Kabotyanski EA, Baxter DA, and Byrne JH. 1998. Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J. Neurophysiol 79: 605–621. doi: 10.1152/jn.1998.79.2.605 [DOI] [PubMed] [Google Scholar]
- Kabotyanski EA, Baxter DA, Cushman SJ, and Byrne JH. 2000. Modulation of fictive feeding by dopamine and serotonin in Aplysia. J. Neurophysiol 83: 374–392. doi: 10.1152/jn.2000.83.1.374 [DOI] [PubMed] [Google Scholar]
- Kebabian PR, Kebabian JW, and Carpenter DO. 1979. Regulation of cyclic AMP in heart and gill of Aplysia by the putative neurotransmitters dopamine and serotonin. Life Sci. 24: 1757–1764. doi: 10.1016/0024-3205(79)90064-x [DOI] [PubMed] [Google Scholar]
- Kemenes I, O’Shea M, and Benjamin PR. 2010. Different circuit and monoamine mechanisms consolidate long-term memory in aversive and reward classical conditioning. Eur. J. Neurosci 33: 143–152. doi: 10.1111/j.1460-9568.2010.07479.x [DOI] [PubMed] [Google Scholar]
- Kerkut GA 1973. Catecholamines in invertebrates. Br. Med. Bull 29: 100–104. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, and Walker RJ. 1961. The effects of drugs on the neurones of the snail Helix aspersa. Comp. Biochem. Physiol. 3: 143–160. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, and Walker RJ. 1962. The specific chemical sensitivity of Helix nerve cells. Comp. Biochem. Physiol. 7: 277–288. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Horn N, and Walker RJ. 1969. Long-lasting synaptic inhibition and its transmitter in the snail Helix aspersa. Comp. Biochem. Physiol 30: 1061–1074. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Sedden CB, and Walker RJ. 1966. The effect of dopa, α-methyldopa and reserpine on the dopamine content of the brain of the snail, Helix aspersa. Comp. Biochem. Physiol 18: 921–930. doi: 10.1016/0010-406x(66)90222-2 [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Sedden CB, and Walker RJ. 1966. The effect of DOPA, a-methyldopa and reserpine on the dopamine content of the brain of the snail, Helix aspersa. Comp. Biochem. Physiol 18: 921–930. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Sedden CB, and Walker RJ. 1967. Uptake of DOPA and 5-hydroxytryptophan by monoamine-forming neurons in the brain of Helix aspersa. Comp. Biochem. Physiol 23: 159–162. [DOI] [PubMed] [Google Scholar]
- Kiehn L, Saleuddin S, and Lange A. 2001. Dopaminergic neurons in the brain and dopaminergic innervation of the albumen gland in mated and virgin Helisoma duryi (Mollusca: Pulmonata). BMC Physiol. 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Hiripi L, Papp N, and Elekes K. 2003. Dopamine and serotonin receptors mediating contractions of the snail, Helix pomatia, salivary duct. Neuroscience 116: 775–790. [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Pan Y-Z, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, and Shipley MT. 2010. Molecular identity of periglomerular and short axon cells. J. Neurosci 30: 1185–1196. doi: 10.1523/jneurosci.3497-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Hataguchi Y, Hama K, Nagatsu I, and Jang-Yen W. 1985. Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb: possible coexistence of gamma-aminobutyric acid (GABA) and dopamine. Brain Res. 343: 166–171. doi: 10.1016/0006-8993(85)91172-2 [DOI] [PubMed] [Google Scholar]
- Kosaka T, and Kosaka K. 2008. Tyrosine hydroxylase-positive GABAergic juxtaglomerular neurons are the main source of the interglomerular connections in the mouse main olfactory bulb. Neurosci. Res 60: 349–354. doi: 10.1016/j.neures.2007.11.012 [DOI] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, and Waddell S. 2009. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139: 416–427. doi: 10.1016/j.cell.2009.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku BS, and Takeuchi H. 1986. Effects of catecholamine and monophenolamine agonists on identifiable giant neurones, sensitive to these amines, of an African giant snail (Achatina fulica Férussac). Eur. J. Pharmacol, 124: 21–29. doi: 10.1016/0014-2999(86)90120-2 [DOI] [PubMed] [Google Scholar]
- Kupfermann I 1974. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav. Biol 10: 1–26. doi: 10.1016/s0091-6773(74)91644-7 [DOI] [PubMed] [Google Scholar]
- Kupfermann I, and Kandel ER. 1969. Neuronal controls of a behavioral response mediated by the abdominal ganglion of Aplysia. Science 164: 847–850. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Pinsker H, Castellucci V, and Kandel ER. 1971. Central and peripheral control of gill movements in Aplysia. Science 174: 1252–1256. doi: 10.1126/science.174.4015.1252 [DOI] [PubMed] [Google Scholar]
- Kyriakides MA, and McCrohan CR. 1989. Effect of putative neuromodulators on rhythmic buccal motor output in Lymnaea stagnalis. J. Neurobiol 20: 635–650. doi: 10.1002/neu.480200704 [DOI] [PubMed] [Google Scholar]
- Lechner HA, Baxter DA, and Byrne JH. 2000a. Classical conditioning of feeding in Aplysia: I. Behavioral analysis. J. Neurosci 20: 3369–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Baxter DA, and Byrne JH. 2000b. Classical conditioning of feeding in Aplysia: II. Neurophysiological correlates. J. Neurosci 20: 3377–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Li S, Cepeda C, Cromwell HC, and Altemus KL. 1996. Neuromodulatory actions of dopamine on synaptically-evoked neostriatal responses in slices. Synapse, 24: 65–78. doi: 10.1002/syn.890240102 [DOI] [PubMed] [Google Scholar]
- Lorenzetti FD, Mozzachiodi R, Baxter DA, and Byrne JH. 2005. Classical and operant conditioning differentially modify the intrinsic properties of an identified neuron. Nature Neurosci. 9: 17–19. doi: 10.1038/nn1593 [DOI] [PubMed] [Google Scholar]
- Lorenzetti FD, Baxter DA, and Byrne JH. 2008. Molecular mechanisms underlying a cellular analog of operant reward learning. Neuron 59: 815–828. doi: 10.1016/j.neuron.2008.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti FD, Baxter DA, and Byrne JH. 2011. Classical conditioning analog enhanced acetylcholine responses but reduced excitability of an identified neuron. J. Neurosci 31: 14789–14793. doi: 10.1523/jneurosci.1256-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzu S, and Castillo PE 2020. Modulation of NMDA receptors by GPCRs: role in synaptic transmission, plasticity and beyond. Neurosci. doi: 10.1016/j.neuroscience.2020.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoski NS, Bauce LG, Syed NI, and Bulloch AG. 1995. Dopaminergic transmission between identified neurons from the mollusk, Lymnaea stagnalis. J. Neurophysiol 74: 1287–1300. doi: 10.1152/jn.1995.74.3.1287 [DOI] [PubMed] [Google Scholar]
- Manger P, Li J, Christensen BM, and Yoshino TP. 1996. Biogenic monoamines in the freshwater snail, Biomphalaria glabrata: Influence of infection by the human blood fluke, Schistosoma mansoni. Comp. Biochem. Physiol. A: Physiol 114: 227–234. doi: 10.1016/0300-9629(95)02131-0 [DOI] [PubMed] [Google Scholar]
- Mansour TA, Habib MR, Rodríguez LCV, Vázquez AH, Alers JM, Ghezzi A, Croll RP, Brown CT, and Miller MW. 2017. Central nervous system transcriptome of Biomphalaria alexandrina, an intermediate host for schistosomiasis. BMC Res.Notes, 10: doi: 10.1186/s13104-017-3018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E And Eisen JS. 1984. Electrically coupled pacemaker neurons respond to same physiological inputs and neurotransmitters. J. Neurophysiol 51: 1362–1374. [DOI] [PubMed] [Google Scholar]
- Marsden C, and Kerkut GA. 1970. The occurrence of monoamines in Planorbis corneus: A fluorescence microscopic and microspectrometric study. Comp. gen. Pharmacol, 1: 101–116.doi: 10.1016/0010-4035(70)90015-7 [DOI] [PubMed] [Google Scholar]
- Martínez-Rubio C, Serrano GE, and Miller MW. 2009. Localization of biogenic amines in the foregut of Aplysia californica: Catecholaminergic and serotonergic innervation. J. Comp. Neurol 514: 329–342. doi: 10.1002/cne.21991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rubio C, Serrano GE, and Miller MW. 2010. Octopamine promotes rhythmicity but not synchrony in a bilateral pair of bursting motor neurons in the feeding circuit of Aplysia. J. Exp. Biol 213: 1182–1194. doi: 10.1242/jeb.040378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Sasaki K, Takashima K, and Sato M. 1987. Desensitization of dopamine receptors observed in Aplysia ganglion cells. Jap. J. Physiol 37: 327–331. [DOI] [PubMed] [Google Scholar]
- McAllister L, Scheller R, Kandel E, and Axel R. 1983. In situ hybridization to study the origin and fate of identified neurons. Science, 222: 800–808. doi: 10.1126/science.6356362 [DOI] [PubMed] [Google Scholar]
- McCaman MW, Ono JK, and McCaman RE. 1979. Dopamine measurements in molluscan ganglia and neurons using a new, sensitive assay. J. Neurochem 32: 1111–1113. doi: 10.1111/j.1471-4159.1979.tb04602.x [DOI] [PubMed] [Google Scholar]
- Miller MW 1997. A cellular approach to the study of complex natural behavior in the ragged sea hare (Bursatella leachii), a marine invertebrate indigenous to Puerto Rico. Puerto Rico Health Sci. J 16: 23–36. [PubMed] [Google Scholar]
- Miller MW, Benson JA, and Berlind A. 1984. Excitatory effects of dopamine on the cardiac ganglia of the crabs Portunus sanguinolentus and Podophthalamus vigil. J. Exp. Biol 108: 97–118. [DOI] [PubMed] [Google Scholar]
- Mink JW 1996. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog. Neurobiol 50: 381–425. doi: 10.1016/s0301-0082(96)00042-1 [DOI] [PubMed] [Google Scholar]
- Montague P, Dayan P, and Sejnowski T. 1996. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci 16: 1936–1947. doi: 10.1523/jneurosci.16-05-01936.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL 2009. On the independent origins of complex brains and neurons. Brain, Behav. and Evol 74: 177–190. doi: 10.1159/000258665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, Lechner HA, Baxter DA, and Byrne JH. 2003. In vitro analog of classical conditioning of feeding behavior in Aplysia. Learn. Mem 10: 478–494. doi: 10.1101/lm.65303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, and Byrne JH. 2008. Plasticity of intrinsic excitability as a mechanism for memory storage. Learning and Memory: A Comprehensive Reference 829–838. doi: 10.1016/b978-012370509-9.00041-3 [DOI] [Google Scholar]
- Mozzachiodi R, Lorenzetti FD, Baxter DA, and Byrne JH. 2008. Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nature Neurosci. 11: 1146–1148. doi: 10.1038/nn.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, and Byrne JH. 2010. More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci. 33: 17–26. 10.1016/j.tins.2009.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, Baxter DA, and Byrne JH. 2013. Comparison of operant and classical conditioning of feeding behavior in Aplysia. Handbook Behav. Neurosci 183–193. doi: 10.1016/b978-0-12-415823-8.00015-0 [DOI] [Google Scholar]
- Mukai ST 2004. Dopamine stimulates snail albumen gland glycoprotein secretion through the activation of a D1-like receptor. J. Exp. Biol 207: 2507–2518. doi: 10.1242/jeb.01052 [DOI] [PubMed] [Google Scholar]
- Murphy AD 2001. The neuronal basis of feeding in the snail, Helisoma, with comparisons to selected gastropods. Prog. Neurobiol 63: 383–408. doi: 10.1016/s0301-0082(00)00049-6 [DOI] [PubMed] [Google Scholar]
- Nargeot R, and Simmers J. 2010. Neural mechanisms of operant conditioning and learning-induced behavioral plasticity in Aplysia. Cell. Molec.. Life Sci 68: 803–816. doi: 10.1007/s00018-010-0570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, and Simmers J. 2012. Functional organization and adaptability of a decision-making network in Aplysia. Front. in Neurosci 6: doi: 10.3389/fnins.2012.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, and Bédécarrats A. 2017. Associative learning in invertebrates. In: The Oxford Handbook of Invertebrate Neurobiology. Byrne JH, Ed. Oxford Handbooks Online. https://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780190456757.001.0001/oxfordhb-9780190456757-e-32. Accessed 8/4/2020 [Google Scholar]
- Nargeot R, Baxter DA, and Byrne JH. 1997. Contingent-dependent enhancement of rhythmic motor patterns: An in vitro analog of operant conditioning. J. Neurosci 17: 8093–8105. doi: 10.1523/jneurosci.17-21-08093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, and Byrne JH. 1999a. In vitro analog of operant conditioning in Aplysia. I. Contingent reinforcement modifies the functional dynamics of an identified neuron. J. Neurosci 19: 2247–2260. doi: 10.1523/jneurosci.19-06-02247.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, and Byrne JH. 1999b. In vitro analog of operant conditioning in Aplysia. II. Modifications of the functional dynamics of an identified neuron contribute to motor pattern selection. J. Neurosci 19: 2261–2272. doi: 10.1523/jneurosci.19-06-02261.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Patterson GW, and Byrne JH. 1999c. Dopaminergic synapses mediate neuronal changes in an analogue of operant conditioning. J Neurophysiol. 81: 1983–1987. doi: 10.1152/jn.1999.81.4.1983 [DOI] [PubMed] [Google Scholar]
- Nargeot R, Petrissans C, and Simmers J. 2007. Behavioral and in vitro correlates of compulsive-like food seeking induced by operant conditioning in Aplysia. J. Neurosci 27: 8059–8070. doi: 10.1523/jneurosci.1950-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Le Bon-Jego M, and Simmers J. 2009. Cellular and network mechanisms of operant learning-induced compulsive behavior in Aplysia. Curr. Biol 19: 975–984. doi: 10.1016/j.cub.2009.05.030 [DOI] [PubMed] [Google Scholar]
- Narusuye K, and Nagahama T. 2002. Cerebral CBm1 neuron contributes to synaptic modulation appearing during rejection of seaweed in Aplysia kurodai. J. Neurophysiol, 88: 2778–2795. doi: 10.1152/jn.00757.2001 [DOI] [PubMed] [Google Scholar]
- Negroni J, Meunier N, Monnerie R, Salesse R, Baly C, Caillol M, and Congar P. 2012. Neuropeptide Y enhances olfactory mucosa responses to odorant in hungry rats. PLoS ONE 7: e45266. doi: 10.1371/journal.pone.0045266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu CL, Costa RM, Homma R, Nagayama S, Baxter DA, and Byrne JH. 2017. Unique configurations of compression and truncation of neuronal activity underlie L-DOPA-induced selection of motor patterns in Aplysia. eNeuro 4: e0206–17.2017 1-18. DoI: 10.1523/ENEURO.0206-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG 2012. Evolution of centralized nervous systems: Two schools of evolutionary thought. Proc. Nat. Acad. Sci, 109 (Supp. 1), 10626–10633. doi: 10.1073/pnas.1201889109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefirowicz TM, and Ewing AG. 1990. Dopamine concentration in the cytoplasmic compartment of single neurons determined by capillary electrophoresis. J. Neurosci. Meth 34: 11–15. doi: 10.1016/0165-0270(90)90036-f [DOI] [PubMed] [Google Scholar]
- Osborne NN, and Cottrell GA. 1970. Occurrence of noradrenaline and metabolites of primary catecholamines in the brain and heart of Helix. Comp. gen, Pharmacol 1: 1–10. [DOI] [PubMed] [Google Scholar]
- Osborne NN, and Cottrell GA. 1971. Distribution of biogenic amines in the slug, Limax maximus. Z. Zellforsch 112: 15–30. doi: 10.1007/bf00665618 [DOI] [PubMed] [Google Scholar]
- Pavlov IP 1927. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. London. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, and Kerr JND. 2008. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci 28: 2435–2446. doi: 10.1523/jneurosci.4402-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar TC 1981a. Voltage-dependent current evoked by dopamine and octopamine in Aplysia. Brain Res. 223: 448–454. doi: 10.1016/0006-8993(81)91163-x [DOI] [PubMed] [Google Scholar]
- Pellmar TC 1981b. Does cyclic 3’,5’ – adenosine monophosphate act as second messenger in a voltage-dependent response to 5-hydroxytrytamine in Aplysia? Br. J. Pharmacol 74: 747–756. doi: 10.1111/j.1476-5381.1981.tb10707.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar TC, and Carpenter DO. 1980. Serotonin induces a voltage-sensitive calcium current in neurons of Aplysia californica. J. Neurophysiol 44: 423–439. doi: 10.1152/jn.1980.44.3.423 [DOI] [PubMed] [Google Scholar]
- Pellmar TC, and Carpenter DO. 1981. Cyclic AMP induces a voltage-dependent current in neurons of Aplysia californica. Neurosci. Lett 22: 151–157. doi: 10.1016/0304-3940(81)90079-3. [DOI] [PubMed] [Google Scholar]
- Pentreath VW, and Berry MS. 1975. Ultrastructure of the terminals of an identified dopamine-containing neurone marked by intracellular injection of radioactive dopamine. J. Neurocytol 4: 249–260. [DOI] [PubMed] [Google Scholar]
- Pentreath VW, Berry MS, and Cottrell GA. 1974. Anatomy of the giant dopamine-containing neurone in the left pedal ganglion of Planorbis corneus. Cell Tissue Res. 151: 369–384. [DOI] [PubMed] [Google Scholar]
- Peretz B, and Estes J. 1974. Histology and histochemistry of the peripheral neural plexus in the Aplysia gill. J. Neurobiol 5: 3–19. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, and Frith CD. 2006. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442: 1042–1045. doi: 10.1038/nature05051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell B, and Cottrell GA. 1974. Dopamine in an identified neuron of Planorbus corneus. J. Neurochem 22: 605–606. doi: 10.1111/j.1471-4159.1974.tb06902.x [DOI] [PubMed] [Google Scholar]
- Prud’homme MJ, Lacroix MC, Badonnel K, Gougis S, Baly C, Salesse R, and Caillol M. 2009. Nutritional status modulates behavioural and olfactory bulb Fos responses to isoamyl acetate or food odour in rats: roles of orexins and leptin. Neuroscience 162: 1287–1298. doi: 10.1016/j.neuroscience.2009.05.043 [DOI] [PubMed] [Google Scholar]
- Puhl JG, and Mesce KA. 2008. Dopamine activates the motor pattern for crawling in the medicinal leech. J. Neurosci 28: 4192–4200. doi: 10.1523/jneurosci.0136-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Arnett BC, and Murphy AD. 1997. Feeding stimulants activate an identified dopaminergic interneuron that induces the feeding motor program in Helisoma. J. Neurophysiol, 78: 812–824. doi: 10.1152/jn.1997.78.2.812 [DOI] [PubMed] [Google Scholar]
- Ramos LJ, Rocafort JLL, and Miller MW. 1995. Behavior patterns of the aplysiid gastropod Bursatella leachii in its natural habitat and in the laboratory. Neurobiol. Learn. Mem, 63: 246–259. doi: 10.1006/nlme.1995.1029 [DOI] [PubMed] [Google Scholar]
- Rathouz MM, and Kirk MD. 1988. Localization of catecholamines in the buccal ganglia of Aplysia californica. Brain Res. 458: 170–175. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, and Gurney K. 1999. The basal ganglia: a vertebrate solution to the selection problem? Neurosci. 89: 1009–1023. doi: 10.1016/s0306-4522(98)00319-4 [DOI] [PubMed] [Google Scholar]
- Rescorla RA 1987. A Pavlovian analysis of goal-directed behavior. Am Psychol. 42: 119–129. doi: 10.1037/0003-066x.42.2.119 [DOI] [Google Scholar]
- Reyes FD, Mozzachiodi R, Baxter DA, and Byrne JH. 2005. Reinforcement in an in vitro analog of appetitive classical conditioning of feeding behavior in Aplysia: Blockade by a dopamine antagonist. Learn. Mem 12: 216–220. doi: 10.1101/lm.92905 [DOI] [PubMed] [Google Scholar]
- Rivard L, Srinivasan J, Stone A, Ochoa S, Sternberg PW, and Loer CM. 2010. A comparison of experience-dependent locomotory behaviors and biogenic amine neurons in nematode relatives of Caenorhabditis elegans. BMC Neurosci. 11: 22. doi: 10.1186/1471-2202-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SC, Teyke T, Miller MW, Weiss KR, and Kupfermann I. 1991. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J. Neurosci 11: 3630–3655. doi: 10.1523/jneurosci.11-11-03630.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben P, and Lukowiak K. 1979. Dopamine modulation of gill reflex behavior in Aplysia. Can. J. Physiol. Pharmacol 57: 329–332. doi: 10.1139/y79-051 [DOI] [PubMed] [Google Scholar]
- Ruben P, and Lukowiak K. 1983. Modulation of the Aplysia gill withdrawal reflex by dopamine. J. Neurobiol 14: 271–284. doi: 10.1002/neu.480140403 [DOI] [PubMed] [Google Scholar]
- Sakharov DA, Borovyagin VL, and Zs.-Nagy L. 1965. Light, fluorescence and electron microscopic studies on “neurosecretion” in Tritonia diomedia Bergh (Mollusca Nudibranchia) Z. Zellforsch 68: 660–673. [DOI] [PubMed] [Google Scholar]
- Sakharov DA, and Zs.-Nagy. L 1968. Localization of biogenic amines in cerebral ganglia of Lymnaea stagnalis. Acta Biol. Acad. Sci. Hung 19: 145–147. [Google Scholar]
- Saleuddin ASM, Mukai ST, Almeida K, and Hatiras G. 2000. Membrane transduction pathway in the neuronal control of protein secretion by the albumen gland in Helisoma (Mollusca). Acta Biol. Hung 51, 243–253. [PubMed] [Google Scholar]
- Salimova NB, Sakharov DA, Miloševic I, Turpaev TM, and Rakic L. 1987a. Monoamine-containing neurons in the Aplysia brain. Brain Res., 400: 285–299. doi: 10.1016/0006-8993(87)90628-7 [DOI] [PubMed] [Google Scholar]
- Salimova NB, Sakharov DA, Milosevic I, and Rakic L. 1987b. Catecholamine-containing neurons in the peripheral nervous system of Aplysia. Acta Biol. Hung 38: 203–212. [PubMed] [Google Scholar]
- Santhanagopalan V, and Yoshino TP. 2000. Monoamines and their metabolites in the freshwater snail Biomphalaria glabrata. Comp. Biochem. Physiol. A: Molec. & Integ. Physiol 125: 469–478. doi: 10.1016/s1095-6433(00)00173-2 [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, and Horvitz HR. 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631. doi: 10.1016/s0896-6273(00)81199-x [DOI] [PubMed] [Google Scholar]
- Schultz W 2007. Behavioral dopamine signals. Trends Neurosci. 30: 203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W 2010. Dopamine signals for reward value and risk: basic and recent data. Behav. Brain Funcs, 6: 24. doi: 10.1186/1744-9081-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan PP, and Montague PR. 1997. A neural substrate of prediction and reward. Science, 275: 1593–1599. doi: 10.1126/science.275.5306.1593 [DOI] [PubMed] [Google Scholar]
- Schwarz M, and Susswein A. 1986. Identification of the neural pathway for reinforcement of feeding when Aplysia learn that food is inedible. J. Neurosci 6: 1528–1536. doi: 10.1523/jneurosci.06-05-01528.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano GE, and Miller MW. 2006. Conditional rhythmicity and synchrony in a bilateral pair of bursting motor neurons in Aplysia. J. Neurophysiol 96: 2056–2071. doi: 10.1152/jn.00282.2006 [DOI] [PubMed] [Google Scholar]
- Sharples SA, Koblinger K, Humphreys JM, and Whelan PJ. 2014. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front. Neural Circuits, 8. doi: 10.3389/fncir.2014.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieling F, Bédécarrats A, Simmers J, Prinz AA, and Nargeot R. 2014. Differential roles of nonsynaptic and synaptic plasticity in operant reward learning-induced compulsive behavior. Current Biol. 24: 941–950. doi: 10.1016/j.cub.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Skinner BF 1938. The behavior of organisms. D. Appleton-Century. New York, NY.. [Google Scholar]
- Sloley BD, and Goldberg JI. 1991. Determination of γ-glutamyl conjugates of monoamines by means of high-performance liquid chromatography with electrochemical detection and application to gastropod tissues. J. Chromatog. B: Biomed. Sci. and Applic 567: 49–56. doi: 10.1016/0378-4347(91)80308-y [DOI] [PubMed] [Google Scholar]
- Stoof JC, De Vlieger TA, and Lodder JC. 1984. Opposing roles for D-1 and D-2 dopamine receptors in regulating the excitability of growth hormone-producing cells in the snail Lymnaea stagnalis. Eur. J. Pharmacol 106: 431–435. doi: 10.1016/0014-2999(84)90735-0 [DOI] [PubMed] [Google Scholar]
- Straub H, and Kuhlmann D. 1984. Identification and quantitative measurements of biogenic monoamines in the central nervous tissue of some gastropods. Comp. Biochem. and Physiol. C: Comp. Pharmacol 78: 319–323. doi: 10.1016/0742-8413(84)90090-2 [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, and Hirth F. 2013. Deep homology of arthropod central complex and vertebrate basal ganglia. Science, 340: 157–161. doi: 10.1126/science.1231828 [DOI] [PubMed] [Google Scholar]
- Svensson E, Woolley J, Wikstrom M, and Grillner S. 2003. Endogenous dopaminergic modulation of the lamprey locomotor network. Brain Res. 970: 1–8. [DOI] [PubMed] [Google Scholar]
- Svensson E, Proekt A, Jing J, and Weiss KR. 2014. PKC-mediated GABAergic enhancement of dopaminergic responses: implication for short-term potentiation at a dual-transmitter synapse. J. Neurophysiol 112: 22–29. doi: 10.1152/jn.00794.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, and Carpenter DO. 1975. Organisation of receptors for neurotransmitters on Aplysia neurones. Nature 258: 751–754. doi: 10.1038/258751a0 [DOI] [PubMed] [Google Scholar]
- Swann JW, Sinback CN, and Carpenter DO. 1978a. Evidence for identified dopamine motor neurons to the gill of Aplysia. Neurosci. Lett, 10: 275–280. doi: 10.1016/0304-3940(78)90239-2 [DOI] [PubMed] [Google Scholar]
- Swann JW, Sinback CN, and Carpenter DO. 1978b. Dopamine-induced muscle contractions and modulation of neuromuscular transmission in Aplysia. Brain Res. 157: 167–172. doi: 10.1016/0006-8993(78)91008-9 [DOI] [PubMed] [Google Scholar]
- Sweeney D 1963. Dopamine: its occurrence in molluscan ganglia. Science 139: 1051. [DOI] [PubMed] [Google Scholar]
- Syed NI, and Winlow W. 1991a. Respiratory behavior in the pond snail Lymnaea stagnalis. I. Behavioral analysis and the identification of motor neurons. J. Comp. Physiol. A 169: 541–555. [Google Scholar]
- Syed NI, and Winlow W. 1991b. Respiratory behavior in the pond snail Lymnaea stagnalis. II. Neural elements of the central pattern generator (CPG). J. Comp. Physiol. A 169: 557–568. [Google Scholar]
- Syed NI, Bulloch AG, and Lukowiak K. 1990. In vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea. Science 250: 282–285. [DOI] [PubMed] [Google Scholar]
- Tamvacakis AN, Senatore A, and Katz PS. 2015. Identification of genes related to learning and memory in the brain transcriptome of the mollusc, Hermissenda crassicornis. Learn. & Mem 22: 617–621. doi: 10.1101/lm.038158.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyke T, Rosen SC, Weiss KR, and Kupfermann I. 1993. Dopaminergic neuron B20 generates rhythmic neuronal activity in the feeding motor circuitry of Aplysia. Brain Res. 630: 226–237. doi: 10.1016/0006-8993(93)90661-6 [DOI] [PubMed] [Google Scholar]
- Ting-A-Kee R, Heinmiller A, and van der Kooy D. 2015. A proposed resolution to the paradox of drug reward: Dopamine’s evolution from an aversive signal to a facilitator of drug reward via negative reinforcement. Neurosci. & Biobehav. Rev 56: 50–61. doi: 10.1016/j.neubiorev.2015.06.016 [DOI] [PubMed] [Google Scholar]
- Trimble DL, and Barker DL. 1984. Activation by dopamine of patterned motor output from the buccal ganglia of Helisoma trivolvis. J. Neurobiol 15: 37–48. doi: 10.1002/neu.480150105 [DOI] [PubMed] [Google Scholar]
- Trimble DL, Barker DL, and Bullard BJ. 1984. Dopamine in a molluscan nervous system: Synthesis and fluorescence histochemistry. J. Neurobiol 15: 27–36. doi: 10.1002/neu.480150104 [DOI] [PubMed] [Google Scholar]
- Tritt SH, and Byrne JH. 1982. Neurotransmitters producing and modulating opaline gland contraction in Aplysia californica. J. Neurophysiol, 48: 1347–1361. doi: 10.1152/jn.1982.48.6.1347 [DOI] [PubMed] [Google Scholar]
- Tritt SH, Posner Lowe I, and Byrne JH. 1983. A modification of the glyoxylic acid induced histofluorescence technique for demonstration of catecholamines and serotonin in tissues of Aplysia californica. Brain Res. 259: 159–162. doi: 10.1016/0006-8993(83)91081-8 [DOI] [PubMed] [Google Scholar]
- Ungless MA 2004. Dopamine: the salient issue. Trends in Neurosci 27: 702–706. doi: 10.1016/j.tins.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Vaasjo LO, Quintana AM, Habib MR, Mendez de Jesus P, Croll RP, and Miller MW. 2018. GABA-like immunoreactivity in Biomphalaria: Colocalization with tyrosine hydroxylase-like immunoreactivity in the feeding motor systems of panpulmonate snails. J. Comp. Neurol 526: 1790–1805. doi: 10.1002/cne.24448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo D, Habib MR, Delgado N, Vaasjo LO, Croll RP, and Miller MW. 2014. Localization of tyrosine hydroxylase-like immunoreactivity in the nervous systems of Biomphalaria glabrata and Biomphalaria alexandrina, intermediate hosts for schistosomiasis. J. Comp. Neurol 522: 2532–2552. doi: 10.1002/cne.23548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RJ 1986. Transmitters and modulators. The Mollusca. Neurobiology and Behavior Willows AOD, Ed., Vol. 9: 279–485. Academic Press. Cambridge, MA. [Google Scholar]
- Walker RJ, and Kerkut GA. 1978. The first family (adrenaline, noradrenaline, dopamine, octopamine, tyramine, phenylethanolamine, and phenylethylamine). Comp. Biochem. Physiol 61C: 261–266. [DOI] [PubMed] [Google Scholar]
- Walker RJ, Woodruff GN, Glaizner B, Sedden CB, and Kerkut GA. 1968. The pharmacology of Helix dopamine receptor of specific neurones in the snail, Helix aspersa. Comp. Biochem. Physiol 24: 455–469. doi: 10.1016/0010-406x(68)90997-3 [DOI] [PubMed] [Google Scholar]
- Walker RJ, Ralph KL, Woodruff GN, and Kerkut GA. 1971. Evidence for a dopamine inhibitory post-synaptic potential in the brain of Helix aspersa. Comp. gen. Pharmacol 2: 15–26. [DOI] [PubMed] [Google Scholar]
- Wentzell MM, Martínez-Rubio C, Miller MW, and Murphy AD. 2009. Comparative neurobiology of feeding in the opisthobranch sea slug, Aplysia, and the pulmonate snail, Helisoma: Evolutionary considerations. Brain, Behav. Evol 74: 219–230. doi: 10.1159/000258668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werkman TR, De Vlieger TA, and Stoof JC. 1990. Indications for a hormonal function of dopamine in the central nervous system of the snail Lymnaea stagnalis. Neurosci. Lett, 108: 167–172. doi: 10.1016/0304-3940(90)90725-o [DOI] [PubMed] [Google Scholar]
- Werkman TR, van Minnen J, Voorn P, Steinbusch HWM, Westerink BHC, De Vlieger TA, and Stoof JC. 1991. Localization of dopamine and its relation to the growth hormone producing cells in the central nervous system of the snail Lymnaea stagnalis. Exp. Brain Res 85: 1–9. doi: 10.1007/bf00229981 [DOI] [PubMed] [Google Scholar]
- White NM 1989. Reward or reinforcement: What’s the difference? Neurosci. & Biobehav. Rev 13: 181–186. doi: 10.1016/s0149-7634(89)80028-4 [DOI] [PubMed] [Google Scholar]
- Wieland S, and Gelperin A. 1983. Dopamine elicits feeding motor program in Limax maximus. J. Neurosci 3: 1735–1745. doi: 10.1523/jneurosci.03-09-01735.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM and Goldman-Rakic PS. 1998. Widespread origin of the primate mesofrontal dopamine system. Cereb. Cortex 8: 321–345. doi: 10.1093/cercor/8.4.321 [DOI] [PubMed] [Google Scholar]
- Wilson W, and Wachtel HH, 1978. Prolonged inhibition in burst firing neurons: synaptic inactivation of the slow regenerative inward current. Science, 202: 772–775. doi: 10.1126/science.715442 [DOI] [PubMed] [Google Scholar]
- Winlow W, and Benjamin PR. 1977. Postsynaptic effects of a multiaction giant interneurone on identified snail neurones. Nature 268: 263–265. [DOI] [PubMed] [Google Scholar]
- Winlow W, Haydon PG, and Benjamin PR. 1981. Multiple postsynaptic actions of the giant dopamine-containing neurone R.Pe.D.1 of Lymnaea stagnalis. J. Exp. Biol 94: 137–148. [Google Scholar]
- Wise RA 2004. Dopamine, learning and motivation. Nature Rev. Neurosci 5: 483–494. doi: 10.1038/nrn1406 [DOI] [PubMed] [Google Scholar]
- Wise RA 2008. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox. Res 14: 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff GN 1971. Dopamine receptors: A review. Comp. Gen. Pharmacol 2: 439–455. doi: 10.1016/0010-4035(71)90041-3 [DOI] [PubMed] [Google Scholar]
- Woodruff GN, and Walker RJ. 1969. The effect of dopamine and other compounds on the activity of neurones of Helix aspersa; Structure-activity relationships. Int. J. Neuropharm 8: 279–289. doi: 10.1016/0028-3908(69)90049-5 [DOI] [PubMed] [Google Scholar]
- Wyeth RC, and Croll RP. 2011. Peripheral sensory cells in the cephalic sensory organs of Lymnaea stagnalis. J. Comp. Neurol 519: 1894–1913. doi: 10.1002/cne.22607 [DOI] [PubMed] [Google Scholar]
- Yavari P, Walker RJ, and Kerkut GA. 1979. The effect of D-tubocurarine on the responses of snail neurones to ACh, 5-HT glutamate and dopamine – receptors or ionophores? Comp. Biochem. Physiol. C 64: 101–114. [DOI] [PubMed] [Google Scholar]