Supplemental Digital Content is available in the text.
Keywords: Alzheimer disease, amyloid-β, autophagy, Drosophila, mitochondria, vitamin K2
Abstract
Objective
Alzheimer disease is characterized by progressive decline in cognitive function due to neurodegeneration induced by accumulation of Aβ and hyperphosphorylated tau protein. This study was conducted to explore the protective effect of vitamin K2 against Aβ42-induced neurotoxicity.
Methods
Alzheimer disease transgenic Drosophila model used in this study was amyloid beta with the arctic mutation expressed in neurons. Alzheimer disease flies were treated with vitamin K2 for 28 days after eclosion. Aβ42 level in brain was detected by ELISA. Autophagy-related genes and NDUFS3, the core subunit of mitochondrial complex I, were examined using real-Time PCR (RT-PCR) and western blot analysis.
Results
Vitamin K2 improved climbing ability (P = 0.0105), prolonged lifespan (P < 0.0001) and decreased Aβ42 levels (P = 0.0267), upregulated the expression of LC3 and Beclin1(P = 0.0012 and P = 0.0175, respectively), increased the conversion of LC3I to LC3II (P = 0.0206) and decreased p62 level (P =0.0115) in Alzheimer disease flies. In addition, vitamin K2 upregulated the expression of NDUFS3 (P = 0.001) and increased ATP production (P = 0.0033) in Alzheimer disease flies.
Conclusion
It seems that vitamin K2 protect against Aβ42-induced neurotoxicity by activation of autophagy and rescue mitochondrial dysfunction, which suggests that it may be a potential valuable therapeutic approach for Alzheimer disease.
Introduction
Alzheimer disease is characterized by progressive decline in cognitive function. The pathological features of Alzheimer disease are deposition of amyloid-β (Aβ) and accumulation of hyperphosphorylated tau protein (pTau) [1]. Accumulating evidence demonstrates that autophagy dysfunction is involved in the pathogenesis of Alzheimer disease. The first direct evidence is that Nixon identified immature autophagy vacuoles accumulated in dystrophic neurons in Alzheimer disease brains [2]. Studies also found that downregulated expressions of some autophagy-related genes occurred in Alzheimer disease, resulting in autophagy dysfunction and reducing the clearance of Aβ [3].
Mitochondria are the main site of ATP production and are considered as ‘powerhouses’ of cells. Many studies have confirmed that mitochondrial dysfunction is involved in Alzheimer disease. In 2004, Swerdlow and Khan [4] proposed a ‘mitochondrial cascade hypothesis’, which declared that mitochondrial dysfunction results in ATP production decline and excessive reactive oxygen species production, which lead to the formulation of Aβ plaques and neurofibrillary tangles. In return, Aβ and pTau interfered with enzyme metabolism and the dynamic system of mitochondria [5]. In addition, autophagy dysfunction leads to reducing the clearance of damaged mitochondria and subsequent accumulation in cells, which in return exacerbates mitochondrial damage [6]. Therefore, a ‘vicious cycle’ was formed among mitochondrial dysfunction, Aβ deposition and autophagy dysfunction.
Vitamin K2 generates from the activity of intestinal bacteria or the conversion of dietary vitamin K1 [7]. It has been reported that vitamin K2 has a variety of biological functions, including anti-inflammation [8], antioxidant stress, antiapoptosis [9], stimulating autophagy [10] and serves as a mitochondrial electron carrier during oxidative respiration [11].
Therefore, based on the pathogenesis of Alzheimer disease and the pharmacological effects of vitamin K2, we hypothesized that vitamin K2 has a protective effect on Alzheimer disease. A study in vitro preliminary has shown that vitamin K2 reduces the Aβ-induced cytotoxicity and improves cell survival [8]. In this study, we set out to establish Alzheimer disease transgenic Drosophila to further demonstrate the protective effect of vitamin K2 and explore its neuroprotective mechanism in vivo.
Materials and methods
Animal strains
Drosophila was cultured on standard medium at 25 °C. The drosophila strains used in this study included: P {UAS-Aβarc} (line for expressing arctic mutant human Aβ42) [12] and wild-type w1118 and [GAL4]A307(drives expression in the giant fiber system). P{UAS-Aβarc} line was crossed to [GAL4]A307 line to receive male filial generation 1(F1) drosophilas expressing arctic mutant Aβ42 in the giant fiber system, [GAL4]A307/{UAS-Aβarc}, which were used for the next series of experiments.
Vitamin K2 treatment paradigm
Vitamin K2 (Sigma, Saint Louis, Missouri, USA) was dissolved in anhydrous ethanol to obtain a 0.4 M stock solution. According to different concentrations, the flies were divided into five groups: (1)wild-type A307/w1118, no treatment with K2, (2)A307/Aβarc, no treatment with K2, (3)A307/Aβarc + 0.1 mM K2, treated with vitamin K2 at 0.1 mM, (4)A307/Aβarc + 0.5 mM K2, treated with vitamin K2 at 0.5 mM and (5)A307/Aβarc + 0.8 mM K2, treated with vitamin K2 at 0.8 mM. A total of 100 μL vitamin K2 solution was added into each vial containing 20 flies daily until 28 days. The flies were transferred into fresh food every 7 days.
Fly behavioral assays
Fly climbing ability
The automatic iterative negative geotaxis (RING) assay was used to detect the fly climbing ability [13]. About 80 flies from each group were divided into different testing tubes. Flies were automatically tapped four consecutive times by the RING apparatus to fell to the bottom of the tubes. Then flies began to climb up along the walls. The climbing behavior of flies was recorded by a digital video. The above process was repeated three times by 1 min intervals. The height of each fly at the tenth second was measured by software RflyDetection2.0 [13], and the average value of three trials was obtained to evaluate the fly climbing ability.
Longevity assay
A total of 80 flies from each group was equally distributed to four vials containing standard fly food and incubated at 25 °C. The food vials were replaced every 3 days and the dead flies were counted until all the flies died. Survival curves were analyzed with the GraphPad Prism 8 software. Fly behavioral assays provided the basis for selecting the optimal concentration of K2 for the following assessments.
Aβ42 detection by ELISA
After dissected on the ice, 30 heads of flies from each group were immediately placed into 50 μL cold ELISA sample buffer containing cocktail protease inhibitors. The heads were thoroughly homogenized and incubated at room temperature for 4 h. The supernatant was collected after centrifugation at 12000 × g and 4 °C for 10 min. A total of 3 μL supernatant was diluted to 60 μL with standard dilution buffer, and 50 μL diluent was taken for ELISA. According to the manufacturer’s instructions, detection of Aβ42 levels was performed using the Aβ42 Human ELISA Kit (Invitrogen, catalog number KHB3441, Carlsbad, California, USA).
Real-time fluorescent quantitative PCR analysis
We used Trizol to extract total RNA. RNA concentration was measured with NanoDrop 2000 (Thermo Scientific, Waltham, Massachusetts, USA). PrimeScriptTM II 1st Strand cDNA Synthesis Kit (TaKaRa, Japan) was used to synthesis cDNA. The sequence of the forward and reverse primers is listed as follows: LC3 Fw primer: 5′-AGGATGCCCTCTTCTTCTTTG-3′, Rev primer: 5′-GAAATAGTCCTCCTCGT GATGTT-3′; Beclin1 Fw primer: 5′-ACAGGAACGACAATGAGT GAG-3′, Rev primer: 5′-TCCGTAGATGGGCAAAGATAAC-3′; NADH dehydrogenase (ubiquinone) ferrithionein 3(NDUFS3) Fw primer: 5′-GCTCG CATCTCTCCGATTT-3′, Rev primer: 5′-AATAAGCACCTCCAGCTCATC-3′.
The real-Time PCR (RT-PCR) reaction system included: 4 ng cDNA, 5 pmol primer, 5 μL Power SYBR Green Master Mix (Thermo Fisher Scientific, USA), 3 μLRNase Freed H2O. SYBR Green was used to detect double-stranded DNA. The PCR amplification was carried out using Applied Biosystems device (7500Fast RT-PCR system, Thermo Fisher Scientific) under the following conditions: 40 cycles of 10 min at 95 °C, 15 s at 95 °C and 1 min at 60 °C. 18 s acted as an endogenous control for data normalization. Relative mRNA expression was determined by the 2−△△Cq method [14]. Relative quantitative analysis of data was conducted with the GraphPad Prism 8 software.
Western blotting
For extracting total protein, 30 heads of flies from each group were homogenized and lysed in RIPA and 1:100 inhibitor proteases and inhibitor phosphatases cocktail (Thermo Fisher Scientific).For each group, a total of 30 μg protein was separated using 12% SDS-PAGE and transferred to a polyvinylidene fuoride membrane. After incubated in 5% BSA (Solarbio, China) at room temperature for 2 h, the membranes were incubated with primary antibodies against β-actin, LC3(Abcam, England), P62(Abcam, England) and NDUFS3(Abcam, England) overnight at 4 °C. After washed with TBST, the membrane was incubated with horseradish peroxidase-conjugated antirabbit or antimouse for 1 h at room temperature. Bands were visualized using an electro-chemi-Luminescence chemiluminescence kit (Thermo Fisher Scientific) on BIO-RAD ChemiDocTM XRS + system (USA), and then the grayscale value of bands were scanned with the Image J software.
ATP measurements
A total of 15 heads of flies from each group was homogenized in 1.5 mL pyrolysis liquid. The ATP content was measured according to the manufacturer’s instructions of the ATP Determination Kit (Beyotime, China). ATP concentrations were determined with TECAN infinite F500 (Switzerland).
Statistical analyses
All data were expressed as mean ± SD. Statistical significance was set at P ≤ 0.05. All statistical analyses were performed using GraphPad Prism 8 software. One-way analysis of variance was used for statistical significance and followed by Dunnett’s post hoc test for comparison between every two groups.
Results
Effects of vitamin K2 on the behavior of Alzheimer disease flies
The automatic RING assay revealed that the climbing ability of A307/Aβarc flies declined compared to wild-type A307/w1118 flies at the same age. This Aβ42-induced locomotor defect was ameliorated by different concentrations of vitamin K2 (from 0.1 to 0.8 mM), but a significant difference was only shown in the 0.5 mM concentration (P = 0.0105) (Fig. 1a).
Fig. 1.
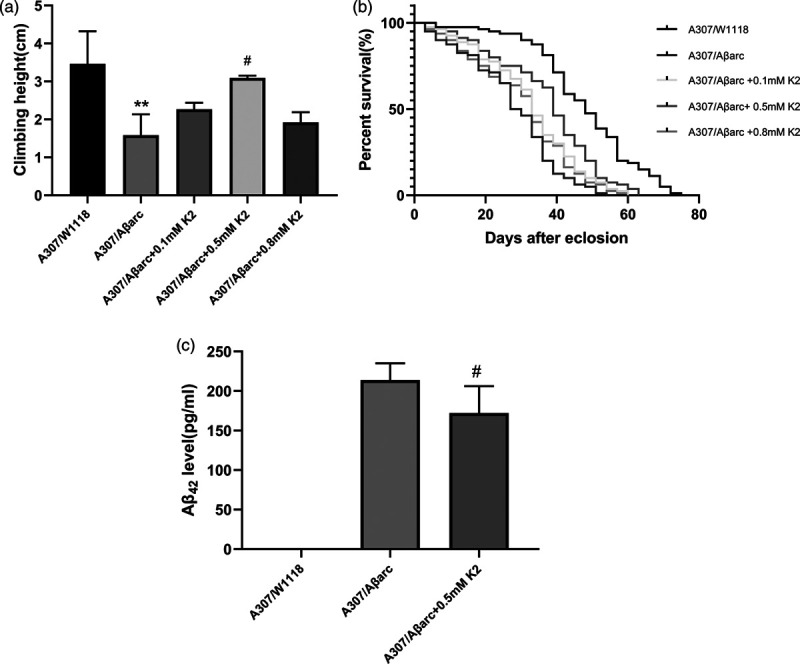
Improvement of climbing ability and lifespan in Alzheimer disease flies by vitamin K2. (a) Analysis of the climbing ability using aRING assay in different groups of flies, one-way ANOVA using Dunnett’s test; **P < 0.01 vs. A307/W1118; # P < 0.05 vs. A307/Aβarc. (b) The lifespan in different groups of flies, n = 80 flies for each line, log-rank test, compared with A307/Aβarc flies, P < for A307/Aβarc + 0.1 mM K2 and A307/Aβarc + 0.5 mM K2 flies was P < 0.05 and P < 0.001, respectively.(c) ELISA analysis human Aβ42 level, one-way ANOVA using Dunnett’s test; #P < 0.05 vs. A307/Aβarc. As expected, the A307/W1118 flies did not express human Aβ42. ANOVA, analysis of variance.
The lifespan of the A307/Aβarc flies was shorter than that of A307/w1118 flies. Vitamin K2 (0.1–0.8 mM) prolonged lifespan of Alzheimer disease flies; significant difference was both shown in the 0.1 mM and 0.5 mM concentration(P = 0.0097 and P < 0.0001, respectively) (Fig 1b).
Based on the above data, the 0.5 mM concentration of vitamin K2 was used as optimal concentration for the following experiments.
ELISA analysis revealed that treatment with vitamin K2 markedly decreased Aβ42 level (P = 0.0267) (Fig. 1c).
Effects of vitamin K2 on autophagy-related gene
To investigate the effects of vitamin K2 on autophagy, LC3, Beclin1 and p62 were detected. RT-PCR analysis showed that treatment with vitamin K2 significantly increased LC3 and Beclin1 (P = 0.0012 and P = 0.0175, respectively) (Fig. 2).
Fig. 2.
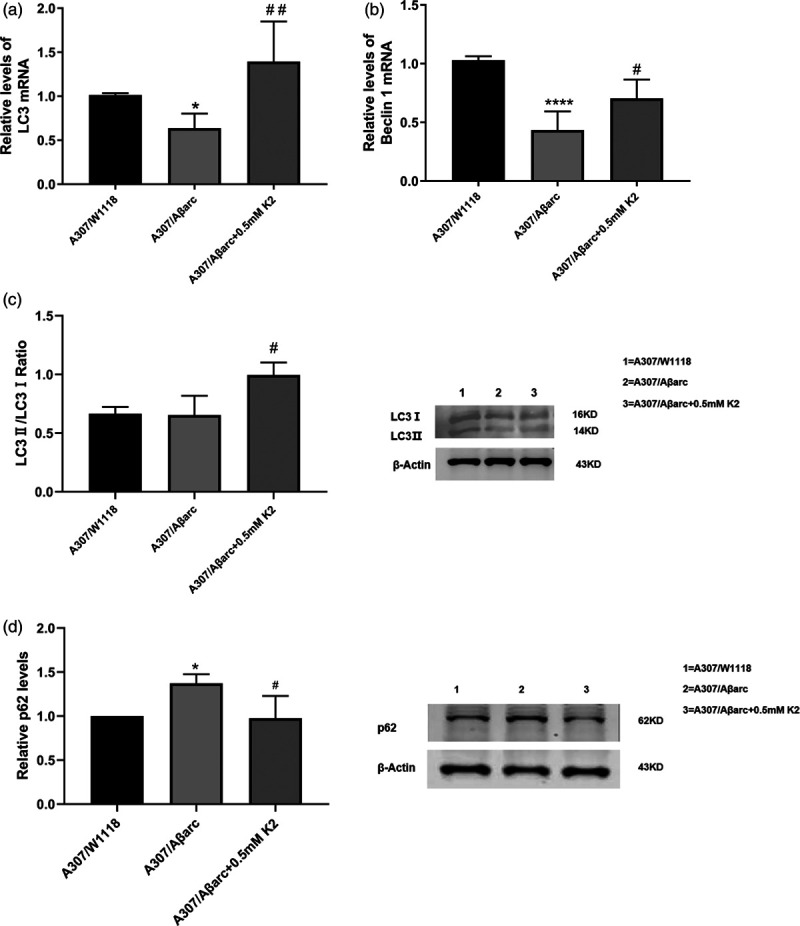
Effects of vitamin K2 on autophagy-related genes. (a,b) Normalized quantification of the LC3 and Beclin1 mRNA in flies, one-way ANOVA using Dunnett’s test, compared with A307/Aβarc flies, P for A307/Aβarc + 0.5 mM K2 flies was ##P < 0.05, and #P < 0.01, respectively. (c,d) Western blot and quantitative analysis show that LC3II/LC3I ratio level were higher in the A307/Aβarc + 0.5 mM K2 flies compared to A307/Aβarc flies (#P < 0.05), and p62 level decreased in the A307/Aβarc + 0.5 mM K2 flies compared to A307/Aβarc flies (#P < 0.05). ANOVA, analysis of variance.
Western blot (WB) analysis showed that treatment with vitamin K2 increased the conversion of LC3 I to LC3 II (P = 0.0206) and decreased p62 level (P = 0.0115) (Fig. 2). The above data revealed that vitamin K2 can activate autophagy.
Vitamin K2 rescued mitochondrial dysfunction
To assess the effect of vitamin K2 on mitochondria, we have measured ATP production and NDUFS3. We found that the ATP level of A307/Aβarc flies were significantly lower than that of A307/W1118 flies (P = 0.0013). Treatment with vitamin K2 significantly increased the ATP level (P = 0.0033) (Fig. 3).
Fig. 3.
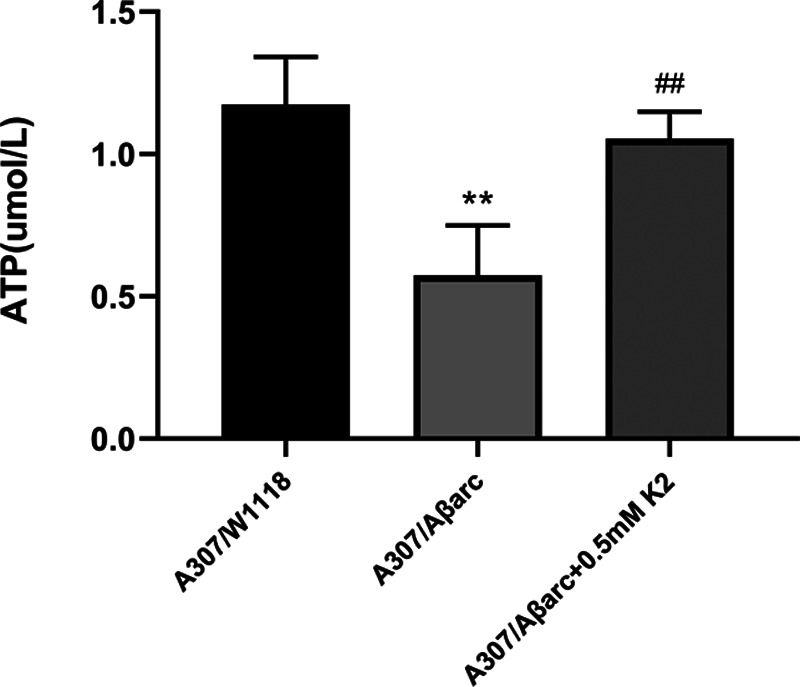
Vitamin K2 increased the ATP level. The supernatant from the brain homogenate was used to assay the ATP level, one-way ANOVA using Dunnett’s test, **P < 0.01 vs. A307/W1118, ##P < 0.01 vs. A307/Aβarc. ANOVA, analysis of variance.
NDUFS3, the core subunit of mitochondrial complex I, participates in the electron transport of the oxidized respiratory chain. RT-PCR and WB revealed that the expression of NDUFS3 declined in A307/Aβarc flies, and vitamin K2 upregulated the expression of NDUFS3 (Fig. 4).
Fig. 4.
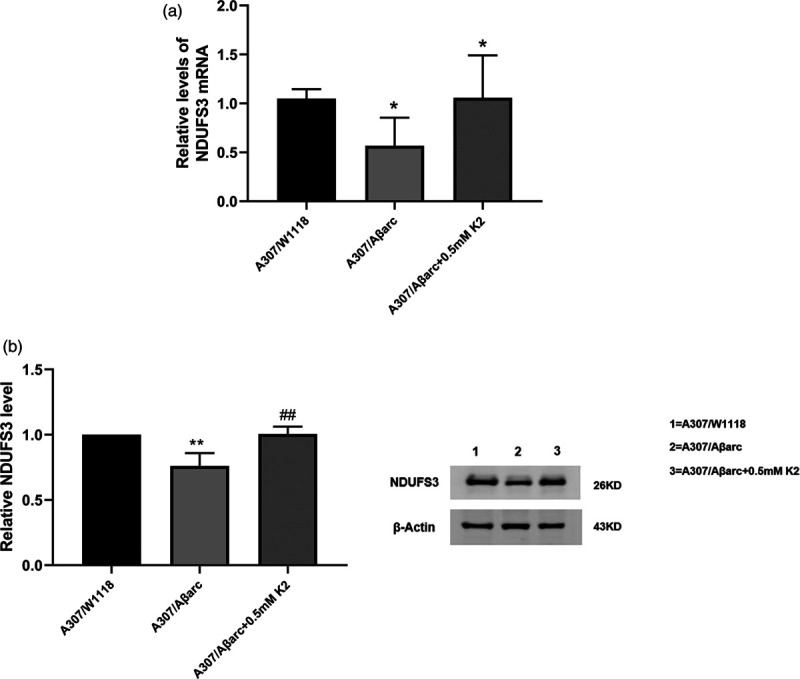
Vitamin K2 increased NDUFS3 expression. (a) Normalized quantification of the NDUFS3 mRNA in flies, one-way ANOVA using Dunnett’s test, *P < 0.05 vs. A307/W1118, #P < 0.05 vs. A307/Aβarc. (b) Western blot and quantitative analysis show the NDUFS3 level in flies, one-way ANOVA using Dunnett’s test, **P < 0.05 vs. A307/W1118, ##P < 0.05 vs. A307/Aβarc. ANOVA, analysis of variance.
Discussion
In this study, we explored the neuronal protective effects of vitamin K2 on Alzheimer disease flies. The results showed that vitamin K2 improved locomotor abilities, prolonged lifespan and significantly decreased Aβ42 level. Further studies showed that vitamin K2 increased the LC3II/LC3I ratio and decreased the p62 level. Moreover, vitamin K2 upregulated the expression of NDUFS3 and increased the ATP level.
The transgenic Drosophila Alzheimer disease model used in this study was [Gal4]A307/P{UAS-Aβarc}. Previous studies have confirmed that compared to wild-type, the climbing ability of A307/Aβarc flies declined and the lifespan shortened [15]. In this study, we obtained the same results. This Aβ42-induced behavioral defect was ameliorated by vitamin K2.
Autophagy is crucial for clearing abnormal proteins in neurodegenerative disorders. Aβ oligomers detected in purified intact autophagosomes confirmed that autophagy plays a direct role in the clearance of Aβ [15]. In the autophagy–lysosomal pathway, the role of LC3 II is to promote phagophore elongation and closure to form a complete autophagosome [16]. Beclin1 plays a key role in the initiation of autophagosome and autophagy regulation [17]. Thus, LC3-II and Beclin1 have been regarded as autophagy markers. P62 plays an important role in the degradation of abnormal protein through two of its functional domains including the ubiquitin-associated domain and LC3-interaction region [18]. P62 aggregates containing autophagy substrates are degraded by proteolytic enzymes in the lysosome; therefore, an increased p62 level reflects lysosome dysfunction. In this study, Alzheimer disease flies showed autophagy dysfunction. Vitamin K2 increased the LC3II/LC3I ratio and Beclin1 level and decreased the p62 level. These findings suggested that vitamin K2 can activate autophagy and maintain autophagy flow, which could contribute to the clearance of Aβ, and thereby reduce the Aβ-induced neurotoxicity. Previous studies have demonstrated that activation of autophagy by different strategies, including genetic intervention, pharmacological intervention and physiological intervention, could reduce Aβ deposition, ameliorate pathological phenotypes and rescue cognitive deficits in Alzheimer disease [19–21].
On the basis of the previous studies, we speculated that the possible mechanisms of vitamin K2 inducing Aβ42-affected autophagy may include: first, electron microscopy revealed the formation of autophagosomes and autolysosomes increased in K2-treated leukemia cells [22], &&which indicated that vitamin K2 treatment could activate autophagy. Second, Aβ can be removed by Aβ degrading enzymes, autophagy and blood–brain barrier (BBB) transport in the brain [23]. Vitamin K2 may promote Aβ clearance by upregulating the mRNA expression of insulin degrading enzyme and NEP1, the Aβ degradation enzymes (Supplementary Data Fig. 5, Supplemental digital content 1, http://links.lww.com/WNR/A618). Transport of Aβ through BBB from brain to blood is mainly mediated by receptors such as low-density lipoprotein receptor (LDLR) and low-density lipoprotein receptor-related protein-1 (LRP1) [24,25]. A study found that MK-4 increased the gene expression of LDLR and LRP1 [26]. So we speculated that vitamin K2 may promote the transport of Aβ through BBB by increasing LDLR and LRP1 expression. Therefore, vitamin K2 may eliminate the effect of Aβ on autophagy by promoting Aβ clearance. In addition, further research is needed to investigate the effects of vitamin K2 on the mechanisms of autophagy regulation, such as the effects of vitamin K2 on the mTOR/TOR pathway and PI3K-Beclin1 pathway.
It has been confirmed that mitochondrial dysfunction is involved in Alzheimer disease. ‘Mitochondrial cascade hypothesis’ states that mitochondrial dysfunction can promote amyloid precursor protein processing towards Aβ production and accumulation, and then trigger amyloid cascade [27]. On the other hand, Aβ accumulation adversely accelerate mitochondrial dysfunction [28,29]. In this study, we found that the expression of NDUFS3 declined in Alzheimer disease flies, which directly affected electron transport of mitochondria oxidize respiratory chain, resulting in reduction of ATP production. Vitamin K2 could increase the expression of NDUFS3 and ATP levels, which suggests that vitamin K2 has a protective effect on mitochondria.
According to our findings and previous reports, we speculated that the mechanisms of vitamin K2 rescue mitochondrial dysfunction may include: first, vitamin K2 serves as electron carrier to transfer electrons in the mitochondrial respiratory chain, increase mitochondrial membrane potential and promote more ATP production [11]. Second, vitamin K2 upregulates the expression of NDUFS3, which directly enhances electron transport. Third, vitamin K2 may promote the clearance of damaged mitochondria by activating autophagy.
Conclusion
In conclusion, this study revealed that Aβ42 may induce neurotoxicity by damaging autophagy and mitochondrial function. The protective effects of vitamin K2 against Aβ42 may be through activating autophagy and improving mitochondrial function. Therefore, vitamin K2 may be a potentially valuable therapeutic approach for Alzheimer disease.
Acknowledgements
The authors thank Dr. Fu-De Huang, Chinese Academy of Sciences for donating P{UAS-Aβarc} and [GAL4]A307 fly strains. This study was supported by the Natural Science Foundation of Guangxi Province (CN) [grant numbers 2018GXNSFAA138110]. Animal strains used in this study are Drosophila.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.
References
- 1.Yee A, Tsui NB, Chang YN, Au CS, Fok M, Lau LT, et al. Alzheimer’s disease: insights for risk evaluation and prevention in the Chinese population and the need for a comprehensive programme in Hong Kong/China. Hong Kong Med J. 2018; 24:492–500. [DOI] [PubMed] [Google Scholar]
- 2.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005; 64:113–122. [DOI] [PubMed] [Google Scholar]
- 3.Lachance V, Wang Q, Sweet E, Choi I, Cai CZ, Zhuang XX, et al. Autophagy protein NRBF2 has reduced expression in Alzheimer’s brains and modulates memory and amyloid-beta homeostasis in mice. Mol Neurodegener. 2019; 14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004; 63:8–20. [DOI] [PubMed] [Google Scholar]
- 5.Lu MH, Zhao XY, Yao PP, Xu DE, Ma QH. The mitochondrion: a potential therapeutic target for Alzheimer’s disease. Neurosci Bull. 2018; 34:1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci. 2017; 40:151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferland G. Vitamin K and brain function. Semin Thromb Hemost. 2013; 39:849–855. [DOI] [PubMed] [Google Scholar]
- 8.Saputra WD, Aoyama N, Komai M, Shirakawa H. Menaquinone-4 suppresses lipopolysaccharide -induced inflammation in MG6 mouse microglia-derived cells by inhibiting the NF-κB Signaling pathway. Int J Mol Sci. 2019; 20:E2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadipour E, Tayarani-Najaran Z, Fereidoni M. Vitamin K2 protects PC12 cells against Aβ (1-42) and H2O2-induced apoptosis via p38 MAP kinase pathway. Nutr Neurosci. 2018; 30:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Miyazawa S, Moriya S, Kokuba H, Hino H, Takano N, Miyazawa K. Vitamin K2 induces non-apoptotic cell death along with autophagosome formation in breast cancer cell lines. Breast Cancer. 2020; 27:225–235. [DOI] [PubMed] [Google Scholar]
- 11.Vos M, Esposito G, Edirisinghe JN, Vilain S, Haddad DM, Slabbaert JR, et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 2012; 336:1306–1310. [DOI] [PubMed] [Google Scholar]
- 12.Alisi L, Cao R, De Angelis C, Cafolla A, Caramia F, Cartocci G, et al. The relationships between Vitamin k and cognition: a review of current evidence. Front Neurol. 2019; 10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Han M, Li Q, Zhang X, Wang WA, Huang FD. Automated rapid iterative negative geotaxis assay and its use in a genetic screen for modifiers of Aβ(42)-induced locomotor decline in Drosophila. Neurosci Bull. 2015; 31:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 15.Zhao XL, Wang WA, Tan JX, Huang JK, Zhang X, Zhang BZ, et al. Expression of beta-amyloid induced age-dependent presynaptic and axonal changes in Drosophila. J Neurosci. 2010; 30:1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard A, Klionsky DJ. Defining the membrane precursor supporting the nucleation of the phagophore. Autophagy. 2014; 10:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill SM, Wrobel L, Rubinsztein DC. Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ. 2019; 26:617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin X, Li S, Zhao Y, Ma X, Zhang K, He X, Wang Z. Interaction domains of p62: a bridge between p62 and selective autophagy. DNA Cell Biol. 2013; 32:220–227. [DOI] [PubMed] [Google Scholar]
- 19.Rocchi A, Yamamoto S, Ting T, Fan Y, Sadleir K, Wang Y, et al. A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer’s disease. Plos Genet. 2017; 13:e1006962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vegh C, Pupulin S, Wear D, Culmone L, Huggard R, Ma D, Pandey S. Resumption of autophagy by Ubisol-Q10 in presenilin-1 mutated fibroblasts and transgenic AD mice: implications for inhibition of senescence and neuroprotection. Oxid Med Cell Longev. 2019; 2019:7404815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Zhou H, Yin T, Gong Y, Yuan G, Chen L, Liu J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J Colloid Interface Sci. 2019; 552:388–400. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama T, Miyazawa K, Naito M, Toyotake J, Tauchi T, Itoh M, et al. Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy. 2008; 4:629–640. [DOI] [PubMed] [Google Scholar]
- 23.Xin SH, Tan L, Cao X, Yu JT, Tan L. Clearance of amyloid beta and tau in Alzheimer’s disease: from mechanisms to therapy. Neurotox Res. 2018; 34:733–748. [DOI] [PubMed] [Google Scholar]
- 24.Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q. Role of blood-brain barrier in Alzheimer’s disease. J Alzheimers Dis. 2018; 63:1223–1234. [DOI] [PubMed] [Google Scholar]
- 25.Yao L, Gu X, Song Q, Wang X, Huang M, Hu M, et al. Nanoformulated alpha-mangostin ameliorates Alzheimer’s disease neuropathology by elevating LDLR expression and accelerating amyloid-beta clearance. J Control Release. 2016; 226:1–14. [DOI] [PubMed] [Google Scholar]
- 26.Rønning SB, Pedersen ME, Berg RS, Kirkhus B, Rødbotten R. Vitamin K2 improves proliferation and migration of bovine skeletal muscle cells in vitro. PLoS One. 2018; 13:e0195432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014; 1842:1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta -amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002; 80:91–100. [DOI] [PubMed] [Google Scholar]
- 29.Hou Y, Ghosh P, Wan R, Ouyang X, Cheng H, Mattson MP, Cheng A, et al. Permeability transition pore-mediated mitochondrial superoxide flashes mediate an early inhibitory effect of amyloid beta1-42 on neural progenitor cell proliferation. Neurobiol Aging. 2014; 35:975–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


