Abstract
Purpose
Patients evaluated in the emergency department (ED) who have concerning symptoms suggestive of a cancer diagnosis are mostly referred to the quick diagnosis unit of our tertiary hospital. This study analyzed the impact of the Covid-19 pandemic on the volume, disease patterns, and accessibility to essential investigations of patients with suspected cancer referred by the ED to this unit.
Methods
Trends in referrals were analyzed from January 1 to July 8, 2020 and the corresponding dates of 2019. Only non-Covid-19 conditions were evaluated. Three time-based cohorts were defined: prepandemic (January 1–February 19), pandemic (February 19–April 22), and postpandemic (April 22–July 8). Along with descriptive statistics, linear regression was used to test for time trends with weekly referrals as the dependent variable.
Results
There were 384, 193, and 450 patients referred during the prepandemic, pandemic, and postpandemic periods, respectively. Following an increasing rate, referrals decreased to unprecedented levels in the pandemic period (average weekly slope: −2.1 cases), then increasing again until near normalization. Waiting times to most diagnostic procedures including radiology, endoscopic, nuclear medicine, and biopsy/cytology during the pandemic period were significantly delayed and time-to-diagnosis was considerably longer (19.72 ± 10.37 days vs. 8.33 ± 3.94 days in prepandemic and 13.49 ± 6.45 days in postpandemic period; P < 0.001 in both). Compared to other cohorts, pandemic cohort patients were more likely to have unintentional weight loss and fever of unknown origin as referral indications while anemia and lymphadenopathy were less common. Patients from the pandemic cohort had a significantly lower rate of malignancies and higher of benign gastrointestinal disorders (40.93% vs. 19.53% and 20.89% in prepandemic and postpandemic periods, respectively; P < 0.001 in both), most notably irritable bowel disease, and of mental and behavioral disorders (15.54% vs. 3.39% and 6.00% in prepandemic and postpandemic periods, respectively; P < 0.001 in both).
Conclusions
As our hospital switched its traditional care to one focused on Covid-19 patients, recognized indicators of healthcare quality of quick diagnosis units were severely disrupted. The clinical patterns of presentation and diagnosis of the pandemic period suggested that mass media-generated mental and behavioral responses with distressing symptoms played a significant role in most of these patients.
Keywords: Covid-19, Quick diagnosis units, Pandemic, Hospital ambulatory medicine, Suspected cancer
Abbreviations: ED, emergency department; Covid-19, coronavirus disease 19; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ICD-10, 10th revision of the International Statistical Classification of Diseases and Related Health Problems; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; CT scan, computed tomography scan; 18F-FDG PET/CT scan, positron emission tomography integrated with CT scan
1. Introduction
Because of potentially serious, time-sensitive consequences, international guidelines recommend expeditious medical evaluation for patients experiencing symptoms suggestive of cancer, including both focal and non-specific but concerning symptoms [[1], [2], [3]]. The management of these patients varies across healthcare systems. Although it depends on various factors such as the severity of the presenting complaints or the tradition of hospital departments, patients are sometimes admitted to hospital and spend several days undergoing investigations until a diagnosis is made [4]. Similar patients with clinically suspected cancer are managed in hospital ambulatory facilities and, once diagnosis is established, they are referred onwards to a specialist for treatment and follow-up [5,6]. A number of healthcare systems have experienced a shift in these practices in recent years. The traditional inpatient-based management model in which patients with serious conditions were hospitalized for workup largely disappeared from public hospitals of the Spanish region of Catalonia in the mid-2000s [7]. The creation of hospital-based, ambulatory units - quick diagnosis units - specifically designed to prevent hospitalization for diagnostic purposes allowed for a full conversion of the classical approach [4,6,7].
Patients evaluated at the quick diagnosis unit of our hospital are referred for investigation from the emergency department (ED) and primary care centers belonging to a healthcare system where the hospital operates as the referral center [8]. Analyses from our group from the last 10 years have disclosed a steady rate of referrals. At an average of 50 weekly patients, the ED constitutes the main source of referrals to the unit. The clinical indications for referral and evaluation mostly include conditions potentially associated with cancer such as severe anemia, unintentional weight loss, abnormal lymphadenopathy or lumps, and fever of unknown origin [[8], [9], [10], [11], [12]]. About 20% of patients are found to have cancer as a final diagnosis and another 20% have gastrointestinal disorders with or without iron-deficiency anemia secondary to blood loss. In addition, functional and mental disorders are diagnosed in 5% of cases approximately. By avoiding inpatient admissions for purely diagnostic reasons, quick diagnosis units have become a new care delivery model with high-quality standards. Studies have shown that these units are cost-effective compared with hospitalization while successfully reducing inappropriate referrals by primary care centers to the ED [5,8,10,11,13].
Anecdotal data from Spain suggest that emergency healthcare seeking among patients presenting with serious conditions suggestive of malignancy has changed during the Covid-19 pandemic and that, given the concern for exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), these patients have largely avoided the EDs of public hospitals. Analyzing the referral pathways for suspected cancer initiated by UK primary care physicians, two population-based modeling studies revealed that Covid-19-related diagnostic and treatment delays were associated with an excess 5-year mortality, mainly attributable to changes in care-seeking behavior and disruption of diagnostic services since the start of national lockdown [14,15]. Along with these collateral effects, the pandemic has taken a toll on the mental health of the population with reports of functionally impaired individuals by fear-based reactions to the outbreak [16,17].
Since the confirmation of the first case of Covid-19 in the country on January 31, 2020 and until late April/early May, there was a sharp increase in the number of diagnosed cases and resultant deaths. To minimize viral spread, social distancing measures were implemented across the country and, on March 15, the government announced the imposition of a national lockdown by which all residents were mandated to stay on their normal residences [[18], [19], [20]]. Although most patients referred to the quick diagnosis unit present first to the ED with non-specific but concerning symptoms or red flags, whether the pandemic changed the processes of care for these patients is unknown.
The purpose of this study was to analyze the impact of the pandemic on the volume, clinical presentation, and diagnostic patterns of non-Covid-19 patients presenting to the ED with concerning symptoms suggestive of cancer and referred to the quick diagnosis unit of our tertiary hospital for diagnostic evaluation. As a central objective of the study, we also analyzed any changes in the accessibility to diagnostic services stemming from the lockdown.
2. Methods
2.1. Study design
This is a retrospective, observational, pre-, during-, and post-event cohort analysis. The study was approved by the Research Ethics Committee of our hospital, which sanctioned a waiver of informed consent. The ethical guidelines of the Declaration of Helsinki were observed. The data that support the findings of this study are available from the corresponding author upon reasonable request by investigators.
2.2. Setting
Ours is a tertiary care, 900-bed public hospital with a reference population of 550,000 in Barcelona, the regional epicenter of the Covid-19 pandemic, and the quick diagnosis unit is placed on the daycare center of the hospital. The structure, criteria for referral, and working procedures of the unit have been reported previously [8,21]. Briefly, it operates as an ambulatory care clinic for patients referred mainly from the ED after presentation with serious specific or non-specific symptoms of cancer, either isolated or combined, whose general condition is acceptable enough to enable them to go to hospital for investigations and appointments, then back to home. The working protocol consists of a first appointment shortly after referral followed by preferential programming of diagnostics tests and subsequent visits until a diagnosis is made. Staff includes a consultant general internist, senior internal medicine residents, family medicine residents, nursing, and administrative staff [11]. It is open 12 h a day, 5 days a week.
2.3. Study population
Trends in referrals by the ED were analyzed from January 1 to July 8, 2020 and the corresponding dates of 2019. We hypothesized that the volume and patterns of patient presentation would differ before and after the week of February 19–26, 2020. This week was selected because the earliest Covid-19 cases in Barcelona and the neighboring cities were announced during this period, conceivably affecting community perception about seeking emergency care as a result of an imminent pandemic. Three time-based cohorts were defined and analyzed: prepandemic, January 1–February 19; pandemic, February 19–April 22; and postpandemic, April 22–July 8. Since the number of new cases and deaths across Spain showed a significant decreasing trend from late April and the government announced a progressive ‘de-escalation’ until normality starting on May 2, we selected April 29–July 8 as the postpandemic period.
Inclusion criteria were age ≥ 18 years and referral by the ED for investigation of serious symptoms suggestive of cancer, either non-specific symptoms or red flags, within the periods indicated. Only non-Covid-19 conditions were evaluated. Accordingly, patients referred who had Covid-19 were excluded. Patients lost to follow or dead before a diagnosis was reached were also excluded.
2.4. Variables
An electronic database was created to enter variables of interest. In addition to trends and clinical indications for referral, variables analyzed included demographics (age, gender, and annual income) and variables related to the routine working activity of the quick diagnosis unit including waiting times from referral to appointment, number of visits to the unit, rate of telehealth visits/total visits, ratio of successive/first visits, and time-to-diagnosis (from first appointment to the unit to date of diagnosis). Consistent with the objective of the study, we also analyzed the frequency and types of diagnostic procedures performed and waiting times between requesting and conducting them, and the final diagnostic categories according to the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10; Version 2019) and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [22,23].
2.5. Interrater agreement and reliability
Diagnostic information in our study population was initially provided by medical history and physical examination and as is common in clinical practice, the diagnostic workup comprised sequential steps with different tests being performed. Although the clinician integrated the assortment of information obtained after each step into a judgment around the probability of a specific disease, a structured diagnostic process was applied according to diagnostic guidelines and algorithms so long as they exist.
To evaluate the reliability of the diagnosis reached, we measured the diagnostic agreement between two independent clinicians after the study was completed. These clinicians were former residents of internal medicine who, as part of the residency program, were trained in the management of patients at quick diagnosis units. They first underwent three 30-min training sessions on the completion of structured abstraction forms and their performance was supervised in between. Then, they were asked to evaluate a number of patients included in the study for whom physicians at the quick diagnosis unit determined different diagnoses. Cases were selected according to initial referral reason and final diagnosis. Clinicians were unaware of the final diagnoses and, for each case, they were presented with demographics, medical history, reasons for referral, presenting symptoms, findings on physical examination and tests performed, extracted from deidentified electronic records. After excluding 9 cases with unavailable tests, there were 60 subjects or 20 with four referral reasons from each of the three periods of the study who had paired independent observations by the two physicians. Referral reasons were unintentional weight loss, fever of unknown origin, change in bowel habit, and anemia. Clinical raters were blinded to the patient's group assignment and to the information obtained by each other. To score each case, physicians were required to select a diagnosis from a list of 7 diagnoses of which one was the actual diagnosis reported in this study. A category labelled ‘other’ was included in the list, in which case the physician was asked to provide their desired diagnosis in a free-text field. The analysis was deemed exempt from the ethics review board of the hospital.
2.6. Statistical analysis
Statistical analyses were performed using GraphPad Prism v8 (GraphPad Software, San Diego, CA). The threshold for statistical significance was established a priori at a P < 0.05. Linear regression was used to test for time trends with weekly referrals as the dependent variable and the calendar week as the independent variable. Trends were considered significant when the slope of the trend was not equal to zero and the P value was <0.05. The estimation of the slope of a linear trend offers a numerical interpretation of the size of the trend, specifically the mean decrease (negative slope) or increase (positive slope) of the measured variable. In addition to slopes, the Y intercepts (β0) and the R2 associated with the regression models were noted.
Categorical and continuous variables are presented as n (%) and mean (standard deviation) or median (interquartile range), respectively. Categorical variables were compared by either χ2 tests or Fisher exact test, depending on the number of observations per cell. Continuous variables were compared using t-tests or the Mann-Whitney U test, as appropriate. To preserve dataset integrity, missing data were not imputed. There were no variables with more than 10% of missing data.
We calculated the interobserver agreement for diagnosis and assessed reliability with the unweighted kappa (κ) statistic with 95% confidence intervals (CI). The interobserver reliability was categorized as poor (κ < 0.20), fair (κ = 0.21–0.40), moderate (κ = 0.41–0.60), good (κ = 0.61–0.80), and excellent (κ = 0.81–1.00). Observations with missing data were excluded from the κ analysis and no imputation was performed.
3. Results
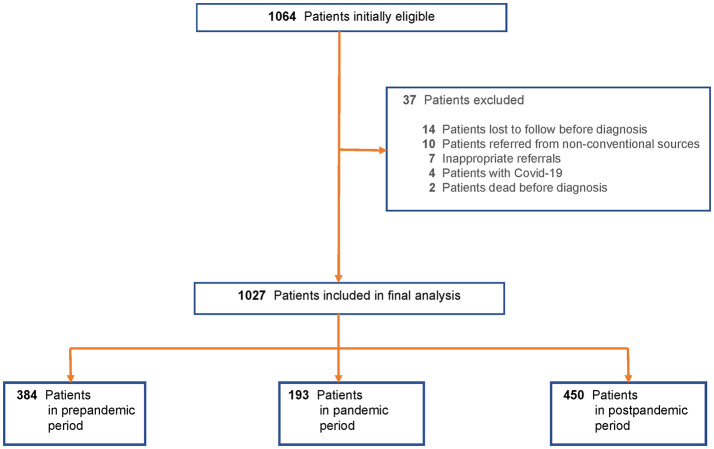
Among 1064 initially eligible patients, 37 (3.48%) were excluded, leaving 1027 patients referred from the ED from January 1 to July 8, 2020 (Fig. 1 ). There were 384 patients referred during the prepandemic period, 193 during the pandemic period, and 450 during the postpandemic period.
Fig. 1.
Flowsheet for the patient selection process.
3.1. Temporal trends in referrals
Temporal trends in referrals to the unit in each of the three study periods of 2020 were analyzed and compared with each other and with the corresponding months of 2019 (Fig. 2, Fig. 3 ). By scatter graphs visualization, we observed time cut points concurrent with changes in referral volumes in 2020. These cut points were then verified by spline analyses and linear regression due to the nonlinearity of data (runs test: P < 0.0001). As shown in Fig. 4 , referrals increased in the prepandemic period of 2020 with a significant linear trend and a slope of 1.190 (95% CI, 0.4930 to 1.888; P value for trend = 0.0058, R2 = 0.7440). However, a significant decline was noticed during the pandemic stage with an average weekly change of −2.117 (95% CI, −4.086 to −0.1470; P for trend = 0.0386, R2 = 0.4798) (Fig. 4). The number of referrals increased again during the postpandemic stage with a weekly slope of 1.500 (95% CI, 1.279 to 1.721; P < 0.0001, R2 = 0.9634).
Fig. 2.
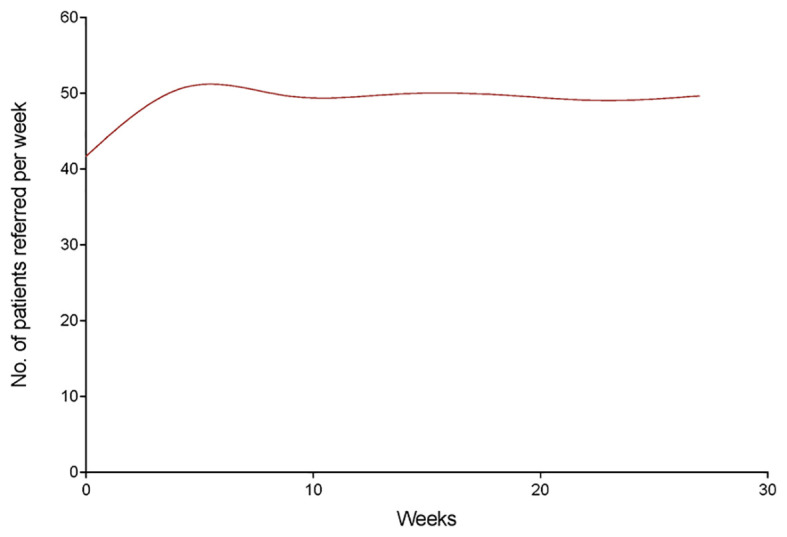
Smoothing spline model of trends in referrals by the emergency department from January 1 to July 9, 2019.
Fig. 3.
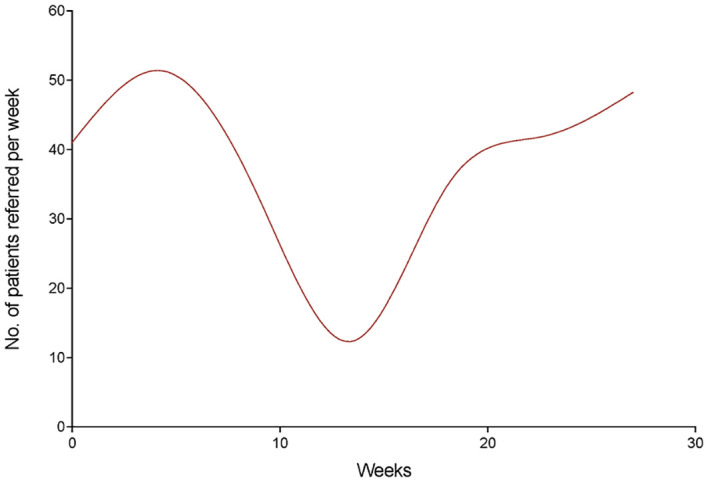
Smoothing spline model of trends in referrals by the emergency department from January 1 to July 8, 2020.
Fig. 4.
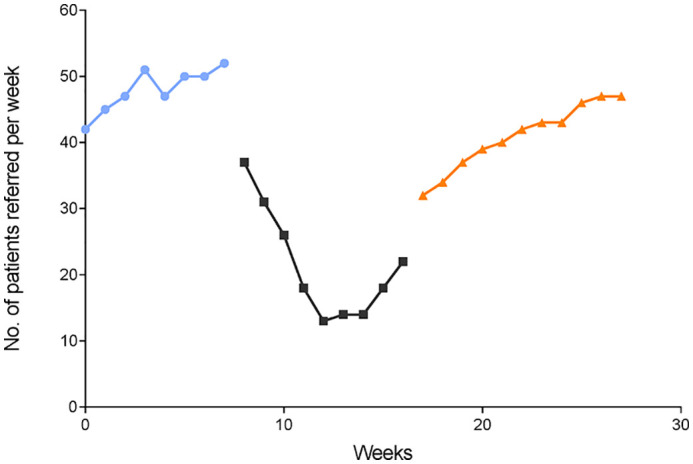
Trends in weekly referrals from January 1 to July 8, 2020. Linear regression models from weeks 0–8 (prepandemic period) (circles), weeks 9–17 (pandemic period) (squares), and weeks 18–28 (postpandemic period) (triangles).
3.2. General characteristics and working activity of the quick diagnosis unit
Table 1 shows the sociodemographic characteristics of patients and the general operating activity of the unit during the three periods. With a mean age of 55.0 years, pandemic patients were significantly younger than patients from the prepandemic (63.6 years) and postpandemic (62.1 years) cohorts. The waiting times between referral and first appointment to the unit were significantly longer in pandemic than prepandemic and postpandemic patients. There was a significant shift from face-to-face to telehealth visits during the pandemic (66.32% rate of telehealth/total visits) and, in a lesser proportion, postpandemic (31.33% rate) periods compared to the prepandemic period (3.13% rate). Although the mean number of visits and the ratio of successive/first visits were lower in pandemic patients, the mean time-to-diagnosis was markedly longer in this cohort (19.72 days vs. 8.33 days in prepandemic and 13.49 days in postpandemic cohorts).
Table 1.
General characteristics of patients and activity of quick diagnosis unit by study period.
| Characteristic | Prepandemic (Jan 1–Feb 19)a |
P for difference | Pandemic (Feb 26–Apr 22) |
P for difference | Postpandemic (Apr 29–Jul 8)b |
P value a vs. b |
|---|---|---|---|---|---|---|
| Referrals (n = 384) | Referrals (n = 193) | Referrals (n = 450) | ||||
| Gender, n (%) | 0.040 | 0.047 | 0.113 | |||
| Female | 186 (48.44) | 105 (54.40) | 227 (50.44) | |||
| Male | 198 (51.56) | 88 (45.60) | 223 (49.56) | |||
| Age (years), mean ± SD | 63.6 ± 13.4 | <0.001 | 55.0 ± 14.1 | <0.001 | 62.1 ± 15.0 | 0.157 |
| Age groups (years), n (%) | <0.001 | <0.001 | 0.176 | |||
| 18–44 | 31 (8.07) | 50 (25.91) | 42 (9.33) | |||
| 45–65 | 199 (51.82) | 85 (44.04) | 230 (51.11) | |||
| >65 | 154 (40.10) | 58 (30.05) | 178 (39.56) | |||
| Annual income (€), n (%)† | 0.064 | 0.096 | 0.239 | |||
| >100,000 | 5 (1.30) | 3 (1.55) | 6 (1.33) | |||
| 18,000–100,000 | 156 (40.63) | 87 (45.08) | 188 (41.78) | |||
| <18,000 | 223 (58.07) | 103 (53.37) | 256 (56.89) | |||
| Wait time to first appointment (days), mean ± SD | 2.75 ± 1.44 | <0.001 | 6.55 ± 2.18 | <0.001 | 4.26 ± 1.72 | <0.001 |
| Visits to quick diagnosis unit, mean ± SD | 3.04 ± 1.08 | 0.029 | 2.68 ± 0.84 | <0.001 | 3.14 ± 1.23 | 0.185 |
| Total number of visits, n (%) | 0.043 | <0.001 | 0.221 | |||
| ≤2 | 222 (57.81) | 122 (63.21) | 252 (56.00) | |||
| ≥3 | 162 (42.19) | 71 (36.79) | 198 (44.00) | |||
| Ratio of successive/first visits, mean ± SD | 1.75 ± 0.44 | 0.038 | 1.53 ± 0.32 | <0.001 | 1.83 ± 0.70 | 0.196 |
| Time-to-diagnosis (days), mean ± SD | 8.33 ± 3.94 | <0.001 | 19.72 ± 10.37 | <0.001 | 13.49 ± 6.45 | <0.001 |
According to the administrative healthcare database of the Health Department of the Government of Catalonia (Catalan Health Surveillance System [CHSS]).
3.3. Diagnostic procedures
Most common procedures performed in patients from the three cohorts and the mean intervals between ordering and conducting them can be seen in Table 2 . Pandemic patients underwent significantly less computed tomography (CT) scans, ultrasonographies, and biopsy/cytology procedures than patients from the other cohorts. However, gastrointestinal endoscopies were more likely to be performed among patients from the pandemic cohort than other patients. In addition, positron emission tomographies integrated with CT scans (18F-FDG PET/CT scans) were more commonly performed among patients from the pandemic and postpandemic cohorts. Analysis of mean intervals revealed significant delays in pandemic and, to a lesser degree, postpandemic patients. In particular, patients from the pandemic cohort had to wait significantly longer than the rest of patients for most of the diagnostic procedures listed in Table 2. The mean waiting time for a CT scan was 13.24 days in pandemic patients vs. 3.54 days in prepandemic patients and 8.67 days in postpandemic patients. Similarly, the mean waiting time for a gastrointestinal endoscopy was 15.45 days in pandemic patients vs. 6.41 days in prepandemic and 10.64 days in postpandemic patients. Waiting times for investigations were also more commonly delayed in postpandemic vs. prepandemic patients.
Table 2.
Diagnostic procedures and times elapsed between requesting and performing them by study period.⁎
| Procedure | Prepandemic (Jan 1–Feb 19)a |
P for difference | Pandemic (Feb 26–Apr 22) |
P for difference | Postpandemic (Apr 29–Jul 8)b |
P value a vs. b |
|---|---|---|---|---|---|---|
| Referrals (n = 384) | Referrals (n = 193) | Referrals (n = 450) | ||||
| CT scan, n (%) | 117 (30.47) | <0.001 | 37 (19.17) | <0.001 | 149 (33.11) | 0.114 |
| Wait time (days), mean ± SD | 3.54 ± 1.14 | <0.001 | 13.24 ± 9.33 | <0.001 | 8.67 ± 5.27 | <0.001 |
| MRI, n (%) | 13 (3.39) | 0.200 | 3 (1.55) | 0.163 | 18 (4.00) | 0.224 |
| Wait time (days), mean ± SD | 6.27 ± 3.41 | <0.001 | 18.66 ± 10.24 | <0.001 | 10.80 ± 5.99 | <0.001 |
| Ultrasonography, n (%) | 89 (23.18) | <0.001 | 26 (13.47) | <0.001 | 113 (25.11) | 0.095 |
| Wait time (days), mean ± SD | 2.53 ± 1.02 | <0.001 | 11.50 ± 8.00 | <0.001 | 6.94 ± 4.51 | <0.001 |
| Gastrointestinal endoscopy and EUS, n (%) | 136 (35.42) | <0.001 | 85 (44.04) | <0.001 | 158 (35.11) | 0.490 |
| Wait time (days), mean ± SD | 6.41 ± 4.30 | <0.001 | 15.45 ± 10.47 | <0.001 | 10.64 ± 11.31 | <0.001 |
| 18F-FDG PET/CT, n (%) | 9 (2.34) | <0.001 | 20 (10.36) | 0.113 | 32 (7.11) | 0.051 |
| Wait time (days), mean ± SD | 3.93 ± 1.88 | <0.001 | 11.31 ± 6.19 | <0.001 | 5.90 ± 2.48 | 0.050 |
| Bronchoscopy and EBUS, n (%) | 13 (3.39) | 0.181 | 4 (2.07) | 0.142 | 19 (4.22) | 0.184 |
| Wait time (days), mean ± SD | 5.37 ± 3.25 | <0.001 | 9.39 ± 4.28 | 0.040 | 7.29 ± 3.82 | 0.060 |
| Bone scintigraphy, n (%) | 10 (2.60) | 0.203 | 2 (1.04) | 0.139 | 14 (3.11) | 0.190 |
| Wait time (days), mean ± SD | 3.24 ± 1.46 | <0.001 | 8.62 ± 5.25 | <0.001 | 4.78 ± 2.17 | 0.115 |
| Biopsy & cytology, n (%)† | 86 (22.40) | <0.001 | 15 (7.77) | <0.001 | 118 (26.22) | 0.072 |
| Wait time (days), mean ± SD | 2.75 ± 0.55 | <0.001 | 5.47 ± 2.34 | 0.012 | 3.41 ± 1.10 | 0.126 |
| Bone marrow aspiration & biopsy, n (%) | 14 (3.65) | 0.146 | 3 (1.55) | 0.223 | 12 (2.67) | 0.149 |
| Wait time (days), mean ± SD | 4.11 ± 2.13 | 0.109 | 5.83 ± 3.08 | 0.252 | 5.26 ± 2.91 | 0.142 |
CT scan, computed tomography scan; MRI, magnetic resonance image; EUS, gastrointestinal endoscopic ultrasonography; 18F-FDG PET/CT, positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro- d-glucose integrated with computed tomography; EBUS, endobronchial ultrasonography.
Examinations not included are, among others, capsule endoscopy, endoscopic retrograde cholangiopancreatography, virtual colonoscopy, echocardiography, high-resolution esophageal manometry, pulmonary function tests, and electromyography.
Mostly including fine-needle aspiration cytology and surgical biopsy of peripheral lymph nodes and lumps, and CT- or ultrasound-guided biopsy of abnormal areas of tissue and organs.
3.4. Reasons for referral
The clinical indications for referral by the ED were conditions raising the suspicion of cancer, with significant differences between cohorts (Table 3 ). The percentage of pandemic patients referred for fever of unknown origin was almost three times as high as the percentage of prepandemic patients, and postpandemic patients were almost twice as likely to be referred for fever of unknown origin as prepandemic patients. Although pandemic patients were also more likely than prepandemic patients to be referred for unintentional weight loss and severe fatigue, they were less likely to be referred for anemia and lymphadenopathy or palpable lumps. Additional descriptions are provided in Table 3.
Table 3.
Clinical reasons for referral to quick diagnosis unit by study period.
| Referral reason, n (%) | Prepandemic (Jan 1–Feb 19)a |
P for difference | Pandemic (Feb 26–Apr 22) |
P for difference | Postpandemic (Apr 29–Jul 8)b |
P value a vs. b |
|---|---|---|---|---|---|---|
| Referrals (n = 384) | Referrals (n = 193) | Referrals (n = 450) | ||||
| Unintentional weight loss | 54 (14.06) | 0.024 | 40 (20.73) | <0.001 | 52 (11.56) | 0.129 |
| Severe fatigue | 8 (2.08) | 0.004 | 17 (8.81) | 0.052 | 22 (4.89) | 0.118 |
| Fever of unknown origin | 20 (5.21) | <0.001 | 28 (14.51) | 0.048 | 46 (10.22) | 0.023 |
| Anemia | 83 (21.61) | <0.001 | 14 (7.25) | <0.001 | 65 (14.44) | <0.001 |
| Persistent nausea/appetite loss | 9 (2.34) | 0.219 | 10 (5.18) | 0.065 | 7 (1.56) | 0.227 |
| Change in bowel habit | 37 (9.64) | 0.088 | 26 (13.47) | 0.030 | 38 (8.44) | 0.255 |
| Abdominal pain | 23 (5.99) | 0.212 | 14 (7.25) | 0.113 | 19 (4.22) | 0.221 |
| Rectal bleeding/hematochezia | 34 (8.85) | <0.001 | 3 (1.55) | 0.072 | 21 (4.67) | 0.013 |
| Dysphagia | 6 (1.56) | 0.098 | 10 (5.18) | 0.190 | 16 (3.56) | 0.106 |
| Abnormal laboratory tests | 10 (2.60) | 0.228 | 2 (1.04) | 0.420 | 6 (1.33) | 0.202 |
| Lymphadenopathy or palpable lumps | 31 (8.07) | 0.001 | 4 (2.07) | <0.001 | 43 (9.56) | 0.187 |
| Lung or mediastinal mass | 8 (2.08) | 0.209 | 1 (0.52) | 0.155 | 14 (3.11) | 0.179 |
| Pulmonary infiltrate | 2 (0.52) | 0.460 | 2 (1.04) | 0.326 | 0 (0.00) | 0.312 |
| Abdominal mass | 14 (3.65) | 0.102 | 1 (0.52) | 0.038 | 25 (5.56) | 0.164 |
| Jaundice | 3 (0.78) | 0.316 | 0 (0.00) | 0.249 | 6 (1.33) | 0.296 |
| Ascites | 2 (0.52) | 0.453 | 2 (1.04) | 0.720 | 5 (1.11) | 0.322 |
| Pain (other than abdominal) | 20 (5.21) | 0.140 | 6 (3.11) | 0.063 | 29 (6.44) | 0.138 |
| Dyspnea | 7 (1.82) | 0.088 | 10 (5.18) | 0.695 | 24 (5.33) | 0.091 |
| Peripheral edema | 2 (0.52) | 0.519 | 2 (1.04) | 0.586 | 4 (0.89) | 0.422 |
| Pleural effusion | 10 (2.60) | 0.144 | 1 (0.52) | 0.401 | 5 (1.11) | 0.156 |
| Hematuria | 1 (0.26) | 0.844 | 0 (0.00) | 0.377 | 3 (0.67) | 0.343 |
3.5. Diagnostic categories
The distribution of final diagnoses across the three periods of the study is provided in Table 4, Table 5 . The rate of malignant diseases among patients from the prepandemic and postpandemic periods was 21.35% and 26.89%, respectively, whereas it was only 5.70% in the pandemic cohort (Table 4). Cancer stage at presentation to the ED was analyzed considering only solid tumors (i.e. other than hematological malignancies). Patients from the three cohorts with solid cancers had a more advanced stage of disease (i.e. stage III–IV or metastatic disease) at the time of diagnosis, ranging from 72.06% (49/68 patients) in the prepandemic cohort to 73.20% (71/97 patients) in the postpandemic cohort to 77.78% (7/9 patients) in the pandemic cohort. Patients from the pandemic cohort were about twice as likely to be diagnosed with nonmalignant gastrointestinal disorders as prepandemic and postpandemic patients, partly due to the significantly higher occurrence of irritable bowel syndrome (17.10% vs. 6.51% and 8.00%, respectively) (Table 5). In addition, pandemic patients had a significantly higher rate of mental and behavioral disorders than prepandemic and postpandemic patients (15.54% vs. 3.39% and 6.00%, respectively). Of note, 5.70% (11/193) patients from the pandemic cohort vs. 0.26% (1/384) and 1.56% (7/450) prepandemic and postpandemic patients, respectively, were categorized as having medically unexplained physical symptoms (P = 0.028 and 0.075, respectively) (see below).
Table 4.
Distribution of malignant diseases by study period.⁎
| Diagnostic categories | Prepandemic (Jan 1–Feb 19)a |
P for difference | Pandemic (Feb 26–Apr 22) |
P for difference | Postpandemic (Apr 29–Jul 8)b |
P value a vs. b |
|---|---|---|---|---|---|---|
| Referrals (n = 384) | Referrals (n = 193) | Referrals (n = 450) | ||||
| Malignant neoplasms,n(%) | 82 (21.35) | <0.001 | 11 (5.70) | <0.001 | 121 (26.89) | 0.004 |
| Pancreatic cancer | 14 (3.65) | 0.117 | 2 (1.04) | 0.082 | 19 (4.22) | 0.190 |
| Lung cancer | 7 (1.82) | 1 (0.52) | 13 (2.89) | |||
| Colorectal cancer | 18 (4.69)† | 0.087 | 2 (1.04)† | 0.073 | 21 (4.67)† | 0.638 |
| Breast cancer | 4 (1.04) | 0 (0.00) | 5 (1.11) | |||
| Ovarian cancer | 6 (1.56) | 1 (0.52) | 9 (2.00) | |||
| Hepatocellular carcinoma | 1 (0.26) | 0 (0.00) | 2 (0.44) | |||
| Renal cell carcinoma | 2 (0.52) | 0 (0.00) | 4 (0.89) | |||
| Prostate cancer | 5 (1.30) | 1 (0.52) | 5 (1.11) | |||
| Mesothelioma | 1 (0.26) | 0 (0.00) | 2 (0.44) | |||
| Head and neck cancer | 2 (0.52) | 1 (0.52) | 3 (0.67) | |||
| Esophageal cancer | 1 (0.26) | 1 (0.52) | 4 (0.89) | |||
| Gastric cancer | 1 (0.26)† | 0 (0.00) | 2 (0.44)† | |||
| Endometrial and cervical cancer | 1 (0.26)† | 0 (0.00) | 1 (0.22) | |||
| Thyroid cancer | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Liposarcoma | 1 (0.26) | 0 (0.00) | 2 (0.44) | |||
| Bone sarcoma | 0 (0.00) | 0 (0.00) | 1 (0.22) | |||
| Neuroendocrine tumor | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Hodgkin and non-Hodgkin lymphoma | 13 (3.39) | 0.143 | 2 (1.04) | 0.072 | 22 (4.89) | 0.169 |
| Multiple myeloma | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Myelodysplastic syndrome | 0 (0.00) | 0 (0.00) | 1 (0.22) | |||
| Cancer of unknown primary site | 2 (0.52) | 0 (0.00) | 2 (0.44) |
Table 5.
Distribution of nonmalignant diseases by study period.⁎
| Diagnostic categories | Prepandemic (Jan 1–Feb 19)a |
P for difference | Pandemic (Feb 26–Apr 22) |
P for difference | Postpandemic (Apr 29–Jul 8)b |
P value a vs. b |
|---|---|---|---|---|---|---|
| Referrals (n = 384) | Referrals (n = 193) | Referrals (n = 450) | ||||
| Benign neoplasms, n (%) | 17 (4.43) | 0.132 | 4 (2.07) | 0.057 | 27 (6.00) | 0.231 |
| Warthin tumor | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Branchial and thyroglossal cyst | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Intestinal polyp | 7 (1.82)† | 2 (1.04)† | 8 (1.78)† | |||
| Gastric polyp | 1 (0.26) | 0 (0.00) | 3 (0.67) | |||
| Pancreatic benign neoplasm | 1 (0.26) | 0 (0.00) | 2 (0.44) | |||
| Benign lipomatous neoplasm | 2 (0.52) | 1 (0.52) | 4 (0.89) | |||
| Leiomyoma of uterus | 1 (0.26)† | 0 (0.00) | 2 (0.44)† | |||
| Benign neoplasm of ovary | 1 (0.26) | 0 (0.00) | 2 (0.44) | |||
| Benign neoplasm of thyroid gland | 2 (0.52) | 1 (0.52) | 4 (0.89) | |||
| Hematological disorders, n (%) | 17 (4.43) | 0.051 | 0 (0.00) | 0.078 | 17 (3.78) | 0.228 |
| Nutritional iron-deficiency anemia | 4 (1.04) | 0 (0.00) | 5 (1.11) | |||
| Anemia of chronic disease | 6 (1.56) | 0 (0.00) | 4 (0.89) | |||
| Multifactorial anemia | 5 (1.30) | 0 (0.00) | 5 (1.11) | |||
| Iron-deficiency anemia secondary to blood loss⁎ | 63 (16.41)† | <0.001 | 14 (7.25)† | 0.049 | 54 (12.00)† | 0.001 |
| Monoclonal gammopathy of undetermined significance | 2 (0.52) | 0 (0.00) | 3 (0.67) | |||
| Nonspecific lymphadenitis, n (%) | 16 (4.17) | 0.162 | 3 (1.55) | 0.139 | 22 (4.89) | 0.212 |
| Gastrointestinal disorders, n (%) | 75 (19.53) | <0.001 | 79 (40.93) | <0.001 | 94 (20.89) | 0.134 |
| Inflammatory bowel disease | 2 (0.52) | 1 (0.52) | 1 (0.22) | |||
| Irritable bowel syndrome | 25 (6.51) | <0.001 | 33 (17.10) | <0.001 | 36 (8.00) | 0.142 |
| Gastroesophageal reflux disease with esophagitis | 6 (1.56)† | 6 (3.11)† | 8 (1.78)† | |||
| Achalasia | 2 (0.52) | 1 (0.52) | 4 (0.89) | |||
| Barrett esophagus | 2 (0.52) | 1 (0.52) | 1 (0.22) | |||
| Peptic ulcer | 5 (1.30)† | 6 (3.11)† | 6 (1.33)† | |||
| Gastroduodenitis | 8 (2.08)† | 0.045 | 13 (6.74)† | 0.039 | 10 (2.22)† | 0.375 |
| Chronic atrophic gastritis | 4 (1.04)† | 4 (2.07) | 6 (1.33)† | |||
| Ischemic colitis | 1 (0.26) | 0 (0.00) | 0 (0.00) | |||
| Diverticulitis | 2 (0.52) | 2 (1.04) | 1 (0.22) | |||
| Angiodysplasia of small intestine and/or colon | 3 (0.78)† | 1 (0.52)† | 3 (0.67)† | |||
| Ulcer of intestine | 2 (0.52)† | 1 (0.52)† | 1 (0.22)† | |||
| Hemorrhoids | 9 (2.34) | 7 (3.63) | 12 (2.67) | |||
| Retroperitoneal fibrosis | 1 (0.26) | 1 (0.52) | 1 (0.22) | |||
| Celiac disease | 2 (0.52) | 1 (0.52) | 2 (0.44) | |||
| Postgastric surgery syndromes | 1 (0.26) | 1 (0.52) | 2 (0.44) | |||
| Liver disorders, n (%) | 17 (4.43) | 0.152 | 4 (2.07) | 0.289 | 6 (1.33) | 0.097 |
| Alcoholic liver disease | 3 (0.78) | 1 (0.52) | 1 (0.22) | |||
| Toxic liver disease | 2 (0.52) | 0 (0.00) | 1 (0.22) | |||
| Chronic hepatitis, unspecified | 5 (1.30) | 1 (0.52) | 1 (0.22) | |||
| Liver cirrhosis | 2 (0.52) | 2 (1.04) | 2 (0.44) | |||
| Nonalcoholic steatohepatitis | 4 (1.04) | 0 (0.00) | 1 (0.22) | |||
| Autoimmune hepatitis | 1 (0.26) | 0 (0.00) | 0 (0.00) | |||
| Pancreaticobiliary disorders, n (%) | 17 (4.43) | 0.222 | 5 (2.59) | 0.494 | 13 (2.89) | 0.155 |
| Cholelithiasis | 6 (1.56) | 2 (1.04) | 5 (1.11) | |||
| Choledocholithiasis | 2 (0.52) | 0 (0.00) | 2 (0.44) | |||
| Calculus of bile duct with cholangitis | 2 (0.52) | 0 (0.00) | 1 (0.22) | |||
| Cholecystitis | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Acute pancreatitis | 2 (0.52) | 1 (0.52) | 0 (0.00) | |||
| Chronic pancreatitis | 2 (0.52) | 1 (0.52) | 2 (0.44) | |||
| Pseudocyst of pancreas | 1 (0.26) | 1 (0.52) | 1 (0.22) | |||
| Primary biliary cholangitis | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Rheumatic and systemic autoimmune diseases, n (%) | 16 (4.17) | 0.167 | 5 (2.59) | 0.703 | 12 (2.67) | 0.148 |
| Rheumatoid arthritis | 2 (0.52) | 0 (0.00) | 2 (0.44) | |||
| Palindromic rheumatism | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Polyarteritis nodosa | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Giant cell arteritis and polymyalgia rheumatica | 4 (1.04) | 1 (0.52) | 2 (0.44) | |||
| Systemic lupus erythematosus | 2 (0.52) | 2 (1.04) | 1 (0.22) | |||
| Dermatopolymyositis | 1 (0.26) | 1 (0.52) | 0 (0.00) | |||
| Sjogren's syndrome | 2 (0.52) | 1 (0.52) | 2 (0.44) | |||
| Cryoglobulinemia | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| ANCA-associated vasculitis | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Diffuse (eosinophilic) fasciitis | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Sarcoidosis, n (%) | 3 (0.78) | 0.634 | 1 (0.52) | 0.605 | 0 (0.00) | 0.200 |
| Bone disorders, n (%) | 5 (1.30) | 0.285 | 1 (0.52) | 0.422 | 4 (0.89) | 0.271 |
| Osteoporosis with vertebral fracture | 3 (0.78) | 1 (0.52) | 2 (0.44) | |||
| Adult osteomalacia | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Fibrous dysplasia (monostotic) | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Autoinflammatory syndromes, n (%) | 1 (0.26) | 0.724 | 0 (0.00) | 0.807 | 1 (0.22) | 0.599 |
| Infectious diseases, n (%) | 35 (9.11) | 0.100 | 10 (5.18) | 0.144 | 36 (8.00) | 0.158 |
| Acute viral infections and viral hepatitis | 17 (4.43) | 3 (1.55) | 20 (4.44) | |||
| Acute lower respiratory infection, unspecified | 6 (1.56) | 2 (1.04) | 4 (0.89) | |||
| Intestinal infectious diseases | 4 (1.04) | 2 (1.04) | 4 (0.89) | |||
| Sexually transmitted diseases | 3 (0.78) | 1 (0.52) | 1 (0.22) | |||
| Tuberculosis | 3 (0.78) | 1 (0.52) | 4 (0.89) | |||
| Visceral leishmaniasis | 1 (0.26) | 0 (0.00) | 0 (0.00) | |||
| Herpes simplex and herpes zoster | 1 (0.26) | 0 (0.00) | 2 (0.44) | |||
| Acute/subacute infective endocarditis | 0 (0.00) | 1 (0.52) | 1 (0.22) | |||
| Endocrine disorders, n (%) | 14 (3.65) | 0.240 | 5 (2.59) | 0.711 | 11 (2.44) | 0.208 |
| Type 2 and 1 diabetes mellitus (uncontrolled) | 5 (1.30) | 2 (1.04) | 4 (0.89) | |||
| Hyperthyroidism | 3 (0.78) | 1 (0.52) | 2 (0.44) | |||
| Hypothyroidism | 2 (0.52) | 2 (1.04) | 4 (0.89) | |||
| Acute thyroiditis | 2 (0.52) | 0 (0.00) | 1 (0.22) | |||
| Primary hyperparathyroidism | 2 (0.52) | 0 (0.00) | 0 (0.00) | |||
| Respiratory diseases, n (%) | 8 (2.08) | 0.874 | 4 (2.07) | 0.451 | 11 (2.44) | 0.230 |
| Pleural effusion, unspecified | 3 (0.78) | 1 (0.52) | 2 (0.44) | |||
| Interstitial pulmonary disease, unspecified | 3 (0.78) | 1 (0.52) | 5 (1.11) | |||
| Chronic obstructive pulmonary disease | 2 (0.52) | 2 (1.04) | 4 (0.89) | |||
| Cardiovascular diseases, n (%) | 16 (4.17) | 0.205 | 6 (3.11) | 0.513 | 15 (3.33) | 0.283 |
| Heart failure, unspecified | 4 (1.04) | 2 (1.04) | 4 (0.89) | |||
| Acute idiopathic pericarditis | 3 (0.78) | 1 (0.52) | 2 (0.44) | |||
| Pulmonary embolism | 2 (0.52) | 0 (0.00) | 3 (0.67) | |||
| Cerebral infarction, unspecified | 3 (0.78) | 1 (0.52) | 2 (0.44) | |||
| Lymphedema | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Phlebitis and thrombophlebitis of lower or upper limbs | 1 (0.26) | 2 (1.04) | 2 (0.44) | |||
| Hypotension, unspecified | 2 (0.52) | 0 (0.00) | 1 (0.22) | |||
| Urologic diseases, n (%) | 8 (2.08) | 0.268 | 5 (2.59) | 0.139 | 6 (1.33) | 0.202 |
| Urinary tract infection including acute prostatitis | 4 (1.04) | 2 (1.04) | 5 (1.11) | |||
| Hydronephrosis with ureteral obstruction | 2 (0.52) | 1 (0.52) | 0 (0.00) | |||
| Renal colic, unspecified | 2 (0.52) | 2 (1.04) | 1 (0.22) | |||
| Dysfunctional uterine bleeding, n (%) | 8 (2.08)† | 0.216 | 2 (1.04)† | 0.211 | 9 (2.00)† | 0.624 |
| Diseases of the nervous system, n (%) | 12 (3.13) | 0.304 | 5 (2.59) | 0.266 | 8 (1.78) | 0.160 |
| Migraine and other headache syndromes | 2 (0.52) | 1 (0.52) | 1 (0.22) | |||
| Degenerative disease of nervous system, unspecified | 3 (0.78) | 1 (0.52) | 2 (0.44) | |||
| Sleep disorders, unspecified | 2 (0.52) | 2 (1.04) | 1 (0.22) | |||
| Trigeminal neuralgia and postherpetic neuralgia | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Nerve root and plexus disorder, unspecified | 2 (0.52) | 0 (0.00) | 2 (0.44) | |||
| Mononeuropathy, unspecified | 0 (0.00) | 0 (0.00) | 0 (0.00) | |||
| Polyneuropathy, unspecified | 1 (0.26) | 0 (0.00) | 1 (0.22) | |||
| Myopathy, unspecified | 1 (0.26) | 1 (0.52) | 0 (0.00) | |||
| Fibromyalgia and chronic fatigue syndrome, n (%) | 3 (0.78) | 0.694 | 2 (1.04) | 0.558 | 3 (0.67) | 0.503 |
| Mental and behavioral disorders, n (%) | 13 (3.39) | <0.001 | 30 (15.54) | <0.001 | 27 (6.00) | 0.119 |
| Anxiety disorder, unspecified | 5 (1.30) | 0.050 | 12 (6.22) | 0.134 | 11 (2.44) | 0.157 |
| Health anxiety/somatic symptom disorder | 1 (0.26) | 0.116 | 8 (4.15) | 0.149 | 7 (1.56) | 0.123 |
| Depression, unspecified | 7 (1.82) | 0.145 | 10 (5.18) | 0.152 | 9 (2.00) | 0.333 |
| Medically unexplained physical symptoms, n (%) | 1 (0.26) | 0.028 | 11 (5.70) | 0.048 | 7 (1.56) | 0.120 |
ANCA, antineutrophil cytoplasmic antibodies.
According to the International Statistical Classification of Diseases and Related Health Problems (ICD-10 Version: 2019) [22].
Patients presenting with iron-deficiency anemia were diagnosed with the gastrointestinal disorders and uterine conditions indicated here and in Table 4 and are not included in the final computation of cases.
3.6. Undisclosed organic disorders
We further analyzed the initial reasons for referral in patients in whom an organic condition was not identified. For each of the selected referral reasons listed in Table 6 , the rate of undisclosed disorders in the pandemic period was significantly higher than in the other periods. Compared to prepandemic patients, those from the postpandemic cohort had also a significantly higher rate of undisclosed disorders for each referral reason. While patients referred for evaluation of unintentional weight loss and severe fatigue without an underlying explanation were more commonly diagnosed with mental and behavioral disorders, those with fever of unknown origin without an apparent reason were mainly included in the group of medically unexplained physical symptoms. Likewise, referrals for evaluation of dysphagia and dyspnea were associated with a significantly higher rate of undisclosed disorders among pandemic and, to a lesser degree, postpandemic patients compared to prepandemic patients (Table 6). As of October 23, after a follow-up via telehealth visits ranging from 3 to 9 months, no patient from the three cohorts included in the categories of mental and behavioral disorders and medically unexplained physical symptoms had developed any explainable medical condition for their initial complaints (unpublished results).
Table 6.
Distribution of patients in whom an organic disorder was not identified according to the initial referral reason by study period.
| Referral reason | Prepandemic (Jan 1–Feb 19)a |
P for difference | Pandemic (Feb 26–Apr 22) |
P for difference | Postpandemic (Apr 29–Jul 8)b |
P value a vs. b |
|---|---|---|---|---|---|---|
| Undisclosed disorder, n (%) | Undisclosed disorder, n (%) | Undisclosed disorder, n (%) | ||||
| Unintentional weight loss | 8/54 (14.81) | <0.001 | 18/40 (45.00) | <0.001 | 12/52 (23.08) | <0.001 |
| Severe fatigue† | 2/8 (25.00) | <0.001 | 11/17 (64.71) | <0.001 | 12/22 (54.55) | <0.001 |
| Fever of unknown origin | 1/20 (5.00) | <0.001 | 7 (25.00) | <0.001 | 5/46 (10.87) | 0.045 |
| Dysphagia | 0/6 (0.00) | <0.001 | 2 (20.00) | <0.001 | 1/16 (6.25) | 0.030 |
| Dyspnea | 0/7 (0.00) | <0.001 | 2 (20.00) | <0.001 | 3/24 (12.50) | <0.001 |
Other than chronic fatigue syndrome.
3.7. Interrater agreement/reliability
Overall diagnostic agreement between the two clinicians for individual diagnoses in each period was excellent, ranging from a κ value of 0.83 (95% CI, 0.74 to 0.93) to 0.91 (95% CI, 0.82 to 0.99) in the prepandemic period, 0.85 (95% CI, 0.75 to 0.94) to 0.92 (95% CI, 0.83 to 1.00) in the pandemic period, and 0.82 (95% CI, 0.71 to 0.92) to 0.90 (95% CI, 0.79 to 0.98) in the postpandemic period. When considering the paired observations in which the clinical raters selected the same diagnosis, the overall coincidence with the diagnoses determined by the quick diagnosis unit physicians was 95% in the prepandemic period, 95% in the pandemic period, and 90% in the postpandemic period.
4. Discussion
In this report, we describe the impact of the Covid-19 pandemic on the accessibility to essential investigations, disease patterns, and volumes of patients with suspected cancer referred by the ED to a quick diagnosis unit of a tertiary public hospital. To have an overall view of the events as they unfolded, we analyzed data in three time intervals: shortly before the initial outbreak, during the highest peak of cases and deaths, and subsequently when lockdown measures were relaxed. Results showed that after an increasing rate as usually observed after Christmas time, referrals by the ED decreased to unprecedented levels during the pandemic period with an average negative slope of −2.1 cases per week, to then increase again until near normalization by the end of the postpandemic period. From the onset of lockdown, the standard activity and operating procedures of the unit experienced a severe setback. Although the disruption was slightly relieved during the postpandemic period, substantial delays persisted due to the backlog of normal work across all hospital services. An assessment of the patterns of clinical presentation and final diagnoses across the three periods of the study suggested that functional and mental disorders played a significant role in the pandemic period.
In addition to having killed 34,000 and afflicted over 1 million as of October 21, 2020, the pandemic caused a drastic change in the emergency care-seeking behavior of the Spanish population and a severe disruption to healthcare services [[24], [25], [26]]. The extent to which one factor has more weight than the other is unknown. The concern is that unintentionally neglecting serious, life-threatening conditions other than Covid-19, most notably cancer, a secondary health crisis may erupt [27,28]. Some experts have suggested a classification of Covid-19-related diagnostic errors which includes ‘acute’ and ‘chronic collateral’ errors [29]. Fear of Covid-19 among patients with life-threatening acute conditions including myocardial infarction and stroke led to a dramatic decline in the volume of ED attendances and admissions in the USA and Europe (‘acute collateral error’) [[30], [31], [32]]. However, collateral harm also includes missed or delayed diagnosis of serious conditions due to cancelations by the health system of essential procedures and appointments (‘chronic collateral error’) [[27], [28], [29], [30]]. A salient finding of our study was the collateral effect of the shutdown on well-known indicators of healthcare quality of quick diagnosis units, most notably waiting times. During the pandemic and, to a lesser degree, postpandemic periods, appointments to the unit and crucial procedures were suspended or deferred, while diagnostic facilities were operating at notably reduced capacity. As a result, mean times to diagnosis were unacceptably long. To our knowledge, this study is the first of its kind to investigate the impact of the Covid-19 lockdown on the ambulatory management of patients referred by the ED due to concerning, serious symptoms suggestive of cancer. Two recent studies evaluated the impact of the pandemic on diagnostic delays and survival since the start of physical distancing measures in the UK in March 2020. While these studies were modeled with respect to primary care urgent pathways for suspected cancer in the UK (i.e., 2-week-wait referral route or direct presentation to the ED) [14,15], descriptions about investigations and waiting times were not provided, preventing us from making direct comparisons with our results.
System- and patient-related factors explain diagnostic delays and decreases in cancer diagnoses during the pandemic. System-related factors including suspension and reduced delivery of diagnostic services are primarily the result of the implementation of measures to prevent SARS-CoV-2 transmission and the allocation of hospital-based resources to Covid-19 patients. Aside from personal assumptions and moral concerns, patient-related factors are mostly linked to anxiety and fear of acquiring Covid-19 in the healthcare setting, explaining the decline in the volumes of ED presentations and emergency admissions of non-Covid-19 patients. Recorded data from our hospital revealed a drop of 39.38% in the number of ED visits in March and April 2020 (n = 10,346) compared with March and April 2019 (n = 17,067). While these differences may account for the significant decline in the number of referrals to the quick diagnosis unit during the pandemic period, waiting times to investigations and time-to-diagnosis are more likely attributable to system-related disruptions (i.e., availability of diagnostic capacity). In the UK, despite fewer patients undergoing diagnostic studies because of the decline in primary care referrals, waiting times increased due to deferments and cessations of procedures considered high-risk for SARS-CoV-2 transmission plus other safety precautions. For instance, in August 2020 the number of patients waiting ≥6 weeks for key tests including CT scans, endoscopies, non-obstetric ultrasonographies, and magnetic resonance imaging was tenfold higher than in August 2019 [33].
The patterns of emergency presentation and referral during the pandemic period of our study were not as those expected and observed during the prepandemic periods of 2020 and 2019. The clinical reasons for referral in non-pandemic conditions partly reflect the rates and distribution of final diagnoses of the unit. The higher rates of referrals for anemia, lymphadenopathy or palpable lumps, and abdominal masses in the prepandemic and postpandemic vs. pandemic periods partly explain the relatively higher occurrence of malignancies such as colorectal, pancreatic, and ovarian cancer and Hodgkin and non-Hodgkin lymphoma in the former two periods [8,10]. However, the rates of referrals for unintentional weight loss, severe fatigue, fever of unknown origin, change in bowel habit, dysphagia and dyspnea in the pandemic vs. prepandemic period were unexpectedly high and not consistent with the final diagnoses. Compared to prepandemic patients, patients from the pandemic cohort had a significantly higher diagnostic rate of benign gastrointestinal disorders, including irritable bowel disease (17.1%) and organic, mainly inflammatory, conditions (23.8%), and they were between three and four times as likely as prepandemic patients to be diagnosed with mental and behavioral disorders, including somatic symptom disorder, anxiety disorder, and depression (according to ICD-10 and DSM-5).
Referrals during the pandemic period included a significant number of patients who presented to the ED with persistent low-grade fever of unknown origin, dysphagia and dyspnea, in whom no recognizable cause was identified after extensive investigations comprising 18F-FDG PET/CT scans, CT scans, high-resolution esophageal manometry, and pulmonary function tests, among others. Unexplained, commonly persistent, physical symptoms may fit criteria of primary functional syndromes such as irritable bowel disease and chronic fatigue syndrome/fibromyalgia or psychiatric disorders such as somatic symptom disorder [34]. The latter was introduced in DSM-5 to designate patients with somatic symptoms that are distressing or result in significant disruption of functioning and disproportionate thoughts and behaviors regarding those symptoms [35]. While ‘medically unexplained symptoms’ are well-recognized by primary care physicians, there is a significant overlap with somatic symptom disorder and other forms of anxiety disorder with somatic symptoms. Although not only explained by anxiety or depression, some patients with somatic symptom disorder and medically unexplained symptoms report that the onset of their symptoms has a chronologic relationship with stressors that include, among others, traumatic events for the individual or community [35,36]. In a study in mainland China that assessed the psychological impact of the Covid-19 outbreak on the general population (n = 1738), unexplained physical symptoms such as persistent fever, dyspnea, myalgia, and gastrointestinal symptoms, alone or in combination were reported by 15.04% of subjects (5.70% in our study) and were significantly associated with stress, anxiety, and depression scores [37]. The authors of the article ‘Do not stay at home: we are ready for you’ used the term ‘dread risk’ to explain the unexpectedly low rates of emergency attendances during the Covid-19 outbreak. Dread risks are high-damage, low-probability shocking events such as terrorist attacks, plane crashes, or earthquakes, in which large numbers of people die at one time. Awareness of such random events in conjunction with repeated stunning images through the media can trigger unfounded fears and behavioral reactions of risk avoidance with distressing symptoms that may mimic organic diseases [[38], [39], [40], [41]].
4.1. Strengths and limitations of this study
We acknowledge several limitations. First, this was a retrospective study with a pre-, during- and post-analysis and was thus limited to the constructs of its design. Second, our cohorts were not comparable in their final diagnostic categories, generating doubts about bias in the conclusions about delays in diagnosis. Nevertheless, despite higher rates of gastrointestinal, mental and behavioral, and unexplained disorders among patients from the pandemic period, only initially undiagnosed patients with suspected cancer met inclusion criteria, which meant that diagnostic decisions including orders for investigations were based on presenting symptoms while waiting times were established by diagnostic services. Third, data were collected from an electronic health record database, thus preventing the level of detail potentially attainable with a manual record review. Finally, we obtained data from a single, urban academic medical center and the study population were patients within the Barcelona metropolitan area. We do not know whether the research findings and conclusions from our study may be extended to the population at large. We recognize that variation is likely in Spain and other countries in terms of emergency access and burden of Covid-19. Spain is a country with 47 million habitants with a nationwide and public healthcare system and a relatively low ‘Gini’ coefficient (an economic inequality measure, in which a low coefficient denotes a homogeneous population) [42]. Hence, the extent of the interruption of essential diagnostic services in patients with suspected cancer from healthcare systems with higher Gini coefficients may be different. Nonetheless, our study reflects an actuality experience, and the reported data may be useful in understanding patient and hospital behaviors in this setting.
4.2. Relevance for clinical practice
In October 2020, seven months after the first outbreak, diagnostic facilities were still working at reduced capacities and the daily working activity of the quick diagnosis unit had not returned to normality. With the pandemic ongoing, waiting times for investigations and times to diagnosis remained excessively long due to the backlog of patients. Although not analyzed in this study, diagnostic delays are expected to have a weight on the prompt initiation of treatment, either surgical or medical, which is essential for ensuring the best outcomes in cancer patients [43]. While public health communities should be informed about the impact of the pandemic on the accessibility to diagnostic services for patients with serious diseases possibly associated with cancer, active interventions are necessary to minimize these undesirable collateral effects. Both technical provision for procedures through which patients with cancer are diagnosed (e.g. imaging and endoscopy) and personnel for specialist assessment should be increased in a timely manner.
5. Conclusions
As a result of the Covid-19 shutdown, the backlog of routine work across all hospital services caused an interruption of the usual operating procedures of the quick diagnosis unit with cancelations and deferrals of essential investigations and excessively long diagnostic times. In addition to the exceptional decrease in the volume of patients presenting to the ED with concerning symptoms suggestive of cancer and referred to the unit for diagnostic evaluation, the patterns of clinical presentation and eventual diagnosis observed during the highest peak of the outbreak were clearly different from those of non-pandemic times. There was an unexpected increase in the rate of functional, mental and behavioral disorders and of medically unexplained physical symptoms including low-grade fever, dysphagia and dyspnea. These unusual patterns suggest that awareness of the pandemic together with continual updates of the death toll and number of cases through the media generated atypical behavioral responses with distressing symptoms.
Read from the prospect of healthcare decision-makers, the findings of our study indicate that, in the midst of the pandemic, the delivery of diagnostic services and the accessibility to outpatients' clinics should be well-balanced. In terms of commonplace practice, clinicians caring for patients may expect an increase in the rates of mental and behavioral health disorders among non-Covid-19 patients seeking care in the ED, including those with symptoms suggestive of cancer.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data. Data reported in this manuscript are available within the article. Additional data from the study are available upon reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
All authors have nothing to declare on conflict of interest.
References
- 1.National Institute for Health and Care Excellence (NICE) Suspected cancer: recognition and referral (NG12) 2015. https://www.nice.org.uk/guidance/ng12 [accessed 11 October 2020] [PubMed]
- 2.Chapman D., Poirier V., Vulkan D., Fitzgerald K., Rubin G., Hamilton W., et al. First results from five multidisciplinary diagnostic centre (MDC) projects for non-specific but concerning symptoms, possibly indicative of cancer. Br J Cancer. 2020;123(5):722–729. doi: 10.1038/s41416-020-0947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenman E., Palmér K., Rydén S., Sävblom C., Svensson I., Rose C., et al. Diagnostic spectrum and time intervals in Sweden’s first diagnostic center for patients with nonspecific symptoms of cancer. Acta Oncol. 2019;58(3):296–305. doi: 10.1080/0284186X.2018.1537506. [DOI] [PubMed] [Google Scholar]
- 4.Pericás J.M., Aibar J., Soler N., López-Soto A., Sanclemente-Ansó C., Bosch X. Should alternatives to conventional hospitalisation be promoted in an era of financial constraint? Eur J Clin Invest. 2013;43(6):602–615. doi: 10.1111/eci.12087. [DOI] [PubMed] [Google Scholar]
- 5.Conley J., O’Brien C.W., Leff B.A., Bolen S., Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693–1702. doi: 10.1001/jamainternmed.2016.5974. [DOI] [PubMed] [Google Scholar]
- 6.Bosch X., Aibar J., Capell S., Coca A., López-Soto A. Quick diagnosis units: a potentially useful alternative to conventional hospitalisation. Med J Aust. 2009;191(9):496–498. doi: 10.5694/j.1326-5377.2009.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 7.Corbella X., Barreto V., Bassetti S., Bivol M., Castellino P., de Kruijf E.J., et al. Hospital ambulatory medicine: a leading strategy for internal medicine in Europe. Eur J Intern Med. 2018;54:17–20. doi: 10.1016/j.ejim.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Bosch X., Jordán A., López-Soto A. Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31(1):114–123. doi: 10.1016/j.ajem.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S., Sukhal S., Agarwal R., Das K. Quick diagnosis units: an effective alternative to hospitalization for diagnostic workup: a systematic review. J Hosp Med. 2014;9(1):54–59. doi: 10.1002/jhm.2129. [DOI] [PubMed] [Google Scholar]
- 10.Bosch X., Escoda O., Nicolás D., Coloma E., Fernández S., Coca A., et al. Primary care referrals of patients with potentially serious diseases to the emergency department or a quick diagnosis unit: a cross-sectional retrospective study. BMC Fam Pract. 2014;15:75. doi: 10.1186/1471-2296-15-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch X., Sanclemente-Ansó C., Escoda O., Monclús E., Franco-Vanegas J., Moreno P., et al. Time to diagnosis and associated costs of an outpatient vs inpatient setting in the diagnosis of lymphoma: a retrospective study of a large cohort of major lymphoma subtypes in Spain. BMC Cancer. 2018;18(1):276. doi: 10.1186/s12885-018-4187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brito-Zerón P., Nicolás-Ocejo D., Jordán A., Retamozo S., López-Soto A., Bosch X. Diagnosing unexplained fever: can quick diagnosis units replace inpatient hospitalization? Eur J Clin Invest. 2014;44(8):707–718. doi: 10.1111/eci.12287. [DOI] [PubMed] [Google Scholar]
- 13.Sanclemente-Ansó C., Bosch X., Salazar A., Moreno R., Capdevila C., Rosón B., et al. Cost-minimization analysis favors outpatient quick diagnosis unit over hospitalization for the diagnosis of potentially serious diseases. Eur J Intern Med. 2016;30:11–17. doi: 10.1016/j.ejim.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sud A., Torr B., Jones M.E., Broggio J., Scott S., Loveday C., et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020 Aug;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor R.C., Wetherall K., Cleare S., McClelland H., Melson A.J., Niedzwiedz C.L., et al. Mental health and well-being during the COVID-19 pandemic: longitudinal analyses of adults in the UK COVID-19 Mental Health & Wellbeing study. Br J Psychiatry. 2020 Oct;21:1–8. doi: 10.1192/bjp.2020.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalin N.H. COVID-19, substance use, anorexia nervosa, 22q11.2 deletion syndrome, and stress. Am J Psychiatry. 2020;177(7):561–563. doi: 10.1176/appi.ajp.2020.20050685. [DOI] [PubMed] [Google Scholar]
- 18.Barba R., Rosado C., Pardo-Moreno J., Rey-Biel J. Managing people, roles, and resources during Covid-19 surge. NEJM Catal Innov Care Deliv. 2020 May 18 doi: 10.1056/CAT.20.0152. [DOI] [Google Scholar]
- 19.Siqueira C.A.D.S., Freitas Y.N.L.D., Cancela M.D.C., Carvalho M., Oliveras-Fabregas A., de Souza D.L.B. The effect of lockdown on the outcomes of COVID-19 in Spain: an ecological study. PLoS ONE. 2020;15(7) doi: 10.1371/journal.pone.0236779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministerio de la Presidencia relaciones con las cortes y memoria democrática. Real Decreto 463/2020, de 14 de marzo, por el que se declara el estado de alarma para la gestión de la situación de crisis sanitaria ocasionada por el COVID-19. BOE no 67 de 14 de marzo de 2020. Boletín Oficial Del Estado. 2020;67(I):25390–25400. [Google Scholar]
- 21.Bosch X., Jordán A., Coca A., López-Soto A. Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7(1):41–47. doi: 10.1002/jhm.931. [DOI] [PubMed] [Google Scholar]
- 22.International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10 Version) 2019. https://icd.who.int/browse10/2019/en [accessed 11 October 2020]
- 23.American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).
- 24.Ministerio de Sanidad Actualización no 225. Enfermedad por el coronavirus (COVID-19) October 9, 2020. https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_225_COVID-19.pdf [accessed 11 October 2020]
- 25.Cano-Valderrama O., Morales X., Ferrigni C.J., Martín-Antona E., Turrado V., García A., et al. Acute care surgery during the COVID-19 pandemic in Spain: changes in volume, causes and complications. A multicentre retrospective cohort study. Int J Surg. 2020;80:157–161. doi: 10.1016/j.ijsu.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alquézar-Arbé A., Piñera P., Jacob J., Martín A., Jiménez S., Llorens P., et al. Impact of the COVID-19 pandemic on hospital emergency departments: results of a survey of departments in 2020 - the Spanish ENCOVUR study. Emergencias. 2020;32(5):320–331. [PubMed] [Google Scholar]
- 27.Petrova D., Pérez-Gómez B., Pollán M., Sánchez M.J. Implicaciones de la pandemia por COVID-19 sobre el cáncer en España [implications of the COVID-19 pandemic for cancer in Spain] Med Clin (Barc) 2020;155(6):263–266. doi: 10.1016/j.medcli.2020.04.011. Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manso L., De Velasco G., Paz-Ares L. Impact of the COVID-19 outbreak on cancer patient flow and management: experience from a large university hospital in Spain. ESMO Open. 2020;4(Suppl. 2) doi: 10.1136/esmoopen-2020-000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi T.K., Singh H. Reducing the risk of diagnostic error in the COVID-19 era. J Hosp Med. 2020;15(6):363–366. doi: 10.12788/jhm.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Leor O., Cid-Álvarez B., Ojeda S., Martín-Moreiras J., Rumoroso J.R., López-Palop R., et al. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España [impact of the COVID-19 pandemic on interventional cardiology activity in Spain] REC Interv Cardiol. 2020;2(2):82–89. Spanish. [Google Scholar]
- 31.Baum A., Schwartz M.D. Admissions to Veterans affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96–99. doi: 10.1001/jama.2020.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jasne A.S., Chojecka P., Maran I., Mageid R., Eldokmak M., Zhang Q., et al. Stroke code presentations, interventions, and outcomes before and during the COVID-19 pandemic. Stroke. 2020;51(9):2664–2673. doi: 10.1161/STR.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwood E., Swanton C. Consequences of COVID-19 for cancer care - a CRUK perspective. Nat Rev Clin Oncol. 2021 Jan;18(1):3–4. doi: 10.1038/s41571-020-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.den Boeft M., Claassen-van Dessel N., van der Wouden J.C. How should we manage adults with persistent unexplained physical symptoms? BMJ. 2017;356:j268. doi: 10.1136/bmj.j268. [DOI] [PubMed] [Google Scholar]
- 35.Kurlansik S.L., Maffei M.S. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49–54. [PubMed] [Google Scholar]
- 36.Croicu C., Chwastiak L., Katon W. Approach to the patient with multiple somatic symptoms. Med Clin North Am. 2014;98(5):1079–1095. doi: 10.1016/j.mcna.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Wang C., Pan R., Wan X., Tan Y., Xu L., McIntyre R.S., et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. 2020;87:40–48. doi: 10.1016/j.bbi.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deerberg-Wittram J., Knothe C. Do not stay at home: we are ready for you. NEJM Catal Innov Care Deliv. 2020 May 5 doi: 10.1056/CAT.20.0146. [DOI] [Google Scholar]
- 39.Gigerenzer G. Out of the frying pan into the fire: behavioral reactions to terrorist attacks. Risk Anal. 2006;26(2):347–351. doi: 10.1111/j.1539-6924.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee S.A., Jobe M.C., Mathis A.A. Mental health characteristics associated with dysfunctional coronavirus anxiety. Psychol Med. 2020 Apr 16 doi: 10.1017/S003329172000121X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S.A. Coronavirus anxiety scale: a brief mental health screener for COVID-19 related anxiety. Death Stud. 2020;44(7):393–401. doi: 10.1080/07481187.2020.1748481. [DOI] [PubMed] [Google Scholar]
- 42.World Bank, Development Research Group Gini index (World Bank estimate): Spain. 1980-2017. https://data.worldbank.org/indicator/SI.POV.GINI?locations=ES [accessed 11 October 2020]
- 43.Sud A., Jones M.E., Broggio J., Loveday C., Torr B., Garrett A., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]