Abstract
Primary lateral sclerosis (PLS) is a rare neurodegenerative disease characterized by progressive degeneration of upper motor neurons (UMNs). Recent studies shed new light onto the cellular events that are particularly important for UMN maintenance including intracellular trafficking, mitochondrial energy homeostasis and lipid metabolism. This review summarizes these advances including the role of Alsin as a gene linked to atypical forms of juvenile PLS, and discusses wider aspects of cellular pathology that have been observed in adult forms of PLS. The review further discusses the prospects of new transgenic upper motor neuron reporter mice, human stem cell-derived UMN cultures, cerebral organoids and non-human primates as future model systems to better understand and ultimately treat PLS.
Keywords: primary lateral sclerosis, Betz cell, upper motor neuron, corticospinal motor neuron, Alsin, ALS2, endosomes, mitochondria, Golgi apparatus, bioenergetics, membrane lipids
Background
Motor neuron disease (MND) is an umbrella term for pathological conditions that affect upper motor neurons (UMNs) in the cerebral motor cortex, lower motor neurons (LMNs) in the spinal cord or brain stem, or both types of motor neurons. Primary lateral sclerosis (PLS) represents a rare form of MND that affects mainly the UMNs and typically results in very slowly-progressive spasticity and paralysis (1-8). By contrast, classical amyotrophic lateral sclerosis (ALS, maladie de Charcot) is characterized by the loss of both upper and lower motor neurons and typically associated with rapidly progressing muscle weakness (9-11). Although the rate of progression and the neuronal involvement are different, PLS and ALS are considered part of a continuum, and part of a broader spectrum including also frontotemporal dementia (FTD) (12,13).
The molecular and cellular mechanisms of UMN degeneration in PLS remain largely unknown. A working group among international PLS experts was therefore constituted on May 3rd and 4th of 2019 in Philadelphia, PA, USA, with the aim to better understand the neurobiology of PLS and to foster collaborative research efforts. Similar working groups were established for PLS diagnostics (14), differential diagnostics (15), disease progression (16), genetics (17), neuroimaging (18), neurophysiology (19), neuropathology (20), as well as PLS clinical care and study design (21).
Anatomy of upper motor neurons
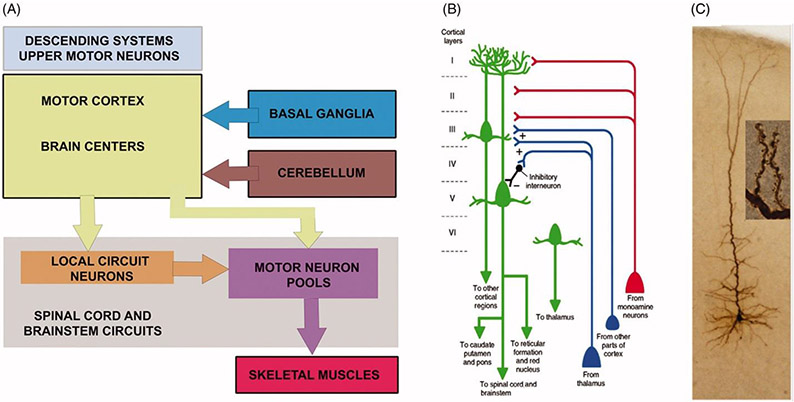
The movement starts in the brain and the motor neuron circuitry is one of the complex circuitries in the central nervous system with components both in the cerebral cortex and the spinal cord (Figure 1(A)). In an effort to better understand the neurobiology of PLS, the cellular properties of UMNs need to be comprehensively analyzed. Numerous mouse models have been generated for this purpose. Corticospinal motor neurons (CSMN) are considered the UMNs in mice, and similar to UMNs in humans they are also located in layer 5 of the motor cortex, their soma size is much larger than that of other excitatory neurons in the cerebral cortex, and they have a long axon projecting to the spinal targets. Even though some aspects of their connectivity may differ among species (24,25), there are remarkable similarities between UMNs at a cellular level (26,27). Therefore, well-defined mouse models with prominent and progressive CSMN loss have been invaluable revealing the underlying causes and pathologies responsible for their vulnerability and progressive degeneration. The UMNs in many different species are distinguished among many different neuron types based on their large size and precise location, their first important characteristic.
Figure 1.
Movement starts in the brain and upper motor neurons are an important component of motor neuron circuitry. (A) Simplified drawing of the basic components of motor neuron circuitry, which has the upper motor neurons and descending paths at the very top of the command chain. Adapted from (22). (B) Schematic representation of a corticospinal motor neuron (CSMN, located in layer V of the motor cortex) in cortical layer V as well as long-distance projection neurons and interneurons that modulate its activity. Also note additional neurons projecting to other cortical regions or thalamus. Adapted from (23). (C) Image of an upper motor neuron, which is located in layer V of the motor cortex, has an apical dendrite which extends toward the top layers of the cortex and spines which are the active site of neuronal integration.
The second important characteristic of UMNs is their axon, which extends toward the target cells in brain stem and spinal cord. UMNs have one of the longest axons in our bodies, reaching up to about a meter in length in the case of Betz cells that project to lumbar and sacral regions of the spinal cord. Therefore, maintaining the integrity and the health of this long axon is challenging. When the neuron shows signs of vulnerability and degeneration, one of the first sites of neuronal dysfunction is thus at the axon, with the longest axon fibers often degenerating first.
The third important characteristic of UMNs is their long apical dendrite that extends toward the top layers of the cortex (Figure 1(B-C)). The apical dendrite is exceptionally important for their neuronal modulation, as this is the site for many different neuron populations to make a synaptic connection with the UMNs (28). The apical dendrite is extensively branched and the branches are adorned with hundreds of thousands, of spines (Figure 1(C)). These spines receive excitatory input from many different neuron types, such as callosal projection neurons, thalamacortical neurons and local circuitry neurons (Figure 1(B)) and (29). Thus, the health and stability of spines are important for these excitatory neurons to convey their information. Especially at the site of layer 2/3, CSMN receive most of their excitatory input, and this is one of their unique characteristics (30,31). The connectivity patterns of both long-distance projection neurons and local circuitry neurons are investigated by novel approaches, revealing the complex connectivity dynamics in the motor cortex and other regions (32,33).
The apical dendrite is however not the only site where CSMN receive excitatory input. There are numerous basal dendrites that emanate from the soma. These basal dendrites are also heavily adorned by spines, which receive input from many different neuron types (Figure 1(B)), mostly callosal projection neurons and other local circuitry neurons (31). CSMN could potentially be receiving thousands of different excitatory synaptic inputs per second, and their ability to converge this information into a single action potential is also determined by the level and the extent of inhibitory input they receive. Inhibitory neurons come in many different types, shapes and function. Likewise, a plethora of inhibitory neurons also act upon CSMN (34) and interestingly, some inhibitory neurons located in layer 2/3 inhibit the inhibitory neurons located in layer 5. Therefore, not all inhibitory neurons’ role should be considered as pure inhibition, as some in fact contribute to the activation of CSMN (Figure 1(B)). The balance of excitation and inhibition is an active area of research, and findings from these studies will help reveal the basis of their mode of electrophysiological modulation (35). Since hyperexcitation contributes to the initial vulnerability of UMNs (36) and hypoexcitation coincides with their progressive degeneration, being able to understand how they are modulated by other cortical neurons is of great importance (37). Imaging techniques were developed to visualize the normal connectivity of Betz cells and their altered connectivity in disease (38). Similar studies are under way to reveal the timing and extent of UMN degeneration in PLS patients (18,19).
Cellular mechanisms relevant to PLS
Loss of dendritic spines is one of the earliest signs of neuronal degeneration in UMNs (27,39). As the neurons fail to retain the integrity of their spines, their input is hampered, leading to alterations in their proper modulation and eventual dysfunction. Because UMNs heavily depend on other cortical neurons for their modulation, the health and stability of their spines are of great importance. Recent evidence reveals that the degenerating CSMN lose their spines, especially at the site of apical dendrite and apical dendrites also fail to maintain their cytoarchitecture (40). This cellular defect is debilitating for CSMN, which depend on the integrity of their apical dendrite and dendritic spines for their proper integration into the circuitry and motor function.
Growing evidence reveals numerous cellular defects that result in CSMN vulnerability. The failure of CSMN to maintain their cytoarchitecture results in a major limitation for retaining the integrity of apical dendrite. Spine loss is one of the early cellular defects that occur in CSMN. Likewise, disintegration of axon or failure of axonal transport machinery is also one of the first cellular defects resulting in neuronal vulnerability. Additionally, increased ER stress (41), mitochondrial dysfunction and perturbations in lipid homeostasis (42) also accounts for the underlying causes of UMN vulnerability. A profound increase in the extent of immune reaction as well as glial activation is also observed in PLS patients (43), and this is recapitulated in many different mouse models of motor neuron disease.
In an effort to better understand the mechanisms of neurodegeneration in PLS, numerous mouse models were generated based on PLS-linked mutations, such as ALS2/Alsin, C9Orf72, DCTN/Dynactin 1, FIG4/Phosphoinositide 5-phosphatase, OPTN/Optineurin, SETX/Senataxin, SPG7/Paraplegin or UBQLN2/Ubiquilin 2 (17). However, generation and characterization of a mouse model for an UMN disease is challenging. Humans heavily depend on their Betz cells for the initiation and modulation of voluntary movement and there are direct projections from Betz cells to the spinal motor neurons. In mice, in addition to the corticospinal tract, the rubrospinal tract also plays an important role and the circuitry within the spinal cord includes an interneuron component (24). Therefore, when humans have defects in their corticospinal tract, they may be paralyzed, whereas mice will be able to move, albeit with loss of their ability for fine movement. During evolution, humans have become more specialized in fine movement in the expense of making themselves vulnerable to significant spinal cord injuries. Rodents, however, have better capabilities to recover from an injury, but they are not as skilled as humans when it comes to dexterity (24,44). Moreover, there are no good outcome measures, which can quantitatively assess the timing and the extent of UMN degeneration in mouse models of PLS. Despite these two limitations the UMNs in mice and human share many common cell biological features and display similar pathologies at a cellular level (26,27,45). Hence, many different labs generated mouse models for genes that had been found mutated in PLS patients. Albeit most mouse lines did not have a prominent phenotype at a species level (46), detailed cellular investigations of their UMNs began to reveal the underlying problems.
Intracellular trafficking in PLS
The precise molecular and cellular mechanisms of UMN degeneration in PLS remained elusive for many years. The importance of intracellular trafficking defects in PLS was first recognized with the identification of mutations in the Alsin/ALS2 gene. Alsin is a large 1657 amino acid (184 kilodalton) protein that harbors three nucleotide exchange factor (GEF) domains for small GTPases: an N-terminal RCC1-like GEF domain for Ran GTPase, a central Dbl-homology and pleckstrin-homology (DH/PH) domain for Rac1 GTPase and a C-terminal VPS9 GEF domain for Rab5 GTPase (47,48).
Recessive loss-of-function mutations in Alsin have been identified in atypical forms of PLS with infantile or juvenile onset (49,50), infantile ascending spastic paraparesis (IAHSP) (51) and hereditary spastic paresis (HSP) (52) with no apparent genotype-phenotype correlation. Five transgenic mouse models were generated by different groups (49,53-56) but none of the mouse models fully recapitulated the motor function defects observed in patients.
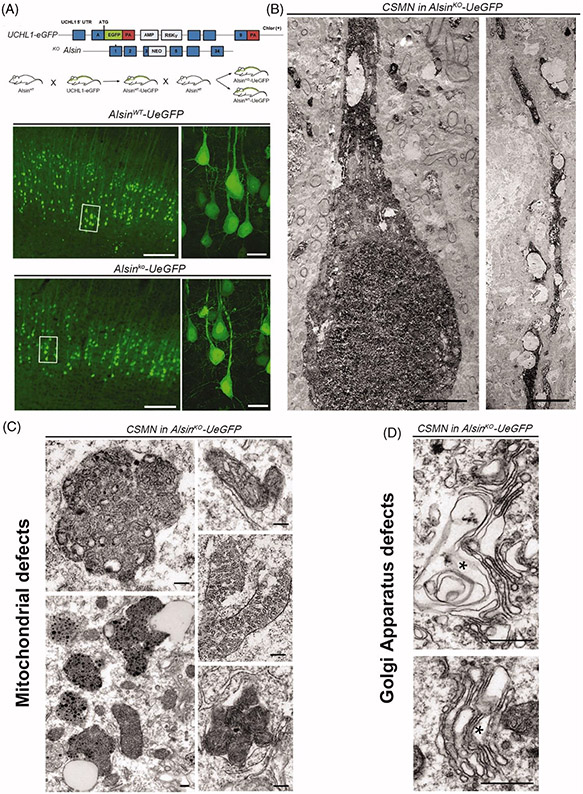
Recent developments in the field allowed genetic labeling of CSMN in the UCHL1-eGFP mice, which labels them with the long-lasting and stable fluorescent reporter eGFP (57). This CSMN reporter line allows visualization and cellular assessment of CSMN within the context of neurodegeneration in many different disease models (Figure 2(A)). Upon crossing Alsin knockout (KO) mice with UCHL1-eGFP, Alsin KO-UeGFP mice were generated, in which CSMN lacked Alsin function and were visualized by eGFP (42). This allowed eGFP immunohistochemistry coupled to electron microscopy to reveal the ultrastructural defects that occur inside CSMN lacking Alsin function. Corticospinal tract axons displayed disintegration at the level of the cervical spinal cord as well as at the pyramidal decussation and the pons, refining earlier studies (54,56). CSMN apical dendrites were disintegrating with numerous vacuoles (Figure 2(B)). There were also profound defects in mitochondria and the Golgi apparatus (42) (Figure 2(C-D)). By contrast, neurons other than CSMN in the Alsin KO mice or CSMN in wild-type (WT) mice were healthy and had structurally intact organelles (42), further documenting the selective vulnerability of UMN in PLS. Being able to shine light to the cellular defects inside a distinct neuron population was very powerful and begun to reveal why UMNs are vulnerable and display progressive degeneration. Profound ultra-structural defects in Golgi apparatus and mitochondria suggest problems with ATP production, energy metabolism, as well as post-translational modification of proteins and lipid homeostasis (42). Beyond Alsin, these data underscore the critical importance of structural and functional Golgi integrity for adult motor neuron maintenance and highlight Golgi pathology as early hallmark of motor neuron degeneration in MND (58,59).
Figure 2.
GFP-labeling of CSMN in Alsin KO mice reveals significant cellular problems in the absence of Alsin function. (A) AlsinKO-UeGFP mice are generated by crossing the UCHL1-eGFP and the AlsinKO mice and in these mice the CSMN are genetically labeled with eGFP that is stable and long-lasting (57). (B–C) The coupled immuno-electron microscopy analyses, reveal that the diseased CSMN cannot maintain the cytoarchitectural integrity of their apical dendrites (B), have massive mitochondrial defects with collapsed mitochondria that are cleared by mitophagy (C). (D) The Golgi apparatus is enlarged and the vesicles may not fuse properly. These cellular defects are not detected in healthy CSMNs.
Alsin localizes to early endosomes in the neuronal soma and to punctuate structures in neurites and neuronal growth cones (47,49,60). In line with the known functions of the Alsin effectors Rac1 and Rab5, Alsin has been implicated in a wide range of cellular functions ranging from endocytosis (47,49,55,60,61), membrane trafficking (62) and macropinocytosis (63) to endolysosomal protein degradation (64). Recently, Alsin was also found to be involved in oxidative stress sensing by endosomes and to catalyze the assembly of the Rab5 endocytic machinery on mitochondria (65). Remarkably, Alsin-deficient spinal motor neurons were defective in relocating Rab5 from endosomes to mitochondria and displayed increased apoptotic signaling from mitochondria upon cellular stress as evidenced by cytochrome c release into the cytosol (65). These observations provide a compelling mechanistic basis for the mitochondrial defects observed in the CSMN of Alsin KO mice (42).
What might be the consequences of Alsin loss of function on neuronal growth and maintenance? Several studies have analyzed cortical neurons from Alsin KO mice in primary culture. These neurons showed normal integrity and survival under baseline conditions and only modest or no susceptibility to cellular stressors, such as free oxygen radicals or excitotoxic agents (49,53,61). More pronounced effects were observed when cultured neurons were subjected to acute RNA interference-mediated (RNAi) knockdown of Alsin, which led to severe defects in axon growth and cell survival (60,66).
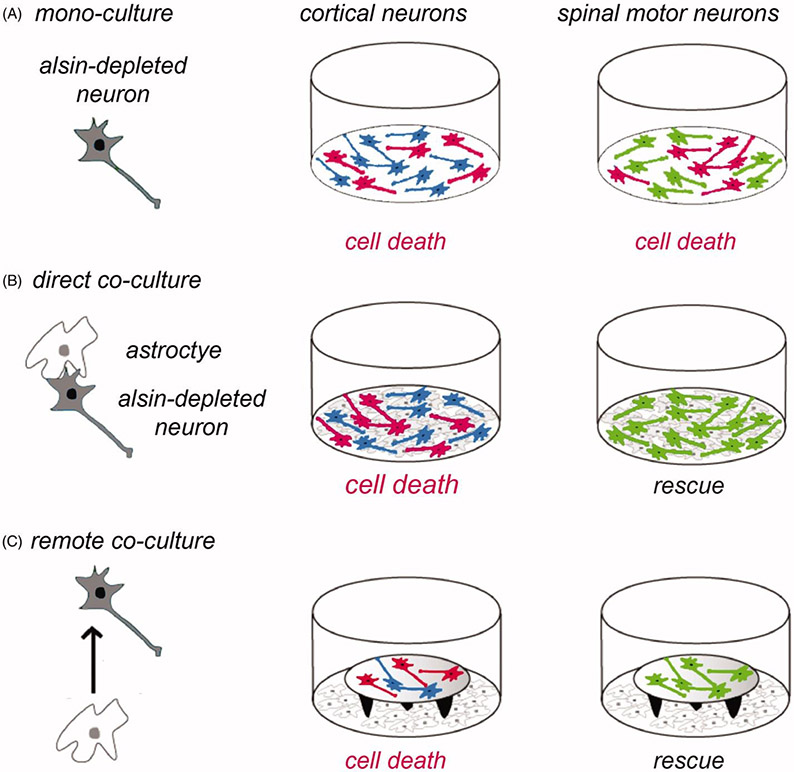
Studies with Alsin knockdown neurons in culture also gave new clues to the selecive vulnerability of CSMN (66). It was demonstrated that several types of neurons including cortical neurons, spinal motor neurons and sensory dorsal root ganglion neurons displayed similar vulnerability to Alsin knockdown as cortical neurons (Figure 3(A)). Remarkably however, in co-culture with astrocytes, Alsin-depleted spinal motor neurons were completely rescued from Alsin RNAi-mediated cell death and defective axon growth (Figure 3(B)). Alsin-depleted cortical neurons showed to such rescue effect (Figure 3(B)). The astrocytic rescue of spinal motor neurons was mediated by a soluble factor (Figure 3(C)) rather than by cellular contact and did not involve Rac1 activation (66). While these data from standard primary cultures of rodent neurons cannot be directly extrapolated to CSMN, they provide a first conceptual hint to the selective vulnerability of CSMN in Alsin-linked PLS.
Figure 3.
Role of astrocytes in the vulnerability of cortical neurons to Alsin knockdown. The schematic shows the experimental design (left panel) using different types of neurons (in grey) in mono-culture or in co-culture with astrocytes (in white). Neurons having undergone cell death are depicted in red (middle and right panels). (A) Cortical neurons (in blue) and spinal motor neurons (in green) cultured each for 2 days in mono-culture show similar vulnerability to cell death (in red) induced by RNAi-mediated Alsin depletion. (B) In direct co-culture with astrocytes, Alsin-depleted cortical neurons display cell death whereas Alsin-depleted spinal motor neurons are completely rescued. (C) The astrocytic rescue of alsin-depleted spinal motor neurons (lower right panel) is mediated by a soluble factor as shown in co-cultures where the neurons are placed on coverslips on top of remote astrocytes. Neuronal viability was analyzed relative to control cultures transduced with a control small interfering RNA and using different types of astroctyes prepared from cerebral cortex or spinal cord (64).
Energy metabolism and oxidative stress in PLS
Mitochondria play a central role in cellular energy metabolism; therefore, functional mitochondria are crucial for the health of cells with high-energy demand, such as neurons. Mitochondrial biogenesis, quality control via mitophagy, and mitochondrial dynamics are fundamental processes that generate ATP at the appropriate time and location within neurons. Mitochondria are also key players in both apoptotic and necrotic cell death processes, cellular calcium homeostasis, and free radical generation. When disrupted, these mechanisms might contribute to neurodegeneration including motor neuron degeneration.
Many lines of evidence highlight mitochondrial abnormalities in degenerative motor neuron diseases. In genetic models, mitochondrial trafficking is affected by mutant SOD1, TDP-43 and truncated Alsin (67-69). Abnormalities in mitochondrial morphology and increased mitophagy were observed in CSMN of Alsin KO mice (42). In humans, hypometabolism measured by FDG-PET (fluorodeoxyglucose positron emission tomography) has been demonstrated in the motor cortex of patients with PLS (33) as well as in a patient with Mills’ syndrome (70), an UMN disease also referred to as unilateral PLS (15,70-72).
In motor neuron diseases, non-neuronal cell types are however also affected (43). Sporadic ALS patients have been shown to have various skin related abnormalities (73-75) with shared pathology with motor neurons (76-79), suggesting skin-derived fibroblasts as potential models for studying motor neuron diseases, as these can offer information on disease mechanisms.
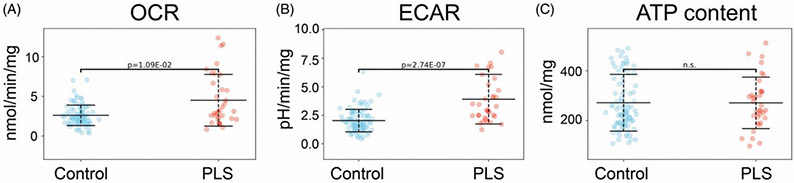
Functional bioenergetic and metabolic readouts have been studied in fibroblasts from motor neuron disease patients (73-75). One such readout is mitochondrial membrane potential, which was found to be elevated in sporadic ALS compared to control lines, and elevated even more significantly in PLS-patient derived fibroblasts compared to ALS (80). As mitochondrial membrane potential is generated by oxidizing reducing equivalents from the Krebs-cycle and dissipated by the ATP-synthase, the high membrane potential in PLS patient-derived cells may signal an elevated metabolic rate, a decreased ATP demand, or a combination of both processes. The production of reactive oxygen species in mitochondria, a well-established contributor to ALS pathology (81), is exponentially dependent on membrane potential. Oxygen consumption rates, glycolytic rates, and cellular ATP contents of fibroblasts derived from healthy control subjects and PLS patients were studied (Figure 4). Both ALS and PLS fibroblasts exhibited elevated oxidative (Figure 4(A)) and glycolytic (Figure 4(B)) rates, with only a modest increase in ATP content in ALS and no differences in ATP levels in PLS compared to controls (Figure 4(C)) (82). These results, together with the elevated mitochondrial membrane potential, suggest that PLS patient-derived cells have elevated ATP demand and consumption, which needs to be matched by enhanced energy metabolism through both oxidative and glycolytic ATP pathways.
Figure 4.
Functional bioenergetics of control versus PLS fibroblasts. Primary skin fibroblasts were cultured in the presence of 5 mM glucose, 4 mM glutamine and 1 mM pyruvate, values were normalized by mg protein. (n = 91 control and 34 PLS). (A) Oxygen consumption rates (OCR) measured by Seahorse flux analyzer, are indicative of mitochondrial respiration. (B) Extracellular acidification rate (ECAR), also measured by Seahorse, are indicative of glycolytic fluxes. (C) Total cellular ATP content measured by a luminescence assay. Experimental details are provided in the study by Konrad and colleagues (82).
The hypermetabolism in PLS could be a source of reactive oxygen species produced as a toxic byproduct. It remains to be determined if these findings in fibroblasts translate to disease-relevant cell types such as glia and neurons in PLS patients. Nevertheless, in support of this hypothesis, clustering analysis of a large number of post mortem sporadic ALS (sALS) cortex samples found the biggest cluster consisting of patients with hallmarks of oxidative and proteotoxic stress (82). Based on our studies, the hypermetabolic phenotype appears to be more pronounced in PLS compared to sALS (Figure 4) and it is possible that some of the differences in the clinical manifestations between these two forms of motor neuron disease are related to different levels of compensation for enhanced energy demands. Further investigations of energy metabolism and oxidative stress in PLS could offer new perspectives on development of PLS-specific biomarkers and therapeutic targets.
Lipid homeostasis in PLS
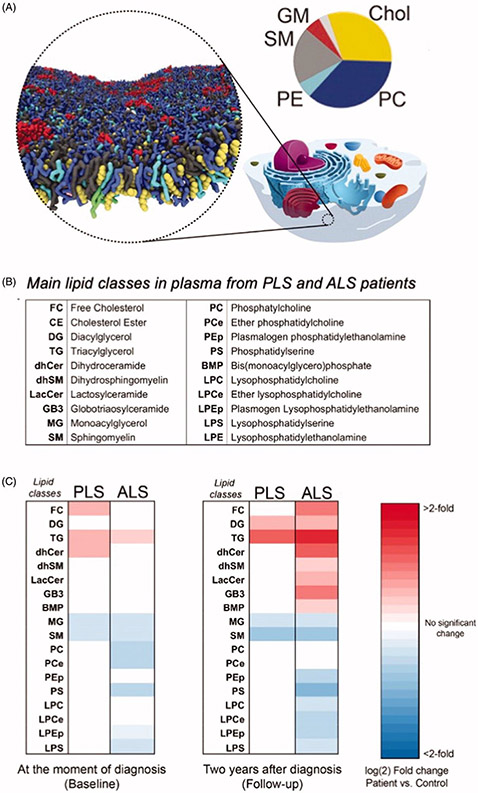
In the nervous system, the lipid composition of neuronal membranes is highly regulated and dynamic, as lipids perform a wide variety of functions, including roles in cell structure, synaptic transmission, and multiple metabolic processes (83). The spatial distribution of lipids in membranes has been investigated by computer simulation (84) and Figure 5(A). Changes in the concentrations of membrane lipids have been reported in several neurodegenerative diseases including Alzheimer’s disease (reviewed in (84)). It is therefore expected that the study of the absolute and relative concentrations of membrane lipids in PLS may shed light into these cellular processes and unravel novel degenerative pathways.
Figure 5.
Pathological changes in the main classes of lipids in plasma from PLS and ALS patients. A. Computer simulation of the spatial distribution of lipids and cholesterol in the outer leaflet of the plasma membrane. Shown are 63 different lipid species, combining 14 types of headgroups and 11 types of tails. Cholesterols are colored yellow, lipid headgroups are colored by type: Phosphatidylcholine PC blue, Sphingomyelin SM gray, Phospatidylethanolamine PE cyan; GM red; Phosphatidylinositolphosphates PIP magenta; Phosphatidylinositol PI pink; Phosphatidylserin PS green; PA, white; CE, ice blue; Diacylglycerol brown; LPC, orange. The pie chart shows the relative distribution of the main lipid in the outer leaflet of the plasma membrane. Modified from (84) with the kind permission of the authors and the publisher. B. Table showing the main classes of lipids in plasma from PLS and ALS patients analyzed by LC-MS. C. Heat map representation of the most significant fold-changes in the concentration of every class of lipids in plasma from ALS and PLS patients compared to controls at the beginning of the study (baseline) and two years after (Follow-up). (n = 40 samples analyzed in triplicate. *<0.05; **<0.01. t-test).
Analysis of the lipid composition of longitudinal plasma samples from PLS and ALS patients showed increased levels of de novo synthesized triglycerides, free-unesterified cholesterol, and specific species of cholesteryl esters, reflecting a significant imbalance in lipoprotein composition (Figure 5(B-C)). Baseline changes in neutral lipids were similar in the two conditions, but there was a differential progressions from baseline to follow-up (two years), consistent with the much more rapid progression of ALS.
In contrast, ALS samples showed marked changes in glycerophospholipids and sphingolipids that were essentially absent in PLS samples (Figure 5(C)). However, given the slow rate of progression of PLS pathogenesis compared to ALS, we cannot exclude the possibility that these changes become significant after longer periods of time in PLS pathogenesis. Those alterations in glycerophospholipids levels have also been detected in spinal cord tissues from ALS animal models with mutations in the superoxide dismutase 1 gene (SOD1G93A) at more advanced disease stages, possibly reflecting loss of MNs (85). Likewise, alterations in sphingolipid levels and in sphingolipid-regulating enzymes have been previously described in tissues from ALS mouse models (86,87) and in cerebrospinal fluid from ALS patients (87). Interestingly, and while the precise causes of these lipid changes in motor neuron disorders are currently unknown, the alterations in the plasma lipid composition of our ALS and PLS samples closely resemble those induced by hypoxic stress (88).
Lipidomics analyses are consistent with the view that PLS and ALS are part of an MND continuum (89). An intriguing possibility is that PLS patients may be protected from the aggressive nature of classical ALS by genetic or environmental factors that buffer, counteract and in some cases stabilize the aforementioned metabolic alterations, thereby slowing the progression of the disease.
Conclusions and perspectives
These are exciting times for fundamental, translational and therapeutic research in PLS. In addition to the information gathered, we believe that novel model systems for PLS will be generated in the future, based on new genetic mutations identified in PLS patients. Non-human primates will also be explored as potential PLS models. Rhesus monkeys, for instance, present some major advantages. First, they have approximately four times more CST axons than rats, display strong functional UMN connections to hand and digit muscles (24) and can be trained to perform precision grips at high success rates (44). Second, PLS-associated gene mutations can be introduced into pyramidal cells of their motor cortex by intrathecal or subpial delivery of AAV-based vectors (90,91). Third, germline transgenesis of rhesus monkeys will be achieved by gene transfer into oocyte (92) or by CRISPR/Cas-mediated gene editing (93). Fourth, technologies will become available to target the expression of PLS mutations temporally or spatially, thereby limiting disease symptoms and lifting ethical concerns. Taken together, these technologies will pave the way for better understanding the basis of UMN pathology in PLS.
AAV-mediated gene therapy approaches allowing direct transduction of UMNs, without affecting other neurons or cells in the cerebral cortex will also provide effective long-term treatment options (40). Last but not least, drug discovery studies are being developed which incorporate UMN survival as a therapeutic readout. Crossing PLS-relevant mouse lines with the UCHL1-eGFP reporter mice will allow the visualization and assessment of the specific cellular responses to drug treatments in vivo and in vitro (45,94).
In addition to primary cultures of rodent cortical neurons, cultures of stem-cell derived human UMNs (95) are currently entering the toolbox for PLS modeling. Indeed, CTIP2-positive (96) and COUP-TF1-negative (97) bona fide UMNs have recently been generated. Future studies will involve UMNs generated either from PLS patient’s iPSc or from normal iPSc that will be engineered by CRISPR/Cas9 to carry PLS-patient linked gene mutations (65).
As a complement to these approaches, cerebral organoids also called “mini-brains” (98) hold promise as intermediate PLS models. Human iPSc-derived cerebral organoids, which contain UMNs, glia and microglia in a 3D cyto-architecture reminiscent of the motor cortex (98-100), can be maintained in bioreactors for long periods of up to one year and are able to form networks responding to physiological stimuli (101). Cerebral organoids thus offer exciting perspectives to study the pathogenic interactions between UMNs, glia, and immune cells which are increasingly recognized as an important contributor to PLS (43).
There are significant achievements for the investigation and identification of novel compounds that improve the health of UMNs, and we think in the near future the first compounds will be identified based on their ability to specifically enhance the health and connectivity of UMNs.
Coupled to further achievements in high-throughput screening, and to efforts to link PLS patients and PLS clinical centers of excellence, these developments offer the unprecedented hope for therapeutic advancements.
Acknowledgements
We thank Drs. Giorgia Quadrato (USC, Los Angeles, USA) and Vincenzo Silani (Dept. Neurology, Milan University, Italy) for helpful comments and suggestions, the Second International PLS Conference, Philadelphia, 2019, was supported by the National Institutes of Neurological Disorders and Stroke (NINDS), the Spastic Paraplegia Foundation (SPF), the Motor Neuron Disease Association (MNDA), The ALS Association, Mr. David Marren and his family, Mitsubishi-Tanabe Pharma, and Biogen. The supplement received financial support from Biogen and SPF. We are grateful to the PLS patients who attended the conference and actively participated in the discussions.
Funding
PHO is supported by NIH-NIA [grant RO1AG061708]. GM is funded by NIH [grant R01NS093872] and the ALS Association. CK is funded by the Muscular Disease Association. GH is supported by Agence Nationale pour la Recherche (ANR) in the frame of an eRARE3 call (grant Repetomics), the Association Française contre les Myopathies (AFM) and the Fédération pour la Recherche sur le Cerveau (FRC). The UCSD Light Microscopy Core is funded by NINDS NS047101.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Erb WH. Ueber einen wenig bekanten spinalen Symptomenkomplex. About a little-known spinal symptom complex. Berliner Klinische Wochenschrift 1875;26:357–9. [Google Scholar]
- 2.Stark FM, Moersch FP. Primary lateral sclerosis. A distinct clinical entity. J Nerv Mental Dis. 1945;102:332–7. [Google Scholar]
- 3.Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain 1992;115: 495–520. [DOI] [PubMed] [Google Scholar]
- 4.Gordon PH, Cheng B, Katz IB, Pinto M, Hays AP, Mitsumoto H, et al. The natural history of primary lateral sclerosis. Neurology 2006;66:647–53. [DOI] [PubMed] [Google Scholar]
- 5.Mitsumoto H, Nagy PL, Gennings C, Murphy J, Andrews H, Goetz R, et al. Phenotypic and molecular analyses of primary lateral sclerosis. Neurol Genet. 2015;1:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazulla J, Ferrer I, Izquierdo-Alvarez S, Alvarez S, Sanchez-Alcudia R, Bestue-Cardiel M, et al. Hereditary primary lateral sclerosis and progressive nonfluent aphasia. J Neurol. 2019;266:1079–90. [DOI] [PubMed] [Google Scholar]
- 7.Statland JM, Barohn RJ, Dimachkie MM, Floeter MK, Mitsumoto H. Primary lateral sclerosis. Neurol Clin. 2015;33:749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier CN, Murphy A, Loci L, Mitsumoto H, Lomen-Hoerth C, Kisanuki Y, et al. Primary lateral sclerosis and early upper motor neuron disease: characteristics of a cross-sectional population. J Clin Neuromuscul Dis. 2016;17:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charcot J-M, Joffroy A. Deux cas d’atrophie musculaire progressive avec lésion de la substance grise et des faisceaux de la moelle épinière. Arch Physiol 1869;2:354–67. 629–49; 744–60. [Google Scholar]
- 10.Charcot JM. Sclérose des cordons latéraux. Gazette hébdomadaire 1865;7:109–110. [Google Scholar]
- 11.Charcot JM. Leçons sur les Maladies du Système Nerveux faites à la Salpêtrière. Paris V: Aux Bureaux du Progrès Médical. Adrien Delahaye. Libraires-Editeurs 1877. [Google Scholar]
- 12.Wais V, Rosenbohm A, Petri S, Kollewe K, Hermann A, Storch A, et al. The concept and diagnostic criteria of primary lateral sclerosis. Acta Neurol Scand. 2017;136:204–11. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S, Highton-Williamson E, Caga J, Matamala JM, Dharmadasa T, Howells J, et al. Primary lateral sclerosis and the amyotrophic lateral sclerosis-frontotemporal dementia spectrum. J Neurol. 2018;265:1819–28. [DOI] [PubMed] [Google Scholar]
- 14.Turner MR, Barohn RJ, Corcia P, Fink JK, Harms MB, Kiernan MC, et al. Primary lateral sclerosis: consensus diagnostic criteria. J Neurol Neurosurg Psychiatry. 2020;91:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barohn RJ, Fink JK, Heiman-Patterson T, Huey ED, Murphy J, Statland JM, et al. The clinical spectrum of primary lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2020. To appear. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore M, Elman L, Babu S, Andres P, Floeter MK. Measuring PLS disease progression. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2020. To appear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silani V, Corcia P, Harms MB, Rouleau G, Siddique T, Ticozzi N. Genetics of primary lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2020. To appear. [DOI] [PubMed] [Google Scholar]
- 18.Pioro E, Turner MR, Bede P. Neuroimaging in primary lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2020. To appear. [DOI] [PubMed] [Google Scholar]
- 19.de Carvalho M, Kiernan MC, Pullman SL, Rezania K, Simmons Z. Neurophysiological features of primary lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. To appear. [DOI] [PubMed] [Google Scholar]
- 20.Mackenzie IRA, Briemberg H. TDP-43 pathology in primary lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2020. July 11:1–7. Crossref [DOI] [PubMed] [Google Scholar]
- 21.Floeter MK, Warden D, Lange D, Wymer J, Paganoni S, Mitsumoto H. Clinical care and therapeutic trials in PLS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2020. To appear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purves D Neuroscience. 3d ed. Sunderland, MA: Sinauer Associates; 2007. [Google Scholar]
- 23.The Thalamus and Cerebral Cortex (Integrative Systems) Part 1: What-when-how. In depth tutorials and information; Available at: http://what-when-how.com/neuroscience/the-thalamus-and-cerebral-cortex-integrative-systems-part-1/. [Google Scholar]
- 24.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. [DOI] [PubMed] [Google Scholar]
- 25.Lemon RN, Kirkwood PA, Maier MA, Nakajima K, Nathan P. Direct and indirect pathways for corticospinal control of upper limb motoneurons in the primate. Prog Brain Res. 2004;143:263–79. [DOI] [PubMed] [Google Scholar]
- 26.Gautam M, Jara JH, Kocak N, Rylaarsdam LE, Kim KD, Bigio EH, et al. Mitochondria, ER, and nuclear membrane defects reveal early mechanisms for upper motor neuron vulnerability with respect to TDP-43 pathology. Acta Neuropathol. 2019;137:47–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genc B, Jara JH, Lagrimas AK, Pytel P, Roos RP, Mesulam MM, et al. Apical dendrite degeneration, a novel cellular pathology for Betz cells in ALS. Sci Rep. 2017;7:41765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd GM. Diversity and complexity in the pyramidal tract projectome. Nat Rev Neurosci. 2014;15:63. [DOI] [PubMed] [Google Scholar]
- 29.Harris KD, Shepherd GM. The neocortical circuit: themes and variations. Nat Neurosci. 2015;18:170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamawaki N, Shepherd GM. Synaptic circuit organization of motor corticothalamic neurons. J Neurosci. 2015;35:2293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooks BM, Mao T, Gutnisky DA, Yamawaki N, Svoboda K, Shepherd GM. Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J Neurosci. 2013;33:748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suter BA, Yamawaki N, Borges K, Li X, Kiritani T, Hooks BM, et al. Neurophotonics applications to motor cortex research. Neurophoton. 2014;1:011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner M, Cosgrove J, Jamieson S, Chowdhury FU. Teaching neuroimages: hypometabolism of the primary motor cortex in primary lateral sclerosis: the stripe sign. Neurology 2016;86:1464. [DOI] [PubMed] [Google Scholar]
- 34.Suter BA, Migliore M, Shepherd GM. Intrinsic electrophysiology of mouse corticospinal neurons: a class-specific triad of spike-related properties. Cereb Cortex. 2013;23:1965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jara JH, Sheets PL, Nigro MJ, Peric M, Brooks C, Heller DB, et al. The electrophysiological determinants of corticospinal motor neuron vulnerability in ALS. Front Mol Neurosci 2020;13:e00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vucic S, Kiernan MC. Transcranial magnetic stimulation for the assessment of neurodegenerative disease. Neurotherapeutics. 2017;14:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal S, Koch G, Hillis AE, Huynh W, Ward NS, Vucic S, et al. Interrogating cortical function with transcranial magnetic stimulation: insights from neurodegenerative disease and stroke. J Neurol Neurosurg Psychiatry. 2019;90:47–57. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Melhem ER. Amyotrophic lateral sclerosis and primary lateral sclerosis: the role of diffusion tensor imaging and other advanced MR-based techniques as objective upper motor neuron markers. Ann NY Acad Sci. 2005;1064:61–77. [DOI] [PubMed] [Google Scholar]
- 39.Fogarty MJ, Klenowski PM, Lee JD, Drieberg-Thompson JR, Bartlett SE, Ngo ST, et al. Cortical synaptic and dendritic spine abnormalities in a presymptomatic TDP-43 model of amyotrophic lateral sclerosis. Sci Rep. 2016;6:37968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jara JH, Villa SR, Khan NA, Bohn MC, Ozdinler PH. AAV2 mediated retrograde transduction of corticospinal motor neurons reveals initial and selective apical dendrite degeneration in ALS. Neurobiol Dis. 2012;47:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jara JH, Genc B, Cox GA, Bohn MC, Roos RP, Macklis JD, et al. Corticospinal motor neurons are susceptible to increased ER stress and display profound degeneration in the absence of UCHL1 function. Cereb Cortex. 2015;25:4259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautam M, Jara JH, Sekerkova G, Yasvoina MV, Martina M, Ozdinler PH. Absence of alsin function leads to corticospinal motor neuron vulnerability via novel disease mechanisms. Hum Mol Genet. 2016;25:1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paganoni S, Alshikho MJ, Zurcher NR, Cernasov P, Babu S, Loggia ML, et al. Imaging of glia activation in people with primary lateral sclerosis. Neuroimage Clin. 2018;17:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemon R Recent advances in our understanding of the primate corticospinal system. F1000Res. 2019;8:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Genc B, Ozdinler PH. Moving forward in clinical trials for ALS: motor neurons lead the way please. Drug Discovery Today. 2014;19:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genc B, Gozutok O, Ozdinler PH. Complexity of generating mouse models to study the upper motor neurons: let us shift focus from mice to neurons. Int J Mol Sci. 2019;20:3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otomo A, Hadano S, Okada T, Mizumura H, Kunita R, Nishijima H, et al. ALS2, a novel guanine nucleotide exchange factor for the small GTPase Rab5, is implicated in endosomal dynamics. Hum Mol Genet. 2003;12:1671–87. [DOI] [PubMed] [Google Scholar]
- 48.Topp JD, Carney DS, Horazdovsky BF. Biochemical characterization of Alsin, a Rab5 and Rac1 guanine nucleotide exchange factor. Methods Enzymol. 2005;403:261–76. [DOI] [PubMed] [Google Scholar]
- 49.Hadano S, Benn SC, Kakuta S, Otomo A, Sudo K, Kunita R, et al. Mice deficient in the Rab5 guanine nucleotide exchange factor ALS2/alsin exhibit age-dependent neurological deficits and altered endosome trafficking. Hum Mol Genet. 2006;15:233–50. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29:160–5. [DOI] [PubMed] [Google Scholar]
- 51.Eymard-Pierre E, Lesca G, Dollet S, Santorelli FM, di Capua M, Bertini E, et al. Infantile-onset ascending hereditary spastic paralysis is associated with mutations in the alsin gene. Am J Hum Genet. 2002;71:518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simone M, Trabacca A, Panzeri E, Losito L, Citterio A, Bassi MT. KIF5A and ALS2 variants in a family with hereditary spastic paraplegia and amyotrophic lateral sclerosis. Front Neurol 2018;9:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai H, Lin X, Xie C, Laird FM, Lai C, Wen H, et al. Loss of ALS2 function is insufficient to trigger motor neuron degeneration in knock-out mice but predisposes neurons to oxidative stress. J Neurosci. 2005;25:7567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng HX, Zhai H, Fu R, Shi Y, Gorrie GH, Yang Y, et al. Distal axonopathy in an alsin-deficient mouse model. Hum Mol Genet. 2007;16:2911–20. [DOI] [PubMed] [Google Scholar]
- 55.Devon RS, Orban PC, Gerrow K, Barbieri MA, Schwab C, Cao LP, et al. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc Natl Acad Sci USA. 2006;103:9595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamanaka K, Miller TM, McAlonis-Downes M, Chun SJ, Cleveland DW. Progressive spinal axonal degeneration and slowness in ALS2-deficient mice. Ann Neurol. 2006;60:95–104. [DOI] [PubMed] [Google Scholar]
- 57.Yasvoina MV, Genc B, Jara JH, Sheets PL, Quinlan KA, Milosevic A, et al. eGFP expression under UCHL1 promoter genetically labels corticospinal motor neurons and a subpopulation of degeneration-resistant spinal motor neurons in an ALS mouse model. J Neurosci. 2013;33:7890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haase G, Rabouille C. Golgi fragmentation in ALS motor neurons. New mechanisms targeting microtubules, tethers, and transport vesicles. Front Neurosci. 2015;9:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabouille C, Haase G. Editorial: golgi pathology in neurodegenerative diseases. Front Neurosci. 2015;9:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacquier A, Buhler E, Schäfer MK, Bohl D, Blanchard S, Beclin C, et al. Alsin/Rac1 signaling controls survival and growth of spinal motoneurons. Ann Neurol. 2006;60:105–17. [DOI] [PubMed] [Google Scholar]
- 61.Lai C, Xie C, Shim H, Chandran J, Howell BW, Cai H. Regulation of endosomal motility and degradation by amyotrophic lateral sclerosis 2/alsin. Mol Brain. 2009;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Topp JD, Gray NW, Gerard RD, Horazdovsky BF. Alsin is a Rab5 and Rac1 guanine nucleotide exchange factor. J Biol Chem. 2004;279:24612–23. [DOI] [PubMed] [Google Scholar]
- 63.Otomo A, Kunita R, Suzuki-Utsunomiya K, Mizumura H, Onoe K, Osuga H, et al. ALS2/alsin deficiency in neurons leads to mild defects in macropinocytosis and axonal growth. Biochem Biophys Res Commun. 2008;370:87–92. [DOI] [PubMed] [Google Scholar]
- 64.Hadano S, Otomo A, Kunita R, Suzuki-Utsunomiya K, Akatsuka A, Koike M, et al. Loss of ALS2/Alsin exacerbates motor dysfunction in a SOD1-expressing mouse ALS model by disturbing endolysosomal trafficking. PLoS One. 2010;5:e9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu F, Spannl S, Ferguson C, Hyman AA, Parton RG, Zerial M. Rab5 and Alsin regulate stress-activated cytoprotective signaling on mitochondria. eLife. 2018;7:e32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacquier A, Bellouze S, Blanchard S, Bohl D, Haase G. Astrocytic protection of spinal motor neurons but not cortical neurons against loss of Als2/alsin function. Hum Mol Genet. 2009;18:2127–39. [DOI] [PubMed] [Google Scholar]
- 67.Magrane J, Cortez C, Gan WB, Manfredi G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum Mol Genet. 2014;23:1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Q, Vande Velde C, Israelson A, Xie J, Bailey AO, Dong MQ, et al. ALS-linked mutant superoxide dismutase 1 (SOD1) alters mitochondrial protein composition and decreases protein import. Proc Natl Acad Sci USA. 2010;107:21146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Millecamps S, Gentil BJ, Gros-Louis F, Rouleau G, Julien JP. Alsin is partially associated with centrosome in human cells. Biochim Biophys Acta. 2005;1745:84–100. [DOI] [PubMed] [Google Scholar]
- 70.Scialo C, Morbelli S, Girtler N, Mandich P, Mancardi GL, Caponnetto C, et al. Bilateral motor and premotor cortex hypometabolism in a case of Mills syndrome. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:414–7. [DOI] [PubMed] [Google Scholar]
- 71.Mills CK. A case of unilateral progressive ascending paralysis, probably representing a new form of degenerative disease. J Nerv Ment Dis 1900;27:195–200. [Google Scholar]
- 72.Gastaut JL, Bartolomei F. Mills’ syndrome: ascending (or descending) progressive hemiplegia: a hemiplegic form of primary lateral sclerosis? J Neurol Neurosurg Psychiatry. 1994;57:1280–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beach RL, Rao JS, Festoff BW, Reyes ET, Yanagihara R, Gajdusek DC. Collagenase activity in skin fibroblasts of patients with amyotrophic lateral sclerosis. J Neurol Sci. 1986;72:49–60. [DOI] [PubMed] [Google Scholar]
- 74.Ono S, Imai T, Tsumura M, Takahashi K, Jinnai K, Suzuki M, et al. Increased serum hyaluronic acid in amyotrophic lateral sclerosis: relation to its skin content. Amyotrophic lateral sclerosis and other motor neuron disorders: official publication of the World Federation of Neurology. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:213–8. [DOI] [PubMed] [Google Scholar]
- 75.Tsukie T, Masaki H, Yoshida S, Fujikura M, Ono S. Decreased Amount of Collagen in The Skin of Amyotrophic Lateral Sclerosis in The Kii Peninsula of Japan. Acta Neurol Taiwan 2014;23:82–9. [PubMed] [Google Scholar]
- 76.Yang S, Zhang KY, Kariawasam R, Bax M, Fifita JA, Ooi L, et al. Evaluation of skin fibroblasts from amyotrophic lateral sclerosis patients for the rapid study of pathological features. Neurotox Res. 2015;28:138–46. [DOI] [PubMed] [Google Scholar]
- 77.Oketa Y, Higashida K, Fukasawa H, Tsukie T, Ono S. Abundant FUS-immunoreactive pathology in the skin of sporadic amyotrophic lateral sclerosis. Acta Neurol Scand. 2013;128:257–64. [DOI] [PubMed] [Google Scholar]
- 78.Watanabe T, Okeda Y, Yamano T, Ono S. An immunohistochemical study of ubiquitin in the skin of sporadic amyotrophic lateral sclerosis. J Neurol Sci. 2010;298:52–6. [DOI] [PubMed] [Google Scholar]
- 79.Suzuki M, Mikami H, Watanabe T, Yamano T, Yamazaki T, Nomura M, et al. Increased expression of TDP-43 in the skin of amyotrophic lateral sclerosis. Acta Neurol Scand 2010;122:367–72. [DOI] [PubMed] [Google Scholar]
- 80.Kirk K, Gennings C, Hupf JC, Tadesse S, D’Aurelio M, Kawamata H, et al. Bioenergetic markers in skin fibroblasts of sporadic amyotrophic lateral sclerosis and progressive lateral sclerosis patients. Ann Neurol. 2014;76:620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pollari E, Goldsteins G, Bart G, Koistinaho J, Giniatullin R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front Cell Neurosci 2014;8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Konrad C, Kawamata H, Bredvik KG, Arreguin AJ, Cajamarca SA, Hupf JC, et al. Fibroblast bioenergetics to classify amyotrophic lateral sclerosis patients. Mol Neurodegener. 2017;12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borroni MV, Valles AS, Barrantes FJ. The lipid habitats of neurotransmitter receptors in brain. Biochim Biophys Acta. 2016;1858:2662–70. [DOI] [PubMed] [Google Scholar]
- 84.Ingolfsson HI, Melo MN, van Eerden FJ, Arnarez C, Lopez CA, Wassenaar TA, et al. Lipid organization of the plasma membrane. J Am Chem Soc. 2014;136:14554–9. [DOI] [PubMed] [Google Scholar]
- 85.Arima H, Omura T, Hayasaka T, Masaki N, Hanada M, Xu D, et al. Reductions of docosahexaenoic acid-containing phosphatidylcholine levels in the anterior horn of an ALS mouse model. Neuroscience 2015;297:127–36. [DOI] [PubMed] [Google Scholar]
- 86.Henriques A, Croixmarie V, Priestman DA, Rosenbohm A, Dirrig-Grosch S, D’Ambra E, et al. Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase. Hum Mol Genet. 2015;24:7390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henriques A, Huebecker M, Blasco H, Keime C, Andres CR, Corcia P, et al. Inhibition of β-Glucocerebrosidase Activity Preserves Motor Unit Integrity in a Mouse Model of Amyotrophic Lateral Sclerosis . Sci Rep. 2017;7:5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Okumiya K, Sakamoto R, Ishimoto Y, Kimura Y, Fukutomi E, Ishikawa M, et al. Glucose intolerance associated with hypoxia in people living at high altitudes in the Tibetan highland. BMJ Open. 2016;6:e009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finegan E, Chipika RH, Shing SLH, Hardiman O, Bede P. Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:133–145. [DOI] [PubMed] [Google Scholar]
- 90.Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23:382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bravo-Hernandez M, Tadokoro T, Navarro MR, Platoshyn O, Kobayashi Y, Marsala S, et al. Spinal subpial delivery of AAV9 enables widespread gene silencing and blocks motoneuron degeneration in ALS. Nat Med. 2020;26:118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, et al. Towards a transgenic model of Huntington’s disease in a non-human primate. Nature 2008;453:921–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cathomen T, Ehl S. Translating the genomic revolution – targeted genome editing in primates. N Engl J Med. 2014;370:2342–5. [DOI] [PubMed] [Google Scholar]
- 94.Ozdinler PH, Silverman RB. Treatment of amyotrophic lateral sclerosis: lessons learned from many failures. ACS Med Chem Lett. 2014;5:1179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo W, Fumagalli L, Prior R, Van Den Bosch L. Current advances and limitations in modeling ALS/FTD in a dish using induced pluripotent stem cells. Front Neurosci. 2017;11:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–86, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Motono M, Ioroi Y, Ogura T, Takahashi J. WNT-C59, a small-molecule WNT inhibitor, efficiently induces anterior cortex that includes cortical motor neurons from human pluripotent stem cells. Stem Cells Transl Med. 2016;5:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Brauninger M, et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci USA. 2015;112:15672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ormel PR, Vieira de Sa R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, et al. Microglia innately develop within cerebral organoids. Nat Commun. 2018;9:4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017;545:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]