Abstract
The management of multiply injured or severely injured patients is a complex and dynamic process. Timely and safe fracture fixation is a critical component of the multidisciplinary care that these patients require. Effective management of these patients, and their orthopaedic injuries, requires a strong understanding of the pathophysiology of the response to trauma and indicators of patient status, as well as an appreciation for the dynamic nature of these parameters. Substantial progress in both clinical and basic science research in this area has advanced our understanding of these concepts and our approach to management of the polytraumatized patient. This article summarizes a symposium on this topic that was presented by an international panel of experts at the 2020 Virtual Annual Meeting of the Orthopaedic Trauma Association.
Keywords: damage control surgery, early appropriate care, inflammation, neutrophil, polytrauma
1. Introduction
The management of polytrauma patients with orthopaedic injuries is a complex and dynamic process that requires a robust understanding of the pathophysiology of the response to trauma and indicators of patient status. There has been a significant evolution in our understanding of these concepts and therefore many changes over time in the way these patients are approached and managed. There has been significant momentum forward with recent clinical and basic science research that has driven these changes. This article summarizes a symposium on this topic that was presented by an international panel of experts at the 2020 Virtual Annual Meeting of the Orthopaedic Trauma Association.
2. Polytrauma management—an update on new aspects
2.1. Technical improvements for reamed nailing
Reamed nailing is the standard of care for the management of long bone fractures. Following certain concerns about the possible side effects of reaming, such as heat generation, issues with reamer flutes, and fat embolization,[1] several companies have made technical changes. To address fat intravasation caused by increased intramedullary pressure, the Reamed Irrigator Aspirator (RIA) device was developed. It reduces the amount of embolization-associated changes.[2,3] Other indications were added, as outlined in Table 1. These new indications have become more important and lead to a more frequent use of the RIA. At the current time, bone graft harvesting is the most frequent indication for RIA use.[4]
Table 1.
Indications for the use of RIA in clinical practice∗
| 1. To reduce the degree of fat embolization |
| 2. To clear the medullary canal of bone marrow/reaming debris |
| 3. To size the medullary canal (IM implant or prosthesis) |
| 4. For bone graft harvesting |
| 5. For removal of tissue from IM canal (e.g. treatment of IM infection) |
The RIA device is approved for these indications by the Food and Drug Administration (FDA) of the USA.
Concurrent with increasing indications and the extended use of the RIA device, certain issues became evident, such as weakening of the medullary canal and technical issues causes by repetitive reaming cycles (Fig. 1).[5] Surgeons also recognized that continuous irrigation and a well cutting device requires a more exact positioning inside the medullary canal than with conventional reamers. Moreover, the size of the existing cutting head appeared to be limiting its use in smaller individuals. Other users reported technical issues with the connection between the drive shaft and the cutting head. For these reasons, a second-generation RIA 2 device has been developed. RIA 2 was launched in 2019 to address the issues mentioned above (Fig. 2).
Figure 1.
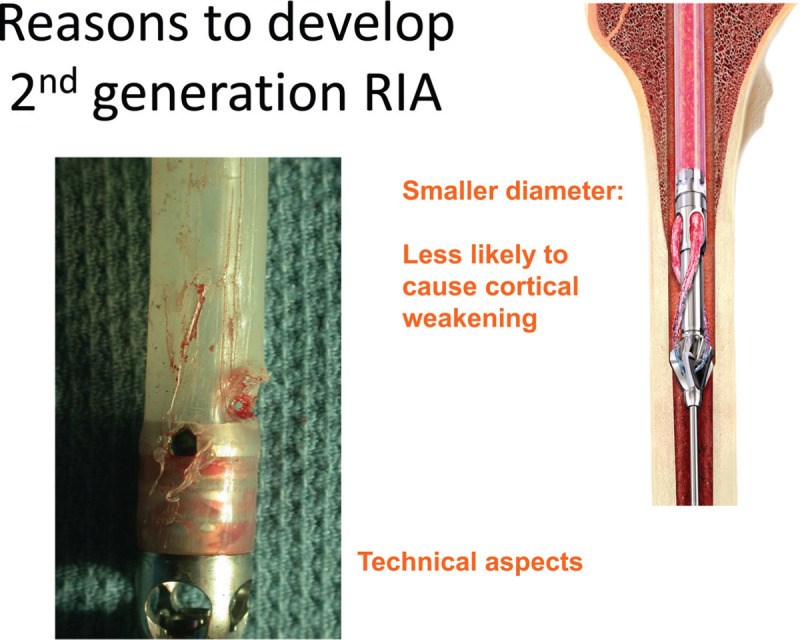
Causes to develop a new, modified reamer irrigator aspirator version (RIA2).
Figure 2.
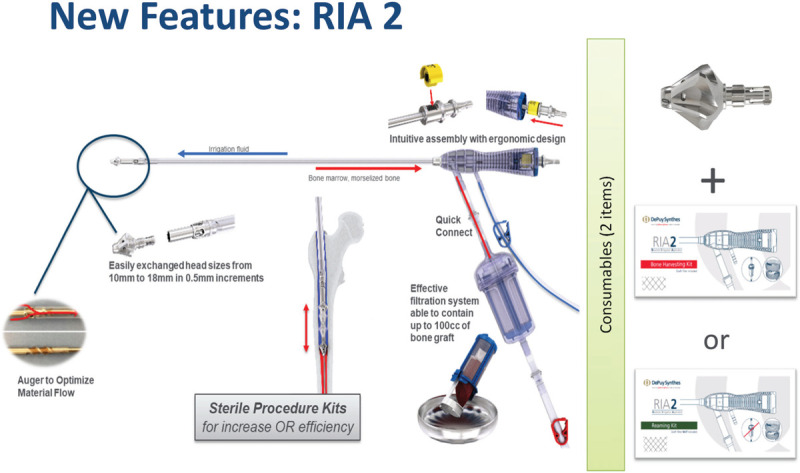
The new features of >RIA2 are depicted in Figure 2. They include a new auger to improve backflow despite a smaller irrigation tube, improved coupling, and a new seal (in yellow), a quick connect for the harvesting tube and, most importantly, exchange options for the reamer head intraoperatively between 10 and 18 mm).
2.2. Aspects of safe definitive surgery (SDS)
To achieve the goals of both timely and safe fracture fixation in severely injured patients, a precise knowledge about the patient's status, and the evolution of that status, is crucial. The Glue Grant project has shown that the trauma-induced (first hit) inflammatory changes can be caused by an acute change in the genetic profile of the circulating immune system (e.g., neutrophils). It may be one reason to explain the spread of inflammatory changes within the entire body even after local injury.[6] Recently, a technique has become available that allows for acute measurement of neutrophil activation.[7] This will ideally be able to demonstrate a more precise risk profiling to avoid the second hit.
In this line, new evidence has been helpful in determining that inflammatory and soft tissue injury induced alterations can be modulated by a surgical impact. In particular, femoral canal reaming in the presence of a chest injury and hemorrhagic shock (performed despite adequate resuscitation) can be associated with pulmonary compromise. In preexisting pulmonary contusions, reaming-associated computed tomography changes were more sustained when compared with those after RIA (i.e., irrigation of the medullary canal with aspiration of its contents).[8] In a similar fashion, cardiac function was shown to be more severely affected after regular reaming when compared with RIA 2. These results clearly underline the importance of a second hit phenomenon, which may not be clinically apparent during surgery.[9,10] They also reinforce the importance of patient assessment to achieve the goal of safe definitive surgery.[11] A recent comparison of existing scores revealed that patient assessment is safer when multiple parameters are used, especially when they cover several pathogenetic cycles.[12] Although acute acid-base changes were predictive of early complications and mortality (<72 hours), the addition of other parameters provided a better prediction overall. Moreover, the combined use was also able to predict late complications in the severely injured patients. If used alone, serial lactate measurements (admission and 24 hours) were also superior to a single measurement in large patient samples, a phenomenon termed “lactate clearance.”[13] We therefore have emphasized using several parameters simultaneously and included all these aspects in the modified criteria for the borderline condition (Table 2).[14] Hopefully, the advances in technical developments for surgeries and for patient assessments will be helpful in making decisions safer in this complex patient population.
Table 2.
Revised parameters to assess the borderline trauma patient in 2020[14]
| Parameters | ||
|---|---|---|
| Static parameters | Injury combination | • Polytrauma ISS > 20 and AIS chest > 2• Thoracic Trauma Score (TTS) > grade 2 |
| Local injury chest | • Bilateral lung contusion: first plain film or• Chest CT:unilateral bisegmental contusionbilateral uni- or bisegmental contusionflail chest | |
| Local injury truncal/extremity | Multiple long bone fractures + truncal injury AIS 2 or more | |
| Truncal | Polytrauma with abdominal/pelvic trauma (SBP <90 mm Hg) (Moore 3 visceral injury and Hemorrhagic Shock) | |
| Major surgery for non-life-saving conditions | Safe definitive surgery (SDS) and damage control (DCO) | |
| Duration of first operative intervention | Presumed operation time > 6 hintraoperative reassessment:• coagulopathy (ROTEM/FIBTEM)• lactate (< 2.0–2.5 mmol/L)• body temperature stable• requirement > 3 pRBC/h | |
| Dynamic parameters | Blood transfusion requirements | Massive transfusion• 10 units RBCs per 6 h(initiation of goal-directed therapy – massive transfusion protocol) |
| Intra/perioperative | • ROTEM/FIBTEM• Lactate clearance < 2.5 mmol/L (24 h) |
AIS = abbreviated injury scale; ISS = injury severity score; ROTEM = rotational thromboelastometry; RR = respiratory rate; SBP = systolic blood pressure.
3. The significance of inflammation in polytraumatized patients with orthopaedic injuries
Patients sustaining multiple fractures frequently sustain multiple injuries and often develop hemorrhagic shock. Multiply injured patients (MIPs) invariably mount a robust immunologic response to injury which includes both pro-inflammatory and anti-inflammatory components.[15] Typically, pro- and anti-inflammatory responses achieve balance and will work toward restoring immunologic homeostasis facilitating wound healing. However, imbalanced inflammation can lead to acute and longer-term complications including isolated or multiple organ dysfunction[16] and failures of healing such as infection[17,18] or nonunion.[17,19]
The immunologic response to injury is notably complex and is largely enacted by circulating and mobilized monocytic and lymphoid cells. The innate response is rapidly mobilized which initiates inflammation and subsequently orchestrates the ensuing adaptive response.[15] Immune cell targeting and function are largely controlled by chemical communication between the cells through cytokines and chemokines in addition to between the cells and injured tissue through local and remote damage-associated molecules and from stimulation via external pathogens. Researchers typically have quantified changes in circulating concentrations of immunologic mediators (cytokines, chemokines) as a surrogate of the immunologic response to injury.[15] These studies have been foundational in identifying the kinetics of the immunologic response with respect to the temporal progression of mediators, how injury magnitude affects mediators and associations of mediators with unremarkable versus complicated outcomes. However, researchers have uniformly recognized that the complexity of the immunologic response to injury precludes any reductionist approaches to affect inflammation-based outcomes after injury.[20]
Leading researchers have largely adopted computational approaches to better understand the trauma inflammatory response.[16,18,21] Rather than focusing on individual or small groups of mediators, computational approaches seek to account for time-dependent and spatial orchestration of immune mediators as a surrogate for the immunologic response. For example, Namas et al[18] demonstrated that networks of pro-inflammatory mediators, quantified using Dynamic Network Analysis, were more highly connected within 24 hours of injury in trauma patients who subsequently developed nosocomial infections. Dynamic networks of mediators within 24 hours of injury were also shown to be distinct in clusters of MIPs who had divergent clinical trajectories of organ dysfunction over the ensuing five days.[16] Pertaining to MIPs with fractures, Almahmoud et al[21] investigated 2 stringently matched cohorts of patients which were demographically homogenous, had equal overall injury severity but were distinguished by the magnitude of extremity injury. Immunologic network coordination was delayed in patients with severe extremity injuries compared with patients with lower magnitude extremity injuries.[21]
In recent work, we identified 2 groups of patients with similar injury magnitude and demographic homogeneity that were either tolerant or sensitive to hemorrhage.[22] Tolerant patients had high-magnitude hemorrhage but minimal subsequent organ dysfunction in contrast to sensitive patients who had little hemorrhage but developed high levels of organ dysfunction. We conducted Dynamic Network Analysis on serially collected immunologic mediators from both groups and documented differences in mediator network connectivity. Consistent with our other studies, shock-tolerant patients demonstrated early robust orchestration of the immunologic response compared with shock-sensitive patients. In addition, we identified 2 notably distinct clusters of mediator orchestration. In shock-tolerant patients, a cluster of 6 cytokines including interleukin-9, 17E/25, 21, 22, 23, and 33 formed very early and were highly connected. In contrast, shock-sensitive patients had minimal connectivity of this same network in the first 24 hours after injury. Shock-sensitive patients subsequently had delayed but robust formation of a largely pro-inflammatory network compared with shock-tolerant patients. Interestingly, recent evidence has shown the 6 mediators in the cluster seen in the shock-tolerant patients are largely cyto-protective and are particularly concentrated in boundary organs including the skin, gut, and lung.[23,24] These findings parallel more recent findings (unpublished) from ongoing work in our group that show distinct differences in temporal progression of all 6 cyto-protective mediators (IL-9, 17E/25, 21, 22, 23, and 33) in MIPs with femur fractures treated with early appropriate care compared with patients treated with damage control methods.
In summary, the immunologic response and associated orchestration of inflammation that occurs after major injury are clearly affected by the magnitude and distribution of injury, and also have significant effects on acute and longer-term outcomes after injury. Computational approaches have begun to decipher the enormous complexities of the immune response to trauma.
4. Early neutrophil changes in polytrauma patients and its prognostic relevance
The primary function of neutrophils is to kill bacteria. However, one of their other main functions is to react to danger signals and aid with the primary processes that follow injury. After injury, a multitude of processes are initiated and many cytokines are produced, the ultimate significance of which is not easily understood. Over the years working with neutrophils, we are more and more convinced that the neutrophil is the natural integrator of all the signals that are elicited after injury. Despite an enormous body of research, much remains unknown regarding the kinetics of the neutrophils after trauma. However, new techniques and point-of-care measurements have made it possible to reveal some of the functions of the neutrophil and its prognostic relevance.
Previous studies revealed that in patients with femur fractures, those with a high level of inflammatory response as measured by neutrophil epitopes showed a high level of subsequent ARDS.[25] Moreover, in an international observational study it was shown that in severe trauma patients whose neutrophils demonstrated less response to bacterial stimuli on admission, severe septic shock was observed 7 days later.[7] In addition, Leliefeld et al[26] demonstrated that there are differences in the bacteria killing capacity of the various subsets of neutrophils. Unexpectedly, banded neutrophils showed the most adequate killing capacity, whereas the hypersegmented cells showed the least adequate killing capacity. It is tempting to speculate that this differential killing capacity is indicative of the susceptibility of trauma patients for infectious complications (see later). It was hypothesized that the level of injury led to differential subsets of neutrophils remaining in the circulation with differential capabilities of bacterial killing. From a certain threshold on, these neutrophils could not withstand the overwhelming power of the bacteria, thus leading to infectious complications or even sepsis.
Measuring neutrophil function however is a tedious process, using hours of manual processing, with many precarious steps, before the final result can be evaluated with flow cytometry (FACS). Recently, we introduced a FACS apparatus that could be used as a point-of-care tool in the trauma bay, with low handling needs. This resulted in a feasibility study in which all trauma patients admitted to the trauma bay of our hospital were measured and neutrophil appearance and epitopes were evaluated.[27] This study showed that the evaluation of neutrophils at the point of care with FACS was feasible, without the requirement of a laboratory technician or substantial burden on the trauma team.
In a second study, it was shown that the severity of trauma was reflected in the appearance of the neutrophil subsets as characterized by different FACS patterns.[28] The appearance of CD16 low neutrophils (associated with the banded neutrophil phenotype) was correlated with injury severity (i.e., tissue damage), whereas the segmented neutrophils characterized by CD62L low appearance in the FACS signified lowered immune capabilities. In very severe polytrauma patients (virtual unsurvivable), the appearance of bone marrow precursor cells was seen as an ominous sign. On top of these findings, it was clear the appearance of more than 8% of CD16 low cells was correlated with the appearance of infectious complications at a later stage, signified by an ROC curve with an area under the curve of 0.9.
In summary, the investigation of neutrophils in the setting of polytrauma represents a novel and exciting avenue of research that may lead to substantial advances in patient care in the future.
5. Management of major fractures in polytrauma— what does the clinical literature tell us?
Major fractures and the strategy for their stabilization are well known to have a significant impact on the clinical course after polytrauma. Beside careful diagnostics and accordingly adjusted surgical techniques, a diligently planned management strategy for the stabilization of major fractures seems to be associated with improved outcomes. In general, different stabilization strategies for major fractures have been introduced over the last decades. This development is characterized by an increasing focus on individualized concepts. In a prospective randomized study, Bone et al[29] found a significantly higher rate of pulmonary complications in case of late stabilization of femoral fractures and therefore recommended early fracture fixation whenever adequate ventilatory support and proper fluid management have been provided (Early Total Care). However, it was noticed that specific groups of patients (borderline patients) were in a compromised condition after prolonged early surgeries. In a prospective randomized study, Pape et al[30] demonstrated that Damage Control Orthopaedics (DCO) with early temporary fixation (e.g., by external fixator) and definitive stabilization once the patient has been stabilized resulted in a significant reduction of acute lung injury in these patients. In the following years, some studies found evidence that the DCO-concept might be overused. Nahm et al[31] reported that the advantages of early fracture fixation outweigh the risks and complications if indicators of the acid–base status (lactate, pH, Base Excess) can be stabilized. Under these conditions, definitive fracture stabilization was associated with decreased rates of pulmonary complication, multiple organ failure, and sepsis. This concept was named Early Appropriate Care (EAC). EAC was supposed to be safely applicable in most multiple trauma patients as long as attention was paid to resuscitation before surgery. Other concepts avoided defining specific time limitations for the duration of the initial surgery or the timing until definitive fracture fixation to allow the development of a patient-specific surgical strategy. This intuitively seems to be important due to an individual response of the patient to the same degree of injury and a dissimilar trauma service provision.
The SDS concept uses routine clinical parameters (e.g., acid–base status, coagulation) as well as injury severity and distribution to estimate potential dynamic changes which might occur in the early phase after trauma.[11] After this first estimate the continual reassessment of patients at risk is the central point of the SDS concept. Therefore, it is suggested to evaluate these patients at multiple time points (e.g., end of resuscitation, during surgical procedures, prior to surgery in intensive care unit). In a similar individualized approach, the concept of Prompt Individualized Safe Management considers patient-specific factors (e.g., age, gender, comorbidities), injury patterns, the physiological status of the patient, and the local resources for trauma management.[32] This approach also requires continuous reassessments and if needed a change of strategy at any time.
In conclusion, it is well accepted that early stabilization of major fractures has to be performed in stable patients, whereas DCO is the treatment of choice in unstable patients. However, the optimal therapy of patients in an unclear condition is still not fully clarified, as parameters to define these patients are not completely characterized. Approaches that consider patient- and injury-specific aspects, as well as local resources for trauma care, represent promising strategies.
6. Putting it all together: when you should intervene based on what we know
In multiply-injured patients, early fixation of mechanically unstable axial and femoral fractures, whether provisional or definitive, reduces morbidity and mortality. Skeletal stability permits upright posture and mobility from bed, relieves pain, and reduces thrombotic complications. Prior literature and experience support this, yet development of safe and expeditious treatment plans, including type and timing of fixation, remains controversial. We propose recommendations, while acknowledging limitations and areas for continued study.
Over 30 years ago many embraced Early Total Care, which later gave way to DCO. Early Total Care arose from the realization that early definitive fixation of femoral shaft fractures provided pulmonary and systemic benefits to most patients. However, compromised physiological status of some polytraumatized patients at the time of major orthopaedic procedures resulted in morbidity and mortality, indicating temporizing stabilization with damage control techniques to minimize surgical insult. The rationale was to avoid long, definitive operations during a robust systemic inflammatory response from injury and surgery.
Early total care was questioned by surgeons noting occasional morbidity and mortality following reamed intramedullary nailing of the femur within 24 hours.[33,34] Contributing factors may have included reaming, presence, and severity of chest injury, or other patient factors. However, resuscitation protocols had not been standardized, and algorithms regarding timing of fixation had not been proposed. Reduction and fixation of mechanically unstable pelvis, acetabulum, and thoracolumbar fractures has also been historically delayed, yet these injuries have similar acute effects on an injured patient, including more pain and recumbent positioning until they are reduced and stabilized.
Although DCO provides an alternative to definitive intramedullary nailing of the femur in severely compromised patients, most femoral shaft fractures can safely be treated on an early basis.[35,36] Potential indications for DCO may include persistent hemodynamic instability, persistent metabolic acidosis, severe head injury with elevated intracranial pressure, or cardiac dysfunction with evolving myocardial ischemia.
The EAC protocol established simple laboratory parameters to guide type and timing of fixation. Multivariate analysis showed 3 primary determinants of early complications to be magnitude and duration of acidosis, severity of chest injury, and timing of fixation, where patients treated with definitive fixation more than 40 hours after injury were at risk for pulmonary demise.[37] The expectation is for definitive fixation of the thoracolumbar spine, pelvic ring, acetabulum, and proximal or diaphyseal femur fractures within 36 hours, when acidosis has improved to recommended levels: pH ≥ 7.25, base excess ≥ −5.5 mmol/L, or lactate < 4.0 mmol/L. Coagulopathy has also been studied, showing resolution similar to acidosis. During surgery, labs may be repeated several times and definitive additional procedures may be undertaken (vs temporized) if the patient continues to respond favorably, based on EAC parameters and cardiopulmonary function. Using EAC guidelines complications and costs are reduced.[38]
Future work calls for modulation of EAC recommendations, potentially in those with cardiac, renal, or pulmonary dysfunction and integration of a patient-specific approach. As discussed above, point-of-care testing for markers of inflammation will identify patients at risk for complications, optimizing timing of definitive fracture care. Markers are elevated after injury, influenced by magnitude of injury, defined by hemorrhage or soft tissue damage, as well as by genetics and medical comorbidities. Additional work may establish consistent clinical associations of elevated inflammatory markers with injury and with complications, as may distinguish causation versus association.
Ultimately, guidelines that incorporate these concepts into a patient-specific approach with the integration of advanced diagnostics to identify the “borderline” or “at-risk” patient, while keeping available resources and the dynamic nature of these parameters in mind, will yield the best care for polytrauma patients in the future.
References
- 1.Miller AN, Deal D, Green J, et al. Use of the reamer/irrigator/aspirator decreases carotid and cranial embolic events in a canine model. J Bone Joint Surg Am. 2016;98:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pape HC, Zelle BA, Hildebrand F, et al. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87:2515–2522. [DOI] [PubMed] [Google Scholar]
- 3.Richards JE, Guillamondegui OD, Archer KR, et al. The association of reamed intramedullary nailing and long-term cognitive impairment. J Orthop Trauma. 2011;25:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiese A, Pape HC. Bone defects caused by high-energy injuries, bone loss, infected nonunions, and nonunions. Orthop Clin North Am. 2010;41:1–4. [DOI] [PubMed] [Google Scholar]
- 5.Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24:42–45. [DOI] [PubMed] [Google Scholar]
- 6.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groeneveld KM, Koenderman L, Warren BL, et al. Early decreased neutrophil responsiveness is related to late onset sepsis in multitrauma patients: an international cohort study. PLoS One. 2017;12:e0180145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halvachizadeh S, Teuben M, Lempert M, et al. Protective effects of new femoral reaming techniques (Reamer irrigator aspirator, RIA I and II) on pulmonary function and posttraumatic contusion (CT morphology)—results from a standardized large animal model. Injury. 2020;S0020-1383(20)30827-5. [DOI] [PubMed] [Google Scholar]
- 9.Lackner I, Weber B, Baur M, et al. Complement activation and organ damage after trauma-differential immune response based on surgical treatment strategy. Front Immunol. 2020;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baur M, Weber B, Lackner I, et al. Structural alterations and inflammation in the heart after multiple trauma followed by reamed versus non-reamed femoral nailing. PLoS One. 2020;15:e0235220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pape HC, Pfeifer R. Safe definitive orthopaedic surgery (SDS): repeated assessment for tapered application of Early Definitive Care and Damage Control?: an inclusive view of recent advances in polytrauma management. Injury. 2015;46:1–3. [DOI] [PubMed] [Google Scholar]
- 12.Halvachizadeh S, Baradaran L, Cinelli P, et al. How to detect a polytrauma patient at risk of complications: a validation and database analysis of four published scales. PLoS One. 2020;15:e0228082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dezman ZD, Comer AC, Smith GS, et al. Failure to clear elevated lactate predicts 24-hour mortality in trauma patients. J Trauma Acute Care Surg. 2015;79:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pape HC, Halvachizadeh S, Leenen L, et al. Timing of major fracture care in polytrauma patients—an update on principles, parameters and strategies for 2020. Injury. 2019;50:1656–1670. [DOI] [PubMed] [Google Scholar]
- 15.Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. 2018;19:327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Namas RA, Vodovotz Y, et al. Unsupervised clustering analysis based on MODS severity identifies four distinct organ dysfunction patterns in severely injured blunt trauma patients. Front Med (Lausanne). 2020;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metsemakers WJ, Handojo K, Reynders P, et al. Individual risk factors for deep infection and compromised fracture healing after intramedullary nailing of tibial shaft fractures: a single centre experience of 480 patients. Injury. 2015;46:740–745. [DOI] [PubMed] [Google Scholar]
- 18.Namas RA, Vodovotz Y, Almahmoud K, et al. Temporal patterns of circulating inflammation biomarker networks differentiate susceptibility to nosocomial infection following blunt trauma in humans. Ann Surg. 2016;263:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrand F, van Griensven M, Huber-Lang M, et al. Is there an impact of concomitant injuries and timing of fixation of major fractures on fracture healing? A focused review of clinical and experimental evidence. J Orthop Trauma. 2016;30:104–112. [DOI] [PubMed] [Google Scholar]
- 20.Spruijt NE, Visser T, Leenen LP. A systematic review of randomized controlled trials exploring the effect of immunomodulative interventions on infection, organ failure, and mortality in trauma patients. Crit Care. 2010;14:R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almahmoud K, Abboud A, Namas RA, et al. Computational evidence for an early, amplified systemic inflammation program in polytrauma patients with severe extremity injuries. PLoS One. 2019;14:e0217577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinley TO, Gaski GE, Zamora R, et al. Early dynamic orchestration of immunologic mediators identifies multiply injured patients who are tolerant or sensitive to hemorrhage. J Trauma Acute Care Surg. 2020;Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Eyerich K, Dimartino V, Cavani A. IL-17 and IL-22 in immunity: driving protection and pathology. Eur J Immunol. 2017;47:607–614. [DOI] [PubMed] [Google Scholar]
- 24.Robinson KM, Ramanan K, Clay ME, et al. Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunol. 2018;11:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hietbrink F, Koenderman L, Althuizen M, et al. Modulation of the innate immune response after trauma visualised by a change in functional PMN phenotype. Injury. 2009;40:851–855. [DOI] [PubMed] [Google Scholar]
- 26.Leliefeld PHC, Pillay J, Vrisekoop N, et al. Differential antibacterial control by neutrophil subsets. Blood Adv. 2018;2:1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spijkerman R, Hesselink L, Hellebrekers P, et al. Automated flow cytometry enables high performance point-of-care analysis of leukocyte phenotypes. J Immunol Methods. 2019;474:112646. [DOI] [PubMed] [Google Scholar]
- 28.Spijkerman R, Hesselink L, Bongers S, et al. Point-of-care analysis of neutrophil phenotypes: a first step toward immuno-based precision medicine in the trauma ICU. Crit Care Explor. 2020;2:e0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bone LB, Johnson KD, Weigelt J, et al. Early versus delayed stabilization of femoral fractures. A prospective randomized study. J Bone Joint Surg Am. 1989;71:336–340. [PubMed] [Google Scholar]
- 30.Pape HC, Rixen D, Morley J, et al. Impact of the method of initial stabilization for femoral shaft fractures in patients with multiple injuries at risk for complications (borderline patients). Ann Surg. 2007;246:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nahm NJ, Como JJ, Wilber JH, et al. Early appropriate care: definitive stabilization of femoral fractures within 24 hours of injury is safe in most patients with multiple injuries. J Trauma. 2011;71:175–185. [DOI] [PubMed] [Google Scholar]
- 32.Giannoudis PV, Giannoudis VP, Horwitz DS. Time to think outside the box: ’Prompt-Individualised-Safe Management’ (PR.I.S.M.) should prevail in patients with multiple injuries. Injury. 2017;48:1279–1282. [DOI] [PubMed] [Google Scholar]
- 33.Harwood PJ, Giannoudis PV, van Griensven M, et al. Alterations in the systemic inflammatory response after early total care and damage control procedures for femoral shaft fracture in severely injured patients. J Trauma. 2005;58:446–452. [DOI] [PubMed] [Google Scholar]
- 34.Pape HC, Griensven MV, Hildebrand FF, et al. Systemic inflammatory response after extremity or truncal fracture operations. J Trauma. 2008;65:1379–1384. [DOI] [PubMed] [Google Scholar]
- 35.O’Toole RV, O’Brien M, Scalea TM, et al. Resuscitation before stabilization of femoral fractures limits acute respiratory distress syndrome in patients with multiple traumatic injuries despite low use of damage control orthopedics. J Trauma. 2009;67:1013–1021. [DOI] [PubMed] [Google Scholar]
- 36.Rixen D, Grass G, Sauerland S, et al. Evaluation of criteria for temporary external fixation in risk-adapted damage control orthopedic surgery of femur shaft fractures in multiple trauma patients: “evidence-based medicine” versus “reality” in the trauma registry of the German Trauma Society. J Trauma. 2005;59:1375–1394. [DOI] [PubMed] [Google Scholar]
- 37.Vallier HA, Moore TA, Como JJ, et al. Complications are reduced with a protocol to standardize timing of fixation based on response to resuscitation. J Orthop Surg Res. 2015;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallier HA, Dolenc AJ, Moore TA. Early appropriate care: a protocol to standardize resuscitation assessment and to expedite fracture care reduces hospital stay and enhances revenue. J Orthop Trauma. 2016;30:306–311. [DOI] [PubMed] [Google Scholar]


