Abstract
Leukotriene B4 (LTB4) is a lipid mediator of inflammation that is generated from arachidonic acid via the 5-lipoxygenase pathway. Previous studies have reported that the receptors of LTB4, BLT1, and BLT2 play mediatory roles in the allergic airway inflammation induced by ovalbumin (OVA). However, considering that house dust mites (HDMs) are the most prevalent allergen and well-known risk factor for asthmatic allergies, we are interested in elucidating the contributory roles of BLT1/2 in HDM-induced allergic airway inflammation. Our aim in this study was to investigate whether BLT1/2 play any roles in HDM-induced allergic airway inflammation. In this study, we observed that the levels of ligands for BLT1/2 [LTB4 and 12(S)-HETE (12(S)-hydroxyeicosatetraenoic acid)] were significantly increased in bronchoalveolar lavage fluid (BALF) after HDM challenge. Block-ade of BLT1 or BLT2 as well as of 5-lipoxygenase (5-LO) or 12-lipoxygenase (12-LO) markedly suppressed the production of TH2 cytokines (IL-4, IL-5, and IL-13) and alleviated lung inflammation and mucus secretion in an HDM-induced eosinophilic airway-inflammation mouse model. Together, these results indicate that the 5-/12-LO-BLT1/2 cascade plays a role in HDM-induced airway inflammation by mediating the production of TH2 cytokines. Our findings suggest that BLT1/2 may be a potential therapeutic target for patients with HDM-induced allergic asthma.
Keywords: BLT1, BLT2, Eosinophilic airway inflammation, HDM, TH2 cytokine
INTRODUCTION
Asthma is a chronic airway inflammatory disease which shows infiltration of immune cells into the airways, airway obstruction, airway hyperresponsiveness (AHR), and mucus hypersecretion (1, 2). A risk factor for developing asthma is exposure to substances that provoke allergic responses, including environmental allergens such as pollen and house dust mites (HDMs) (2, 3). HDMs are the most prevalent allergen and a risk factor for asthmatic allergies (4, 5). In total, 45-85% of asthma patients are allergic to HDMs, with geographical differences (2, 6). Previous studies using mouse models reported that chronic exposure to HDMs induces eosinophilic airway inflammation and elevation of TH2-associated cytokines in the bronchoalveolar lavage fluid (BALF) (3, 4, 7).
Leukotriene B4 (LTB4) is a major lipid mediator of inflammatory processes and immune responses. LTB4 is derived via the arachidonic-acid pathway and works as a chemoattractant molecule for leukocytes, such as granulocytes, monocytes, and T lymphocytes, to sites of acute inflammation (8-13). This proinflammatory molecule has two receptors, BLT1 and BLT2 (14). These receptors are members of the G protein-coupled receptor (GPCR) protein and are expressed on cell surface (14-16). BLT1, a high-affinity receptor of LTB4, is exclusively expressed on the surface of leukocytes. On the other hand, BLT2 shows a low-affinity for LTB4, and it is expressed in various tissues, including the spleen, lung, and liver (14, 17). BLT2 was shown to interact with various arachidonic acid-derived metabolites, such as 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) and 12-hydroxyheptadecatreinoic acid (12-HHT), in addition to LTB4 (14). Our previous research showed that the LTB4 receptors BLT1 and BLT2 play critical mediatory roles in the development of allergic asthma (8, 18-21). Although LTB4 receptors were suggested to play roles in OVA-induced asthmatic airway inflammation, their roles in HDM-induced asthmatic mouse models have not been previously studied.
In this study, we examined the mediatory role of the BLT1/2-linked cascade in HDM-driven airway inflammation. Inhibition of BLT1/2 markedly reduced the production of TH2 cytokines, IL-4, IL-5, and IL-13, in the BALF of HDM-induced model mice. We also observed that BLT1/2 inhibition reduced lung inflammation and mucus secretion. In addition, the production of TH2 cytokines, lung inflammation, and mucus secretion are inhibited by the blockade of 5-/12-LO, which are enzymes that catalyze the production of ligands for BLT1/2. Collectively, these results suggest that the 5-/12-LO-BLT1/2-linked cascade contributes to the development of HDM-induced eosinophilic airway inflammation via TH2 cytokine production. Thus, we propose that BLT1/2 may be a potential therapeutic target for HDM-sensitive asthmatic patients.
RESULTS
HDM-induced TH2 cytokine synthesis is dependent on BLT1/2
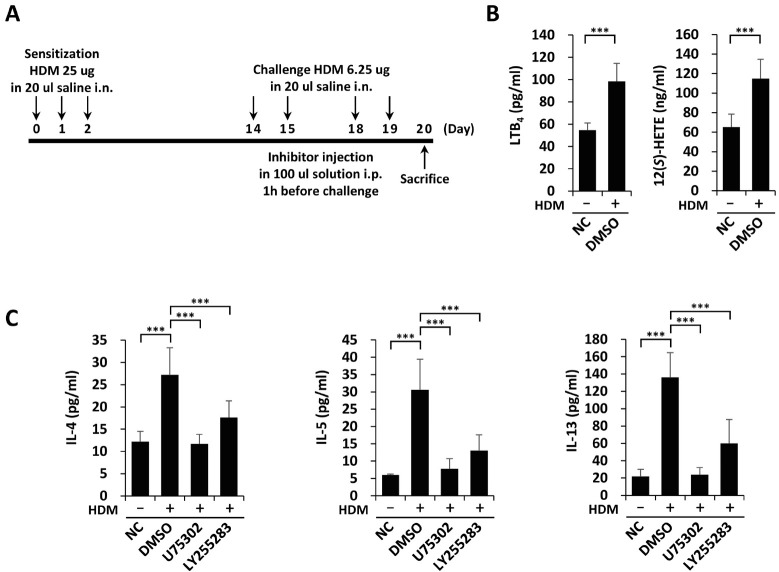
We established HDM-induced eosinophilic airway inflammation as previously described (3) with some modifications. Mice were intranasally sensitized with 25 µg of HDMs on days 0, 1 and 2 and then challenged with 6.25 µg HDMs on days 14, 15, 18, and 19. Mice were sacrificed on day 20. Inhibitors were intraperitoneally injected 1 h before every challenge (Fig. 1A). To study the roles of BLT1/2 signaling in HDM-induced airway inflammation, we measured the levels of their ligands (LTB4 and 12(S)-HETE) in BALF. As shown in Fig. 1B, the levels of LTB4 and 12(S)-HETE in BALF were markedly elevated after HDM challenge. The levels of the TH2 cytokines, IL-4, IL-5 and IL-13 were also increased in BALF as a result of HDM challenge (Fig. 1C). These increased cytokine levels were significantly abolished by pretreatment with U75302 or LY255283, an inhibitor of BLT1 or BLT2 (Fig. 1C). Together, these results indicate that BLT1 and BLT2 contribute to TH2 cytokine synthesis in HDM-induced airway inflammation.
Fig. 1.
HDM-induced TH2 cytokine synthesis is dependent on BLT1 and BLT2. (A-C) We randomly divided mice into four groups, gave them HDM sensitization and challenge, and sacrificed them 24 h after the last challenge. We collected BALF for ELISA. (A) Schematic of the HDM-induced eosinophilic airway inflammation model. We induced airway inflammation by sensitization with HDM (25 µg) and challenge with HDM (6.25 µg). We administered inhibitors via i.p. injection 1 h before every challenge. (B) We analyzed the levels of LTB4 and 12(S)-HETE in BALF using ELISA. Data are shown as the mean ± SD (n = 5-7 per group). ***P < 0.001 versus the control group. (C) We analyzed the levels of IL-4, IL-5, and IL-13 in BALF using ELISA. Data are shown as the mean ± SD (n = 7-12 per group). ***P < 0.001 versus the negative (NC) or positive (DMSO) control group.
BLT1/2 contributes to HDM-induced eosinophilic airway inflammation
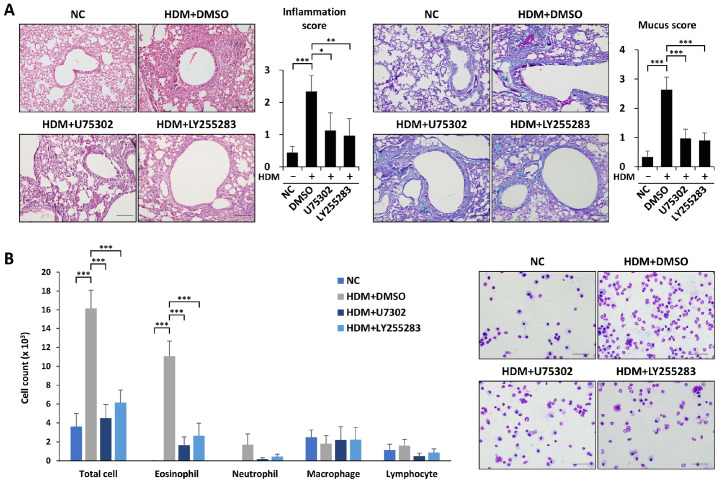
To examine whether BLT1/2 have roles in HDM-induced eosinophilic airway inflammation, we investigated lung histology and cell populations. Histopathological analysis and quantitative analysis of inflammation scores with H&E staining showed that lung inflammation induced by HDM administration was markedly attenuated by BLT1 antagonist U75302 or BLT2 antagonist LY255283 (Fig. 2A). In addition, increased mucus secretion induced by HDMs was also attenuated by U75302 and LY255283 (Fig. 2A). HDM administration markedly increased the influx of total cells in BALF. The increased total cell influx in BALF by HDM administration was markedly abolished by pretreatment with U75302 or LY255283 (Fig. 2B). However, the numbers of neutrophils, macrophages, and lymphocytes were not affected by HDMs (Fig. 2B). Taken together, these results suggest that BLT1 and BLT2 contribute to HDM-induced eosinophilic airway inflammation.
Fig. 2.
BLT1 and BLT2 contribute to HDM-induced eosinophilic airway inflammation. (A, B) Mice were intraperitoneally injected with U75302 or LY255283 1 h before every challenge. We harvested lung tissue 24 h after the last challenge, fixed them in formalin and embedded them in paraffin for section staining. (A) Lung tissue sections were stained with H&E (upper panel) or PAS (lower panel). We measured and scored peribronchial and perivascular lung inflammation or mucus secretion . Data are shown as the mean ± SD (n = 4-5 per group). *P < 0.05, ***P < 0.001 versus each control group. Scale bar, 100 µm. (B) Total immune cells, eosinophils, neutrophils, monocytes and lymphocytes in BALF were obtained using cytospin and stained with Diff-quik. Data are shown as the mean ± SD (n = 4-5 per group). ***P < 0.001 versus each control group. Scale bar, 50 µm.
5-/12-lipoxygeneases play roles in HDM-induced TH2 cytokine synthesis
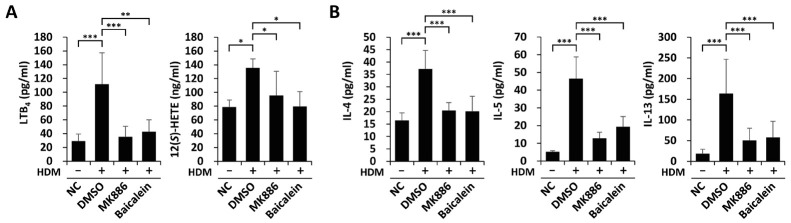
To further elucidate the contributory roles of BLT1 and BLT2 in the HDM-induced eosinophilic airway inflammation model, we investigated whether 5-LO and 12-LO, the enzymes catalyzing the synthesis of BLT1/2 ligands, are also involved. The levels of LTB4 and 12(S)-HETE in BALF were highly elevated by HDM-induced inflammation and were markedly reduced by MK886 or baicalein, an inhibitor of 5-LO or 12-LO (Fig. 3A). The increased TH2 cytokine secretion in HDM-induced airway inflammation was also attenuated by pretreatment with MK886 or baicalein (Fig. 3B). Thus, these results suggest that HDM-induced TH2 cytokine production is dependent on 5-/12-LO, the enzymes catalyzing the synthesis of BLT1/2 ligands.
Fig. 3.
5-/12-lipoxygeneases play roles in HDM-induced TH2 cytokine synthesis. (A, B) Mice were intraperitoneally injected with MK886 or baicalein 1 h before every challenge. We collected BALF for ELISAs. (A) We analyzed the levels of LTB4 and 12(S)-HETE in BALF using ELISA. Data are shown as the mean ± SD (n = 5-7 per group). *P < 0.05, ***P < 0.001 versus the control group. (B) We analyzed the levels of IL-4, IL-5 and IL-13 in BALF using ELISA. Data are shown as the mean ± SD (n = 7-12 per group). ***P < 0.001 versus the negative (NC) or positive (DMSO) control group.
HDM-induced eosinophilic airway inflammation is suppressed by 5-/12-lipoxygenase inhibition
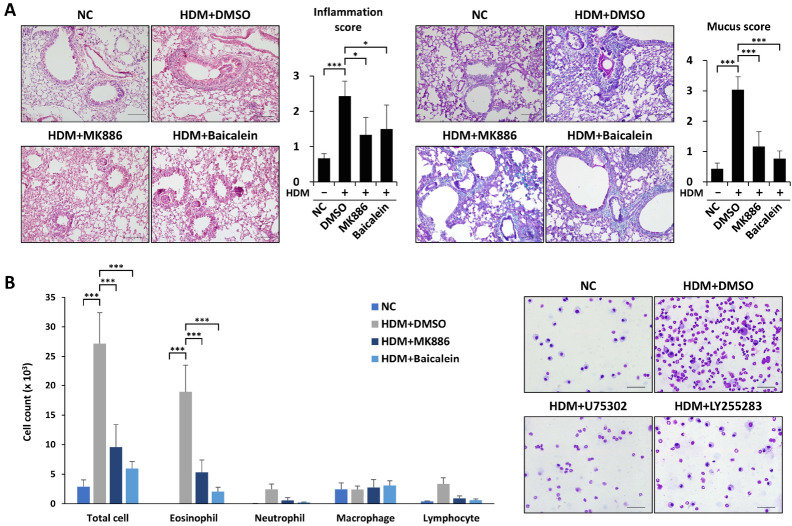
Finally, we investigated the effect of 5-/12-LO inhibition on HDM-induced eosinophilic airway inflammation. MK886 or baicalein treatment clearly attenuated alveolar hemorrhage and the influx of immune cells into the airway (Fig. 4A). Additionally, the administration of MK886 or baicalein significantly reduced mucus secretion (Fig. 4A). The numbers of total cells and eosinophils were also decreased by treatment with these inhibitors (Fig. 4B). Together, these results suggest that the 5-/12-LO-BLT1/2 cascade contributes to HDM-induced eosinophilic airway inflammation.
Fig. 4.
HDM-induced eosinophilic airway inflammation is suppressed by 5-/12-lipoxygenase blockade. (A, B) Mice were intraperitoneally injected with MK886 or baicalein 1 h before every challenge. We harvested lung and liver tissues 24 h after the last challenge, fixed them in formalin and embedded them in paraffin for section staining. (A) Lung tissue sections were stained with H&E (upper panel) or PAS (lower panel). We measured and scored peribronchial and perivascular lung inflammation or mucus secretion. Data are shown as the mean ± SD (n = 4-5 per group). *P < 0.05, ***P < 0.001 versus each control group. Scale bar, 100 µm. (B) We obtained total immune cells, eosinophils, neutrophils, monocytes, and lymphocytes in BALF using cytospin and stained them with Diff-quik. Data are shown as the mean ± SD (= 4-5 per group). ***P < 0.001 versus each control group. Scale bar, 50 µm.
DISCUSSION
In this study, we found that the levels of LTB4 and 12(S)-HETE, the ligands of BLT1/2, are highly elevated in BALF. In addition, we found that BLT1/2 contributes to the production of the TH2 cytokines, IL-4, IL-5 and IL-13, in HDM-induced airway inflammation. Furthermore, BLT1/2 inhibition suppressed airway inflammation and mucus secretion in the lung tissues of mice with HDM-induced allergic asthma, thus together suggesting that BLT1/2 contributes to eosinophilic airway inflammation in HDM-induced asthma by producing TH2 cytokines. Blockade of 5-LO or 12-LO, the synthesizing enzymes for LTB4 or 12(S)-HETE, also reduced cytokine secretion, airway inflammation, and mucus secretion. Our findings indicate the mediatory role of the 5-/12-LO-BLT1/2 cascade in HDM-induced eosinophilic airway inflammation.
Previous studies reported that repeated exposure to HDMs induces eosinophilic airway inflammation with greater TH2-associated humoral immune responses and airway remodeling (4, 7). The critical roles of TH2 cytokines in the HDM-induced allergic asthma mouse model are already known (2, 7, 22-24). These cytokines have been reported to induce eosinophil influx, airway smooth muscle hyperplasia, and mucus secretion (25). Recent studies also revealed that HDM treatment induces the release of lipoxygenase-derived lipid mediators in BALF (26). In addition, HDM-induced airway inflammation showed increased levels of cysteinyl leukotrienes and 12/15-LO metabolites (27). However, the role of BLT1/2, receptors for LTB4 or 12(S)-HETE, in HDM-induced airway inflammation has not yet been studied. Our results clearly demonstrate that BLT1/2 play mediatory roles in HDM-induced eosinophilic airway inflammation (Fig. 2). In addition, we conducted ELISA with serum to identify any changes in cytokine levels. Similar to the levels in the BALF, the levels of TH2-related cytokines (especially IL-5 and IL-13) in the serum were also upregulated by HDM administration and reduced by the treatment of BLT1/2 antagonists (Supplementary Fig. 1). Although intranasal HDM administration induces local airway inflammation, we think that the effect involved the complex systemic responses as well (such as the adaptive immune response and elevated TH2 cells). In support of the contributory roles of BLT1/2, we also demonstrate that 5-LO and 12-LO, the enzymes catalyzing the synthesis of BLT1/2 ligands, are necessary for the development of HDM-induced airway inflammation (Figs. 3 and 4). The detailed mechanism by which BLT2 cascade mediates HDM-driven eosinophilic airway inflammation is not elucidated yet. However, our previous studies have shown that BLT2 significantly contributes to reactive oxygen species (ROS) generation via NADPH oxidase (NOX) stimulation (28, 29). We also demonstrated that the BLT2 cascade activates redox-sensitive NF-κB in mouse macrophages (30). BLT2 was also shown to stimulate NF-κB signaling in allergen-stimulated mast cells, thus mediating TH2 cytokine production (19, 21). Taken these results together, we propose that BLT2 mediates NOX-ROS-NF-κB signaling to contribute to the synthesis of TH2-related cytokines and other inflammatory mediators in the airway.
Eosinophilia is one characteristic of HDM-induced allergic airway inflammation (Fig. 2). Nonetheless, we cannot rule out the contribution of other types of immune cells including macrophages, mast cells, and T lymphocytes (31). For example, the mediatory role of mast cells in the exacerbation of asthma has been well reported (32-36), and we previously demonstrated the role of BLT2 in the mast-cell activation and secretion of TH2 cytokines in vitro, as well as in an OVA-induced mouse model (18, 19, 21, 37). Thus, we speculate that BLT2 may act on mast cells to contribute to HDM-induced airway inflammation; clearly, further studies are necessary to elucidate the role of immune cells in the BLT1/2-linked cascade induced by HDM challenge.
In summary, our results suggest that the 5-/12-LO-BLT1/2 cascade clearly contributes to the development of HDM-induced eosinophilic airway inflammation via TH2 cytokine production. This is the first report on the role of BLT1/2 in an HDM-induced asthmatic mouse model, and our results may provide a potential therapeutic target for HDM allergic asthma.
MATERIALS AND METHODS
Reagents
We obtained:
HDM-induced eosinophilic airway inflammation mouse model
We obtained female C57BL/6 mice (8-9 weeks old) from Young-Bio (Seongnam, Korea). HDM extract (Greer Laboratories, Lenoir, NC), derived from Dermatophagoides pteronyssinus (Der p), was resuspended in saline and used to induce airway inflammation, as previously described with some modifications (Fig. 1A) (3). Briefly, we anesthetized mice with isoflurane intranasally sensitized with 25 µg of HDMs in 20 µl of saline on days 0, 1 and 2. We did challenges with 6.25 µg of HDMs in 20 µl of saline on days 14, 15, 18, and 19. Negative controls received 20 µl of saline intranasally on each injection. The mice were sacrificed on day 20. For the inhibition experiments, we administered LY255283 (10 mg/kg), U75302 (0.5 mg/kg), MK886 (0.5 mg/kg), baicalein (20 mg/kg) or a vehicle control (DMSO) intraperitoneally (i.p.) 1 h before every challenge. The doses of the inhibitors used in this study were within the concentration ranges used in a previous study (38). Mice were maintained in a temperature-controlled facility under a 12-h light-dark cycle with free access to water and food. We treated all experimental animals used in this study according to guidelines approved by the Institutional Animal Care and Use Committee of Korea University (KU-IACUC), and the experimental protocols were approved by KU-IACUC (Approval no. KU-IACUC-2019-0056).
Measurement of IL-4, IL-5, IL-13, LTB4 and 12(S)-HETE
We quantified the levels of IL-4, IL-5, IL-13, LTB4 and 12(S)-HETE in the supernatants of the BALF using an ELISA kit (R&D Systems for IL-4, IL-5 and IL-13; Enzo Life Sciences for LTB4 and 12(S)-HETE) according to the manufacturer’s instructions. We obtained BALF from mice by tracheal cannulation using 0.65 ml of PBS; we centrifuged the collected BALF at 1000 × g for 3 min (20). Then, we collected the supernatant for ELISA.
Analysis of BAL cells and lung histology
Inflammatory cells collected from BALF by centrifugation (1,000 × g for 3 min) were washed with PBS. Next, cytocentrifuge slides of BAL cells were fixed and stained with Diff-Quik. We harvested lung tissues, fixed them in 10% formaldehyde for 3 weeks, and embedded them in paraffin. We mounted Lung sections (4.5 µm thickness) onto SuperfrostTM Plus glass slides (Fisher Scientific, Pittsburgh, PA, USA) and deparaffinized and stained them with H&E and periodic acid-schiff (PAS). We did a quantitative histological analysis by five blinded investigators. We evaluated the degree of peribronchial and perivascular lung inflammation on a subjective scale from 0 to 3, as previously described (8). For the quantification of goblet cells in the airway, we used a five-point grading system: 0 < 0.5% PAS positive cells; 1 < 25%; 2, 25-50%; 3, 50-75%; and 4 > 75% (39). All images were acquired using a BX51 microscope (Olympus, Tokyo, Japan) equipped with a DP71 digital camera (Olympus).
Statistical analysis
We did all statistical analyses with one way analysis of variance, followed by Tukey’s post hoc test. We used SPSS software (IBM SPSS Statistics for Windows, version 21.0; IBM Corp., Armonk, NY, USA) for statistical analysis. The results are presented as the mean ± SD; P < 0.05 indicated statistical significance.
Supplementary Materials
ACKNOWLEDGEMENTS
This work was supported by a Bio & Medical Technology Development Program Grant (2017M3A9D8063317) and a Mid-Career Researcher Program Grant (2020R1A2B5B01002046) through the National Research Foundation funded by the Ministry of Science, Information, and Communication Technologies (ICT) and Future Planning, Republic of Korea. This work was also supported by Korea University grant.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Choi J, Choi BK, Kim JS, et al. Picroside II attenuates airway inflammation by downregulating the transcription factor GATA3 and Th2-related cytokines in a mouse model of HDM-induced allergic asthma. PLoS One. 2016;11:e0167098. doi: 10.1371/journal.pone.0167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer JD, Yang J, van den Boogaard FE, et al. Mast cell-deficient kit mice develop house dust mite-induced lung inflammation despite impaired eosinophil recruitment. J Innate Immun. 2014;6:219–226. doi: 10.1159/000354984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JR, Wiley RE, Fattouh R, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169:378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 5.Ulrik CS, Backer V. Markers of impaired growth of pulmonary function in children and adolescents. Am J Respir Crit Care Med. 1999;160:40–44. doi: 10.1164/ajrccm.160.1.9806059. [DOI] [PubMed] [Google Scholar]
- 6.Platts-Mills TAE, de Weck AL, Aalberse RC, et al. Dust mite allergens and asthma-a worldwide problem. J Allergy Clin Immunol. 1989;83:416–427. doi: 10.1016/0091-6749(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 7.Raemdonck K, Baker K, Dale N, et al. CD4+ and CD8+ T cells play a central role in a HDM driven model of allergic asthma. Respir Res. 2016;17:45. doi: 10.1186/s12931-016-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho KJ, Seo JM, Shin Y, et al. Blockade of airway inflammation and hyperresponsiveness by inhibition of BLT2, a low-affinity leukotriene B4 receptor. Am J Respir Cell Mol Biol. 2010;42:294–303. doi: 10.1165/rcmb.2008-0445OC. [DOI] [PubMed] [Google Scholar]
- 9.Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B4-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelfand EW, Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. J Allergy Clin Immunol. 2006;117:577–582. doi: 10.1016/j.jaci.2005.12.1340. [DOI] [PubMed] [Google Scholar]
- 11.Silbaugh SA, Stengel PW, Williams GD, Herron DK, Gallagher P, Baker SR. Effects of leukotriene B4 inhalation. Airway sensitization and lung granulocyte infil-tration in the guinea pig. Am Rev Respir Dis. 1987;136:930–934. doi: 10.1164/ajrccm/136.4.930. [DOI] [PubMed] [Google Scholar]
- 12.Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- 13.Taube C, Miyahara N, Ott V, et al. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol. 2006;176:3157–3164. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- 14.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B4 receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:123–134. doi: 10.1016/S0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 15.Jang JH, Wei JD, Kim M, Kim JY, Cho AE, Kim JH. Leukotriene B4 receptor 2 gene polymorphism (rs1950504, Asp196Gly) leads to enhanced cell motility under low-dose ligand stimulation. Exp Mol Med. 2017;49:e402. doi: 10.1038/emm.2017.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundeen KA, Sun B, Karlsson L, Fourie AM. Leukotriene B4 receptors BLT1 and BLT2: expression and function in human and murine mast cells. J Immunol. 2006;177:3439–3447. doi: 10.4049/jimmunol.177.5.3439. [DOI] [PubMed] [Google Scholar]
- 17.Kim GY, Lee JW, Cho SH, Seo JM, Kim JH. Role of the low-affinity leukotriene B4 receptor BLT2 in VEGF-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:915–920. doi: 10.1161/ATVBAHA.109.185793. [DOI] [PubMed] [Google Scholar]
- 18.Ro M, Lee AJ, Kim JH. 5-/12-Lipoxygenase-linked cascade contributes to the IL-33-induced synthesis of IL-13 in mast cells, thus promoting asthma development. Allergy. 2018;73:350–360. doi: 10.1111/all.13294. [DOI] [PubMed] [Google Scholar]
- 19.Cho KJ, Seo JM, Lee MG, Kim JH. BLT2 Is upregulated in allergen-stimulated mast cells and mediates the synthesis of Th2 cytokines. J Immunol. 2010;185:6329–6337. doi: 10.4049/jimmunol.1001213. [DOI] [PubMed] [Google Scholar]
- 20.Ro M, Kwon SY, Kim JH. Leukotriene B4 receptors mediate the production of IL-17, thus contributing to neutrophil-dominant asthmatic airway inflammation. Allergy. 2019;74:1797–1799. doi: 10.1111/all.13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AJ, Ro M, Cho KJ, Kim JH. Lipopolysaccharide/TLR4 stimulates IL-13 production through a MyD88-BLT2-linked cascade in mast cells, potentially contributing to the allergic response. J Immunol. 2017;199:409–417. doi: 10.4049/jimmunol.1602062. [DOI] [PubMed] [Google Scholar]
- 22.Reithofer M, Jahn-Schmid B. Allergens with protease activity from house dust mites. Int J Mol Sci. 2017;18:1368. doi: 10.3390/ijms18071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JY. The innate immune response in house dust mite-induced allergic inflammation. Allergy Asthma Immunol Res. 2013;5:68–74. doi: 10.4168/aair.2013.5.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108–116. doi: 10.1038/35100503. [DOI] [PubMed] [Google Scholar]
- 25.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolmert J, Pineiro-Hermida S, Hamberg M, et al. Prominent release of lipoxygenase generated mediators in a murine house dust mite-induced asthma model. Prostaglandins Other Lipid Mediat. 2018;137:20–29. doi: 10.1016/j.prostaglandins.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Henkel FDR, Friedl A, Haid M, et al. House dust mite drives proinflammatory eicosanoid reprogramming and macrophage effector functions. Allergy. 2019;74:1090–1101. doi: 10.1111/all.13700. [DOI] [PubMed] [Google Scholar]
- 28.Cho KJ, Seo JM, Kim JH. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol Cells. 2011;32:1–5. doi: 10.1007/s10059-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim C, Kim JY, Kim JH. Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep. 2008;41:555–559. doi: 10.5483/BMBRep.2008.41.8.555. [DOI] [PubMed] [Google Scholar]
- 30.Lee AJ, Cho KJ, Kim JH. MyD88-BLT2-dependent cascade contributes to LPS-induced interleukin-6 production in mouse macrophage. Exp Mol Med. 2015;47:e156. doi: 10.1038/emm.2015.8. [DOI] [PubMed] [Google Scholar]
- 31.Elieh Ali Komi D, Bjermer L. Mast cell-mediated orchestration of the immune responses in human allergic asthma: current insights. Clin Rev Allergy Immunol. 2019;56:234–247. doi: 10.1007/s12016-018-8720-1. [DOI] [PubMed] [Google Scholar]
- 32.Yu CK, Chen CL. Activation of mast cells is essential for development of house dust mite Dermatophagoides farinae-induced allergic airway inflammation in mice. J Immunol. 2003;171:3808–3815. doi: 10.4049/jimmunol.171.7.3808. [DOI] [PubMed] [Google Scholar]
- 33.Schmit D, Le DD, Heck S, et al. Allergic airway inflammation induces migration of mast cell populations into the mouse airway. Cell Tissue Res. 2017;369:331–340. doi: 10.1007/s00441-017-2597-9. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Aliyeva M, Daphtary N, et al. Antigen-induced mast cell expansion and bronchoconstriction in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol. 2014;306:L196–206. doi: 10.1152/ajplung.00055.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibilano R, Frossi B, Pucillo CE. Mast cell activation: a complex interplay of positive and negative signaling pathways. Eur J Immunol. 2014;44:2558–2566. doi: 10.1002/eji.201444546. [DOI] [PubMed] [Google Scholar]
- 36.Waern I, Lundequist A, Pejler G, Wernersson S. Mast cell chymase modulates IL-33 levels and controls allergic sensitization in dust-mite induced airway inflammation. Mucosal Immunol. 2013;6:911–920. doi: 10.1038/mi.2012.129. [DOI] [PubMed] [Google Scholar]
- 37.Lee AJ, Ro M, Kim JH. Leukotriene B4 receptor 2 is critical for the synthesis of vascular endothelial growth factor in allergen-stimulated mast cells. J Immunol. 2016;197:2069–2078. doi: 10.4049/jimmunol.1502565. [DOI] [PubMed] [Google Scholar]
- 38.Kwon SY, Ro M, Kim JH. Mediatory roles of leukotriene B4 receptors in LPS-induced endotoxic shock. Sci Rep. 2019;9:5936. doi: 10.1038/s41598-019-42410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kujur W, Gurram RK, Haleem N, Maurya SK, Agrewala JN. Caerulomycin A inhibits Th2 cell activity: a possible role in the management of asthma. Sci Rep. 2015;5:15396. doi: 10.1038/srep15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.