Abstract
Objective
Pharmacotherapy is established as an effective method for reducing symptoms of panic disorder (PD). However, about 20–40% of PD patients are treatment-resistant. Predictors of pharmacotherapy outcomes for PD patients are needed.
Methods
This study included 152 PD patients to measure the clinical severities of PD symptoms and used the Early Trauma Inventory (ETI) to measure early trauma. Treatment response was defined as a 40% reduction in the total Panic Disorder Severity Scale score from baseline. We measured the treatment responses at 8 weeks and 6 months. Binary logistic regression was used to predict treatment response after controlling for confounding variables.
Results
Early sexual trauma alone was associated with poor treatment response at 8 weeks. However, at 6 months, the total ETI score was associated with an unfavorable treatment response.
Conclusion
Therefore, our study suggests that clinicians need to be aware of a history of early trauma to optimize treatment outcomes for PD patients.
Keywords: Panic disorder, Early trauma, Predictors, Pharmacotherapy, Treatment response
INTRODUCTION
Panic disorder (PD) is an anxiety disorder characterized by recurrent and unexpected panic attacks associated with anticipatory anxiety, and physical symptoms including the sensation of shortness of breath, palpitation, or fear of dying [1]. PD has a one-year and lifetime prevalence of approximately 2.4% and 3.8%, respectively, in the United States [2]. In Korea, the oneyear and lifetime prevalence are 0.2% and 0.5%, respectively, and the rate is increasing [3]. In addition, PD is associated with other psychiatric and medical comorbidities, significant daily functional impairments, poorer work performance, decreased quality of life, and various social burdens [4].
Pharmacotherapy and cognitive-behavioral therapy (CBT) are well-established as effective methods to reduce the symptoms of patients with PD. Medications include first-line agents such as selective serotonin reuptake inhibitors (SSRIs) venlafaxine, the serotonin-norepinephrine reuptake inhibitor (SNRI), and second-line agents such as tricyclic antidepressants (TCAs) and benzodiazepines (BDZs) [5]. However, even when treated in clinical settings, the pharmacological treatment response may not be completely satisfactory; approximately 20–40% of patients with PD are non-responsive to treatment with drugs such as SSRIs, SNRI, TCAs, and BDZs [6,7]. In some clinical trials, PD patients were treated with SSRIs (fluvoxamine, sertraline, citalopram, and escitalopram) and clonazepam for a period of 8 to 12 weeks, and 17–61% of PD patients did not respond to treatment [8,9]. Therefore, early detection of patients with treatmentresistant PD is important as well as finding alternative treatments. Moreover, further studies are needed to identify response predictors of pharmacological treatment for patients with PD.
Several baseline predictors that might contribute to the pharmacological treatment response in PD have been suggested. Previous short-term or long-term research has suggested the symptom severities of PD, duration of the illness, presence of agoraphobia, comorbidities with other anxiety, depressive and personality disorders, personality traits, and the female gender as predictors [10-16]. In addition, some studies found that medical comorbidities and recent emergency room visits were predictors of unfavorable treatment responses in patients with PD [17].
Various kinds of early trauma also appear to influence some characteristics such as age at onset, symptom severities, personality traits, and poor treatment response in patients with PD [18]. Negative effects of early trauma have ongoing impacts that can last a lifetime in individuals with PD [19]. A previous study showed that early physical trauma is related to the symptom severity of PD [20]. Another study demonstrated that an association between early sexual trauma and personal vulnerability traits, such as neuroticism, and poor long-term treatment outcomes in individuals with PD [21]. However, further research is needed due to the lack of studies on responses to long-term pharmacological treatment for early trauma.
Therefore, our study aims to examine whether early trauma subtypes are associated with short-term and long-term pharmacological treatment responses in PD patients using a relatively larger sample size compared to previous studies. We hypothesized that PD patients with early trauma will have poor responses to pharmacological treatments in short-term and long-term follow-ups.
METHODS
Participants
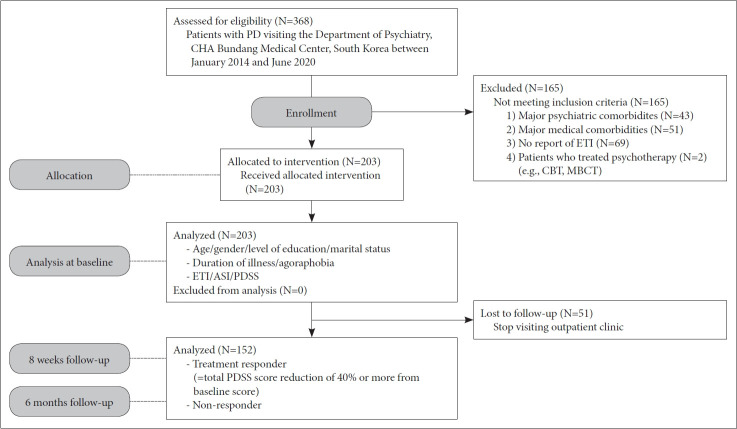
From January 2014 to June 2020, 368 participants were recruited as part of the PD patients treated in the Department of Psychiatry at CHA Bundang Medical Center. All participants were Koreans aged between 17 and 75 years old. Personal and family histories of PD were established through interviews. Only 152 PD patients who finished the Early Trauma Inventory (ETI) and 8-weeks and 6-months follow-up were included in this study analysis (Figure 1).
Figure 1.
Flow diagram of patient recruitment. ETI: Early Trauma Inventory, ASI: Anxiety Sensitivity Inventory, PDSS: Panic Disorder Severity Scale, CBT: Cognitive Behavioral Therapy, MBCT: Mindfulness-Based Cognitive Therapy.
Participants in PD with or without agoraphobia met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Axis I Disorder, as diagnosed by practiced psychiatrists using the Structured Clinical Interview for DSM-IV-TR. Only patients primarily diagnosed as PD were included. PD patients with additional major medical comorbidities were excluded. Participants with a primary diagnosis of any schizophrenia, bipolar disorder, major depressive disorder, personality disorder, anxiety disorders other than PD, substance use disorder, mental retardation, and pregnancy were also excluded. In addition, all patients with PD who had been treated with psychotherapy such as CBT or mindfulness-based cognitive therapy were excluded.
All PD patients had pharmacotherapy with SSRIs including escitalopram, paroxetine, and sertraline [escitalopram equivalence dosage=11.01±1.81 (mean±SD) mg/day] [22], and BDZs were primarily permitted on a pro re nata (PRN; as required) basis. All PD patients were undergoing pharmacotherapy with antidepressants such as SSRIs and anxiolytics such as BDZs according to the Korean Mediation Algorithm for PD [23] or the Clinical Practice Guidelines: Treatment of PD [24].
All study procedures adhered to the Institutional Review Board regulations and principles of Good Clinical Practice at the CHA Bundang Medical Center. After sufficiently explaining the study process to participants, their written informed consent was acquired (2019-05-030, 2018-06-029, 2011-11-164).
Early trauma and clinical measures
Early trauma was assessed using the Korean version of the Early Trauma Inventory Self Report-Short Form (ETISR-SF) at baseline. Cronbach’s α for the Korean version of the ETISR-SF was 0.869. This instrument is significantly correlated higher with the scores on the Childhood Trauma Questionnaire-Short Form (r=0.691) [25] which consists of 27 “yes” or “no” questionnaires in total assessing the four domains of general, physical, emotional, and sexual trauma experiences before the age of 18 years. Each of the domains is assessed using 11, 5, 5, and 6 questions, respectively.
To evaluate potential trait markers in PD at baseline, the Korean version of the Anxiety Sensitivity Inventory-Revised (ASI-R) [26,27] was used. The ASI-R is the most commonly used measure of anxiety sensitivity (AS), consisting of fears of respiratory symptoms, cardiovascular symptoms, publicly observable anxiety reactions, and cognitive dyscontrol.
To measure the clinical severities of the participants’ anxiety and depressive symptoms at baseline, we used the Panic Disorder Severity Scale (PDSS) [28] and the Beck Depression Inventory-II (BDI-II) [29] simultaneously, respectively.
Treatment response to pharmacotherapy was measured after a minimum of 8 weeks and 6 months of treatment in an adequate dose. It was defined as a total PDSS score reduction of 40% or more from baseline score after a minimum of 8 weeks and 6 months treatment in an adequate dose [30-32].
Statistical analyses
To analyze the relationship between treatment response and demographic and clinical data about participants with PD, Student t-test, and chi-square tests were used. Pearson’s correlation was applied to find out association among continuous variables such as age, duration of illness, ETI, ASI-R, and PDSS. In addition, binary logistic regression model with treatment response as dependent variables and with those that differed significantly between the good and poor treatment response at 8 weeks and in 6 months on Student t-tests or chi-square tests as covariates were performed. All statistical analyses used the IBD SPSS Statistics 26 software (IBM Corp., Armonk, NY, USA).
RESULTS
Sociodemographic characteristics and clinical variables at baseline
Sociodemographic characteristics and clinical variables of the 152 patients with PD are summarized in Table 1. PD patients with agoraphobia were 78.9% and without agoraphobia were 21.1%. The average duration of illness was 3.7 years [44.62±72.88 (mean±SD) months]. The average total sum of ETI was 4.88±4.00. Among the early trauma subtypes, physical trauma showed the highest mean score (1.89±1.64) among the subtypes of trauma (general: 1.36±1.52, emotional: 1.29±1.68, and sexual: 0.35±0.87). The average scores in the ASI-R were 46.48±26.06, and on the PDSS were 12.52±5.98.
Table 1.
Sociodemographic characteristics and clinical variables of patients with panic disorder at baseline
| Patients with panic disorder |
|
|---|---|
| N=152 | |
| Demographics | |
| Age (years) | 37.91±11.65 |
| Gender | |
| Male | 69 (45.4) |
| Female | 83 (54.6) |
| Level of education | |
| High school or less | 51 (33.6) |
| College or higher | 101 (66.4) |
| Marital status | |
| Married | 84 (55.3) |
| Single | 68 (44.7) |
| Clinical variables | |
| Duration of illness (months) | 44.62±72.88 |
| Agoraphobia | |
| Yes | 120 (78.9) |
| No | 32 (21.1) |
| ETI (total sum) | |
| All subtypes | 4.88±4.00 |
| General subtype | 1.36±1.52 |
| Physical subtype | 1.89±1.64 |
| Emotional subtype | 1.29±1.68 |
| Sexual subtype | 0.35±0.87 |
| ASI (total sum) | 46.48±26.06 |
| PDSS (total sum) | 12.52±5.98 |
| BDI-II (total sum) | 15.67±9.74 |
| SSRIs (escitalopram equivalence dose) | 11.01±1.81 |
Values represent count (percent), mean±SD, and median (interquartile range). SD: standard deviation, ETI: Early Traumatic Inventory, ASI: Anxiety Sensitivity Inventory, PDSS: Panic Disorder Severity Scale, BDI: Beck Depression Inventory, SSRI: selective serotonin receptor inhibitor
Changes in scores of panic disorder symptom severity over time and pharmacological treatment response at 8 weeks and 6 months
Over time, the PDSS scores gradually decreased from 12.52±5.98 (mean±SD) at baseline to 10.07±4.71 at 8 weeks, then 8.98±4.49 at 6 months. Assuming a treatment response of 40% or more reduction in PDSS scores compared to baseline, the number of patients with treatment response was 29 (19.2%) at 8 weeks. In 6 months, the score increased to 57 (37.5%) (Table 2).
Table 2.
Changes in scores of panic disorder severity scale and pharmacological treatment response at 8 weeks and 6 months
| Baseline | 8 weeks | 6 months | |
|---|---|---|---|
| PDSS | 12.52±5.98 | 10.7±4.71 | 8.98±4.49 |
| Treatment response | 152 (100.0) | 151 (100.0) | 152 (100.0) |
| Yes | - | 29 (19.2) | 57 (37.5) |
| No | - | 122 (80.8) | 95 (62.5) |
Values represent count (percent) and mean±SD. Criteria for treatment response: when the PDSS score at each time point is reduced by 40% or more compared to the PDSS score at the beginning of treatment, it is set as a treatment response. SD: standard deviation, PDSS: Panic Disorder Severity Scale
Comparison of patients with or without pharmacological treatment response at 8 weeks and 6 months
After 8 weeks from baseline, there were no significant differences between PD patients with treatment response and without treatment response according to sociodemographic characteristics (age, gender, level of education, and marital status), ratio of agoraphobia, and duration of illness (Table 3). There were no statistically significant differences in symptom severity measured by the ASI and the PDSS regardless of whether or not treatment responses presented. However, regarding early trauma, those with more sexual trauma (0.138±0.082) (mean±SD) showed more negative treatment response than those with less sexual trauma (0.405±0.936) (t=2.261, p=0.026).
Table 3.
Comparison of patients with or without pharmacological treatment response at 8-week and 6-month period stratified by sociodemographic characteristics and clinical variables
| Treatment response |
||||||
|---|---|---|---|---|---|---|
| 8 weeks |
χ2 or t (p) | 6 months |
χ2 or t (p) | |||
| Yes (N=29) | No (N=122) | Yes (N=57) | No (N=95) | |||
| Demographics | ||||||
| Age (years) | 40.07±14.44 | 37.54±10.84 | -0.885 (0.382) | 39.42±12.58 | 37.01±11.03 | -1.237 (0.218) |
| Gender | 1.299 (0.302) | 0.087 (0.867) | ||||
| Male | 16 (23.2) | 53 (76.8) | 25 (36.2) | 44 (63.8) | ||
| Female | 13 (15.9) | 69 (84.1) | 32 (38.6) | 51 (61.4) | ||
| Level of education | 0.030 (1.000) | 4.723 (0.034*) | ||||
| High school or less | 10 (20.0) | 40 (80.0) | 13 (25.5) | 38 (74.5) | ||
| College or higher | 19 (18.8) | 82 (81.2) | 44 (43.6) | 57 (56.4) | ||
| Marital status | 0.003 (1.000) | 0.255 (0.736) | ||||
| Married | 16 (19.0) | 68 (81.0) | 33 (39.3) | 51 (60.7) | ||
| Single | 13 (19.4) | 54 (80.6) | 24 (35.3) | 44 (64.7) | ||
| Clinical variables | ||||||
| Duration of illness (months) | 49.90±88.79 | 43.43±69.25 | -0.427 (0.670) | 48.91±89.29 | 42.04±61.35 | -0.561 (0.575) |
| Agoraphobia | 0.879 (0.448) | 0.169 (0.838) | ||||
| Yes | 21 (17.6) | 98 (82.4) | 46 (38.3) | 74 (61.7) | ||
| No | 8 (25.0) | 24 (75.0) | 11 (34.4) | 21 (65.6) | ||
| ETI (total sum) | ||||||
| All subtypes | 4.16±3.98 | 5.06±4.01 | 1.020 (0.310) | 3.96±3.90 | 5.41±3.98 | 2.126 (0.035*) |
| General subtype | 1.50±1.79 | 1.34±1.46 | -0.489 (0.626) | 1.26±1.57 | 1.41±1.50 | 0.598 (0.551) |
| Physical subtype | 1.59±1.47 | 1.95±1.68 | 1.023 (0.308) | 1.44±1.57 | 2.15±1.63 | 1.607 (0.010*) |
| Emotional subtype | 1.25±1.71 | 1.31±1.69 | 0.173 (0.863) | 1.07±1.61 | 1.42±1.72 | 1.235 (0.219) |
| Sexual subtype | 0.14±0.08 | 0.41±0.94 | 2.261 (0.026*) | 0.28±0.73 | 0.39±0.94 | 0.776 (0.439) |
| ASI (total sum) | 47.36±25.51 | 46.13±26.35 | -0.212 (0.832) | 44.75±25.54 | 47.50±26.46 | 0.602 (0.548) |
| PDSS (total sum) | 14.28±6.49 | 12.12±5.83 | -1.753 (0.082) | 13.82±5.72 | 11.73±6.03 | -2.105 (0.037*) |
| BDI-II (total sum) | 17.00±9.13 | 15.26±9.85 | -0.841 (0.402) | 14.33±9.05 | 16.46±10.10 | 1.291 (0.199) |
Values represent count (percent), mean±SD, and median (interquartile range).
p<0.05.
Criteria for treatment response: when the PDSS score at each time point is reduced by 40% or more compared to the PDSS score at the beginning of treatment, it is set as a treatment response. SD: standard deviation, ETI: Early Traumatic Inventory, ASI: Anxiety Sensitivity Inventory, PDSS: Panic Disorder Severity Scale, BDI: Beck Depression Inventory
After 6 months, the level of education, the severity of PD symptoms, and the early trauma experience demonstrated significant differences in the distribution of treatment response (Table 3). Regarding the level of education, those with a college education or more had better treatment responses (43.6%) than those with a high school education or less (25.5%) (χ2=4.723, p=0.034). Those with higher PDSS scores (13.82±5.72) had significantly better treatment responses than those with lower PDSS scores (11.73±6.03) (t=-2.105, p=0.037). Finally, those with higher total sum of ETI (5.41±3.98) had poorer treatment responses than those with lower total sum (3.96± 3.90) (t=2.126, p=0.035). Among subtypes of ETI, the higher scores of physical trauma (2.15±1.63) significantly reduced the proportion of pharmacological long-term treatment responses than lower scores (1.44±1.57) (t=1.607, p=0.010). On the other hand, there were no significant differences in the scores of general trauma, emotional trauma, and sexual trauma between patients with and without treatment response.
Pearson’s correlation analyses among continuous clinical variables
Table 4 presents the correlations among continuous variables. Higher ETI scores were significantly correlated with younger age (r=-0.295, p<0.000) and increased clinical symptoms in ASI-R (r=0.403, p<0.001) and PDSS (r=0.188, p=0.024). In addition, significant positive correlations were found between the total sum of ETI and the scores of subtypes such as general ETI (r=0.724, p<0.001), physical ETI (r=0.698, p<0.001), emotional ETI (r=0.797, p<0.001), and sexual ETI (r=0.477, p<0.001). Higher ASI scores were significantly correlated with younger age (r=-0.189, p=0.026) and higher PDSS scores (r=0.495, p<0.001). However, there were no significant correlations between duration of illness and other major variables (ETI, ASI, and PDSS), except for age (r=0.267, p=0.001).
Table 4.
Pearson’s correlation among continuous variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 Age (years) | - | ||||||||
| 2 Duration of illness (months) | 0.267† | - | |||||||
| 3 ETI (total sum) – All subtypes | -0.295‡ | 0.034 | - | ||||||
| 4 General | -0.115 | 0.015 | 0.724‡ | - | |||||
| 5 Physical | -0.215† | 0.032 | 0.698‡ | 0.305‡ | - | ||||
| 6 Emotional | -0.317‡ | 0.078 | 0.797‡ | 0.396‡ | 0.380‡ | - | |||
| 7 Sexual | -0.095 | -0.045 | 0.477‡ | 0.227† | 0.068 | 0.326‡ | - | ||
| 8 ASI (total sum) | -0.189* | 0.021 | 0.403‡ | 0.268† | 0.160 | 0.445† | 0.203* | - | |
| 9 PDSS (total sum) | -0.168* | 0.019 | 0.188* | 0.125 | 0.213† | 0.122 | 0.015 | 0.495‡ | - |
| 10 BDI-II (total sum) | -0.137 | 0.053 | 0.345† | 0.189* | 0.164* | 0.427† | 0.161* | 0.703† | 0.382† |
p<0.05,
p<0.005,
p<0.001.
ETI: Early Traumatic Inventory, ASI: Anxiety Sensitivity Inventory, PDSS: Panic Disorder Severity Scale, BDI: Beck Depression Inventory
Binary logistic regression results predicting pharmacological treatment response
Only sexual trauma was associated with poorer treatment response at 8 weeks (Table 3). To evaluate which factors contribute to the presence of treatment response at 6 months, a binary logistic regression analysis was performed (Table 5). In this model, only the essential variables, i.e., ETI, level of education, and PDSS at baseline, that exhibited significant differences in treatment response at 6 months were included. This research model proved to be significant at the p=0.001 level, with the χ2 value for the -2 log likelihood difference between the null model, in which the independent variable was excluded, and the model with the independent variable. The explanatory power of this model was 11.1% based on the Cox and Snell’s R2, and overall percentage was 64.1%.
Table 5.
Binary logistic regression results predicting pharmacological treatment response at 6 months
| Variables | B | p | Odds ratio (CI 95%) |
|---|---|---|---|
| ETI–All subtypes (total sum) | -0.121 | 0.016* | 0.886 (0.803–0.977) |
| Level of education (high school or more) | 1.022 | 0.015* | 2.778 (1.217–6.344) |
| PDSS at baseline (total sum) | 0.084 | 0.010* | 1.088 (1.020–1.160) |
| Constant | -1.772 | 0.003 | 0.170 |
-2 log likelihood=173.575, χ2 (df)=16.818(3) (p=0.001), Cox & Snell R2=0.111, overall percentage=64.1%, reference group is the level of education (high school or more).
p<0.05.
Criteria for treatment response: when the PDSS score at each time point is reduced by 40% or more compared to the PDSS score at the beginning of treatment, it is set as a treatment response. ETI: Early Traumatic Inventory, PDSS: Panic Disorder Severity Scale, CI: confidence interval
After 6 months, early trauma appeared to be significantly associated with the treatment response in PD patients (B= -0.121, p=0.016). Higher total scores of ETI of all subtypes reduced the possibility of pharmacological treatment response (OR=0.886, 95% CI=0.803–0.977) when the level of education and PD severity at baseline were controlled. Regarding the level of education, a high school education or more was associated with a significantly better response to treatment (B=1.022, p=0.015, OR=2.778, 95% CI=2.778–6.344). On the other hand, a higher level of PDSS was significantly associated with higher possibility of treatment response after 6 months (B=0.084, p=0.010, OR=1.088, 95% CI=1.020–1.160). In another model which included the scores of physical subtype of ETI instead of total sum of the ETI subtypes, the results were also statistically significant (Cox & Snell’s R2=0.121, overall percentage= 69.1%) (Supplementary Table 1 in the online-only Data Supplement). Experiences of early physical trauma significantly lowered the possibility of treatment response (B=-0.343, p= 0.004, OR=0.709, 95% CI=0.562–0.895). The level of education (B=0.954, p=0.020, OR=2.595, 95% CI=1.161–5.800) and the symptom severities of PD at baseline (B=0.089, p=0.007, OR=1.093, 95% CI=1.025–1.166) were significantly associated with the treatment response.
DISCUSSION
This is the first study to demonstrate that early trauma can affect the short term and long-term unfavorable pharmacological treatment response in patients with PD. Our study suggests that PD patients who had been experience early trauma may be more vulnerable and have poorer pharmacological treatment responses. This may be lead to a chronic course of illness.
In addition to early trauma, several risk factors contribute to worse pharmacological treatment responses of patients with PD [10,12,13,15]. Especially, studies have proposed AS as a predictor of panic-related pathology that directly affects the frequency of panic attacks [33,34]. However, there was no observed correlation between ASI and treatment response in the short-term and long-term in our study. Although ASI may affect some symptoms of PD and increase the PDSS score, which is the criterion for measuring treatment response, it is unlikely that it has a direct relationship with the pharmacological treatment response. Therefore, further studies are needed to examine if ASI directly contributes to poorer treatment responses.
Our results showed that the level of education was a potential sociodemographic predictor of treatment response. However, it has not been widely reported in previous studies [16]. We assume that people with a higher level of education generally tend to have good compliance after education, resulting in better treatment responses after long-term follow-up [35].
Studies suggest that high symptom severities can be associated with poor treatment responses and remission [12,36]. It is unclear why higher PDSS scores were associated with poor treatment responses in our findings. However, we assume that the participants whose symptom severity scores were high at baseline and easily declined over time might be included in this study. Since we recruited PD patients in the acute care hospital equipped with an emergency room, this kind of environment may have affected the treatment outcome in this study.
Other variables such as confounding factors known to potentially have a significant effect on long-term treatment responses such as the duration of the illness [13], the presence of agoraphobia [15], and female gender were not correlated or associated with the treatment response. The ratio of treatment responders at 8 weeks and 6 months in our study was relatively lower than in other studies. The difference between our findings and previous studies is due to the number of participants and the kind of pharmacotherapy that might affect the treatment response. In addition, our study observed poorer treatment response proportions than other studies because we only included more vulnerable PD patients who reported early trauma associated with the poor treatment response.
Notably, previous studies suggested an association between early trauma and the frequency of panic attacks or the age of onset in patients with PD. One previous study demonstrated that early physical and sexual trauma are risk factors associated with increased frequency of panic attacks in patients with PD [37]. Another study showed that a history of early trauma was associated with an earlier onset of symptoms in patients with PD [18]. Our findings indicated no significant correlation between early trauma and frequency of panic attacks or the age of onset in PD. However, early trauma may have a significant negative effect on the onset of PD and chronic outcomes in patients.
It is not clear why early trauma is related to poor pharmacological treatment response in PD patients. However, it is known that early trauma can cause the changes of brain regions and neurotransmitters such as glutamate. Neuroanatomically, early trauma can also adversely affect brain development, resulting in dysfunction of the hippocampus, amygdala, fornix, cerebral cortex, cerebellum, tapetum, and corpus callosum (CC) [21,38,39]. In particular, a previous study showed that early sexual trauma may be associated with higher FA values in the regions of tapetum in PD patients, and that these higher FA values are correlated with poor long-term pharmacological treatment outcome [21]. These findings suggest that the possibility of a relationship between axon and myelin microalterations in the tapetum may be related to early sexual trauma and the poor pharmacological treatment response in patients with PD. From a neurochemical perspective, increased glutamate and monoamine release induced by early traumatic events can damage neuronal resilience [40,41]. These changes in PD patients can be presumed to be related with the poor pharmacological treatment response.
Our findings showed the response rate of pharmacological treatment to be relatively low—about 19% at 8 weeks and 37% at 6 months of treatment. In our study, treatment response evaluation was performed at 8 weeks, which was a rather rapid period for this. Generally, treatment response evaluation is performed between 8 and 12 weeks. Furthermore, because the hospitals visited by the patients included in our study were not primary care hospitals, but rather general hospitals referred by other hospitals, it is highly likely that many patients with high symptoms severities were included in the study sample.
There are several limitations to our study. First, we could not completely rule out the possibility of recall bias in evaluating early trauma because our findings relied on retrospective selfreports. However, the use of self-reports in trauma studies may be an advantage, as self-reports may be more reliable than faceto-face clinical interviews for reporting sensitive and reluctant information such as trauma. In addition, ETISR-SF is designed to make it easier to report its own trauma, making trauma reporting more reliable. Second, we recruited PD patients in an acute care hospital and some PD patients might have depressed episodes in the long-term follow-up as usually shown in the natural course of PD. Thus, sampling bias may have affected the response to pharmacological treatment.
In conclusion, the current findings suggest that early trauma could be associated with poor pharmacological treatment responses in patients with PD short- and long-term. Therefore, our study highlights the need for clinicians to recognize a history of early trauma to help determine the direction of medical treatment and predict pharmacotherapy for patients with PD. In the future, the optimization of pharmacological treatments is a medical necessity for patients with PD.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by Ministry of Science and ICT (NRF-2019M3C7A1032262) and by Healthcare AI Convergence Research & Development Program through the National IT Industry Promotion Agency of Korea (NIPA) funded by the Ministry of Science and ICT (No. S1601-20-1034) to S.H.Lee.
Footnotes
The authors have no potential conflicts of interest to disclose.
Authors’ contribution
Conceptualization: Hyun-Ju Kim, Sang-Hyuk Lee. Data curation: Ji Eun Kim, Sang-Hyuk Lee. Formal analysis: Ji Eun Kim, Sang-Hyuk Lee. Funding acquisition: Sang-Hyuk Lee. Investigation: all authors. Methodology: all authors. Project administration: all authors. Resources: Sang-Hyuk Lee. Software: all authors. Supervision: Sang-Hyuk Lee. Validation: all authors. Visualization: all authors. Writing—original draft: all authors. Writing—review & editing: all authors.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2020.0380.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) Washington DC: American Psychiatric Pub; 2013. [Google Scholar]
- 2.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong J, Lee D, Ham B, Lee S, Sung S, Yoon T. The Survey of Mental Disorders in Korea 2016. Seoul: Ministry of Health and Welfare; 2017. [Google Scholar]
- 4.Bystritsky A, Kerwin L, Niv N, Natoli JL, Abrahami N, Klap R, et al. Clinical and subthreshold panic disorder. Depress Anxiety. 2010;27:381–389. doi: 10.1002/da.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19:93. doi: 10.31887/DCNS.2017.19.2/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perna G, Caldirola D. Management of treatment-resistant panic disorder. Curr Treat Options Psychiatry. 2017;4:371–386. doi: 10.1007/s40501-017-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandelow B, Rüther E. Treatment-resistant panic disorder. CNS Spectr. 2004;9:725–739. doi: 10.1017/s1092852900022379. [DOI] [PubMed] [Google Scholar]
- 8.Bandelow B, Behnke K, Lenoir S, Hendriks G, Alkin T, Goebel C, et al. Sertraline versus paroxetine in the treatment of panic disorder: an acute, double-blind noninferiority comparison. J Clin Psychiatry. 2004;65:405. doi: 10.4088/jcp.v65n0317. [DOI] [PubMed] [Google Scholar]
- 9.Freire RC, Hallak JE, Crippa JA, Nardi AE. New treatment options for panic disorder: clinical trials from 2000 to 2010. Expert Opin Pharmacother. 2011;12:1419–1428. doi: 10.1517/14656566.2011.562200. [DOI] [PubMed] [Google Scholar]
- 10.Albus M, Scheibe G, Scherer J. Panic disorder with or without concomitant depression 5 years after treatment: a prospective follow-up. J Affect Disord. 1995;34:109–115. doi: 10.1016/0165-0327(95)00007-a. [DOI] [PubMed] [Google Scholar]
- 11.Maier W, Buller R. One-year follow-up of panic disorder. Eur Arch Psychiatry Neurol Sci. 1988;238:105–109. doi: 10.1007/BF00452785. [DOI] [PubMed] [Google Scholar]
- 12.Noyes Jr R, Garvey MJ, Cook BL. Follow-up study of patients with panic disorder and agoraphobia with panic attacks treated with tricyclic antidepressants. J Affect Disord. 1989;16:249–257. doi: 10.1016/0165-0327(89)90080-3. [DOI] [PubMed] [Google Scholar]
- 13.Noyes R, Reich J, Christiansen J, Suelzer M, Pfohl B, Coryell WA. Outcome of panic disorder: relationship to diagnostic subtypes and comorbidity. Arch Gen Psychiatry. 1990;47:809–818. doi: 10.1001/archpsyc.1990.01810210017003. [DOI] [PubMed] [Google Scholar]
- 14.Scheibe G, Albus M. Predictors and outcome in panic disorder: a 2-year prospective follow-up study. Psychopathology. 1997;30:177–184. doi: 10.1159/000285045. [DOI] [PubMed] [Google Scholar]
- 15.Sharp D, Power K. Predicting treatment outcome for panic disorder and agoraphobia in primary care. Clin Psychol Psychother. 1999;6:336–348. [Google Scholar]
- 16.Slaap BR, den Boer JA. The prediction of nonresponse to pharmacotherapy in panic disorder: a review. Depress Anxiety. 2001;14:112–122. doi: 10.1002/da.1053. [DOI] [PubMed] [Google Scholar]
- 17.Chavira DA, Stein MB, Golinelli D, Sherbourne CD, Craske MG, Sullivan G, et al. Predictors of clinical improvement in a randomized effectiveness trial for primary care patients with panic disorder. J Nerv Ment Dis. 2009;197:715–721. doi: 10.1097/NMD.0b013e3181b97d4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kipper L, Blaya C, Wachleski C, Dornelles M, Salum GA, Heldt E, et al. Trauma and defense style as response predictors of pharmacological treatment in panic patients. Eur Psychiatry. 2007;22:87–91. doi: 10.1016/j.eurpsy.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Horesh N, Amir M, Kedem P, Goldberger Y, Kotler M. Life events in childhood, adolescence and adulthood and the relationship to panic disorder. Acta Psychiatr Scand. 1997;96:373–378. doi: 10.1111/j.1600-0447.1997.tb09932.x. [DOI] [PubMed] [Google Scholar]
- 20.Zou Z, Huang Y, Wang J, He Y, Min W, Chen X, et al. Association of childhood trauma and panic symptom severity in panic disorder: exploring the mediating role of alexithymia. J Affect Disord. 2016;206:133–139. doi: 10.1016/j.jad.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Song C, Bang M, Lee SH. Early sexual trauma is related with the tapetum in patients with panic disorder. J Affect Disord. 2020;267:107–113. doi: 10.1016/j.jad.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Hayasaka Y, Purgato M, Magni LR, Ogawa Y, Takeshima N, Cipriani A, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179–184. doi: 10.1016/j.jad.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Gim M, Kim MK, Lee JH, Kim W, Moon E, Seo HJ, et al. Korean guidelines for the treatment of panic disorder 2018: Psychosocial treatment strategies. Anxiety Mood. 2019;15:13–19. [Google Scholar]
- 24.Simon N, Hollander E, Rothbaum BO, Stein DJ. The American Psychiatric Association Publishing Textbook of Anxiety, Trauma, and OCD-Related Disorders. American Psychiatric Pub; 2020. [Google Scholar]
- 25.Jeon JR, Lee EH, Lee SW, Jeong EG, Kim JH, Lee D, et al. The early trauma inventory self report-short form: psychometric properties of the Korean version. Psychiatry Investig. 2012;9:229–235. doi: 10.4306/pi.2012.9.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim YJ, Yu BH, Kim JH. Korean anxiety sensitivity index—revised: its factor structure, reliability, and validity in clinical and nonclinical samples. Depress Anxiety. 2007;24:331–341. doi: 10.1002/da.20210. [DOI] [PubMed] [Google Scholar]
- 27.Taylor S, Cox BJ. An expanded anxiety sensitivity index: evidence for a hierarchic structure in a clinical sample. J Anxiety Disord. 1998;12:463–483. doi: 10.1016/s0887-6185(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 28.Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, et al. Multicenter collaborative panic disorder severity scale. Am J Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Steer RA, Brown GK. Beck Depression Inventory (BDI-II) San Antonio: TX: Psychological Corp; 1996. [Google Scholar]
- 30.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 31.Aaronson CJ, Shear MK, Goetz RR, Allen LB, Barlow DH, White KS, et al. Predictors and tie course of response among panic disorder patients treated with cognitive-behavioral therapy. J Clin Psychiatry. 2008;69:418–424. doi: 10.4088/jcp.v69n0312. [DOI] [PubMed] [Google Scholar]
- 32.Furukawa TA, Katherine Shear M, Barlow DH, Gorman JM, Woods SW, Money R, et al. Evidence‐based guidelines for interpretation of the Panic Disorder Severity Scale. Depress Anxiety. 2009;26:922–929. doi: 10.1002/da.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart SH, Taylor S, Jang KL, Cox BJ, Watt MC, Fedoroff IC, et al. Causal modeling of relations among learning history, anxiety sensitivity, and panic attacks. Behav Res Ther. 2001;39:443–456. doi: 10.1016/s0005-7967(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 34.McNally RJ. Anxiety sensitivity and panic disorder. Biol Psychiatry. 2002;52:938–946. doi: 10.1016/s0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- 35.Kim HS, Kim HY. Factors predicting medication compliance among elderly visitors of public health centers. J Korean Acad Community Health Nurs. 2007;18:5–13. [Google Scholar]
- 36.Kim B, Cho SJ, Lee KS, Lee JY, Choe AY, Lee JE, et al. Factors associated with treatment outcomes in mindfulness-based cognitive therapy for panic disorder. Yonsei Med J. 2013;54:1454–1462. doi: 10.3349/ymj.2013.54.6.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin RD, Fergusson DM, Horwood LJ. Childhood abuse and familial violence and the risk of panic attacks and panic disorder in young adulthood. Psychol Med. 2005;35:881–890. doi: 10.1017/s0033291704003265. [DOI] [PubMed] [Google Scholar]
- 38.Yu ST, Lee KS, Lee SH. Fornix microalterations associated with early trauma in panic disorder. J Affect Disord. 2017;220:139–146. doi: 10.1016/j.jad.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 39.Grimm S, Pestke K, Feeser M, Aust S, Weigand A, Wang J, et al. Early life stress modulates oxytocin effects on limbic system during acute psychosocial stress. Soc Cogn Affect Neurosci. 2014;9:1828–1835. doi: 10.1093/scan/nsu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey BH, McEwen BS, Stein DJ. Neurobiology of antidepressant withdrawal: implications for the longitudinal outcome of depression. Biol Psychiatry. 2003;54:1105–1117. doi: 10.1016/s0006-3223(03)00528-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.