Abstract
The emergence of hair is a defining event during mammalian skin development, but the cellular mechanisms leading to the opening of the hair follicle canal remain poorly characterized. Our previous studies have shown that early hair buds possess a central column of differentiated keratinocytes expressing Keratin 79 (K79), which marks the future hair follicle opening. Here, we report that during late embryogenesis and early postnatal development, K79+ cells at the distal tips of these columns downregulate E-cadherin, change shape, recede and undergo cell death. These changes likely occur independently of sebaceous glands and the growing hair shaft, and serve to create an orifice for hair to subsequently emerge. Defects in this process may underlie phenomena such as ingrown hair, or may potentially contribute to upper hair follicle pathologies including acne, hidradenitis suppurativa and infundibular cysts.
Keywords: Krt79, hair canal, infundibulum, skin pore, epidermis
Background
Our skin is the largest and most visible organ in the body, and studies over the past few decades have uncovered many of the cellular and molecular mechanisms that underlie epidermal and hair follicle development, maintenance and renewal (1, 2). In spite of this, the processes that enable our skin to transform during development from a continuous epithelial sheet into one that is perforated at the surface and pierced by regularly spaced hair shafts remain enigmatic. Simplistically, one might imagine that the physical pressure imparted by an outward growing hair might be sufficient to rupture the skin’s surface. However, since pelage hairs first appear in mice at postnatal day ~3–4 (3), such a scenario would require the hair shaft to breach a mature epithelium that, by this point, possesses a rigid outer cornified layer secured by corneodesmosomes and extensive covalent cross-links (4, 5). Thus, the seemingly simple act of sprouting hair likely belies a more complicated cellular process.
Early studies in different mammals have suggested a variety of mechanisms for creating the hair follicle opening. In opossum, Gibbs et al., noted that a plug of cells connected to the sebaceous glands (SGs) gets pushed distally by the growing hair fiber (6). Unable to penetrate the stratum corneum, these cells deflect horizontally beneath the cornified layer and subsequently keratinize, leaving behind a hair canal. Similar observations have also been made by Wildman et al., in sheep, where a column of SG-derived cells disintegrates to create a passageway in advance of the growing hair shaft (7).
In contrast to these findings, Hardy et al., reported in explant cultures of mouse embryonic skin that the formation of the hair canal does not appear to depend on SGs (8). Rather, this process is initiated by cells that keratinize within the epidermal spinous layer and reorient themselves perpendicular to the skin surface, similar to observations originally reported by Pinkus (9). In human skin, Maximow and Bloom simply noted that cells in the epidermis flatten and move aside to form the hair follicle opening (10). Critically, ultrastructural studies by Hashimoto (11), and later by Breathnach and Robins (12), have suggested that the hair canal is comprised of adjoining epidermal and follicular compartments, and that interior cells become eliminated to create a lumen prior to the maturation of the hair shaft.
Our previous studies in mice have shown that Keratin 79 (K79) identifies the earliest terminally differentiated cells within embryonic hair buds (13, 14). K79+ cells fill the interior, suprabasal compartment of hair germs prior to dermal papilla engulfment, and form a column that extends out into the developing epidermis (Figure 1A). Later in more mature follicles, K79+ cells line the inner surface of the hair follicle opening, known as the infundibulum (14). How the initial column of K79+ cells transforms into the hair canal remains unclear, especially as this occurs while the overlying epidermis is also rapidly changing and gaining barrier function (Figure 1A). Here, we delineate some of the complex cellular events that help create the hair follicle opening in mice. In doing so, we identify certain aspects of this process that are consistent with early observations from opossum, sheep, mouse and human follicles, as well as notable differences.
Figure 1. The tips of K79+ cell columns express both hair follicle and epidermal differentiation markers.
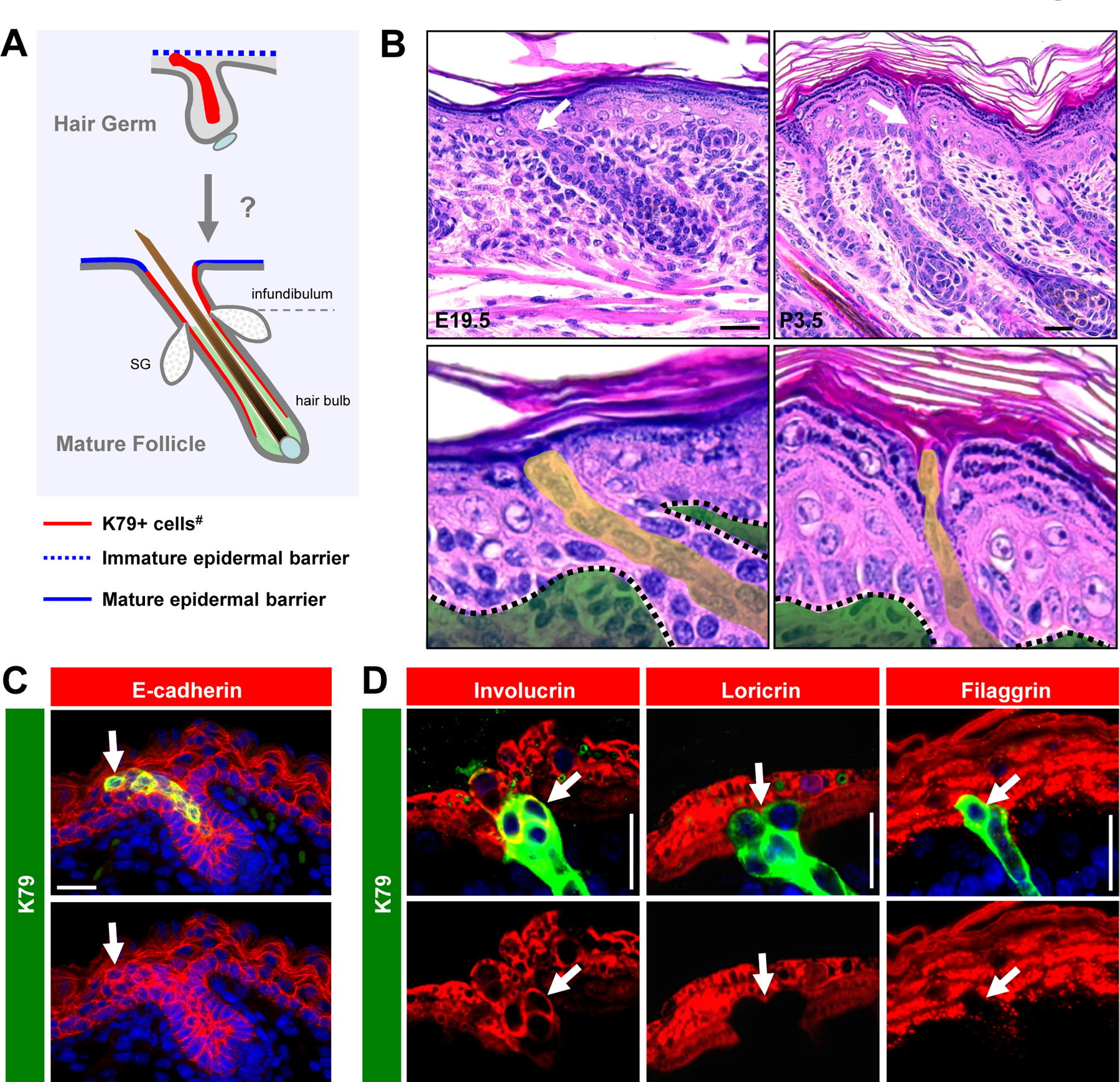
A. Schematic showing the localization of K79+ cells (red) in the embryonic hair germ and in a mature postnatal follicle. Beneath the sebaceous glands (SG), K79 is also expressed in the companion layer, although its expression in this layer becomes reduced in the mature follicle (13, 14, 50). Light green, inner root sheath. #, K79 is also expressed in the SG and sebaceous duct (not highlighted here). Images not drawn to scale. B. H&E sections of E19.5 skin (left panels) and P3.5 skin (right panels). In bottom magnified images, green shading identifies the dermis, while yellow shading identifies cell columns protruding from hair follicles. C. E16.5 hair germ containing a K79+ cell column with cuboidal tip that expresses E-cadherin (arrows). D. The tips of K79+ cell columns (arrows) express involucrin, but not other epidermal differentiation markers in P0.5 skin. Scale bars, 50 μm.
Experimental Design
Tissue harvest and staining:
Embryonic and newborn C57BL/6 mouse skin were excised at the indicated times, fixed overnight in formalin and processed for paraffin sectioning. For frozen sections, samples were fixed in cold 3.7% paraformaldehyde for 1 hour, sunk in 30% sucrose overnight at 4° C, and embedded the next day in Tissue-Tek OCT. Paraffin sections were re-hydrated, antigen-retrieved by boiling in 1 mM EDTA pH 8.0, and stained with antibodies against the following antigens: involucrin (PRB-140C, Covance), loricrin (PRB-145P, Covance), filaggrin (PRB-417P, Covance) and E-cadherin (24E10, Cell Signaling; and Clone 36, BD Biosciences). For K79 staining, we used clone Y-17, lot J2412, Santa Cruz Biotechnology; and clone ab7195, Abcam, which was previously shown to recognize K79 (14). TUNEL staining was performed on 7 μm frozen sections using the Apoptag Red kit (S7165, Millipore), followed by immunostaining using the ab7195 antibody.
Quantitation:
For quantitation of K79+ column morphology, over 100 columns were scored for all timepoints besides E19.5 where 44 columns were scored. To classify column morphology, only the distal-most K79+ cells were considered. Columns were scored as “cuboidal” when the horizontal axis of the cell body exceeded the vertical axis, and cells at the tip of the column were well-integrated with the adjacent epidermis. Columns were scored as “pointed” if the vertical axis of the cell body exceeded the horizontal axis, which was also typically accompanied by a slight depression or divot above the nascent hair canal. Columns were scored as “mature” when K79+ cells were entirely localized to the suprabasal lining of the infundibulum. For TUNEL staining, columns were counted if K79+ cells were observed at the follicle-epidermal interface in the section. A column was scored as TUNEL+ if at least one K79+ cell was also TUNEL+. Over 50 columns were scored for E19.5, P0.5 and P3.5 skin samples, while 15 columns were scored for E16.5.
Results
We began our studies by examining the overall morphology of the upper hair follicle domain during development. In hematoxylin and eosin (H&E)-stained sections of Stage 6–7 hair pegs (15), a column of cells protruding out from the hair follicle and integrating into the interfollicular epidermis (IFE) was readily observed (Figure 1B). This column persisted in more mature Stage 8–9 follicles but appeared noticeably narrower, while the adjacent surface epithelium appeared folded inwards, marking the future site of the hair follicle opening (Figure 1B).
Previously, we have shown that this column of cells expresses K79 (Figure 1C) and arises directly from Shh+ progenitors that initiate terminal differentiation in Stage 4 hair germs (13, 14). Since K79+ cells arise from follicles but also appear to integrate into the IFE, we wondered whether these keratinocytes might express IFE differentiation markers. Indeed, we observed that the tips of these cells extended up into the junction between the granular and cornified layers, and expressed involucrin, but not later stage differentiation markers such as loricrin and filaggrin (Figure 1D). Thus, the tips of K79+ cell columns uniquely exhibit features of both hair follicle and early epidermal differentiation.
Since the formation of the hair canal likely involves extensive cellular remodeling, we next assessed the shape of K79+ cell columns in embryonic and newborn skin. Between E16.5 - E19.5, the tips of these columns largely possessed a cuboidal morphology and expressed E-cadherin, similar to adjacent IFE cells (Figures 1C, 2A). However, soon after birth, these columns took on a “pointed” morphology and appeared to recede, losing E-cadherin and partially evacuating a space above the developing hair follicle (Figure 2A). At the same time, suprabasal IFE cells adjacent to K79+ pointed columns appeared compacted. Nearly all K79+ cell columns continued to exhibit a pointed morphology until P4.5, when unobstructed hair canals were observed (Figure 2A–B). At this stage, K79+ cells now occupied the suprabasal layer of the infundibulum. Overall, these observations indicate that K79+ columns undergo extensive changes in cell shape and organization prior to the opening of the hair canal (Figure 2B).
Figure 2. The tips of K79+ cell columns change shape, recede and lose E-cadherin prior to hair canal opening.
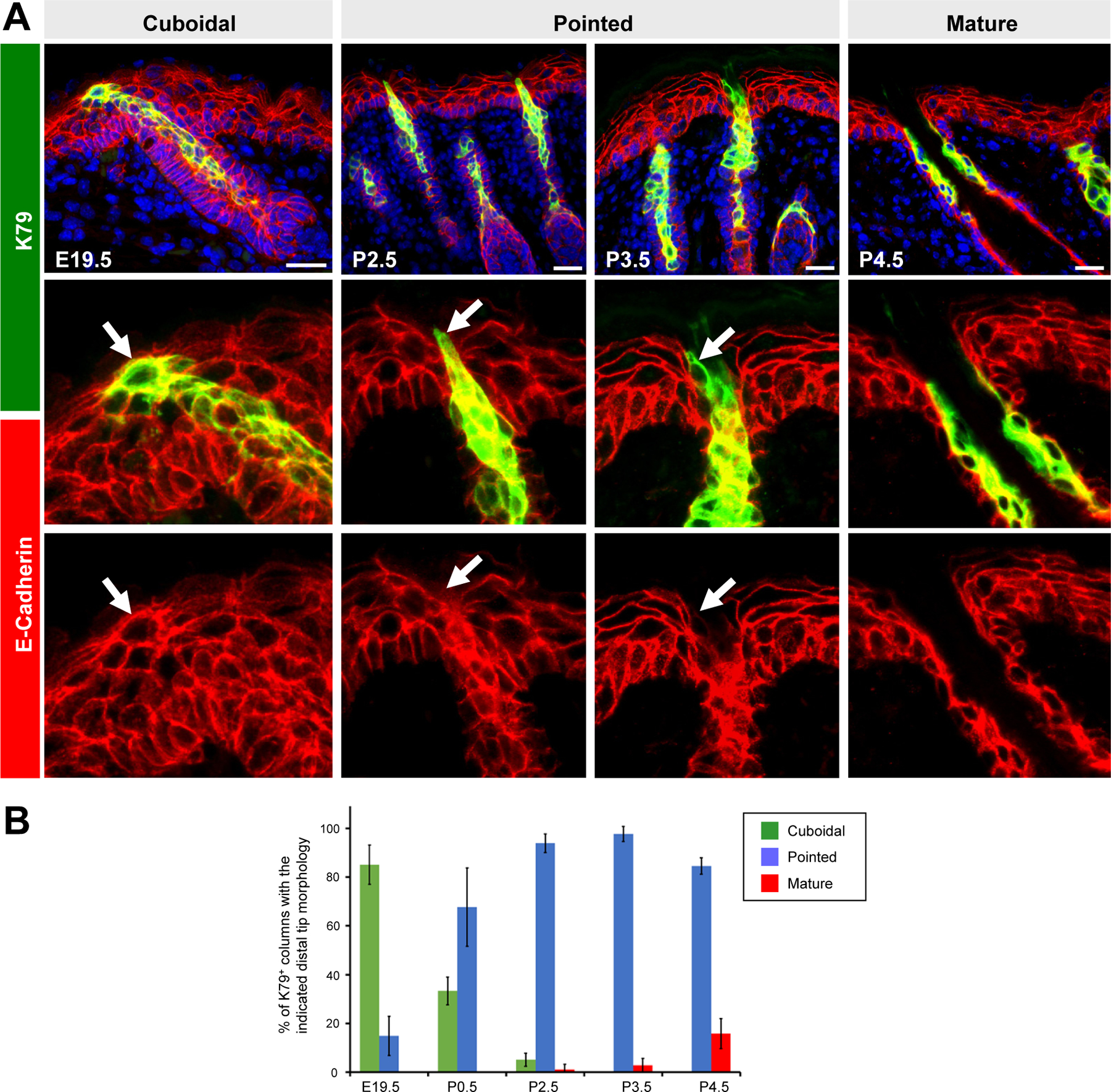
A. Top panels, representative images of K79+ cell columns, with tips that possess a “cuboidal,” “pointed” or “mature” morphology. Middle and bottom panels, magnified images showing that the tips of K79+ cell columns lose E-cadherin between E19.5-P2.5 (arrow). B. Quantitation showing transition of cuboidal K79+ cell columns into pointed and mature columns over time. Error bars, SD. Scale bars, 50 μm.
We lastly investigated whether cell death might be an underlying driver of the cellular changes noted above. In E16.5 - E19.5 embryos, we observed that while the acanthotic layers of the epidermis were often marked by TUNEL staining, the majority of K79+ cell columns were negative (Figure 3A–B). Within hours after birth, however, K79+ columns containing TUNEL+ cells were frequently observed (Figure 3A–B). While increased cell death generally coincided with the appearance of pointed K79+ columns, we also occasionally observed TUNEL+ cells in cuboidal columns postnatally (Figure 3C). Finally, since changes in cell shape and viability first appeared in E19.5 - P0.5 skin—at least 3 days prior to the specification of SGs (16)—this strongly suggests that these changes proceed independently of SG input. Altogether, we have shown here that K79+ cell columns extend out into the epidermis during early hair follicle development, and that the tips of these columns change shape, recede, lose E-cadherin and undergo cell death as a potential mechanism to create the hair follicle opening (Figure 3D).
Figure 3. The tips of K79+ cell columns undergo cell death.
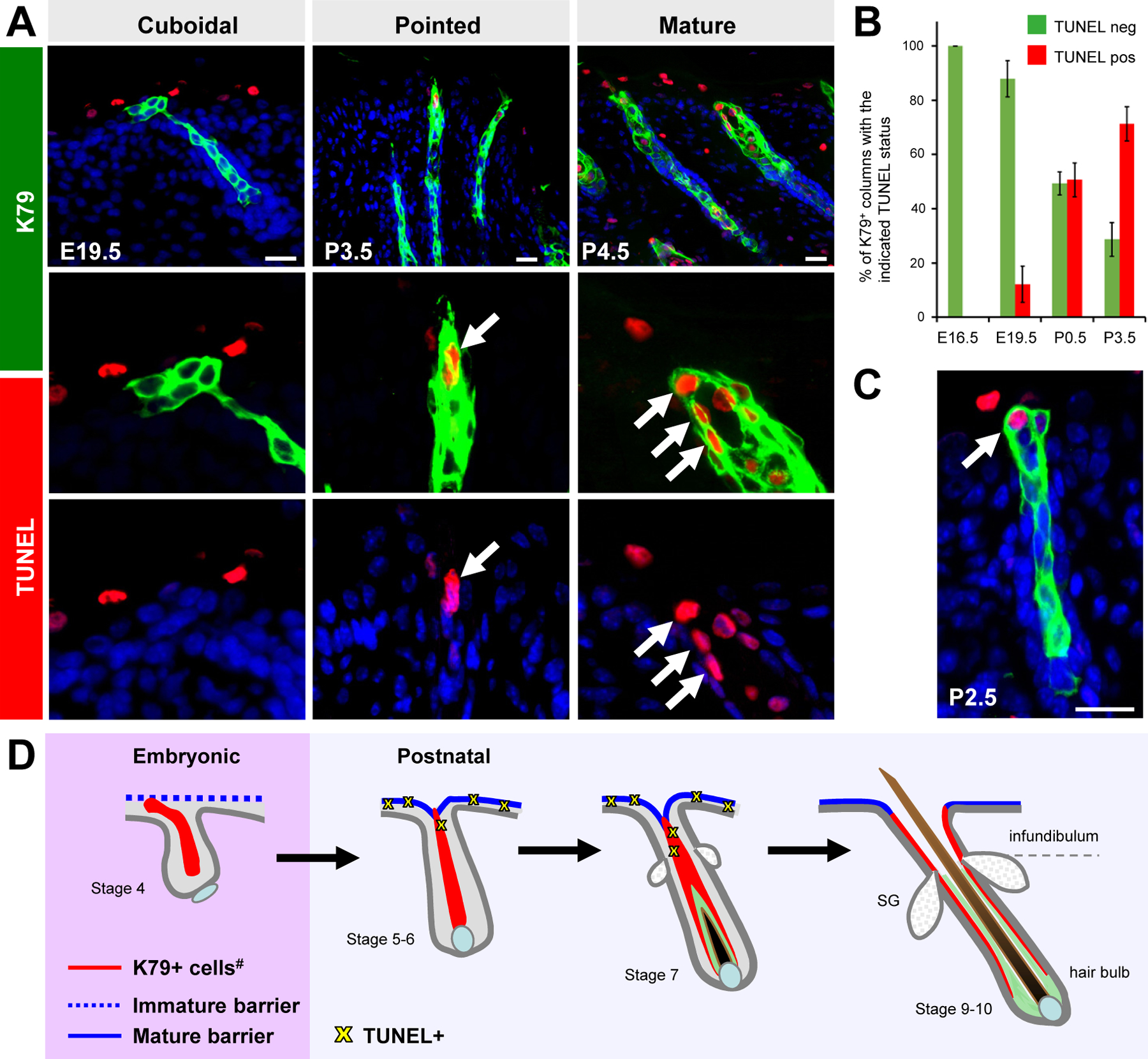
A. TUNEL+ cells (red) in postnatal K79+ cell columns (arrows). B. Quantitation of TUNEL+/K79+ cell columns. C. Rare cuboidal K79+ cell column with TUNEL+ tip at P2.5. D. Summary of findings. Over time, the tips of K79+ cell columns change shape, recede, lose E-cadherin, and undergo cell death prior to the opening of the hair canal. Note that the newborn acanthotic epidermis is also frequently TUNEL+. Beneath the SG, K79 is expressed in the companion layer, although its expression in this layer becomes reduced in the mature follicle (13, 14, 50). Light green, inner root sheath. #, K79 is also expressed in the SG and sebaceous duct (not highlighted here). Images not drawn to scale. Error bars, SD. Scale bars, 50 μm.
Conclusions & Perspectives
The hair follicle canal acts as a critical interface between the surface epidermis and the deeper pilosebaceous unit (17). Not only does it enable hair to emerge, but it also creates a passageway by which oily secretions from the SG, known as sebum, are released onto the skin’s surface. Sebum is thought to possess numerous functions, including acting as a moisturizer, an anti-oxidant, and a modulator of the skin’s microflora (18, 19). Indeed, certain skin-resident commensals preferentially colonize the upper hair follicle (20–22), which in turn may recruit immune cells such as regulatory T cells (23).
Early studies from different mammals have suggested at least two mechanisms by which the hair follicle opening is formed. In opossum and sheep, SG-derived cells have been reported to be pushed outwards (6, 7), whereas studies in mouse and human have suggested that keratinocytes within the epidermal spinous layer reorient themselves to form the hair canal (8, 9). Consistent with the first mechanism, we observed that K79+ cell columns arising from the hair follicle extend out into the epidermis; however, this occurs prior to SG specification. Somewhat intriguingly, K79 is also highly expressed in SGs (24), although we have been unable to find any other commonalities between K79+ cell columns and K79+ sebocytes thus far (data not shown). Consistent with the second mechanism, we found that the tips of K79+ columns can express some epidermal differentiation markers, and change shape and organization during hair canal formation; however, once again, these K79+ cells, while present in the epidermis, in fact arise from hair follicle progenitors (13). We should note that some discrepancies in these mechanisms may reflect species-specific differences.
Our observation that K79+ cells frequently undergo cell death postnatally aligns well with data from ultrastructural studies in human skin (11, 12) and mice (25). These studies have suggested that cells within the upper-most region of the hair canal, at the level of the epidermis, are removed following keratinization and desquamation. For cells within the nascent infundibulum, both keratinization-dependent (12) and -independent forms of cell death (11) have been proposed as mechanisms for creating a lumen. It is important to note that TUNEL+ cells in the developing hair follicle often do not exhibit typical morphological features of apoptosis, as previously noted by Magerl et al., (25), suggesting that K79+/TUNEL+ cells may be undergoing differentiation-induced cell death (26).
In Sox9-deficient mice, both SGs and mature hair shafts fail to form, yet hair follicle openings are still visible in post-natal skin, again arguing that these structures arise independently (27, 28). Nonetheless, it has been proposed that SG-derived proteases may enable the hair shaft to separate from the inner root sheath, as suggested by observations in asebia mice with hypoplastic SGs and in cathepsin L (CTSL) mutants (29, 30). In both cases, the hair shaft becomes encased by the inner root sheath, causing a blockage that impairs hair shaft eruption. Taken together, these studies suggest that SGs may play a pivotal role in hair emergence, subsequent to the formation of the hair canal.
Many questions still remain. In particular, it is unclear how different forms of cell death, including apoptosis, necrosis, autophagy and cornification, act in concert with cellular rearrangements to form the hair canal. In addition, the role of proteases in shaping this process requires further examination, especially given the prominent role of proteolysis in regulating cellular organization, differentiation, desquamation and survival (31–33). Finally, the cellular signals that trigger increased cell death in K79+ cells after birth remain to be identified. Answers to some of these questions may come from detailed analyses of mice that possess hair canal defects, including hairless (Hrhr) and rhino (Hrrh-J) mice (34); mutants lacking proteases such as MMP9, matriptase and CTSL (35–37); and mice treated with anti-PDGFR-α antibodies (38). The transcription factor Gata6, which is expressed in the upper hair follicle domain, has been reported to specify sebaceous duct identity (39, 40), and our own studies have shown that mice lacking Gata6 in the skin possess dilated hair canals (41). In contrast, K79-null mice do not manifest overt defects in hair canal formation or hair cycling (13), even though these mutants possess SGs that are structurally compromised (24).
In humans, mutations in matriptase have been linked to autosomal recessive ichthyosis with hypotrichosis (42). It remains to be seen whether the relative absence of hair in these patients is caused by defects in the hair canal and/or hair shaft, although matriptase-deficient newborn mice possess fully mature vibrissae that nonetheless fail to erupt (36). On the other hand, ingrown hairs seen in pseudofolliculitis barbae patients are likely caused by structural defects in the hair shaft (43, 44). Acne, the most common skin disorder, manifests as comedonal lesions that appear in the hair canal (45). We have previously reported that these comedones often lack K79 expression (14), but the causes of acne are complex and likely multi-factorial, and remain poorly understood. Notably, hidradenitis suppurativa is a painful skin condition associated with occlusion of the upper hair follicle, leading to the formation of cysts that often rupture and cause inflammation (46, 47). Several other cystic skin pathologies are also known to affect the infundibulum (48, 49). Altogether, these disorders indicate that even after the hair canal has formed during development, other cellular processes must continue to operate throughout adult life in order to properly maintain this critical domain of the hair follicle.
Questions Asked.
How is the hair follicle canal formed during development?
What cellular changes take place to convert Keratin 79+ cell columns into an open-ended structure?
Acknowledgements
S.Y.W. acknowledges the generous support of the NIH (R01AR065409); the American Cancer Society; the Leo Foundation; the Biological Sciences Scholars Program; and the Center for Organogenesis.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009: 10: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rognoni E, Watt FM. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol 2018: 28: 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paus R, Müller-Röver S, van der Veen D, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 1999: 113: 523–532. [DOI] [PubMed] [Google Scholar]
- 4.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 2005: 6: 328–340. [DOI] [PubMed] [Google Scholar]
- 5.Eckhart L, Lippens S, Tschachler E, et al. Cell death by cornification. Biochim Biophys Acta 2013: 1833: 3471–3480. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs HF A study of the development of the skin and hair of the Australian opossum, Trichosurus vulpecula. Proc Zool Soc Lond 1938: 108B: 611–648. [Google Scholar]
- 7.Wildman AB Coat and fibre development in some British sheep. Proc Zool Soc Lond 1932: 1: 257–285. [Google Scholar]
- 8.Hardy MH The development of mouse hair in vitro with some observations on pigmentation. J Anat 1949: 83: 364–384. [PMC free article] [PubMed] [Google Scholar]
- 9.Pinkus H Embryology of hair. New York: Academic Press: 1958. [Google Scholar]
- 10.Maximow AA, Bloom W. Textbook of histology. Philadelphia: W.B. Saunders Company: 1948. [Google Scholar]
- 11.Hashimoto K The ultrastructure of the skin of human embryos. Arch klin exp Derm 1970: 238: 333–345. [DOI] [PubMed] [Google Scholar]
- 12.Breathnach AS, Robins EJ. Ultrastructure of developing hair tract and hair canal in the human fetus. In: Orfanos CE, Montagna W, Stüttgen G, eds. Hair Research. Heidelberg: Springer, 1981: 12–17. [Google Scholar]
- 13.Mesler AL, Veniaminova NA, Lull MV, et al. Hair follicle terminal differentiation is orchestrated by distinct early and late matrix progenitors. Cell Rep 2017: 19: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veniaminova NA, Vagnozzi AN, Kopinke D, et al. Keratin 79 identifies a novel population of migratory epithelial cells that initiates hair canal morphogenesis and regeneration. Development 2013: 140: 4870–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena N, Mok KW, Rendl M. An updated classification of hair follicle morphogenesis. Exp Dermatol 2019: 28: 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinde E, Haslam IS, Schneider MR, et al. A practical guide for the study of human and murine sebaceous glands in situ. Exp Dermatol 2013: 22: 631–637. [DOI] [PubMed] [Google Scholar]
- 17.Schneider MR, Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res 2014: 358: 697–704. [DOI] [PubMed] [Google Scholar]
- 18.Niemann C, Horsley V. Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol 2012: 23: 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi T, Voisin B, Kim DY, et al. Homeostatic Control of Sebaceous Glands by Innate Lymphoid Cells Regulates Commensal Bacteria Equilibrium. Cell 2019: 176: 982–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange-Asschenfeldt B, Marenbach D, Lang C, et al. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol Physiol 2011: 24: 305–311. [DOI] [PubMed] [Google Scholar]
- 21.Montes LF, Wilborn WH. Anatomical location of normal skin flora. Arch Dermatol 1970: 101: 145–159. [PubMed] [Google Scholar]
- 22.Alexeyev OA, Lundskog B, Ganceviciene R, et al. Pattern of tissue invasion by Propionibacterium acnes in acne vulgaris. J Dermatol Sci 2012: 67: 63–66. [DOI] [PubMed] [Google Scholar]
- 23.Scharschmidt TC, Vasquez KS, Pauli ML, et al. Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe 2017: 21: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veniaminova NA, Grachtchouk M, Doane OJ, et al. Niche-specific factors dynamically regulate sebaceous gland stem cells in the skin. Dev Cell 2019: 51: 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magerl M, Tobin DJ, Müller-Rover S, et al. Patterns of proliferation and apoptosis during murine hair follicle morphogenesis. J Invest Dermatol 2001: 116: 947–955. [DOI] [PubMed] [Google Scholar]
- 26.Lippens S, Denecker G, Ovaere P, et al. Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ 2005: 12: 1497–1508. [DOI] [PubMed] [Google Scholar]
- 27.Nowak JA, Polak L, Pasolli HA, et al. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 2008: 3: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal VP, Chaboissier MC, Lützkendorf S, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol 2005: 15: 1340–1351. [DOI] [PubMed] [Google Scholar]
- 29.Tobin DJ, Foitzik K, Reinheckel T, et al. The lysosomal protease cathepsin L is an important regulator of keratinocyte and melanocyte differentiation during hair follicle morphogenesis and cycling. Am J Pathol 2002: 160: 1807–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundberg JP, Boggess D, Sundberg BA, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol 2000: 156: 2067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turk B, Stoka V. Protease signalling in cell death: caspases versus cysteine cathepsins. FEBS Lett 2007: 581: 2761–2767. [DOI] [PubMed] [Google Scholar]
- 32.Zeeuwen PL, Cheng T, Schalkwijk J. The Biology of Cystatin M/E and its Cognate Target Proteases. J Invest Dermatol 2009: 129: 1327–1338. [DOI] [PubMed] [Google Scholar]
- 33.Furio L, Pampalakis G, Michael IP, et al. KLK5 inactivation reverses cutaneous hallmarks of Netherton Syndrome. PLoS Genet 2015: 11: e1005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann SJ Hair loss and cyst formation in hairless and rhino mutant mice. Anat Rec 1971: 170: 485–499. [DOI] [PubMed] [Google Scholar]
- 35.Sharov AA, Schroeder M, Sharova TY, et al. Matrix metalloproteinase-9 is involved in the regulation of hair canal formation. J Invest Dermatol 2011: 131: 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.List K, Haudenschild CC, Szabo R, et al. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene 2002: 21: 3765–3779. [DOI] [PubMed] [Google Scholar]
- 37.Roth W, Deussing J, Botchkarev VA, et al. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J 2000: 14: 2075–2086. [DOI] [PubMed] [Google Scholar]
- 38.Takakura N, Yoshida H, Kunisada T, et al. Involvement of platelet-derived growth factor receptor-alpha in hair canal formation. J Invest Dermatol 1996: 107: 770–777. [DOI] [PubMed] [Google Scholar]
- 39.Donati G, Rognoni E, Hiratsuka T, et al. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat Cell Biol 2017: 19: 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oulès B, Rognoni E, Hoste E, et al. Mutant Lef1 controls Gata6 in sebaceous gland development and cancer. EMBO J 2019: 38: e100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson JB, Vagnozzi AN, Veniaminova NA, et al. Loss of Gata6 causes dilation of the hair follicle canal and sebaceous duct. Exp Dermatol 2019: 28: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, et al. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet 2007: 80: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol 2004: 122: 652–657. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Jaeger K, Den Z, et al. Mice expressing a mutant Krt75 (K6hf) allele develop hair and nail defects resembling pachyonychia congenita. J Invest Dermatol 2008: 128: 270–279. [DOI] [PubMed] [Google Scholar]
- 45.Guy R, Kealey T. Modelling the infundibulum in acne. Dermatology 1998: 196: 32–37. [DOI] [PubMed] [Google Scholar]
- 46.Janse IC, Blok JL, Diercks GFH, et al. Hidradenitis suppurativa: a disease of infundibular epidermis rather than pilosebaceous units? Br J Dermatol 2017: 176: 1659–1661. [DOI] [PubMed] [Google Scholar]
- 47.Negus D, Ahn C, Huang W. An update on the pathogenesis of hidradenitis suppurativa: implications for therapy. Expert Rev Clin Immunol 2018: 14: 275–283. [DOI] [PubMed] [Google Scholar]
- 48.Thomas VD, Snavely NR, Lee KK, et al. Cysts of epidermal origin. In: Goldsmith LA, Katz SI, Gilchrest BA, et al. , eds. Fitzpatrick’s dermatology in general medicine. Chicago: McGraw Hill Medical, 2012: 1333–1336. [Google Scholar]
- 49.Gruber R, Sugarman JL, Crumrine D, et al. Sebaceous gland, hair shaft, and epidermal barrier abnormalities in keratosis pilaris with and without filaggrin deficiency. Am J Pathol 2015: 185: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joost S, Annusver K, Jacob T, et al. The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 2020: 26: 441–457. [DOI] [PubMed] [Google Scholar]


