Abstract
Chronic inflammation due to inappropriate immune cell activation can have significant effects on a variety of organ systems, reducing lifespan and quality of life. As such, highly targeted control of immune cell activation is a major therapeutic goal. Vagus nerve stimulation (VNS) has emerged as a therapeutic modality that exploits neuro-immune communication to reduce immune cell activation and consequently inflammation. Although vagal efferent fibers were originally identified as the primary driver of anti-inflammatory actions, the vagus nerve in most species of animals is predominantly comprised of afferent fibers. Stimulation of vagal afferent fibers can also reduce inflammation; it is however uncertain how these two neuro-immune circuits diverge. Here we show that afferent VNS induces a distinct mechanism from efferent VNS, ameliorating LPS-induced inflammation independent from T-cell derived acetylcholine (ACh) that is required by efferent VNS. Using a β2-adrenergic receptor antagonist (β2-AR), we find that immune regulation induced by intact, afferent, or efferent VNS occur in a β2-AR-dependent manner. Together, our findings indicate that intact VNS activates at least two distinct neuroimmune circuits each with unique mechanisms of action. Selective targeting of either the vagal efferent or afferent fibers may provide more personalized, robust, and effective control over inappropriate immune responses.
Keywords: Inflammation, Neuroimmunology, vagal afferent, vagal nerve stimulation, peripheral nervous system, Vagus nerve
Introduction
The nervous and immune systems are highly integrated with neuronal signaling capable of modulating numerous immunological outcomes. Neuronal control of immune function can occur in response to physiological or targeted electrical stimulation of the nerves that comprise the circuit (Borovikova et al., 2000; Martelli et al., 2014b; Reardon et al., 2018; Murray et al., 2019; Ramirez et al., 2020b). Various discrete neuroimmune circuits are now appreciated to regulate diverse functions ranging from host responses to pathogens (Pinho-Ribeiro et al., 2018; Lai et al., 2020; Lankadeva et al., 2020; Ramirez et al., 2020a; Ramirez et al., 2020b), to the inhibition of overexuberant immune responses (Borovikova et al., 2000; Koopman et al., 2016).
Induction of vagus nerve activity has been long demonstrated to reduce the severity of experimental septic shock induced by LPS administration (Borovikova et al., 2000; Luyer et al., 2005; Wang et al., 2016; Somann et al., 2019). This proposed “Cholinergic anti-inflammatory pathway” (CAIP) relies on efferent vagal nerve fiber activation to induce activation of sympathetic neurons within the superior mesenteric/celiac ganglion, that project into the spleen and mesenteric lymph nodes (MLN) (Borovikova et al., 2000; Rosas-Ballina et al., 2008; Murray et al., 2019; Kressel et al., 2020). The CAIP hypothesis proposes that in these secondary lymphoid organs, NE released by these nerve fibers binds to the β2-adrenergic receptor (β2AR) on a unique subset of T cells that express the enzyme choline acetyltransferase (ChAT). Release of ACh from CD4+ ChAT+ T cells in turn activates the nicotinic acetylcholine α7 receptor (α7 nAChR) on macrophages to ultimately reduce macrophage activation and inhibit TNFα production (Wang et al., 2003; Rosas-Ballina et al., 2011; Reardon et al., 2018). As the vagus nerve is composed of approximately ~80% afferent and 20% efferent fibers, depending on the animal species (Prechtl & Powley, 1990), it should not be surprising that the proposed circuitry and mechanisms of action are contentious (Martelli et al., 2014a). Alternative pathways have demonstrated inhibition of inflammation in the spleen through processes that are independent of CAIP. Optogenetic stimulation of the C1 region in the brain activated both vagal efferents and sympathetic neurons, although only the sympathetic pathway reduced ischemia reperfusion injury of the kidney independent of the vagus nerve (Abe et al., 2017). The direct connection and ability of vagal efferents to activate sympathetic neurons projecting to the spleen have also been called into question, with electrical vagal efferent stimulation in rats failing to activate the splenic nerve (Bratton et al., 2012). Together these studies suggest that the anti-inflammatory effect of intact VNS could be entirely derived from afferent vagal fiber activation. Discrete pathways of immune inhibition elicited by afferent or efferent vagal nerve fibers were further demonstrated with intact VNS preventing LPS-induced inflammation in an α7nAChR independent manner, while efferent stimulation was without effect in α7nAChR KO mice (Vida et al., 2011). It is unclear if immune regulation induced by intact, afferent, or efferent selective VNS use the same pathways and cellular components or are discrete neuro-immune circuits that converge on the spleen.
Here we describe that afferent and efferent vagal nerve stimulation (VNS) activate discrete neuroimmune circuits that inhibit inflammation through unique mechanisms of action. Selective electrical stimulation of the afferent or efferent vagus nerve significantly reduced LPS-induced proinflammatory cytokine production in the serum, spleen, and MLN. Using mice with conditional ablation of ChAT in T-cells, we show that immune regulation induced by efferent but not afferent VNS requires T-cell derived ACh. As the afferent pathway was previously demonstrated to be dependent on activation of sympathetic innervation, we assessed the role of β2AR. Using a highly selective antagonist of this receptor we demonstrate that intact, afferent and efferent VNS requires signaling through the β2AR. Together these results provide strong evidence for at least two discrete neuroimmune circuits that serve to downregulate immune cell function, each using divergent mechanisms of control.
Although there is a great deal of excitement at the potential to use VNS clinically to mediate immune regulation in various chronic inflammatory diseases including rheumatoid arthritis (Koopman et al., 2016) and inflammatory bowel disease (Bonaz et al., 2016; Sinniger et al., 2020), our data indicate that neural stimulation devices and therapeutics must be carefully designed as dramatically different mechanisms of action could be induced. In addition, these findings suggest that novel technologies could be developed to deliver a personalized therapeutic regime for immunopathologies.
METHODS
Mice
Male and female C57BL/6, LCK.cre, and ChATf/f mice were originally purchased from the Jackson Laboratories (Bar Harbor ME) and used to establish a breeding colony. Mice with conditional ablation of ChAT in T-cells (LCK.Cre+ ChATf/f) have been characterized previously (Ramirez et al., 2019). Adult mice aged 8–10 weeks old were used in these studies. For LPS-induced TNFα production with VNS, mice were first anesthetized using 1–2% isoflurane and the right cervical vagus exposed. For intact VNS, the exposed vagus nerve was placed directly on a bipolar hook electrode (FHC, Bowdoin, ME). To stimulate afferent vagal neurons, the cervical vagus nerve was transected, and a hook electrode was placed above the transection point. To stimulate efferent vagal neurons, the cervical vagus nerve was again transected, with the hook electrode placed below the transection point. Mice were then unstimulated (sham condition) or subjected to 20 minutes of VNS (5 V, 2 ms, monophasic square wave, 5 Hz) using a Grass stimulator S88 with a stimulus isolation module (Murray et al., 2019). LPS (Ultrapure LPS from E. coli 05:B5, Invivogen, 4 mg/Kg) was administered i.v. (retroorbital) after the first 10 minutes of stimulation and 1 hour post LPS challenge, serum was collected via a cardiac puncture from deeply anesthetized mice, and euthanasia by cervical dislocation, with the spleen and MLNs were placed in Trizol (Invitrogen, Carlsbad, CA).
For experiments assessing the role of the β2AR, mice were anesthetized using 1–2% isoflurane, and the selective antagonist ICI 118 551 (1 mg/Kg) or PBS as a vehicle was administered by i.v. injection 5 minutes prior to VNS or sham stimulation. The VNS parameters outlined above were followed. All animals had ad libitum access to food and water, and all experimental protocols and procedures were approved by the UC Davis Institutional Animal Care and Use Committee, protocol number 21873.
Quantitative PCR
Gene expression analysis was performed by quantitative real-time PCR (qRT-PCR). RNA was extracted from the spleen, MLN and inguinal LN using 5 mm stainless steel beads in a bead beater (Qiagen), and homogenized in Trizol (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. An iSCRIPT reverse transcriptase kit (Bio-Rad, Hercules, CA) was used to synthesize cDNA. qRT-PCR was then performed for the following targets using the indicated primer pairs from Primerbank (Spandidos et al., 2010): Actb forward 5’-GGCTGTATTCCCCTCCATCG-3’ reverse 5’-CCAGTTGGTAACAATGCCATGT-3’ Il6 forward 5’-TAGTCCTTCCTACCCCAATTTCC-3’ reverse 5’-TTGGTCCTTAGCCACTCCTTC-3’ Tnf forward 5’-CCTCACACTCAGATCATCTTCT-3’, reverse 5’-GCTACGACGTGGGCTACAG-3’. Amplification and data acquisition were conducted using a QuantStudio6 (Thermo Fisher Scientific, Waltham, MA). Gene expression was assessed using Actb as the reference gene for each sample by the 2(-ΔΔCT) method.
ELISA
A standard sandwich ELISA (Thermo Fisher Scientific, Waltham, MA) was used to determine serum concentrations of TNFα. Following manufacturer instructions, 96 well maxisorp microtiter plates (NUNC, Thermofisher scientific) coated with capture antibody were incubated with samples diluted in assay diluent and washed extensively with PBS-Tween20 before addition of the biotin conjugated capture antibody. After incubation and further washing, streptavidin HRP was added, and incubated again to allow binding, followed by a final wash to remove unbound enzyme. Plates were developed using TMB substrate with the reaction stopped after 15 minutes by addition of 1 N H2SO4. Optical density at 450 nm and 570 nm were determined using a plate reader, and sample concentrations were determined by regression using a standard curve.
Results
Afferent VNS reduces systemic pro-inflammatory cytokine release
To distinguish between the effects of afferent and efferent VNS, the vagus nerve was transected to allow for selective stimulation of either ending. Intact, efferent, and afferent VNS or a sham stimulation were performed for 20 minutes, with an administration of LPS or vehicle after the first 10 minutes (Figure 1A). Serum TNFα one-hour post-LPS administration was significantly reduced in mice receiving intact VNS, efferent VNS, or afferent VNS (Figure 1B). As the spleen and MLNs are innervated by the sympathetic nervous system, and these nerves are presumed to be activated through the CAIP, we assessed proinflammatory cytokine gene expression in these tissues. Transcription of Tnf was significantly reduced in both the spleen and MLN (Figure 1C–D) in mice that received LPS with either intact, efferent, or afferent VNS, compared to LPS (sham VNS). Expression of IL-6 was also significantly reduced within the spleen in mice that received intact VNS but not afferent or efferent VNS (Figure 1E). In contrast, no inhibition of IL-6 was observed in the MLN (Figure 1F). Together our data suggests that afferent VNS can suppress LPS-induced pro-inflammatory cytokine production as effectively as efferent VNS, and that targeting the vagal sensory or motor fibers may provide comparable protection compared to stimulation of the intact vagus nerve.
Figure 1. Afferent and efferent VNS reduces pro-inflammatory cytokine release.
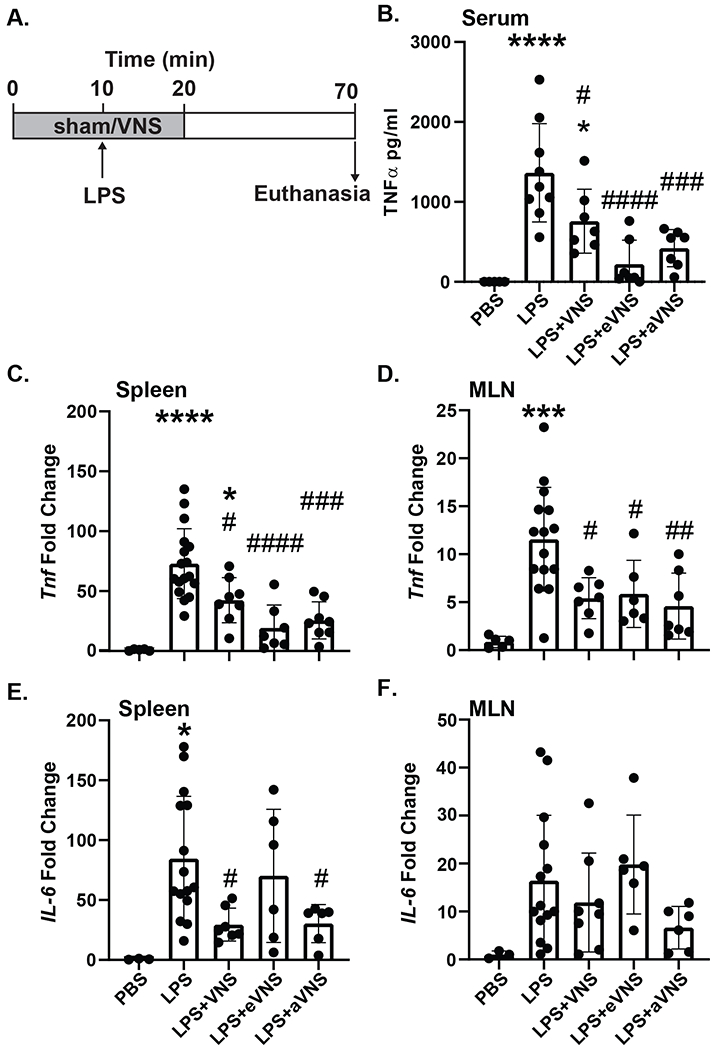
Mice were subjected to 20 min of sham (no stimulation), intact, afferent, or efferent VNS, with LPS or sterile PBS injection (i.v.) after the first 10 min (A). After 60 minutes post-LPS injection, mice were euthanized and TNFα was assessed in serum by ELISA (LPS ****P<0.0001; LPS + VNS *P=0.0236, # P=0.0411; LPS + eVNS ####P<0.0001; LPS + aVNS ###P=0.0006) (B). Expression of Tnf and Il6 were determined in these mice by qPCR in the spleen (Tnf: LPS ****P<0.0001; LPS + VNS *P=0.0178, #P=0.0224; LPS + eVNS #### P<0.0001; LPS + aVNS ###P=0.0001) (Il6: LPS *P=0.0128; LPS + VNS #P=0.0238; LPS + aVNS #P=0.0389) (C, E), and MLN (Tnf: LPS ***P=0.0001; LPS + VNS #P=0.0168; LPS + eVNS #P=0.0453; LPS + aVNS ##P=0.0052) (D, F). n=6–16 per group; * compared with PBS, # compared with LPS. Data are presented mean ± SD, one-way ANOVA followed by Tukey’s test.
Afferent VNS is independent of ACh-producing ChAT+ T-cells
The classical CAIP is thought to be dependent on ACh release from ChAT+ T-cells, however it is unknown if this unique set of T-cells are required for the anti-inflammatory effects induced by afferent VNS. To determine if afferent VNS mediates inflammation through a similar mechanism, we used ChAT-T-cell conditional knock out (LCK.Cre+ ChATf/f, “T-cell cKO”) mice. In T-cell cKO mice, the reduction of LPS-induced serum TNFα production by efferent VNS was eliminated, with no statistical difference between mice that received LPS (sham stimulation) (Figure 2B). Afferent VNS still significantly reduced LPS-induced TNFα production, implying a distinct mechanism of action from the CAIP. This loss of protection afforded by efferent VNS in T-cell cKO mice was in stark contrast to the inhibition of TNF production observed in WT mice (Figure 2B). In assessing tissue specific Tnf and Il6 mRNA transcripts, only intact and afferent VNS in T-cell cKO mice resulted in suppression of LPS-indued Tnf in the spleen and MLN (Figure 2C–D). Once again, suppression of LPS-induced inflammation as evidenced by reduced Tnf transcription, was present in WT mice receiving intact, afferent, and efferent VNS. Transcription of Il6 was also significantly reduced in the spleen and MLN in the intact, efferent, and afferent VNS groups in WT (LCK.Cre- ChATf/f) mice. Reduced Il6 expression induced by efferent VNS however was eliminated in the spleen and MLN in T-cell cKO mice, while afferent VNS remained effective, implying that each pathway has distinct mechanisms of action (Figure 2E–F).
Figure 2. Afferent VNS does not require ACh from ChAT+ T cells.
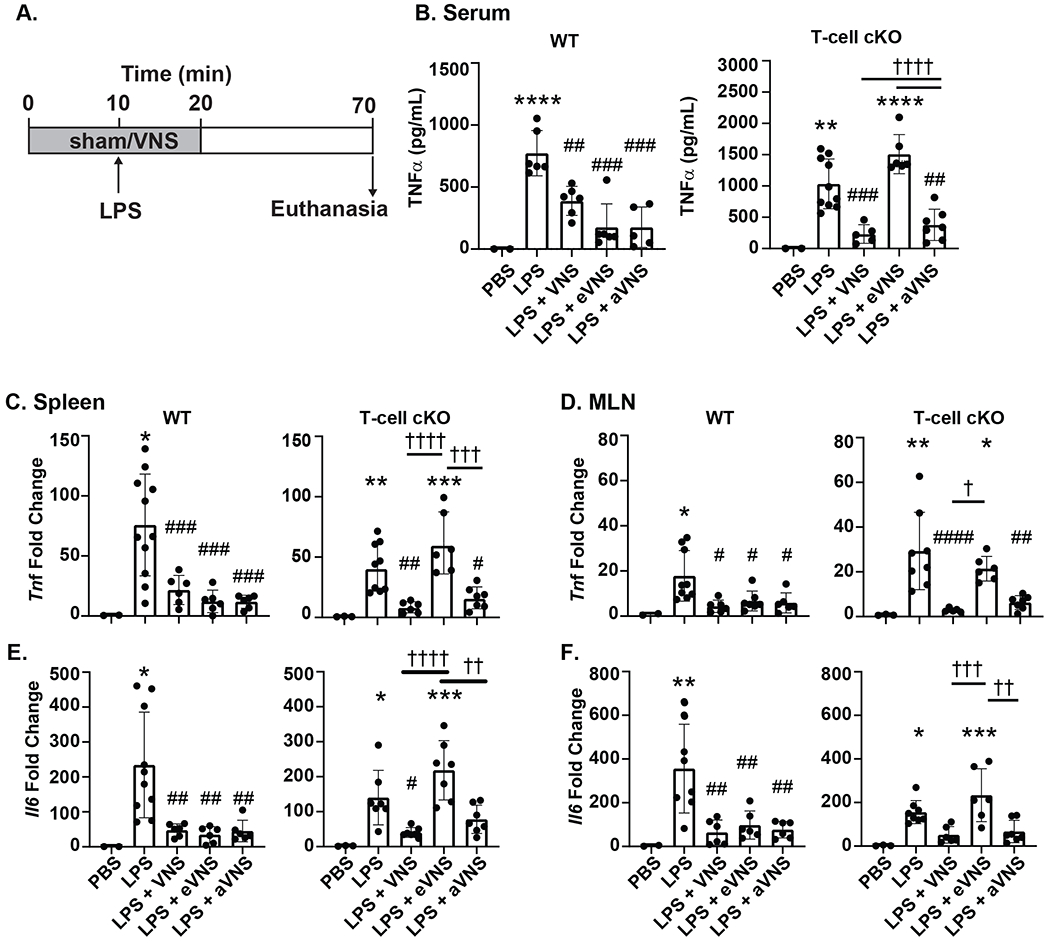
The requirement of T-cell derived ChAT was assessed by subjecting WT (LCK.Cre- ChATf/f) and ChAT T-cell cKO (LCK.Cre+ ChATf/f) mice to 20 minutes of sham (no stimulation), intact, afferent or efferent VNS with an injection of LPS (i.v.) or sterile PBS after the first 10 minutes (A). LPS-induced inflammation was assessed by serum TNFα (B) (WT: LPS ****P<0.0001; LPS + VNS ##P=0.0042; LPS + eVNS ###P=0.0001; LPS + aVNS ###P=0.0001) (T-cell cKO: LPS **P=0.0018; LPS + VNS ###P=0.0006, †††† P<0.0001; LPS + eVNS ****P<0.0001, †††† P<0.0001; LPS + aVNS ##P=0.0019) and expression of Tnf (C) and Il6 (E) in the spleen (Tnf WT: LPS *P=0.0107; LPS + VNS ##P=0.0048; LPS + eVNS ###P=0.0008; LPS + aVNS ###P=0.0008; Tnf T-cell cKO: LPS **P=0.0077; LPS + VNS ##P=0.006, †††† P<0.0001; LPS + eVNS ***P=0.0002, ††† P=0.0003; LPS + aVNS #P=0.0366) (Il6 WT: LPS *P=0.245; LPS + VNS ##P=0.0053; LPS + eVNS ##P=0.0027; LPS + aVNS ##P=0.0046; Il6 T-cell cKO: LPS *P=0.0174; LPS + VNS #P=0.0297,††††P<0.0001; LPS + eVNS ***P=0.0001, ††P=0.0013) and MLN (D, F) (Tnf WT: LPS *P=0.0368; LPS + VNS #P=0.0111; LPS + eVNS #P=0.0341; LPS + aVNS #P=0.0285; Tnf T-cell cKO: LPS **P=0.002; LPS+VNS #### P<0.0001; † P=0.0336; LPS + eVNS *P=0.0459; LPS + aVNS ##P=0.0011) (Il6 WT: LPS **P=0.009; LPS + VNS ##P=0.0014; LPS + eVNS ##P=0.0048; LPS + aVNS ##P=0.0022; Il6 T-cell cKO: LPS *P=0.021; LPS + VNS †††P=0.0009, LPS + eVNS ***P=0.0006, ††P=0.0015). n=5–10 per group; * compared with PBS, # compared with LPS. † significance as indicated. Data are presented mean ± SD, one-way ANOVA followed by Tukey’s test.
Afferent VNS is dependent on the β2AR
The β2AR on splenic macrophages is generally thought to be anti-inflammatory and a component of the sympathetic anti-inflammatory pathway (Bellinger & Lorton, 2014). To determine whether afferent VNS represses LPS-induced inflammatory cytokine release through the β2AR, we administered the β2AR antagonist ICI 118 551 and performed a similar series of experiments. Mice that received the β2AR antagonist and intact, afferent, or efferent VNS did not have reduced LPS-induced TNFα (Figure 3B). In line with the serum results, Tnf (Figure 3C&D) and Il6 transcripts (Figure 3E&F) from the spleen and MLN showed no difference between the LPS (sham VNS) and the experimental VNS groups. These data suggest that the anti-inflammatory reflex mediated by afferent and intact VNS are both dependent on β2AR signaling.
Figure 3. Afferent VNS is dependent on β2AR.
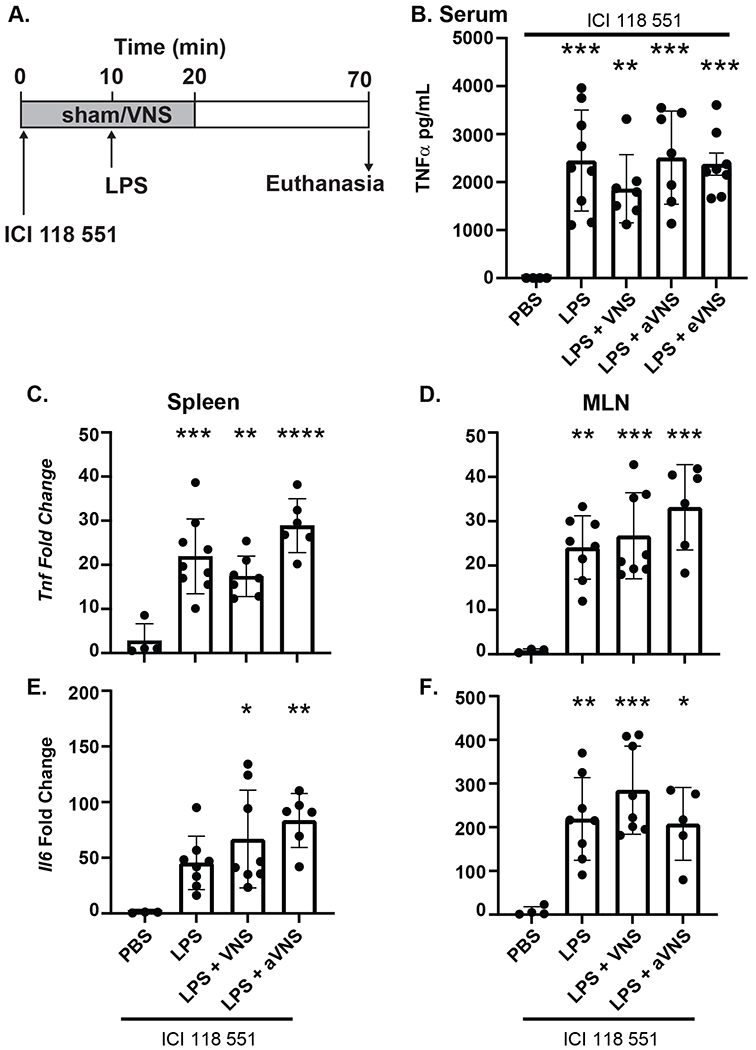
Mice were injected (i.v.) with β2AR antagonist ICI 118 551 and subjected to 20 minutes of sham (no stimulation), intact, afferent, or efferent VNS with LPS (i.v.) or sterile PBS injected after the first 10 min (A). TNFα in serum collected from mice treated with ICI 118 551 ± LPS with sham, intact VNS (VNS) afferent VNS (aVNS) or efferent VNS (eVNS) was assessed by ELISA (B) (LPS ***P=0.0003; LPS + VNS **P=0.0094; LPS + aVNS ***P=0.0003; LPS + eVNS ***P=0.0005). Expression of Tnf and Il6 mRNA expression following LPS challenge in mice receiving intact or afferent VNS in the spleen (C, E) (Tnf: LPS ***P=0.0004; LPS + VNS **P=0.0083; LPS + aVNS ****P<0.0001; Il6: LPS+VNS *P=0.0249; LPS + aVNS **P=0.0061) and MLN (D, F) (Tnf: LPS **P=0.0027; LPS + VNS ***P=0.0009; LPS + aVNS ***P=0.0001; Il6: LPS **P=0.004; LPS + VNS ***P=0.0002; LPS + aVNS *P=0.0134) was determined by qPCR. n=5–7 per group; * compared with PBS treatment. Data are presented mean ± SD, one-way ANOVA followed by Tukey’s post-hoc test.
Discussion
Although VNS has long been described to inhibit aberrant immune activation in a variety of experimental and clinical immunopathologies, there remains little consensus on the neuro-immune circuitry and mechanisms of action. These disputes seem to arise in part due to a lack of neural tracing and anatomical studies in mice, and perhaps more importantly the ability of VNS to activate both vagal efferent and afferent pathways. Consequently, at least two discrete models have emerged positing that VNS drives activation of efferent fibers, and inhibition of macrophage activation by T-cell derived ACh (Rosas-Ballina et al., 2008; Rosas-Ballina et al., 2011), and a model whereby afferent activation triggers sympathetic outflow instead (Vida et al., 2011; Komegae et al., 2018). With these disparities in mind, our studies were designed to ascertain the neuroimmune circuitry of afferent vs efferent VNS, in the control of immune activation during a model of sepsis.
In accordance with both models, our results demonstrate that stimulation of afferent and efferent VNS significantly reduces immune cell activation as evidenced by proinflammatory cytokine production. In dissecting the components of this vagal-spleen neuroimmune circuitry we identified that while T-cell derived ACh is a critical component of vagal efferent stimulation, it is not required to mediate the immune inhibitory effects of afferent VNS. Efferent VNS effectively suppressed LPS-induced pro-inflammatory cytokine production in wildtype mice, however this effect was lost in mice with conditional knockout of ChAT in T-cells. These data are in keeping with the literature and extend these observations to confirm the importance of these unique T-cells in a vagal efferent neuro-immune circuit (Rosas-Ballina et al., 2011). Critically, immune regulation afforded by afferent VNS remained intact in ChAT T-cell conditional knockout mice as indicated by suppressed pro-inflammatory cytokines in the serum, spleen, and MLN. These data suggest that at least two discrete immune regulatory pathways converge in these tissues: one elicited by efferent VNS that is dependent on T-cell derived ACh, and one mediated by afferent VNS that is independent of ChAT T-cells. These findings are in agreement with the literature indicating suppression of immune activation can occur through sympathetic innervation either elicited through local stimulation of sympathetic neurons in the SMG/CG complex (Murray et al., 2019), the splenic nerve (Buijs et al., 2008; Rosas-Ballina et al., 2008), or central activation (Abe et al., 2017). We have summarized these findings as a proposed schematic model in Figure 4.
Figure 4. Afferent VNS vs efferent VNS schematic.
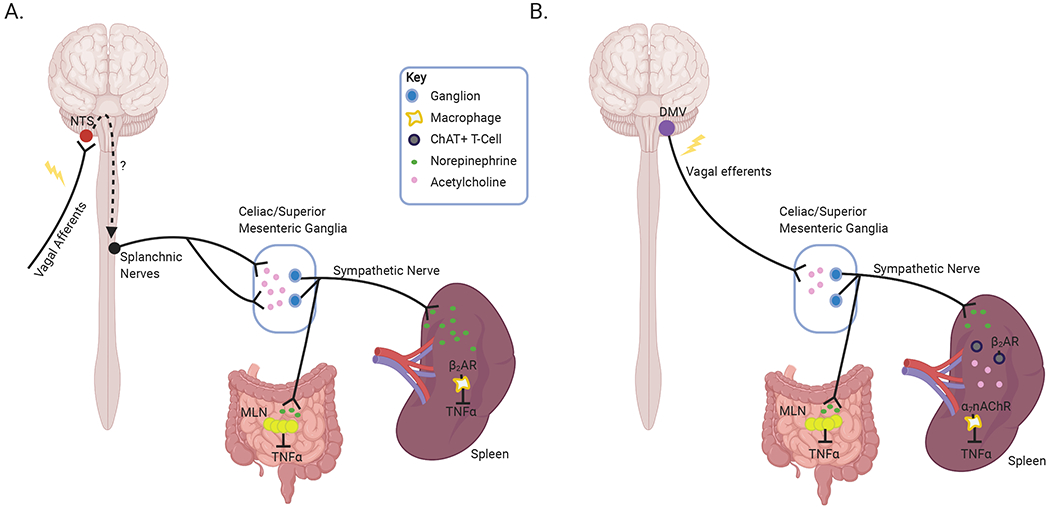
Proposed mechanisms of action elicited by afferent (A) and efferent (B) VNS based on our reported findings and current literature. Afferent VNS induces activation of sympathetic neurons, in the celiac/superior mesenteric plexus. These sympathetic nerves innervate the spleen and MLN, and their activation causes NE released that can act on β2AR on immune cells, such as macrophages, reducing activation and inhibition of TNFα release (A). In contrast, activation of vagal efferent neurons induces sympathetic innervation originating in the celiac/superior mesenteric ganglia, to release NE release in the spleen and MLN. NE in these secondary lymphoid organs elicits Ach release from ChAT+ T-cells in a β2AR dependent manner, to inhibit TNFα production (Created with BioRender.com).
As both the CAIP and sympathetic pathways are proposed to require NE-induced activation of β2AR, we assessed if the immune suppression from afferent VNS occurs in a β2AR dependent manner. Our experiments using a highly selective β2AR antagonist showed loss of immune suppression with intact, afferent or efferent VNS, confirming that stimulation elicited NE release and consequently β2AR activation is a key mechanism of these pathways. In accordance with previous in vitro (Rosas-Ballina et al., 2011) studies where NE stimulated ACh release from T-cells, and in vivo studies where efferent VNS acted in a β2AR dependent manner (Vida et al., 2011), our results demonstrate that immune inhibition induced by intact and efferent VNS requires β2AR signaling in vivo. These data would support the contention that efferent VNS can induce the release of NE from sympathetic neurons and consequently ACh from ChAT+ T-cells. Additional experiments using tools such as conditional ablation of β2AR restricted to ChAT+ cells are however required to provide additional insight and formal proof of this pathway in vivo. Such studies are clearly complicated by the ability of T-cells to transiently express ChAT (Reardon et al., 2013), and the lack of commercially available β2AR cKO mice. With respect to the afferent VNS pathway, at this point it is uncertain if signaling elicited through β2AR acts directly on immune cells or indirectly on another cell population to exert an anti-inflammatory effect. In part this is due to the well described role of β2AR expressed by numerous immune and non-immune cells that can exert changes in immune outcomes (Elenkov et al., 2000).
Vagal afferent fibers terminate in the nucleus tractus solitarius (NTS), an area that serves as the primary reflex center of the autonomic nervous system and relays information from the periphery to various brain regions, including the ventrolateral medulla. Highlighting the anti-inflammatory capabilities of this center, optogenetic activation of C1 neurons in the RVLM reduces inflammation in a mouse model of kidney ischemia-reperfusion injury (Abe et al., 2017). As the RVLM sends multiple projections through the spinal cord to execute sympathetic function, the splanchnic nerves are most likely a part of the afferent VNS circuitry. This notion is supported by previous studies that show activation of vagal afferent neurons can increase splanchnic nerve activity and promote anti-inflammatory actions (Martelli et al., 2014b). Our results would therefore demonstrate that the CAIP and the sympathetic immune regulatory pathway use discrete components and converge on secondary lymphoid organs such as the spleen and MLN. Although we previously identified the sympathetic innervation of the MLN and spleen that originate in the SMG/CG complex can be activated by intact VNS to inhibit immune responses, it seems that based on the requirement for ChAT+ T-cells in the efferent but not afferent pathway that these ganglia may not be the point of convergence. Mapping of both the afferent and efferent VNS neuro-immune circuit at higher resolution could usher in further refinements or the potential for organ specific immune regulation. Such targeted applications could be delivered using next-generation technologies such as adeno-associated vectors to deliver optogenetic proteins, new microelectrodes, and nanotechnologies (All et al., 2019; Wu et al., 2019).
Electrical VNS of either the left or the right vagus nerve can block LPS- induced immune activation, despite clear differences between the organs innervated by each of these branches (Bernik et al., 2002). Despite these collected works, there is a clear need for continued mapping studies to be performed to clearly identify the entire neuroimmune circuit elicited by right and left VNS. Clinically, VNS is performed on the left vagus to lessen the effect of VNS on cardiac function as this branch innervates the atrioventricular node of the heart while the right vagus innervates the sinoatrial node (Bonaz et al., 2017). Despite this presumption, pre-clinical studies have shown limited differences in cardiac function during left vs right VNS (Yamakawa et al., 2014). Clinical use of VNS in the treatment of immunopathologies has progressed despite the uncertainties in the mechanisms of action and ability to selectively target desired organ systems (Bonaz et al., 2016; Koopman et al., 2016; Addorisio et al., 2019; Sinniger et al., 2020). While reduction of off-target effects has been achieved with frequency dependent activation of distinct vagal fibers (Bonaz et al., 2017) or kilohertz frequency blockade (Patel et al., 2017), the standard therapeutic VNS parameters activate both vagal efferent and afferent pathways (Cao et al., 2017). Our studies would suggest that while activation of both pathways could elicit two discrete mechanisms of immune regulation in previously healthy animals, such a modality could be detrimental to patients with longstanding inflammation and possible increased expression of adrenergic receptors (Straub & Härle, 2005; Pongratz & Straub, 2012). The existence of multiple neuro-immune circuits could also complicate recent hypotheses that activation of vagal efferents to restore normal organ function could best be achieved through vagal afferent stimulation. Preclinical studies using VNS in gastroparesis have identified vagal afferent stimulation as more effective at driving gastric motility compared to direct efferent activation alone (Ward et al., 2020). Based on our data, in the context of immune regulation it would seem that an approach to use vagal afferent activation to drive vagal efferent signaling would likely elicit unique pathways compared to those activated by direct efferent stimulation. This would further suggest that although VNS is a promising therapeutic in the treatment of inflammatory diseases, neuro-immune circuits in each organ system should be carefully evaluated in preclinical studies prior to advancing to clinic.
Key Points:
It has previously been shown that afferent and efferent vagal nerve stimulation potently inhibits LPS-induced inflammation
Our data show inhibition of inflammation by efferent but not afferent vagal nerve stimulation requires T-cell derived acetylcholine
We show that afferent and efferent neuroimmune circuits require β2-adrenergic receptor signaling
Acknowledgements.
Funding
This work was funded in part by an NIH grant to CR (OT2OD023871), and KM was funded in part by a NIH NIGMS-funded Pharmacology Training Program grant (T32GM099608).
Footnotes
Data availability
All pertinent data is either included in this manuscript or are available from the corresponding author upon reasonable request.
Competing Interests
All authors have no conflicts of interest to declare.
References
- Abe C, Inoue T, Inglis MA, Viar KE, Huang L, Ye H, Rosin DL, Stornetta RL, Okusa MD & Guyenet PG. (2017). C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nature Neuroscience 20, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addorisio ME, Imperato GH, de Vos AF, Forti S, Goldstein RS, Pavlov VA, van der Poll T, Yang H, Diamond B, Tracey KJ & Chavan SS. (2019). Investigational treatment of rheumatoid arthritis with a vibrotactile device applied to the external ear. Bioelectronic Medicine 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- All AH, Zeng X, Teh DBL, Yi Z, Prasad A, Ishizuka T, Thakor N, Hiromu Y & Liu X. (2019). Expanding the Toolbox of Upconversion Nanoparticles for In Vivo Optogenetics and Neuromodulation. Adv Mater 31, e1803474. [DOI] [PubMed] [Google Scholar]
- Bellinger DL & Lorton D. (2014). Autonomic regulation of cellular immune function. Autonomic Neuroscience 182, 15–41. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ & Tracey KJ. (2002). Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg 36, 1231–1236. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, Cracowski J-L & Pellissier S. (2016). Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterology & Motility 28, 948–953. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Sinniger V & Pellissier S. (2017). The Vagus Nerve in the Neuro-Immune Axis: Implications in the Pathology of the Gastrointestinal Tract. Frontiers in Immunology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW & Tracey KJ. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458. [DOI] [PubMed] [Google Scholar]
- Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, and McAllen RM. (2012). Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol 97, 1180–1185. [DOI] [PubMed] [Google Scholar]
- Buijs RM, van der Vliet J, Garidou M-L, Huitinga I & Escobar C. (2008). Spleen Vagal Denervation Inhibits the Production of Antibodies to Circulating Antigens. PLOS ONE 3, e3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Lu KH, Powley TL & Liu Z. (2017). Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS One 12, e0189518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP & Vizi ES. (2000). The Sympathetic Nerve—An Integrative Interface between Two Supersystems: The Brain and the Immune System. Pharmacological reviews 52, 595–638. [PubMed] [Google Scholar]
- Komegae EN, Farmer DGS, Brooks VL, McKinley MJ, McAllen RM & Martelli D. (2018). Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun 73, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ & Tak PP. (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proceedings of the National Academy of Sciences 113, 8284–8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressel AM, Tsaava T, Levine YA, Chang EH, Addorisio ME, Chang Q, Burbach BJ, Carnevale D, Lembo G, Zador AM, Andersson U, Pavlov VA, Chavan SS & Tracey KJ. (2020). Identification of a brainstem locus that inhibits tumor necrosis factor. Proceedings of the National Academy of Sciences , 202008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai NY, Musser MA, Pinho-Ribeiro FA, Baral P, Jacobson A, Ma P, Potts DE, Chen Z, Paik D, Soualhi S, Yan Y, Misra A, Goldstein K, Lagomarsino VN, Nordstrom A, Sivanathan KN, Wallrapp A, Kuchroo VK, Nowarski R, Starnbach MN, Shi H, Surana NK, An D, Wu C, Huh JR, Rao M & Chiu IM. (2020). Gut-Innervating Nociceptor Neurons Regulate Peyer’s Patch Microfold Cells and SFB Levels to Mediate Salmonella Host Defense. Cell 180, 33–49. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankadeva YR, May CN, McKinley MJ, Neeland MR, Ma S, Hocking DM, Robins-Browne R, Bedoui S, Farmer DGS, Bailey SR, Martelli D & McAllen RM. (2020). Sympathetic nerves control bacterial clearance. Scientific Reports 10, 15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH & Buurman WA. (2005). Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. The Journal of experimental medicine 202, 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli D, McKinley MJ & McAllen RM. (2014a). The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci 182, 65–69. [DOI] [PubMed] [Google Scholar]
- Martelli D, Yao ST, McKinley MJ & McAllen RM. (2014b). Reflex control of inflammation by sympathetic nerves, not the vagus. The Journal of Physiology 592, 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, Barboza M, Rude KM, Brust-Mascher I & Reardon C. (2019). Functional circuitry of neuro-immune communication in the mesenteric lymph node and spleen. Brain Behav Immun 82, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel YA, Saxena T, Bellamkonda RV & Butera RJ. (2017). Kilohertz frequency nerve block enhances anti-inflammatory effects of vagus nerve stimulation. Scientific Reports 7, 39810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR & Chiu IM. (2018). Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection. Cell 173, 1083–1097. e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz G & Straub RH. (2012). Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nature Reviews Rheumatology 9, 117. [DOI] [PubMed] [Google Scholar]
- Prechtl JC & Powley TL. (1990). The fiber composition of the abdominal vagus of the rat. Anat Embryol (Berl) 181, 101–115. [DOI] [PubMed] [Google Scholar]
- Ramirez V, Swain S, Murray K & Reardon C. (2020a). Neural Immune Communication in the Control of Host-Bacterial Pathogen Interactions in the Gastrointestinal Tract. Infect Immun 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez VT, Godinez DR, Brust-Mascher I, Nonnecke EB, Castillo PA, Gardner MB, Tu D, Sladek JA, Miller EN, Lebrilla CB, Bevins CL, Gareau MG & Reardon C. (2019). T-cell derived acetylcholine aids host defenses during enteric bacterial infection with Citrobacter rodentium. PLoS Pathog 15, e1007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez VT, Sladek J, Godinez DR, Rude KM, Chicco P, Murray K, Brust-Mascher I, Gareau MG & Reardon C. (2020b). Sensory nociceptive neurons contribute to host protection during enteric infection with Citrobacter rodentium. J Infect Dis. 221, 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon C, Duncan GS, Brustle A, Brenner D, Tusche MW, Olofsson P, Rosas-Ballina M, Tracey KJ & Mak TW. (2013). Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon C, Murray K & Lomax AE. (2018). Neuroimmune Communication in Health and Disease. Physiological Reviews 98, 2287–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S & Tracey KJ. (2008). Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proceedings of the National Academy of Sciences of the United States of America 105, 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW & Tracey KJ. (2011). Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinniger V, Pellissier S, Fauvelle F, Trocmé C, Hoffmann D, Vercueil L, Cracowski JL, David O & Bonaz B. (2020b). A 12-month pilot study outcomes of vagus nerve stimulation in Crohn’s disease. Neurogastroenterol Motil , e13911. [DOI] [PubMed] [Google Scholar]
- Somann JP, Wasilczuk KM, Neihouser KV, Sturgis J, Albors GO, Robinson JP, Powley TL & Irazoqui PP. (2019). Characterization of plasma cytokine response to intraperitoneally administered LPS & subdiaphragmatic branch vagus nerve stimulation in rat model. PLoS One 14, e0214317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H & Seed B. (2010). PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Research 38, D792–D799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH & Härle P. (2005). Sympathetic neurotransmitters in joint inflammation. Rheum Dis Clin North Am 31, 43–59, viii. [DOI] [PubMed] [Google Scholar]
- Vida G, Peña G, Deitch EA & Ulloa L. (2011). α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol 186, 4340–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida G, Peña G, Kanashiro A, Thompson-Bonilla Mdel R, Palange D, Deitch EA, Ulloa L. (2011). β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system . FASEB J 25, 4476–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DW, Yin YM & Yao YM. (2016). Vagal Modulation of the Inflammatory Response in Sepsis. Int Rev Immunol 35, 415–433. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ & Tracey KJ. (2003). Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- Ward MP, Gupta A, Wo JM, Rajwa B, Furness JB, Powley TL & Nowak TV. (2020). An emerging method to noninvasively measure and identify vagal response markers to enable bioelectronic control of gastroparesis symptoms with gastric electrical stimulation. J Neurosci Methods 336, 108631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhu X, Chong P, Liu J, Andre LN, Ong KS, Brinson K Jr., Mahdi AI, Li J, Fenno LE, Wang H & Hong G. (2019). Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proceedings of the National Academy of Sciences of the United States of America 116, 26332–26342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K, So EL, Rajendran PS, Hoang JD, Makkar N, Mahajan A, Shivkumar K & Vaseghi M. (2014). Electrophysiological effects of right and left vagal nerve stimulation on the ventricular myocardium. Am J Physiol Heart Circ Physiol 307, H722–H731. [DOI] [PMC free article] [PubMed] [Google Scholar]


