Abstract
Background
Pituitary tumours are usually benign and relatively common intracranial tumours, with under- and overexpression of pituitary hormones and local mass effects causing considerable morbidity and increased mortality. While most pituitary tumours are sporadic, around 5% of the cases arise in a familial setting, either isolated [familial isolated pituitary adenoma, related to AIP or X-linked acrogigantism], or in a syndromic disorder, such as multiple endocrine neoplasia type 1 or 4, Carney complex, McCune–Albright syndrome, phaeochromocytoma/paraganglioma with pituitary adenoma, DICER1 syndrome, Lynch syndrome, and USP8-related syndrome. Genetically determined pituitary tumours usually present at younger age and show aggressive behaviour, and are often resistant to different treatment modalities.
Subject
In this practical summary, we take a practical approach: which genetic syndromes should be considered in case of different presentation, such as tumour type, family history, age of onset and additional clinical features of the patient.
Conclusion
The identification of the causative mutation allows genetic and clinical screening of relatives at risk, resulting in earlier diagnosis, a better therapeutic response and ultimately to better long-term outcomes.
Keywords: Pituitary, Tumour, Genetics, FIPA, MEN
Introduction
Consideration of genetic abnormalities in a patient with a pituitary tumour has only recently entered the clinical thinking of the practising endocrinologist. While in families with multiple endocrine neoplasia type 1 (MEN1) syndrome it was long recognised that members can develop pituitary adenomas with incomplete penetrance, the flurry of conditions we can now list with a genetic cause of pituitary adenoma would have been well beyond imagination 20 years ago. Indeed, most pituitary tumours are sporadic, but approximately 5% can be due to a hereditary disease. Pituitary tumours can occur in a familial setting, either isolated [Familial Isolated Pituitary Adenoma (FIPA), for example aryl hydrocarbon receptor-interacting protein (AIP) mutation-positive FIPA, or in patients with X-linked acrogigantism (XLAG)], or as part of a syndromic condition, such as MEN1 or MEN4, Carney complex (CNC), McCune–Albright syndrome (MAS), phaeochromocytoma/paraganglioma with pituitary adenoma (3P associations, 3Pa), DICER1 syndrome and a USP8-related syndrome and some other rare conditions where the nature of association with pituitary adenomas needs further studies (Table 1).
Table 1.
Genetic alterations in pituitary tumours
| Abnormality | Germline | Mosaic mutation | Somatic (tumour only) | |
|---|---|---|---|---|
| Isolated | AIP | x | ||
| GPR101 | x | x | ||
| Unknown germline alterations gene(s) in AIP-negative FIPA | x | |||
| Syndromic | MEN1, CDKN1B, CDC73a | x | x | |
| PRKAR1A, PRKACBa | x | |||
| SDHx | x | x | ||
| MAX | x | |||
| DICER1 | x | |||
| USP8 | xa | x | ||
| USP48 | x | |||
| MLH, PMS2 | x | |||
| GNAS | x | x | ||
| VHL | xa | |||
| RET | xa | |||
| TSC1 | xa | |||
| Somatic | SF3B1 | x | ||
| ATRX | x |
aCase reports/further study needed.
Genetic testing might lead to the recognition of a syndromic disease and therefore beneficial effects of timely identification of other aspects of the disease, or it can diagnose disease in family members at an early stage leading to earlier diagnosis and treatment, and ultimately to better outcomes.
Here we approach this issue from the practical point of view, centring the discussion on the presentation of the patient.
Gigantism
The most common cause of a germline genetic abnormality in a patient with a pituitary adenoma is growth hormone (GH) excess, especially childhood-onset GH excess leading to gigantism. While the usual cause of acromegalic gigantism is a GH-secreting pituitary adenoma, resulting in elevated GH- and insulin-like growth factor 1 (IGF-1) levels, we should remember other conditions with tall stature, either as a normal variant or part of a syndrome with no abnormalities in the GH axis [1].
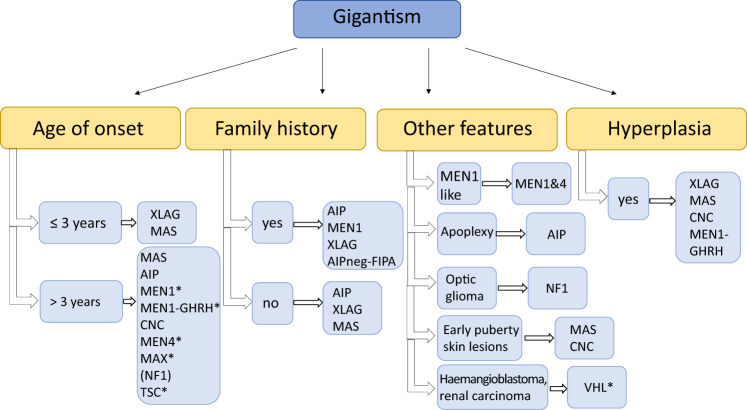
For true acromegalic gigantism we need to consider the age of onset of rapid growth. In case of very early onset, XLAG (<2 years of age) and MAS (from 3 years of age) might be the diagnosis.
XLAG is an infant-onset gigantism syndrome caused by germline or somatic mosaic duplication of the GPR101 gene, which encodes a G-protein coupled receptor [2, 3]. The onset of accelerated growth is always observed before the age of 5 years, and in most patients during the first year of life. Apart from rapid growth, these children have acromegalic features, such as acral enlargement and facial coarsening and other abnormalities, like increased appetite and hyperinsulinemia [4]. Their pituitary MRI can show a tumour, a diffusely enlarged gland suggesting hyperplasia or a normal MRI. The tumours often have an unusual histological appearance (sinusoidal, lobular and acinar architecture, microcalcification and pseudo-follicles are characteristic), while hyperplasia can be seen on histology in some cases [3]. Pituitary hyperplasia can be also observed in same cases of MAS, CNC and in GH-releasing hormone (GHRH)-induced GH excess. GPR101 variants, other than gene duplication, have not been associated with GH-secreting or other type of pituitary tumours [3, 5–8].
AIP-related gigantism is the most common genetic cause of pituitary gigantism [3, 9]. Patients typically show signs of the disease during the second decade but several cases have been described with accelerated growth already in the first decade. These patients usually have large invasive tumours needing multiple treatment modalities [10, 11], although microadenoma cured after surgery has also been described [12]. Pituitary apoplexy is a characteristic phenomenon in some AIP mutation-positive patients [11–13]. Male predominance is observed in AIP mutation-positive gigantism, although the physiologically later puberty and ascertainment bias due to taller stature in males could play a part in this.
MEN1 syndrome extremely rarely manifests as gigantism. A 5-year-old boy was described with a mammosomatotroph macroadenoma causing gigantism [14], while another case of MEN1-related gigantism was due to a pancreatic GHRH-secreting tumour [15].
MAS is a mosaic disease, caused by a mutation in the GNAS gene at an early post-zygotic stage of development. The variable phenotype depends on what tissues are affected by the mutation. MAS is characterised by fibrous dysplasia, cafè-au-lait spots of the skin and different endocrinopathies. Acromegaly affects around 20% of MAS patient. The mean age at diagnosis of acromegaly is 24.4 years; however, the disease can start in early childhood as well [16].
In the case of disease onset at >3 years of age but still in childhood, AIP, MEN1 and 4, CNC, MAS, 3Pa and a non-pituitary condition neurofibromatosis type 1 (NF1), should be considered. In NF1, approximately 10% of cases with optic glioma have GH excess often manifesting in childhood and causing accelerated growth; the exact mechanism of the GH excess is unknown (Fig. 1).
Fig. 1.
Diseases to be considered in cases of gigantism. AIP aryl hydrocarbon receptor-interacting protein, AIPneg-FIPA AIP mutation-negative Familial Isolated Pituitary Adenoma (this group does not represent XLAG families), CNC Carney complex, GHRH growth hormone-releasing hormone, MAS McCune–Albright syndrome, MEN multiple endocrine neoplasia, NF1 neurofibromatosis type 1, TSC tuberous sclerosis, VHL von Hippel–Lindau syndrome, XLAG X-linked acrogigantism. *Case reports/further study needed [69]
Acromegaly
Apart from age of onset, the other major significant feature for GH excess patients is the family history. A positive family history is very suggestive of a genetic disease, but we should be aware of phenocopies—the same phenotype by chance and not due to common genetic background; this has been described in several AIP- and succinate dehydrogenase (SDHx)-positive families already [17–19]. In the case of a positive family history, the most common diseases are related to AIP or MEN1 mutations. If there is no family history of pituitary tumours, this could be due to (i) a lack of genetic background, (ii) low penetrance, (iii) lack of information regarding a positive family history, (iv) a de novo germline or mosaic mutation and (v) imprinting complicating penetrance (SDHD for example).
FIPA is defined as two or more members of a family who develop pituitary adenoma with no other clinical manifestation. In FIPA families, either heterogeneous—with different pituitary tumour subtypes, or homogeneous—the most frequent pituitary adenoma subtypes are somatotrophinomas or prolactinomas. Germline mutations in the AIP gene have been identified in 10% of FIPA families, while in most cases the causative gene(s) remain unknown. It is important to note that AIP mutations can also occur in subjects with apparently sporadic early-onset somatotrophinomas or prolactinomas, as a consequence of incomplete penetrance, while de novo mutations are extremely rare. AIP mutation-positive tumours show a distinct phenotype, with younger age at diagnosis (usually age of onset under 30 years), tumour invasiveness and relative resistance to treatment with first-generation somatostatin receptor ligands. AIP mutation-positive patients usually need a multimodal therapeutic approach. In AIP mutation-negative patients somatotrophinoma is also the most common subtype (although not as common as in AIP mutation-positive subjects, around 78% compared to 58%) [10, 12, 18].
MEN1 is an autosomal dominantly inherited disease characterised by hyperparathyroidism, gastro-enteropancreatic neuroendocrine (NET) and pituitary tumours, with associated other endocrine and non-endocrine tumours. Mutations of the MEN1 tumour suppressor gene are detected in 90% patients with the MEN1 phenotype. De novo germline or mosaic mutations occur in approximately 10% of cases. A pituitary tumour develops in 30–40% of the patients [20]. Prolactinomas are the most common clinically presenting pituitary tumour subtype, sometimes large and arising at a younger age. Patients with MEN1 may have plurihormonal adenomas. Non-functioning pituitary adenomas (NFPAs), somatotrophinomas and, rarely, corticotrophinomas or thyrotrophinomas have also been described. However, during systematic screening of MEN1 mutation carriers, small non-functioning lesions, not dissimilar to incidentalomas, can often be found.
Around 10% of cases with a MEN1-like syndrome do not have a mutation in MEN1. Some of these patients harbour germline changes in the CDKN1B gene coding for the cyclin-dependent kinase inhibitor p27. This clinically overlapping rare condition is termed MEN4 [21, 22]. In a group of 24 patients with acromegaly and at least one other manifestation of MEN1 syndrome, but without pathogenic MEN1 or CDKN1B variants, a CDC73 missense mutation was found in one case [23].
In cases of the MEN1 syndrome, a GHRH-secreting pancreatic [24] or thymic [25] NET tumour might be part of the syndrome and the source of GHRH, which causes pituitary hyperplasia and thus acromegaly. A similar case has now been described in a patient with germline MAX mutation and multiple tumours including a GHRH-positive phaeochromocytoma [26].
CNC is a clinical diagnosis based on characteristic skin pigmentation, cardiac myxomas, primary pigmented nodular adrenocortical disease and pituitary tumour or hyperplasia, testicular lesions, melanotic schwannomas and others. Although around 75% of patients have an abnormal GH axis in CNC, only 10% of the patients show clinically acromegaly, and less frequently gigantism. Most patients (67%) have pituitary hyperplasia affecting the somatolactotroph cells, while only 10–12% of them have a real GH-producing pituitary tumour [27]. Genetic testing for inactivating germline mutations in the protein kinase A regulatory subunit 1-α (PRKAR1A) coding gene is available, and large deletions are known to cause more severe disease. There is a second locus located at 2p16, which is still unknown, but cytogenetic changes of the 2p16 chromosomal region are frequently observed in tumours from CNC patients [28, 29].
Phaeochromocytoma/paraganglioma can be associated with pituitary tumours (the 3Pa), even carcinoma [30], due to mutations in SDHx or MAX genes. The pituitary tumours include somatotrophinomas, prolactinomas and NFPAs. Recently MAX mutation has been described in families with phaeochromocytoma, pituitary adenoma and other endocrine- and non-endocrine tumours [26]. Acromegaly associated with phaeochromocytoma can be the consequence of a GHRH-secreting phaeochromocytoma without any pituitary adenoma [19, 31] (Table 2).
Table 2.
Familial pituitary tumours or alterations, showing the syndrome, the background genetic alteration, the typical pituitary adenoma subtype and the other main clinical features in a syndromic setting
| Isolated | Syndromic | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Syndrome | FIPA | MEN1, 4 | Carney complex | McCune–Albright | 3Pa | Dicer syndrome | Neurofibromatosis | USP8-related syndrome | Lynch syndrome |
| Gene | AIP, GPR101 | MEN1, CDKN1B, MAX*, CDC73* | PRKAR1A PRKACB* | GNAS | SDHx, MAX | DICER1 | NF1 | USP8* | MLH*, PMS2* |
| Type of pituitary tumour (secreted hormone) | GH GH&PRL PRL, NFPA, TSH* | PRL, NFPA, GH (ACTH, TSH) | GH, GH&PRLPRL, ACTH | GH, GH&PRL | PRL, GH, NFPA | ACTH |
GH excess PRL*,# ACTH*,# NFPA*,# |
ACTH | ACTH |
| Other main conditions | None | pHPT, pancreatic tumour, angiofibromas, adrenal adenomas, lipomas, meningiomas | Myxoma, PPNAD, pigmented lesions of the skin and mucosae | Fibrous dysplasia, hyper-functioning endocrinopathies, cafè-au-lait spots | Renal cell carcinoma, PGL/phaeo, GIST | PPB, goitre/thyroid cc, cystic nephroma, Sertoli–Leydig cell tumour | Neurofibromas, cafè-au-lait spots, optic glioma, phaeo | Developmental delay, dysmorphic features, lung and kidney disease | Colon, brain, uterine, pancreatic, ovarian, stomach tumour |
ACTH adrenocorticotropic hormone, GH growth hormone, GIST gastrointestinal neuroendocrine tumour, NFPA non-functioning pituitary adenoma, 3Pa 3P (phaeochromocytoma, paraganglioma and pituitary adenoma) association, phaeo, phaeochromocytoma, pHPT primary hyperparathyroidism, PGL paraganglioma, PPB pleuropulmonary blastoma, PPNAD primary pigmented nodular adrenocortical disease, PRL prolactin, TSH thyroid-stimulating hormone.*, case reports, #, could be coincidences
NF1-associated GH excess should be considered in patients with characteristic clinical signs and symptoms of dermal neurofibromas, café-au-lait spots, axillary or inguinal freckling and hamartomas of the iris as well as brain neoplasms due to inactivating mutation of NF1 gene. Around 10% of children with NF1 and optic pathways glioma show GH excess [32]. While tuberous sclerosis has been described with pituitary adenomas, the causative link between the two diseases is uncertain [33, 34].
Acromegalic features and GH hypersecretion have been recently described in patients with mutations in the X-linked immunoglobulin superfamily member 1 (IGSF1) gene. The exact mechanism of GH excess is unclear [35].
Somatic mutations can lead to acromegaly as well. The most frequent genetic alteration is somatic GNAS mutation, found in 40% of somatotrophinomas [36]. These tumours show favourable clinical features, such as older age at diagnosis, less invasiveness and better response to therapy (Fig. 2).
Fig. 2.
Diseases to be considered in cases of acromegaly. AIP aryl hydrocarbon receptor-interacting protein, AIPneg-FIPA AIP mutation-negative Familial Isolated Pituitary Adenoma (this group does not represent XLAG families), CNC Carney complex, FIPA Familial Isolated Pituitary Adenoma, GH growth hormone, GHRH growth hormone-releasing hormone, MEN multiple endocrine neoplasia, NET neuroendocrine tumour, phaeo phaeochromocytoma, SDH succinate dehydrogenase. *Case reports/further study needed [25, 70]
Prolactinoma
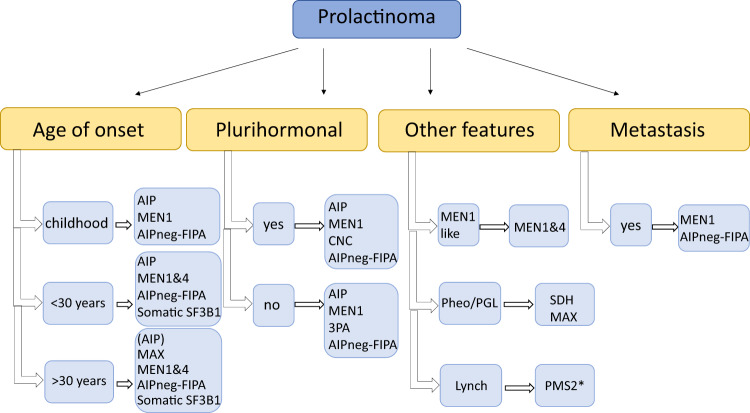
Prolactinomas are the most common pituitary adenoma subtype in MEN1, and the second most common in MEN4 and FIPA, both in AIP-positive (even as a homogenous prolactinoma family [37]) and AIP-negative FIPA, but can occur in SDHx cases as well (Fig. 3). Metastatic prolactinoma case has been described in MEN1 [38]. A large childhood-onset prolactinoma can be the first manifestation of MEN1 or AIP-related disease. Recently, two families with MAX mutations with multiple tumour types were described, including a patient with prolactinoma and parathyroid tumour in one of them [26].
Fig. 3.
Diseases to be considered in cases of prolactinoma. AIP aryl hydrocarbon receptor-interacting protein, AIPneg-FIPA AIP mutation-negative Familial Isolated Pituitary Adenoma (this group does not represent XLAG families), MEN multiple endocrine neoplasia, NF1 neurofibromatosis type 1, 3Pa 3P (phaeochromocytoma, paraganglioma and pituitary adenoma) association, SDH succinate dehydrogenase. *Case reports/further study needed [45]
Recently, a somatic mutation in the SF3B1 gene has been described in 20% of prolactinoma patients, showing higher prolactin (PRL) levels [39].
Cushing’s disease
Infant-onset Cushing’s disease can occur in DICER1 syndrome with very low penetrance due to a pituitary blastoma, but this is pathognomic to the disease. Pituitary blastomas are aggressive tumours arising in young children, presenting clinically with often severe ACTH-dependent hypercortisolism. It is locally destructive, severe disease with high mortality [40]. A family history of young-onset large goitre, pleuropulmonary blastoma, Sertoli–Leydig cell tumours, nodular thyroid hyperplasia or differentiated cancer of the thyroid, cystic nephroma and renal sarcoma can be associated [41]. Cushing’s disease is rare (5–10%) in MEN1 [42], and to date no convincing AIP mutation-positive patient has been found with a corticotrophinoma. More recently, a few cases of CDKN1B mutations were described in children with corticotrophinoma with or without other features of MEN4 [43]. Germline potentially pathogenic CABLES1 variants were identified in 2% of the cases in a patient cohort of Cushing’s disease [44]. Aggressively growing corticotroph tumours has been described as part of Lynch syndrome in patients with DNA mismatch repair genes MSH6, PMS2 mutations and associated colorectal, endometrial, ovarian, urinary tract, small bowel, gastric, hepatobiliary, adrenocortical or malignant brain tumours [45]. Cushing’s disease has been described in patients with RET mutation as part of MEN2A or MEN2B syndrome. It is unclear whether these cases are coincidences, or RET mutation plays a causative role in pituitary tumour formation ([46, 47] and references within [19]).
Somatic USP8 mutations can be observed in corticotrophinomas in a high (around 30%) percentage. Patients harbouring mutations in this gene are predominantly female, and they have more frequently microadenomas with better therapeutic outcome [48, 49]. Germline USP8 mutation has been found in a paediatric case with recurrent Cushing’s disease and developmental delay, representing a syndromic form of pituitary adenomas [50]. Somatic GNAS mutation might occur very rarely in ACTH-producing pituitary adenomas [51, 52]. The differential diagnosis of Cushing’s syndrome is often challenging, but two recent case reports complicated this further in CNC: in addition to the typical adrenal Cushing’s syndrome, pituitary Cushing’s disease have been also described [53, 54]. Recently aggressive corticotroph pituitary tumours and carcinomas harbouring somatic mutations in ATRX [55] have been described (Table 2) (Fig. 4).
Fig. 4.
Diseases to be considered in cases of Cushing’s disease. AIP aryl hydrocarbon receptor-interacting protein, AIPneg-FIPA AIP mutation-negative Familial Isolated Pituitary Adenoma (this group does not represent XLAG families), CDKN1B cyclin-dependent kinase inhibitor 1B, CNC Carney complex, FIPA Familial Isolated Pituitary Adenoma, TSC tuberous sclerosis. *Case reports/further study needed [19, 45–47, 50, 52–54, 71–73]
Non-functioning pituitary adenoma
NFPAs can occur in MEN1 and mostly AIP mutation-negative FIPA patients. NFPA can be part of AIP mutation-negative homogenous NFPA kindreds—12% in our cohort of 318 FIPA kindreds, or part of heterogenous families—31% in our AIP mutation-negative cohort. AIP mutation-positive clinically NFPA cases are often microadenomas identified in asymptomatic carriers or if operated show positive GH and PRL immunostaining [56]. NFPA might be part of the 3Pa as well, and interestingly, the pituitary carcinoma case with an SDHB mutation was a non-functioning pituitary tumour [30]. NFPAs are most often clinically silent tumours, most often of gonadotroph origin (Fig. 5).
Fig. 5.
Diseases to be considered in cases of non-functioning pituitary adenoma (NFPA). AIP aryl hydrocarbon receptor-interacting protein, AIPneg-FIPA AIP mutation-negative Familial Isolated Pituitary Adenoma (this group does not represent XLAG families), FIPA Familial Isolated Pituitary Adenoma, GH growth hormone, MEN multiple endocrine neoplasia, PRL prolactin, SDH succinate dehydrogenase. *Case reports/further study needed [45, 74]
Thyrotrophinoma
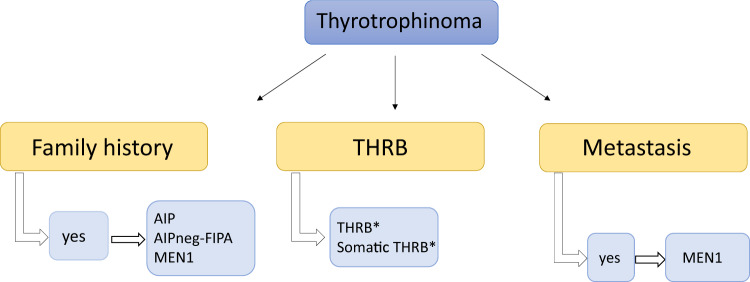
TSH-producing pituitary tumours are very rare in a familial setting. There is one case described with an AIP mutation and a few cases with MEN1 mutation [57] including an MEN1 mutation-positive metastatic case [58]. In MEN1 syndrome PIT1-positive plurihormonal adenomas can also be identified, sometimes with silent or clinically relevant TSH positivity [59–61]. Somatic mutation has been described in TRβ (THRB) [62] and a patient with TSHoma and germline THRB mutation [63], corresponding to data from a THRB-deficiency animal model where TSH-secreting adenomas have been observed [64] (Fig. 6).
Fig. 6.
Diseases to be considered in cases of thyrotrophinomas. AIP aryl hydrocarbon receptor-interacting protein, AIPneg-FIPA AIP mutation-negative Familial Isolated Pituitary Adenoma (this group does not represent XLAG families), FIPA Familial Isolated Pituitary Adenoma, MEN multiple endocrine neoplasia, THRB thyroid hormone receptor beta. *Case reports/further study needed [62, 63]
Clinical considerations
Pituitary tumours in a familial setting are relatively rare disorders, and identification of the genetic cause can lead to several advantages. Discovery of a syndromic disease might help to identify other aspects of the proband’s condition with beneficial effects through earlier treatment. Genetic screening might help to identify family members in an earlier stage of the disease, when the tumour responds better to therapy and a better outcome can predicted.
First of all, a detailed family history should be obtained from all patients with a pituitary tumour. In case of FIPA, screening for AIP mutations should be considered, as mutations are identified in about 10% of unselected families and 20% of those with familial acromegaly. Screening for AIP mutations should also be considered in patients, primarily with GH or prolactin-secreting adenomas, with age at onset ≤18 years and patients with macroadenomas and age at onset ≤30 years. Genetic and clinical screening show clear benefits in AIP mutation-positive patients; prospectively diagnosed patients have smaller, less invasive lesions controlled less frequently with multimodal treatment, compared with clinically presenting patients [65]. XLAG should also be considered in case of early-onset gigantism starting during the first 2 years of life.
In the case of MEN1, the additional main clinical features, such as hyperparathyroidism and enteropancreatic NET present in the proband or other family members, can help in the decision regarding genetic testing. However, pituitary tumours may represent the first disease manifestation, and considering the possibility of de novo mutations, screening for MEN1 should be considered in patients with childhood-onset pituitary macroadenomas (especially prolactinomas) [66]. In patients with a MEN1 phenotype but without MEN1 mutation, the rare MEN4 should be considered and genetic testing for CDKN1B mutation is suggested. Case reports of patients with multiple endocrine tumours and CDC73 or MAX variants have been described.
Other less frequent syndromes should be considered in the presence of associated features, such as testing for SDHx mutation in case of phaeochromocytoma/paraganglioma with pituitary adenoma or testing for PRKAR1A in case of symptoms characteristic of CNC.
For patients with pituitary diseases there are a few questions to consider:
-
Which patient needs genetic testing?
Genetic testing is recommended in patients (i) with a family history of pituitary tumour, (ii) with early-onset pituitary tumour and (iii) with additional clinical features which predispose to pituitary tumour in a syndromic setting.
-
What change will bring a genetic diagnosis in the treatment or the follow-up of the proband?
Identifying a mutation helps to understand the nature of the disease and be more vigilant regarding tumour behaviour, can draw attention to possible hyperplasia, but in general would not necessarily change the treatment algorithm of the proband.
-
Which family members need genetic screening?
Genetic testing should be offered to first-degree relatives (children, siblings and parents) of a gene carrier.
-
How should carrier family members be followed up?
It depends on the specific syndromic condition, but in general baseline clinical, biochemical and imaging assessment is needed with follow-up depending on the specific syndrome and patient age.
In the case of AIP mutation carriers, it is advised to monitor growth starting in early childhood and yearly biochemical testing (GH, IGF-1 and prolactin) not later than at the age of 10 years. A baseline pituitary MRI is advised around the age of 10 and should be repeated every 5 years until the age of 30, although between 20 and 30 follow-up could be gradually relaxed to facilitate patient cooperation. In carriers identified as adults, if a baseline clinical and biochemical assessments with pituitary MRI has not identified any abnormality, then screening can be reduced after the age of 30, as most patients with AIP mutation start their disease before this age.
In case of MEN1 patients, published guidelines should be followed. For pituitary disease, starting at the age of 5 with yearly clinical and biochemical assessments (prolactin and IGF-1). A pituitary MRI should be performed every 3 years [67, 68].
If the categorisation of an identified variant is unclear, i.e., a variant of uncertain significance, what policy should be followed for the proband and family members?
In this case, genetic testing of family members is not recommended. However, attention should be focused on any family member developing typical signs or symptoms; that person should be clinically evaluated, and if affected, genetic testing performed. As data on genetic variants are constantly evolving, patients and their clinicians should be later notified if the status of the variant has changed based on novel clinical, experimental or genetic data.
In conclusion, genetic testing not only helps us to identify patients with an increased risk of developing pituitary or other tumours, and to achieve early diagnosis and better therapeutic outcome, but also leads to better understanding of pituitary tumorigenesis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marques P, Korbonits M. Pseudoacromegaly. Front. Neuroendocrinol. 2019;52:113–143. doi: 10.1016/j.yfrne.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Trivellin G, Daly A, Faucz F, Yuan B, Rostomyan L, Larco D, Schernthaner-Reiter M, Szarek E, Leal L, Caberg JH, Castermans E, Villa C, Dimopoulos A, Chittiboina P, Xekouki P, Shah N, Metzger D, Lysy P, Ferrante E, Strebkova N, Mazerkina N, Zatelli M, Lodish M, Horvath A, de Alexandre RB, Manning A, Levy I, Keil M, de la Luz Sierra M, Palmeira L, Coppieters W, Georges M, Naves L, Jamar M, Bours V, Wu T, Choong C, Bertherat J, Chanson P, Kamenický P, Farrell W, Barlier A, Quezado M, Bjelobaba I, Stojilkovic S, Wess J, Costanzi S, Liu P, Lupski J, Beckers A, Stratakis C. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N. Engl. J. Med. 2014;371(25):2363–2374. doi: 10.1056/NEJMoa1408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D. Iacovazzo, R. Caswell, B. Bunce, S. Jose, B. Yuan, L.C. Hernández-Ramírez, S. Kapur, F. Caimari, J. Evanson, F. Ferraù, M.N. Dang, P. Gabrovska, S.J. Larkin, O. Ansorge, C. Rodd, M.L. Vance, C. Ramírez-Renteria, M. Mercado, A.P. Goldstone, M. Buchfelder, C.P. Burren, A. Gurlek, P. Dutta, C.S. Choong, T. Cheetham, G. Trivellin, C.A. Stratakis, M.B. Lopes, A.B. Grossman, J. Trouillas, J.R. Lupski, S. Ellard, J.R. Sampson, F. Roncaroli, M. Korbonits, Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study. Acta Neuropathol. Commun. 4 (2016). 10.1186/s40478-016-0328-1 [DOI] [PMC free article] [PubMed]
- 4.Beckers A, Lodish MB, Trivellin G, Rostomyan L, Lee M, Faucz FR, Yuan B, Choong CS, Caberg JH, Verrua E, Naves LA, Cheetham TD, Young J, Lysy PA, Petrossians P, Cotterill A, Shah NS, Metzger D, Castermans E, Ambrosio MR, Villa C, Strebkova N, Mazerkina N, Gaillard S, Barra GB, Casulari LA, Neggers SJ, Salvatori R, Jaffrain-Rea ML, Zacharin M, Santamaria BL, Zacharieva S, Lim EM, Mantovani G, Zatelli MC, Collins MT, Bonneville JF, Quezado M, Chittiboina P, Oldfield EH, Bours V, Liu P, De Herder W, Pellegata N, Lupski JR, Daly AF, Stratakis CA. X-linked acrogigantism (X-LAG) syndrome: clinical profile and therapeutic responses. Endocr. Relat. Cancer. 2015;22(3):353–367. doi: 10.1530/erc-15-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lecoq AL, Bouligand J, Hage M, Cazabat L, Salenave S, Linglart A, Young J, Guiochon-Mantel A, Chanson P, Kamenický P. Very low frequency of germline GPR101 genetic variation and no biallelic defects with AIP in a large cohort of patients with sporadic pituitary adenomas. Eur. J. Endocrinol. 2016;174(4):523–530. doi: 10.1530/eje-15-1044. [DOI] [PubMed] [Google Scholar]
- 6.Ferraù F, Romeo PD, Puglisi S, Ragonese M, Torre ML, Scaroni C, Occhi G, De Menis E, Arnaldi G, Trimarchi F, Cannavò S. Analysis of GPR101 and AIP genes mutations in acromegaly: a multicentric study. Endocrine. 2016;54(3):762–767. doi: 10.1007/s12020-016-0862-4. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto R, Izawa M, Fukuoka H, Iguchi G, Odake Y, Yoshida K, Bando H, Suda K, Nishizawa H, Takahashi M, Inoshita N, Yamada S, Ogawa W, Takahashi Y. Genetic and clinical characteristics of Japanese patients with sporadic somatotropinoma. Endocr. J. 2016;63(11):953–963. doi: 10.1507/endocrj.EJ16-0075. [DOI] [PubMed] [Google Scholar]
- 8.Trivellin G, Correa RR, Batsis M, Faucz FR, Chittiboina P, Bjelobaba I, Larco DO, Quezado M, Daly AF, Stojilkovic SS, Wu TJ, Beckers A, Lodish M, Stratakis CA. Screening for GPR101 defects in pediatric pituitary corticotropinomas. Endocr. Relat. Cancer. 2016;23(5):357–365. doi: 10.1530/erc-16-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, Lecumberri B, Trivellin G, Salvatori R, Moraitis AG, Holdaway I, Kranenburg-van Klaveren DJ, Chiara Zatelli M, Palacios N, Nozieres C, Zacharin M, Ebeling T, Ojaniemi M, Rozhinskaya L, Verrua E, Jaffrain-Rea ML, Filipponi S, Gusakova D, Pronin V, Bertherat J, Belaya Z, Ilovayskaya I, Sahnoun-Fathallah M, Sievers C, Stalla GK, Castermans E, Caberg JH, Sorkina E, Auriemma RS, Mittal S, Kareva M, Lysy PA, Emy P, De Menis E, Choong CS, Mantovani G, Bours V, De Herder W, Brue T, Barlier A, Neggers SJ, Zacharieva S, Chanson P, Shah NS, Stratakis CA, Naves LA, Beckers A. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr. Relat. Cancer. 2015;22(5):745–757. doi: 10.1530/erc-15-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly AF, Tichomirowa MA, Petrossians P, Heliövaara E, Jaffrain-Rea ML, Barlier A, Naves LA, Ebeling T, Karhu A, Raappana A, Cazabat L, De Menis E, Montañana CF, Raverot G, Weil RJ, Sane T, Maiter D, Neggers S, Yaneva M, Tabarin A, Verrua E, Eloranta E, Murat A, Vierimaa O, Salmela PI, Emy P, Toledo RA, Sabaté MI, Villa C, Popelier M, Salvatori R, Jennings J, Longás AF, Labarta Aizpún JI, Georgitsi M, Paschke R, Ronchi C, Valimaki M, Saloranta C, De Herder W, Cozzi R, Guitelman M, Magri F, Lagonigro MS, Halaby G, Corman V, Hagelstein MT, Vanbellinghen JF, Barra GB, Gimenez-Roqueplo AP, Cameron FJ, Borson-Chazot F, Holdaway I, Toledo SP, Stalla GK, Spada A, Zacharieva S, Bertherat J, Brue T, Bours V, Chanson P, Aaltonen LA, Beckers A. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J. Clin. Endocrinol. Metab. 2010;95(11):E373–E383. doi: 10.1210/jc.2009-2556. [DOI] [PubMed] [Google Scholar]
- 11.Dutta P, Reddy KS, Rai A, Madugundu AK, Solanki HS, Bhansali A, Radotra BD, Kumar N, Collier D, Iacovazzo D, Gupta P, Raja R, Gowda H, Pandey A, Devgun JS, Korbonits M. Surgery, octreotide, temozolomide, bevacizumab, radiotherapy, and pegvisomant treatment of an AIP mutation-positive child. J. Clin. Endocrinol. Metab. 2019;104(8):3539–3544. doi: 10.1210/jc.2019-00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández-Ramírez LC, Gabrovska P, Dénes J, Stals K, Trivellin G, Tilley D, Ferraù F, Evanson J, Ellard S, Grossman AB, Roncaroli F, Gadelha MR, Korbonits M, Agha A, Akker SA, Aflorei ED, Alföldi S, Arlt W, Atkinson B, Aulinas-Masó A, Aylwin SJ, Backeljauw PF, Badiu C, Baldeweg S, Bano G, Barkan A, Barwell J, Bernal-González C, Besser GM, Bevan JS, Blair J, Bouloux P, Bradley L, Buchfelder M, Cakir M, Canham N, Carroll P, Chahal HS, Cheetham T, Chentli F, Clayton RN, Cohen M, Cole T, Courtney H, Crowne E, Cuthbertson D, Dal J, Dalantaeva N, Daousi C, Darzy K, Dattani M, Davies JH, Davis J, De Castro M, De Marinis L, Drake W, Dutta P, Dzeranova L, Edén-Engström B, Eeles R, Elfving M, Elston M, Emmerson L, Fersht N, Fica S, Fischli S, Flanagan D, Fleseriu M, Freda PU, Friedman T, Frohman LA, Gallego P, Gevers E, Gláz E, Goldman JA, Goldstone AP, Goth M, Greenhalgh L, Grieve J, Guitelman M, Gürlek A, Gurnell M, Horvath K, Howlett TA, Höybye C, Hunter S, Iacovazzo D, Igaz P, Inder WJ, Iwata T, Izatt L, Jagadeesh S, Kaltsas G, Kaplan F, Karavitaki N, Kastelan D, Katz M, Kearney T, Khoo B, Kiraly-Borri C, Knispelis R, Kovács GL, Kumar AV, Laws ER, Lechan RM, Levy MJ, Lewandowski K, Lo J, Maartens N, Matsuno A, McGowan B, McQuaid SE, Medic-Stojanoska M, Mercado-Atri M, Mezősi E, Miljic D, Miller KK, Modenesi S, Molitch ME, Monson J, Morris DG, Morrison PJ, Munir A, Murray RD, Musat M, Musolino N, Nachtigall L, Newell-Price J, Ogilvie A, Orme SM, Paşcanu I, Patócs A, Patterson C, Pearce SH, Pecori Giraldi F, Pfeifer M, Popovic V, Poplawski N, Powell M, Pullan P, Quinton R, Radian S, Randeva H, Ribeiro-Oliveira A, Rodd C, Ryan F, Salvatori R, Schöfl C, Shears D, Shotliff K, Soares BS, Somasundaram N, Spada A, Sperber J, Spoudeas HA, Stewart S, Storr H, Strasburger C, Street ME, Swords F, Thakker RV, Tham E, Thompson C, Thorner DMO, Tóth M, Trainer PJ, Tsagarakis S, Tzanela M, Vadász J, Vaks V, Verkauskiene R, Wass JA, Webb SM, Weber A, Yamada S, Yarman S, Yeoh P, Yoshimoto K, Zammitt NN. Landscape of familial isolated and young-onset pituitary adenomas: prospective diagnosis in AIP mutation carriers. J. Clin. Endocrinol. Metab. 2015;100(9):E1242–E1254. doi: 10.1210/jc.2015-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villa C, Lagonigro MS, Magri F, Koziak M, Jaffrain-Rea ML, Brauner R, Bouligand J, Junier MP, Di Rocco F, Sainte-Rose C, Beckers A, Roux FX, Daly AF, Chiovato L. Hyperplasia-adenoma sequence in pituitary tumorigenesis related to aryl hydrocarbon receptor interacting protein gene mutation. Endocr. Relat. Cancer. 2011;18(3):347–356. doi: 10.1530/erc-11-0059. [DOI] [PubMed] [Google Scholar]
- 14.Farrell WE, Azevedo MF, Batista DL, Smith A, Bourdeau I, Horvath A, Boguszewski M, Quezado M, Stratakis CA. Unique gene expression profile associated with an early-onset multiple endocrine neoplasia (MEN1)-associated pituitary adenoma. J. Clin. Endocrinol. Metab. 2011;96(11):E1905–E1914. doi: 10.1210/jc.2011-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srirangam Nadhamuni V, Iacovazzo D, Evanson J, Trouillas J, Kurzawinski T, Bhattacharya S, Korbonits M. Unusual cause of gigantism—growth hormone releasing hormone (GHRH)-secreting pancreatic neuroendocrine tumour in a patient with multiple endocrine neoplasia type 1 (MEN1) Endocrine Abstracts. 2019;67:O36. [Google Scholar]
- 16.Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. J. Clin. Endocrinol. Metab. 2014;99(6):1955–1969. doi: 10.1210/jc.2013-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igreja S, Chahal HS, King P, Bolger GB, Srirangalingam U, Guasti L, Chapple JP, Trivellin G, Gueorguiev M, Guegan K, Stals K, Khoo B, Kumar AV, Ellard S, Grossman AB, Korbonits M. Characterization of aryl hydrocarbon receptor interacting protein (AIP) mutations in familial isolated pituitary adenoma families. Hum. Mutat. 2010;31(8):950–960. doi: 10.1002/humu.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312(5777):1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 19.Dénes J, Swords F, Rattenberry E, Stals K, Owens M, Cranston T, Xekouki P, Moran L, Kumar A, Wassif C, Fersht N, Baldeweg SE, Morris D, Lightman S, Agha A, Rees A, Grieve J, Powell M, Boguszewski CL, Dutta P, Thakker RV, Srirangalingam U, Thompson CJ, Druce M, Higham C, Davis J, Eeles R, Stevenson M, O’Sullivan B, Taniere P, Skordilis K, Gabrovska P, Barlier A, Webb SM, Aulinas A, Drake WM, Bevan JS, Preda C, Dalantaeva N, Ribeiro-Oliveira A, Jr., Garcia IT, Yordanova G, Iotova V, Evanson J, Grossman AB, Trouillas J, Ellard S, Stratakis CA, Maher ER, Roncaroli F, Korbonits M. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: results from a large patient cohort. J. Clin. Endocrinol. Metab. 2015;100(3):E531–E541. doi: 10.1210/jc.2014-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4) Mol. Cell Endocrinol. 2014;386(1-2):2–15. doi: 10.1016/j.mce.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alrezk R, Hannah-Shmouni F, Stratakis CA. MEN4 and CDKN1B mutations: the latest of the MEN syndromes. Endocr. Relat. Cancer. 2017;24(10):T195–t208. doi: 10.1530/erc-17-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Graw J, Atkinson MJ. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc. Natl Acad. Sci. USA. 2006;103(42):15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nachtigall LB, Guarda FJ, Lines KE, Ghajar A, Dichtel L, Mumbach G, Zhao W, Zhang X, Tritos NA, Swearingen B, Miller KK, Thakker RV. Clinical MEN-1 among a large cohort of patients with acromegaly. J. Clin. Endocrinol. Metab. 2020;105(6):e2271–e2281. doi: 10.1210/clinem/dgaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borson-Chazot F, Garby L, Raverot G, Claustrat F, Raverot V, Sassolas G. Acromegaly induced by ectopic secretion of GHRH: a review 30 years after GHRH discovery. Ann. Endocrinol. (Paris) 2012;73(6):497–502. doi: 10.1016/j.ando.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Boix E, Picó A, Pinedo R, Aranda I, Kovacs K. Ectopic growth hormone-releasing hormone secretion by thymic carcinoid tumour. Clin. Endocrinol. (Oxf) 2002;57(1):131–134. doi: 10.1046/j.1365-2265.2002.01535.x. [DOI] [PubMed] [Google Scholar]
- 26.A.J Seabrook, J.E. Harris, S.B. Velosa, E. Kim, A.M. McInerney-Leo, T. Dwight, J.I. Hockings, N.G. Hockings, J. Kirk, P.J. Leo, A.J. Love, C. Luxford, M. Marshall, O. Mete, D.J. Pennisi, M.A. Brown, A.J. Gill, G.I. Hockings, R.J. Clifton-Bligh, E.L. Duncan, Multiple endocrine tumors associated with germline MAX mutations: multiple endocrine neoplasia type 5? J. Clin. Endocrinol. Metab. (2021). 10.1210/clinem/dgaa957 [DOI] [PubMed]
- 27.Correa R, Salpea P, Stratakis CA. Carney complex: an update. Eur. J. Endocrinol. 2015;173(4):M85–M97. doi: 10.1530/eje-15-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J. Clin. Endocrinol. Metab. 2001;86(9):4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 29.Matyakhina L, Pack S, Kirschner LS, Pak E, Mannan P, Jaikumar J, Taymans SE, Sandrini F, Carney JA, Stratakis CA. Chromosome 2 (2p16) abnormalities in Carney complex tumours. J. Med. Genet. 2003;40(4):268–277. doi: 10.1136/jmg.40.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tufton N, Roncaroli F, Hadjidemetriou I, Dang MN, Dénes J, Guasti L, Thom M, Powell M, Baldeweg SE, Fersht N, Korbonits M. Pituitary carcinoma in a patient with an SDHB mutation. Endocr. Pathol. 2017;28(4):320–325. doi: 10.1007/s12022-017-9474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xekouki P, Szarek E, Bullova P, Giubellino A, Quezado M, Mastroyannis SA, Mastorakos P, Wassif CA, Raygada M, Rentia N, Dye L, Cougnoux A, Koziol D, Sierra Mde L, Lyssikatos C, Belyavskaya E, Malchoff C, Moline J, Eng C, Maher LJ, 3rd, Pacak K, Lodish M, Stratakis CA. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in humans and mice. J. Clin. Endocrinol. Metab. 2015;100(5):E710–E719. doi: 10.1210/jc.2014-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cambiaso P, Galassi S, Palmiero M, Mastronuzzi A, Del Bufalo F, Capolino R, Cacchione A, Buonuomo PS, Gonfiantini MV, Bartuli A, Cappa M, Macchiaiolo M. Growth hormone excess in children with neurofibromatosis type-1 and optic glioma. Am. J. Med. Genet. A. 2017;173(9):2353–2358. doi: 10.1002/ajmg.a.38308. [DOI] [PubMed] [Google Scholar]
- 33.Barry S, Korbonits M. Update on the genetics of pituitary tumors. Endocrinol. Metab. Clin. North Am. 2020;49(3):433–452. doi: 10.1016/j.ecl.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Dworakowska D, Grossman AB. Are neuroendocrine tumours a feature of tuberous sclerosis? A systematic review. Endocr. Relat. Cancer. 2009;16(1):45–58. doi: 10.1677/erc-08-0142. [DOI] [PubMed] [Google Scholar]
- 35.Joustra SD, Roelfsema F, van Trotsenburg ASP, Schneider HJ, Kosilek RP, Kroon HM, Logan JG, Butterfield NC, Zhou X, Toufaily C, Bak B, Turgeon MO, Brûlé E, Steyn FJ, Gurnell M, Koulouri O, Le Tissier P, Fontanaud P, Duncan Bassett JH, Williams GR, Oostdijk W, Wit JM, Pereira AM, Biermasz NR, Bernard DJ, Schoenmakers N. IGSF1 deficiency results in human and murine somatotrope neurosecretory hyperfunction. J. Clin. Endocrinol. Metab. 2020;105(3):e70–e84. doi: 10.1210/clinem/dgz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallar L, Spada A, Giannattasio G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987;330(6148):566–568. doi: 10.1038/330566a0. [DOI] [PubMed] [Google Scholar]
- 37.D.M. Carty, R. Harte, R.S. Drummond, R. Ward, K. Magid, D. Collier, M. Owens, M. Korbonits, AIP variant causing familial prolactinoma. Pituitary (2020). 10.1007/s11102-020-01085-5 [DOI] [PMC free article] [PubMed]
- 38.Gordon MV, Varma D, McLean CA, Bittar RG, Burgess JR, Topliss DJ. Metastatic prolactinoma presenting as a cervical spinal cord tumour in multiple endocrine neoplasia type one (MEN-1) Clin. Endocrinol. (Oxf) 2007;66(1):150–152. doi: 10.1111/j.1365-2265.2006.02697.x. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Xie W, Rosenblum JS, Zhou J, Guo J, Miao Y, Shen Y, Wang H, Gong L, Li M, Zhao S, Cheng S, Zhu H, Jiang T, Ling S, Wang F, Zhang H, Zhang M, Qu Y, Zhang Q, Li G, Wang J, Ma J, Zhuang Z, Zhang Y. Somatic SF3B1 hotspot mutation in prolactinomas. Nat. Commun. 2020;11(1):2506. doi: 10.1038/s41467-020-16052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.A.P.Y. Liu, M.M. Kelsey, N. Sabbaghian, S.H. Park, C.L. Deal, A.J. Esbenshade, O. Ploner, A. Peet, H. Traunecker, Y.H.E. Ahmed, M. Zacharin, A. Tiulpakov, A.M. Lapshina, A.W. Walter, P. Dutta, A. Rai, M. Korbonits, L. de Kock, K.E. Nichols, W.D. Foulkes, J.R. Priest, Clinical outcomes and complications of pituitary blastoma. J. Clin. Endocrinol. Metab. 106(2), 351–363 (2021). 10.1210/clinem/dgaa857 [DOI] [PMC free article] [PubMed]
- 41.Schultz KAP, Williams GM, Kamihara J, Stewart DR, Harris AK, Bauer AJ, Turner J, Shah R, Schneider K, Schneider KW, Carr AG, Harney LA, Baldinger S, Frazier AL, Orbach D, Schneider DT, Malkin D, Dehner LP, Messinger YH, Hill DA. DICER1 and associated conditions: identification of at-risk individuals and recommended surveillance strategies. Clin. Cancer Res. 2018;24(10):2251–2261. doi: 10.1158/1078-0432.Ccr-17-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonds WF, Varghese S, Marx SJ, Nieman LK. Cushing’s syndrome in multiple endocrine neoplasia type 1. Clin. Endocrinol. (Oxf) 2012;76(3):379–386. doi: 10.1111/j.1365-2265.2011.04220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chasseloup F, Pankratz N, Lane J, Faucz FR, Keil MF, Chittiboina P, Kay DM, Hussein Tayeb T, Stratakis CA, Mills JL, Hernández-Ramírez LC. Germline CDKN1B loss-of-function variants cause pediatric Cushing’s disease with or without an MEN4 phenotype. J. Clin. Endocrinol. Metab. 2020;105(6):1983–2005. doi: 10.1210/clinem/dgaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernández-Ramírez LC, Gam R, Valdés N, Lodish MB, Pankratz N, Balsalobre A, Gauthier Y, Faucz FR, Trivellin G, Chittiboina P, Lane J, Kay DM, Dimopoulos A, Gaillard S, Neou M, Bertherat J, Assié G, Villa C, Mills JL, Drouin J, Stratakis CA. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing’s disease. Endocr. Relat. Cancer. 2017;24(8):379–392. doi: 10.1530/erc-17-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bengtsson D, Joost P, Aravidis C, Askmalm Stenmark M, Backman AS, Melin B, von Salomé J, Zagoras T, Gebre-Medhin S, Burman P. Corticotroph pituitary carcinoma in a patient with Lynch syndrome (LS) and pituitary tumors in a nationwide LS cohort. J. Clin. Endocrinol. Metab. 2017;102(11):3928–3932. doi: 10.1210/jc.2017-01401. [DOI] [PubMed] [Google Scholar]
- 46.Naziat A, Karavitaki N, Thakker R, Ansorge O, Sadler G, Gleeson F, Cranston T, McCormack A, Grossman AB, Shine B. Confusing genes: a patient with MEN2A and Cushing’s disease. Clin. Endocrinol. (Oxf) 2013;78(6):966–968. doi: 10.1111/cen.12072. [DOI] [PubMed] [Google Scholar]
- 47.Kasturi K, Fernandes L, Quezado M, Eid M, Marcus L, Chittiboina P, Rappaport M, Stratakis CA, Widemann B, Lodish M. Cushing disease in a patient with multiple endocrine neoplasia type 2B. J. Clin. Transl. Endocrinol. Case Rep. 2017;4:1–4. doi: 10.1016/j.jecr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T, Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, Tanaka K, Wieland T, Graf E, Saeger W, Ronchi CL, Allolio B, Buchfelder M, Strom TM, Fassnacht M, Komada M. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 2015;47(1):31–38. doi: 10.1038/ng.3166. [DOI] [PubMed] [Google Scholar]
- 49.Wanichi IQ, de Paula Mariani BM, Frassetto FP, Siqueira SAC, de Castro Musolino NR, Cunha-Neto MBC, Ochman G, Cescato VAS, Machado MC, Trarbach EB, Bronstein MD, Fragoso M. Cushing’s disease due to somatic USP8 mutations: a systematic review and meta-analysis. Pituitary. 2019;22(4):435–442. doi: 10.1007/s11102-019-00973-9. [DOI] [PubMed] [Google Scholar]
- 50.Cohen M, Persky R, Stegemann R, Hernández-Ramírez LC, Zeltser D, Lodish MB, Chen A, Keil MF, Tatsi C, Faucz FR, Buchner DA, Stratakis CA, Tiosano D. Germline USP8 mutation associated with pediatric cushing disease and other clinical features: a new syndrome. J. Clin. Endocrinol. Metab. 2019;104(10):4676–4682. doi: 10.1210/jc.2019-00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williamson EA, Ince PG, Harrison D, Kendall-Taylor P, Harris PE. G-protein mutations in human pituitary adrenocorticotrophic hormone-secreting adenomas. Eur. J. Clin. Invest. 1995;25(2):128–131. doi: 10.1111/j.1365-2362.1995.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 52.Riminucci M, Collins MT, Lala R, Corsi A, Matarazzo P, Gehron Robey P, Bianco P. An R201H activating mutation of the GNAS1 (Gsalpha) gene in a corticotroph pituitary adenoma. Mol. Pathol. 2002;55(1):58–60. doi: 10.1136/mp.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiefer FW, Winhofer Y, Iacovazzo D, Korbonits M, Wolfsberger S, Knosp E, Trautinger F, Höftberger R, Krebs M, Luger A, Gessl A. PRKAR1A mutation causing pituitary-dependent Cushing disease in a patient with Carney complex. Eur. J. Endocrinol. 2017;177(2):K7–k12. doi: 10.1530/eje-17-0227. [DOI] [PubMed] [Google Scholar]
- 54.Hernández-Ramírez LC, Tatsi C, Lodish MB, Faucz FR, Pankratz N, Chittiboina P, Lane J, Kay DM, Valdés N, Dimopoulos A, Mills JL, Stratakis CA. Corticotropinoma as a component of Carney complex. J. Endocr. Soc. 2017;1(7):918–925. doi: 10.1210/js.2017-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O. Casar-Borota, H.B. Boldt, B.E. Engström, M.S. Andersen, B. Baussart, D. Bengtsson, K. Berinder, B. Ekman, U. Feldt-Rasmussen, C. Höybye, J.O.L. Jørgensen, A.J. Kolnes, M. Korbonits, Å.K. Rasmussen, J.R. Lindsay, P.B. Loughrey, D. Maiter, E. Manojlovic-Gacic, J. Pahnke, P.L. Poliani, V. Popovic, O. Ragnarsson, C. Schalin-Jäntti, D. Scheie, M. Tóth, C. Villa, M. Wirenfeldt, J. Kunicki, P. Burman, Corticotroph aggressive pituitary tumours and carcinomas frequently harbour ATRX mutations. J. Clin. Endocrinol. Metab. (2020). 10.1210/clinem/dgaa749
- 56.C.E. Stiles, M. Korbonits, in Familial Isolated Pituitary Adenoma, ed. by K.R. Feingold, B. Anawalt, A. Boyce, G. Chrousos, W.W. de Herder, K. Dungan, A. Grossman, J.M. Hershman, H.J. Hofland, G. Kaltsas, C. Koch, P. Kopp, M. Korbonits, R. McLachlan, J.E. Morley, M. New, J. Purnell, F. Singer, C.A. Stratakis, D.L. Trence, D.P. Wilson. Endotext. MDText.com, Inc.Copyright © 2000-2020, MDText.com, Inc., South Dartmouth (MA), 2000.
- 57.Taylor TJ, Donlon SS, Bale AE, Smallridge RC, Francis TB, Christensen RS, Burma KD. Treatment of a thyrotropinoma with octreotide-LAR in a patient with multiple endocrine neoplasia-1. Thyroid. 2000;10(11):1001–1007. doi: 10.1089/thy.2000.10.1001. [DOI] [PubMed] [Google Scholar]
- 58.Scheithauer BW, Kovacs K, Nose V, Lombardero M, Osamura YR, Lloyd RV, Horvath E, Pagenstecher A, Bohl JE, Tews DS. Multiple endocrine neoplasia type 1-associated thyrotropin-producing pituitary carcinoma: report of a probable de novo example. Hum. Pathol. 2009;40(2):270–278. doi: 10.1016/j.humpath.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Erickson D, Scheithauer B, Atkinson J, Horvath E, Kovacs K, Lloyd RV, Young WF., Jr Silent subtype 3 pituitary adenoma: a clinicopathologic analysis of the Mayo Clinic experience. Clin. Endocrinol. (Oxf) 2009;71(1):92–99. doi: 10.1111/j.1365-2265.2008.03514.x. [DOI] [PubMed] [Google Scholar]
- 60.Mete O, Gomez-Hernandez K, Kucharczyk W, Ridout R, Zadeh G, Gentili F, Ezzat S, Asa SL. Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Mod. Pathol. 2016;29(2):131–142. doi: 10.1038/modpathol.2015.151. [DOI] [PubMed] [Google Scholar]
- 61.Trouillas J, Labat-Moleur F, Sturm N, Kujas M, Heymann MF, Figarella-Branger D, Patey M, Mazucca M, Decullier E, Vergès B, Chabre O, Calender A. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am. J. Surg. Pathol. 2008;32(4):534–543. doi: 10.1097/PAS.0b013e31815ade45. [DOI] [PubMed] [Google Scholar]
- 62.Ando S, Sarlis NJ, Oldfield EH, Yen PM. Somatic mutation of TRbeta can cause a defect in negative regulation of TSH in a TSH-secreting pituitary tumor. J. Clin. Endocrinol. Metab. 2001;86(11):5572–5576. doi: 10.1210/jcem.86.11.7984. [DOI] [PubMed] [Google Scholar]
- 63.Teng X, Jin T, Brent GA, Wu A, Teng W, Shan Z. A patient with a thyrotropin-secreting microadenoma and resistance to thyroid Hormone (P453T) J. Clin. Endocrinol. Metab. 2015;100(7):2511–2514. doi: 10.1210/jc.2014-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furumoto H, Ying H, Chandramouli GV, Zhao L, Walker RL, Meltzer PS, Willingham MC, Cheng SY. An unliganded thyroid hormone beta receptor activates the cyclin D1/cyclin-dependent kinase/retinoblastoma/E2F pathway and induces pituitary tumorigenesis. Mol. Cell. Biol. 2005;25(1):124–135. doi: 10.1128/mcb.25.1.124-135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marques P, Caimari F, Hernández-Ramírez LC, Collier D, Iacovazzo D, Ronaldson A, Magid K, Lim CT, Stals K, Ellard S, Grossman AB, Korbonits M. Significant benefits of AIP testing and clinical screening in familial isolated and young-onset pituitary tumors. J. Clin. Endocrinol. Metab. 2020;105(6):e2247–e2260. doi: 10.1210/clinem/dgaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cuny T, Pertuit M, Sahnoun-Fathallah M, Daly A, Occhi G, Odou MF, Tabarin A, Nunes ML, Delemer B, Rohmer V, Desailloud R, Kerlan V, Chabre O, Sadoul JL, Cogne M, Caron P, Cortet-Rudelli C, Lienhardt A, Raingeard I, Guedj AM, Brue T, Beckers A, Weryha G, Enjalbert A, Barlier A. Genetic analysis in young patients with sporadic pituitary macroadenomas: besides AIP don’t forget MEN1 genetic analysis. Eur. J. Endocrinol. 2013;168(4):533–541. doi: 10.1530/eje-12-0763. [DOI] [PubMed] [Google Scholar]
- 67.Korbonits M, Storr H, Kumar AV. Familial pituitary adenomas—who should be tested for AIP mutations? Clin. Endocrinol. (Oxf) 2012;77(3):351–356. doi: 10.1111/j.1365-2265.2012.04445.x. [DOI] [PubMed] [Google Scholar]
- 68.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J. Clin. Endocrinol. Metab. 2012;97(9):2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 69.Tudorancea A, François P, Trouillas J, Cottier JP, Girard JJ, Jan M, Gilbert-Dussardier B, Richard S, Lecomte P. Von Hippel-Lindau disease and aggressive GH-PRL pituitary adenoma in a young boy. Ann. Endocrinol. (Paris) 2012;73(1):37–42. doi: 10.1016/j.ando.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Miljic D, Pekic S, Doknic M, Nikolic-Djurovic M, Stojanovic M, Manojlovic-Gacic E, Korbonits M, Trouillas J, Vasiljevic A, Popovic V, Petakov M. Changes in pituitary tumour biology and behaviour in FIPA patient with GH secreting aggressive pituitary macro adenoma. Endocrine Abstracts. 2017;49:EP954. doi: 10.1530/endoabs.49.EP954. [DOI] [Google Scholar]
- 71.Tigas S, Carroll PV, Jones R, Bingham E, Russell-Jones D, Powell M, Scobie IN. Simultaneous Cushing’s disease and tuberous sclerosis; a potential role for TSC in pituitary ontogeny. Clin. Endocrinol. (Oxf) 2005;63(6):694–695. doi: 10.1111/j.1365-2265.2005.02374.x. [DOI] [PubMed] [Google Scholar]
- 72.Nandagopal R, Vortmeyer A, Oldfield EH, Keil MF, Stratakis CA. Cushing’s syndrome due to a pituitary corticotropinoma in a child with tuberous sclerosis: an association or a coincidence? Clin. Endocrinol. (Oxf) 2007;67(4):639–641. doi: 10.1111/j.1365-2265.2007.02941.x. [DOI] [PubMed] [Google Scholar]
- 73.Uraki S, Ariyasu H, Doi A, Furuta H, Nishi M, Sugano K, Inoshita N, Nakao N, Yamada S, Akamizu T. Atypical pituitary adenoma with MEN1 somatic mutation associated with abnormalities of DNA mismatch repair genes; MLH1 germline mutation and MSH6 somatic mutation. Endocr. J. 2017;64(9):895–906. doi: 10.1507/endocrj.EJ17-0036. [DOI] [PubMed] [Google Scholar]
- 74.Voisin MR, Almeida JP, Perez-Ordonez B, Zadeh G. Recurrent undifferentiated carcinoma of the sella in a patient with Lynch syndrome. World Neurosurg. 2019;132:219–222. doi: 10.1016/j.wneu.2019.08.180. [DOI] [PubMed] [Google Scholar]