Abstract
The aim of this study was to reveal the histological features of oxyntic gland adenomas and gastric adenocarcinoma of the fundic-gland type (GA-FG). We retrospectively examined the histological features of 126 lesions of oxyntic gland adenoma and/or GA-FG in 116 patients. The prevalence of oxyntic gland adenomas and GA-FG was approximately equal. The majority of the lesions were resected by endoscopic mucosal resection using a diathermic snare (EMR, n = 42) or endoscopic submucosal dissection (ESD, n = 72). Histologically, there were no lesions with invasion at the level of the muscularis propria or deeper, and lymphovascular invasion was present in 1.6%. Of the ESD and EMR specimens, there were no lesions that were positive for vertical margins. Among the eight GA-FG patients with deep (≥ 500 μm) submucosal invasion, six were treated with endoscopic resection alone, and no recurrence was documented. No patients died of the disease during the median follow-up period of 14.5 months. In conclusion, all lesions were confined to the mucosa or submucosa and were negative for vertical margins. Lymphovascular invasion was present in only 1.6% of the patients. Thus, we believe that endoscopic resection is a suitable initial treatment method for oxyntic gland adenoma and GA-FG.
Subject terms: Cancer, Cancer therapy, Gastrointestinal cancer
Introduction
Gastric adenocarcinoma of the fundic gland type (GA-FG) is an epithelial neoplasm composed of columnar cells with differentiation to chief cells, parietal cells, or both. Since GA-FG was first reported as a single case report in 20071, histological, clinical, endoscopic, and genetic features have been investigated and documented in multiple case reports and case series2–21. With the increasing popularity of the disease concept, GA-FG has been formally cited as a variant of gastric adenocarcinoma in the latest version of the classification of gastric neoplasms issued by the World Health Organization (WHO)22. Historically, this disease entity was variably named gastric adenocarcinoma with chief cell differentiation1,5, gastric neoplasia of the fundic gland (chief cell-predominant) type2,7,9, gastric fundic gland-associated neoplasms/polyps13, gastric adenocarcinoma of the fundic gland (chief cell predominant type)14,16, and chief-cell predominant gastric polyps15. According to the WHO classification, a neoplasm confined to the mucosa is called an oxyntic gland adenoma, while a neoplasm with submucosal invasion is classified as GA-FG22. Due to their infrequency, treatment strategies for oxyntic gland adenoma and GA-FG have not yet been established. In this study, we included 126 lesions of oxyntic gland adenoma and/or GA-FG from 116 patients across 10 institutions and retrospectively examined the histological features, which is an essential step in the determination of an appropriate initial treatment strategy. The clinical and endoscopic features of this disease have also been summarized.
Methods
Letters of inquiry were sent to nine collaborating institutions with patients with histologically diagnosed oxyntic gland adenoma and GA-FG, from the Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences. Histological diagnoses were made based on endoscopic biopsy, endoscopic mucosal resection, endoscopic submucosal dissection, and/or surgical resection. The diagnoses of oxyntic gland adenoma and GA-FG were made based on the presence of intramucosal proliferation of differentiated columnar cells with pale basophilic cytoplasm and mild nuclear atypia, which have been found to mimic the oxyntic (fundic) gland22. The diagnoses were supported by positive immunohistochemical staining for pepsinogen I and MUC6 in some patients. We identified 116 patients who were diagnosed with oxyntic gland adenoma and/or GA-FG between October 2008 and February 2020. These patients were retrospectively enrolled in the study.
We retrospectively examined the patients’ sex, age at diagnosis, Helicobacter pylori infection status, endoscopic features, histological features, treatments, and prognoses. In patients with two or more lesions, a second or third primary lesion identified within 12 months of the detection of the first lesion were defined as “synchronous” multifocal lesions, while those identified more than 12 months after the detection of the first lesion were classified as “metachronous” multifocal lesions. H. pylori infection status was examined by urea breath tests, rapid urease tests, microscopic observations or culture tests on endoscopically biopsied specimens, stool antigen tests, serum or urine antibody tests, or a combination of these methods. Based on these test results, H. pylori infection status was classified into one of three groups: (i) uninfected (the gastric mucosa has never been infected by H. pylori), (ii) active gastritis (the gastric mucosa is currently infected by H. pylori), and (iii) inactive-gastritis (the gastric mucosa was previously infected by H. pylori, but H. pylori was absent following eradication treatment or spontaneous loss due to advanced atrophy).
Lesion morphologies and depth of invasion were classified according to the Japanese Classification of Gastric Carcinoma23,24. Briefly, polypoid-protruding tumors were classified as type 0–I. Slightly elevated superficial tumors were defined as type 0–IIa. Superficial flat tumors without elevation or depression were classified as type 0–IIb. Slightly depressed tumors were defined as type 0–IIc. Excavated tumors with depressions were classified as type 0–III. In terms of the invasion depth, T1a represents a tumor confined to the mucosa, T1b1 indicates a tumor with invasion < 500 μm from the muscularis mucosa, and T1b2 is a tumor invading ≥ 500 μm from the muscularis mucosa. The follow-up period was defined as the time from endoscopic or surgical resection of gastric lesions to death from any cause or the last hospital visit. The endoscopic follow-up period was defined as the time from endoscopic or surgical resection of gastric lesions to the last esophagogastroduodenoscopy examination.
The primary purpose of this study was to measure the prevalence of submucosal (T1b1/T1b2), vascular, and lymphatic invasion in oxyntic gland adenoma and GA-FG patients in order to determine the appropriate initial treatment strategy. Patients’ clinical and endoscopic characteristics, as well as their prognostic outcomes, were also reviewed.
This study was approved by the Ethics Committees of Okayama University Hospital and other institutions and adhered to the Declaration of Helsinki. Written informed consent was waived because of the observational, non-interventional, and retrospective design. All investigations were performed in accordance with the relevant guidelines and regulations.
Results
The patient characteristics are listed in Table 1. This study included 75 men and 41 women. The mean age at diagnosis of oxyntic gland adenoma or GA-FG was 66.4 years (range 46–91 years), and 101 patients were 60 years or older (87.1%). Most patients (n = 108, 93.1%) had a single lesion, six patients (5.2%) had two lesions, and two patients (1.7%) had three lesions. Among the 8 patients with multifocal lesions, synchronous occurrence of oxyntic gland adenoma or GA-FG was observed in 7 patients, while the remaining patients presented with two metachronously developed lesions that were diagnosed 13 months apart. Thus, 126 lesions of oxyntic gland adenoma or GA-FG were included in this study.
Table 1.
Clinical characteristics of the study population.
| N | % | |
|---|---|---|
| Sex | ||
| Male | 75 | 64.7 |
| Female | 41 | 35.3 |
| Mean age (range), years | 66.4 (46–91) | |
| No. of lesions | ||
| 1 | 108 | 93.1 |
| 2 | 6 | 5.2 |
| 3 | 2 | 1.7 |
| H. pylori infection status | ||
| Uninfected | 38 | 30.2 |
| Active gastritis | 13 | 10.3 |
| Inactive gastritis | 60 | 47.6 |
| Undeterminable | 15 | 11.9 |
| Treatments | ||
| ESD | 72 | 57.1 |
| EMR | 42 | 33.3 |
| Surgery | 3 | 2.4 |
| ESD followed by surgery | 2 | 1.6 |
| None | 7 | 5.6 |
| Median follow-up period (range), months | 14.5 (0–107) | |
| Median endoscopic follow-up period (range), months* | 11 (0–107) | |
| Outcome | ||
| Alive | 112 | 96.6 |
| Died of other cause | 3 | 2.6 |
| Unknown | 1 | 0.9 |
*n = 113. ESD, endoscopic submucosal dissection; EMR endoscopic mucosal resection using a diathermic snare.
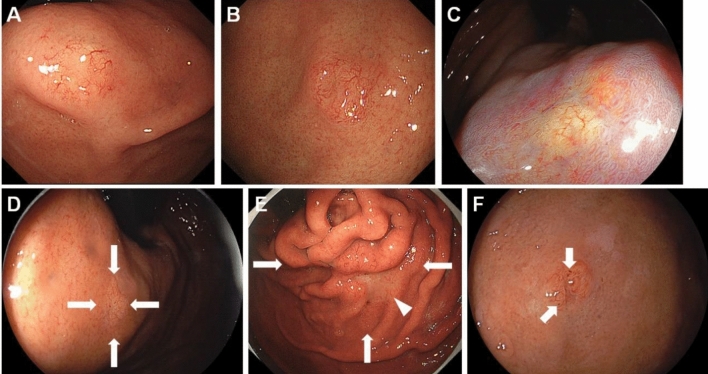
Representative endoscopic images of oxyntic gland adenoma and GA-FG are shown in Fig. 1, and the endoscopic features of the 126 lesions are listed in Table 2. Oxyntic gland adenoma and GA-FG were most frequently observed in the gastric body (n = 66, 52.4%), followed by the fornix (n = 38, 30.2%) and cardia (n = 21, 16.7%). Among the lesions developing in the gastric body, 34 lesions were found in the upper third portion, 24 lesions in the middle third portion, and 8 lesions in the lower third portion. No lesions were identified in the gastric antrum or pylorus. The mean lesion size was 6.2 mm (range 2–25 mm). Notably, the lesion size was 10 mm or less in 117 lesions (92.9%). Oxyntic gland adenoma and GA-FG appeared as 0–IIa (n = 72, 57.1%), 0–IIb (n = 39, 31.0%), 0–IIc (n = 9, 7.1%), 0–IIa + IIc (n = 4, 3.2%), or 0–I (n = 2, 1.6%). Macroscopically, 64 lesions (50.8%) exhibited a subepithelial lesion. The color of the lesion was similar to that of the peripheral mucosa (n = 49, 38.9%), yellowish-white (n = 34, 27.0%), whitish (n = 22, 17.5%), reddish (n = 14, 11.1%), or yellowish (n = 7, 5.6%). Vascular dilatation was present on the surface of 81 lesions (64.3%), and black pigmentation was observed in 18 lesions (14.3%).
Figure 1.
Representative endoscopic images of oxyntic gland adenoma and/or gastric adenocarcinoma of fundic-gland type. (A) A typical image of oxyntic gland adenoma showing a slightly whitish, elevated lesion of 5 mm, with vascular dilatation on the surface. (B) A representative image of gastric adenocarcinoma of fundic-gland type (T1b1) presented as a slightly yellowish, elevated lesion of 7 mm, with vascular dilatation. (C) An oxyntic gland adenoma showing a flat, whitish lesion (linked color imaging). (D) A gastric adenocarcinoma of fundic-gland type (pT1b1) showing a flat lesion. The color of the lesion is similar to that of the peripheral gastric mucosa. (E) Gastric adenocarcinoma of fundic-gland type (T1b2) presented as the largest tumor (25 mm, arrows). The lesion is composed of swollen gastric folds and a whitish, depressed area in the center (arrowhead). Vascular dilatation is observed throughout the lesion. (F) A gastric adenocarcinoma of fundic-gland type (pT1b1) accompanying black pigmentation (arrows).
Table 2.
Endoscopic characteristics of the study population.
| N | % | |
|---|---|---|
| Location | ||
| Fornix | 38 | 30.2 |
| Cardia | 21 | 16.7 |
| Body | 66 | 52.4 |
| Upper third of the body | 34 | |
| Middle third of the body | 24 | |
| Lower third of the body | 8 | |
| Angle | 1 | 0.8 |
| Antrum | 0 | 0.0 |
| Pylorus | 0 | 0.0 |
| Mean size (range), mm | 6.2 (2–25) | |
| Morphology | ||
| 0–I | 2 | 1.6 |
| 0–IIa | 72 | 57.1 |
| 0–IIa + IIc | 4 | 3.2 |
| 0–IIb | 39 | 31.0 |
| 0–IIc | 9 | 7.1 |
| 0–III | 0 | 0.0 |
| Macroscopic appearance | ||
| SEL-like | 64 | 50.8 |
| Non SEL-like | 62 | 49.2 |
| Color | ||
| Similar to the peripheral mucosa | 49 | 38.9 |
| Reddish | 14 | 11.1 |
| Whitish | 22 | 17.5 |
| Yellowish-white | 34 | 27.0 |
| Yellowish | 7 | 5.6 |
| Vascular dilatation on the surface | ||
| Present | 81 | 64.3 |
| Absent | 45 | 35.7 |
| Black pigmentation | ||
| Present | 18 | 14.3 |
| Absent | 108 | 85.7 |
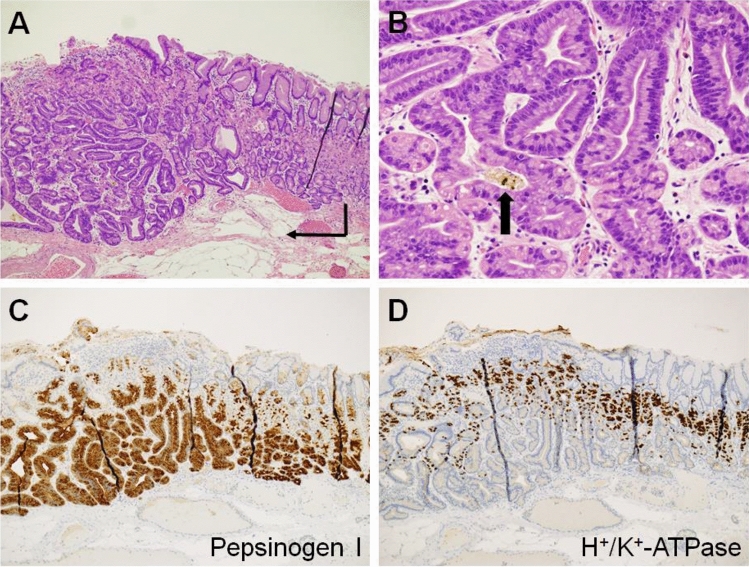
The histological features of oxyntic gland adenoma and GA-FG are listed in Table 3, and typical histological images of GA-FG are shown in Fig. 2. Information on the depth of invasion was available for 107 lesions; 51 lesions were T1a (40.5%), 48 lesions were T1b1 (38.1%), and 8 lesions were T1b2 (6.3%). No lesions invaded the muscularis propria or deeper. Vascular invasion was present in 1/104 lesions (0.8%), and lymphatic invasion was present in 1/106 lesions (0.8%). Immunohistochemistry showed that 67/71 lesions were positive for pepsinogen I (94.4%), 71/82 lesions were positive for MUC6 (86.6%), of 24/39 lesions were positive for H+/K+-ATPase (61.5%), and 9/73 lesions were positive for MUC5AC (12.3%). No lesions were positive for MUC2 (0/23, 0.0%) or CD10 (0/15, 0.0%).
Table 3.
Histological characteristics of the study population.
| N | % | |
|---|---|---|
| Depth of invasion | ||
| T1a | 51 | 40.5 |
| T1b1 | 48 | 38.1 |
| T1b2 | 8 | 6.3 |
| Not available | 19 | 15.1 |
| Vascular invasion | ||
| Present | 1 | 0.8 |
| Absent | 103 | 81.7 |
| Not available | 22 | 17.5 |
| Lymphatic invasion | ||
| Present | 1 | 0.8 |
| Absent | 105 | 83.3 |
| Not available | 20 | 15.9 |
| Pepsinogen I | ||
| Positive | 67 | 94.4 |
| Negative | 4 | 5.6 |
| MUC6 | ||
| Positive | 71 | 86.6 |
| Negative | 11 | 13.4 |
| H+/K+-ATPase | ||
| Positive | 24 | 61.5 |
| Negative | 15 | 38.5 |
| MUC5AC | ||
| Positive | 9 | 12.3 |
| Negative | 64 | 87.7 |
| MUC2 | ||
| Positive | 0 | 0.0 |
| Negative | 23 | 100.0 |
| CD10 | ||
| Positive | 0 | 0.0 |
| Negative | 15 | 100.0 |
Figure 2.
Typical histological images of a gastric adenocarcinoma of fundic-gland type (pT1b1). Endoscopic submucosal dissection specimen of Fig. 1F. (A) Neoplastic cells form a more complex gland structure (hematoxylin and eosin staining, × 4.2, arrow), compared to their normal oxyntic counterparts (right portion of image). The neoplastic cells are mainly observed in the deeper portion of the mucosa and covered with foveolar epithelium. (B) Tumor cells are composed of highly differentiated columnar cells with mild nuclear atypia and a pale basophilic cytoplasm (hematoxylin and eosin staining, × 20). Brown to black pigments are observed in the dilated glands lined by the neoplastic cells (arrow). (C) The tumor cells are positive for pepsinogen-I. (D) The tumor cells are partially positive for H+/K+-ATPase.
Oxyntic gland adenoma and GA-FG were resected by endoscopic submucosal dissection (ESD, n = 72, 57.1%), endoscopic mucosal resection using a diathermic snare (EMR, n = 42, 33.3%), surgery (n = 3, 2.4%), or ESD followed by surgery (n = 2, 1.6%) (Table 1). EMR in one patient resulted in a partially positive horizontal margin, while none of the patients had positive vertical margins. One patient with T1b2 GA-FG underwent additional surgery after ESD according to the attending physician’s recommendation. The other patient with T1b1 GA-FG underwent surgical resection after ESD at the patient’s request. No lymph node metastases were observed in the surgically resected specimens. During the median follow-up period of 14.5 months (range 0–107 months), 112 patients were still alive (96.6%), while three patients died of causes other than oxyntic gland adenoma or GA-FG (2.6%), including cecal cancer, small intestine cancer, and recurrence after cholangiocellular carcinoma. The outcomes of the remaining patients were unavailable. Repeat esophagogastroduodenoscopy was performed in 113 lesions (89.7%), with a median endoscopic follow-up period of 11 months (range 0–107 months). Local recurrence was identified in 2 patients. Although the initial lesion was confined to the mucosa (T1a), EMR in one patient resulted in a positive horizontal margin, and recurrence of oxyntic gland adenoma was noted on the stomach scar 45 months after the initial treatment. The other patient also had recurrence of oxyntic gland adenoma on the stomach scar, despite curative resection of the initial lesion by ESD with negative horizontal and vertical margins and no lymphatic or vascular invasion. Both patients underwent no additional treatment and were followed up with endoscopic and radiological examinations.
When considering the 8 patients with T1b2 GA-FG (Table 4), one patient underwent surgical resection and another patient underwent ESD followed by surgery, while 5 patients were treated with ESD alone, and the remaining patient was treated with EMR alone. In the 6 patients who underwent ESD or EMR alone, no distant metastases or recurrences were identified during the median endoscopic follow-up period of 10.5 months (0–54 months).
Table 4.
Characteristics of the patients with submucosal invasion of 500 μm or more (T1b2).
| Case no | Sex | Age at diagnosis (years) | Morphology | Size (mm) | Treatment | Submucosal invasion (μm) | Endoscopic follow-up period (mo.) | Recurrence | Follow-up period (mo.) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 76 | 0–IIa | 7 | ESD | 870 | 29 | None | 61 | Alive |
| 2 | F | 66 | 0–IIa + IIc | 15 | ESD | 2700 | 54 | None | 64 | Dead by other cause |
| 3 | M | 59 | 0–IIa + IIc | 10 | ESD | 880 | 19 | None | 21 | Alive |
| 4 | M | 86 | 0–IIa | 15 | ESD | 900 | 0 | None | 2 | Alive |
| 5 | M | 64 | 0–IIa | 12 | ESD | 649 | 0 | None | 0 | Alive |
| 6 | M | 55 | 0–I | 5 | EMR | 640 | 2 | None | 2 | Alive |
| 7 | M | 46 | 0–IIa + IIc | 25 | Surgery | > 500 | N/A | None | 0 | Alive |
| 8 | M | 61 | 0–IIa + IIc | 15 | ESD followed by surgery | > 500 | 14 | None | 14 | Alive |
F female, M male, ESD endoscopic submucosal dissection, EMR endoscopic mucosal resection using a diathermic snare, N/A not applicable.
Discussion
To our knowledge, this study included the largest number of patients with oxyntic gland adenoma and/or GA-FG. Based on histological analyses, 51 lesions were confined to the muscularis propria, and submucosal invasion was identified in 56 lesions. Thus, the prevalence of oxyntic gland adenoma and GA-FG was approximately equal in this study. In 111 reported cases, Benedict et al. showed that the invasion depth reached the mucosa (n = 31), muscularis mucosae (n = 1), submucosa (n = 63), serosa (n = 1), muscularis propria (n = 1), and undetermined (n = 14)25. Thus, the incidence rates of oxyntic gland adenoma and GA-FG were estimated to be 1:222. The discrepancy in the prevalence between our study and previous reports may have been caused by publication biases because more lesions with submucosal invasion (GA-FG) may be published than those confined within the mucosa (oxyntic gland adenoma). In the present study, most of the lesions were resected using either ESD or EMR (n = 114, 90.5%). There were no lesions with invasion at the level of the muscularis propria or deeper, and lymphovascular invasion was present in only 1.6% of lesions. Although EMR resulted in a positive horizontal margin in one patient, there were no lesions with exposed tumor cells in the vertical margins of the endoscopic resection specimens. These results indicate that endoscopic resection is a suitable initial treatment strategy for oxyntic gland adenoma and GA-FG.
Notably, 6 of the 8 GA-FG patients with deep submucosal invasion (T1b2) were followed up without additional surgical resection after endoscopic treatment. No recurrence was documented at a median endoscopic follow-up period of 10.5 months. In Japanese gastric cancer treatment guidelines, endoscopically resected-T1b2 tumors are defined as endoscopic curability C-2 (eCuraC-2), and gastrectomy with lymphadenectomy should be considered23,24. Despite the recommendations described in these guidelines, most attending physicians followed up with the patients. These decisions were probably supported by the fact that the neoplastic cells in the submucosa can be regarded as “prolapse-type” misplaced glands showing minimal desmoplastic changes, rather than true submucosal invasion21,25. However, further investigations with longer observation periods are required to elucidate the appropriate treatment strategies for oxyntic gland adenoma and GA-FG.
Although some debate remains regarding the biological behavior of oxyntic gland adenoma and GA-FG, it is widely accepted that this gastric neoplasm rarely affects lymphatic and vascular systems and grows slowly8,9,12,14,15. Lymphatic and/or vascular invasion was positive in 7/94 reported cases (7.4%)25 and 2/126 in the present study (1.6%). Additionally, no patients died of oxyntic gland adenoma or GA-FG in our study or in previous reports. These data suggest that oxyntic gland adenoma and GA-FG are neoplasms with low malignant potential, resulting in a favorable prognosis. Thus, discussions are still ongoing on the correct nomenclature25 and some pathologists favor terms, such as “oxyntic gland adenomas,” “fundic gland adenoma,” or “fundic gland polyps with chief or parietal cell differentiation,” in place of “adenocarcinoma.” In contrast, a patient with GA-FG infiltrating into the subserosa with lymphatic and venous invasion has been reported10. Ushiku et al. proposed that GA-FG with atypical cellular differentiation, such as mucous neck cells and foveolar epithelium in addition to chief cells and parietal cells, be subcategorized as “adenocarcinoma of fundic gland mucosa type” as they are probably a more aggressive phenotype21. Since lesions of oxyntic gland adenoma and GA-FG are likely to be heterogeneous, it is necessary to establish appropriate histological subcategorization and terminology.
We revealed that these neoplasms developed in patients aged 60 years or older in most cases (87.1%), with a male-to-female ratio of approximately 2:1. Male predominance and frequent occurrence in patients aged 60 years or older has been previously reported14,22,25. Oxyntic gland adenoma and GA-FG mostly occurred solely (93.1%), with the development of two or three lesions occurring in a limited number of patients. As the second and third lesions were identified within 12 months from the diagnosis of the initial lesion, except in one patient, careful observation is recommended during esophagogastroduodenoscopy to avoid overlooking other lesions of oxyntic gland adenoma and GA-FG. H. pylori infection reportedly has no association with the development of these lesions22,25. Chiba et al. reported that among 20 patients with oxyntic gland adenoma or GA-FG, 5 patients were uninfected with H. pylori (25.0%), while the remaining 15 patients had active or inactive gastritis (75.0%)14. We also revealed that the H. pylori infection status varied from inactive gastritis (47.6%) and uninfected (30.2%) to active gastritis (10.3%). These results reinforce the hypothesis that oxyntic gland adenoma or GA-FG develops irrespective of H. pylori infection status.
The typical endoscopic features of oxyntic gland adenoma or GA-FG are known as a whitish small elevation of < 10 mm in diameter, with irregular branching vessels, predominantly occurring in the upper third portion of the stomach14,22,25. In the present study, 73.8% of the lesions were found in the upper third portion of the stomach (i.e., the fornix, cardia, and upper third of the body), while the other lesions were identified in the middle third portion (i.e., the middle to lower third of the body and angle). No lesions were observed in the lower third portion of the stomach. The lesion size was 10 mm or less in most cases (92.9%). A slightly elevated superficial morphology accounted for more than half of the lesions (57.1%), and a flat tumor, without elevation or depression, represented approximately one-third of the lesions (31.0%). Half of the lesions showed a subepithelial lesion (50.8%). Vascular dilatation was present on the surface of approximately two-thirds of the lesions (64.3%). Although the color of the lesions in oxyntic gland adenoma and GA-FG varied from a color similar to the peripheral mucosa, to yellowish-white, white, reddish, and yellowish, the macroscopic features observed in the present study were consistent with those reported previously22,25. Brownish or black pigmentation has been described in several case reports12,17,19, which are considered to reflect pigmented substances contained in the dilated glands of neoplastic cells (Fig. 2B). Such pigmentation was identified in 14.3% of lesions in this study.
This study had several limitations. First, endoscopic and histological analyses were performed at various institutions. It is possible that interobserver variations and differences in methodologies between the participating institutions may have resulted in heterogeneous patient data. However, our results could be generalized to institutions worldwide. Second, the follow-up period following diagnosis of oxyntic gland adenoma and/or GA-FG was relatively short (median 14.5 months), resulting in insufficient analysis of patient outcomes. Complete follow-up with a longer observation period should be considered in future investigations. Third, immunohistochemical studies were not performed for all patients. Although diagnosis can essentially be made according to the WHO classification criteria based on the presence of intramucosal proliferation of differentiated columnar cells with pale basophilic cytoplasm and mild nuclear atypia that mimics the oxyntic gland, it is preferably, however, confirmed by positivity for both pepsinogen I and MUC622. Cell differentiation markers, such as H+/K+-ATPase (parietal cell) and MUC5AC (foveolar epithelium), will help in the diagnosis of the adenocarcinoma of the fundic gland mucosa subtype21. In addition, D2-40-based assessment improves the histopathological detection of lymphovascular invasion. Fourth, histological and patient information was incomplete due to the retrospective nature of this study; the mucosa adjacent to oxyntic gland adenoma or GA-FG is reportedly normal without any intestinal metaplasia or atrophy9. Additionally, several researchers have suggested a possible relationship between acid suppressive therapy and the pathogenesis of oxyntic gland adenoma and GA-FG. Future studies incorporating such histological and patient information may reveal a more detailed pathophysiology of these gastric neoplasms.
In conclusion, we retrospectively investigated 116 patients with 126 lesions of oxyntic gland adenoma or GA-FG. The majority of oxyntic gland adenomas and GA-FG cases were resected using ESD or EMR. Histologically, there were no lesions with invasion at the level of the muscularis propria or deeper, and lymphovascular invasion was present in only 1.6% of lesions. Of the ESD/EMR specimens, no lesions were positive for vertical margins. Based on these results, we believe that endoscopic resection is a suitable initial treatment strategy for oxyntic gland adenoma and GA-FG. Although most of the T1b2 GA-FG patients were treated with endoscopic resection alone and no recurrence was documented, further investigations are required to elucidate the optimal treatment strategy for T1b2 GA-FG.
Abbreviations
- eCuraC-2
Endoscopic curability C-2
- EMR
Endoscopic mucosal resection using a diathermic snare
- ESD
Endoscopic submucosal dissection
- GA-FG
Gastric adenocarcinoma of the fundic gland type
Author contributions
M.I. conceived the study and wrote the manuscript; C.K., M.N., S.K., M.Y., T.I., T. Toyokawa, S.H., S.T., and K.M. collected the data; and T. Tanaka and H.O. contributed to revising the obtained data. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsukamoto T, et al. Gastric adenocarcinoma with chief cell differentiation. Pathol. Int. 2007;57:517–522. doi: 10.1111/j.1440-1827.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- 2.Ueyama H, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): Proposal for a new entity of gastric adenocarcinoma. Am. J. Surg. Pathol. 2010;34:609–619. doi: 10.1097/PAS.0b013e3181d94d53. [DOI] [PubMed] [Google Scholar]
- 3.Fukatsu H, Miyoshi H, Ishiki K, Tamura M, Yao T. Gastric adenocarcinoma of fundic gland type (chief cell predominant type) treated with endoscopic aspiration mucosectomy. Dig. Endosc. 2011;23:244–246. doi: 10.1111/j.1443-1661.2011.01125.x. [DOI] [PubMed] [Google Scholar]
- 4.Terada T. Well differentiated adenocarcinoma of the stomach composed of chief cell-like cells and parietal cells (Gastric adenocarcinoma of fundic gland type) Int. J. Clin. Exp. Pathol. 2011;4:797–798. [PMC free article] [PubMed] [Google Scholar]
- 5.Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: A proposal for reclassification as oxyntic gland polyp/adenoma. Am. J. Surg. Pathol. 2012;36:1030–1035. doi: 10.1097/PAS.0b013e31825033e7. [DOI] [PubMed] [Google Scholar]
- 6.Park ES, et al. Gastric adenocarcinoma of fundic gland type: Report of three cases. Korean J. Pathol. 2012;46:287–291. doi: 10.4132/KoreanJPathol.2012.46.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidaka Y, et al. Alteration in the Wnt/β-catenin signaling pathway in gastric neoplasias of fundic gland (chief cell predominant) type. Hum. Pathol. 2013;44:2438–2448. doi: 10.1016/j.humpath.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Abe T, et al. Long-term follow-up of gastric adenocarcinoma with chief cell differentiation using upper gastrointestinal tract endoscopy. Intern. Med. 2013;52:1585–1588. doi: 10.2169/internalmedicine.52.0361. [DOI] [PubMed] [Google Scholar]
- 9.Ueyama H, et al. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type) Endoscopy. 2014;46:153–157. doi: 10.1055/s-0034-1364955. [DOI] [PubMed] [Google Scholar]
- 10.Ueo T, Yonemasu H, Ishida T. Gastric adenocarcinoma of fundic gland type with unusual behavior. Dig. Endosc. 2014;26:293–294. doi: 10.1111/den.12212. [DOI] [PubMed] [Google Scholar]
- 11.Nomura R, et al. GNAS mutation as an alternative mechanism of activation of the Wnt/β-catenin signaling pathway in gastric adenocarcinoma of the fundic gland type. Hum. Pathol. 2014;45:2488–2496. doi: 10.1016/j.humpath.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Takeda S, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type) with unique endoscopic appearance curatively treated by endoscopic submucosal resection. Acta Gastroenterol. Belg. 2015;78:340–343. [PubMed] [Google Scholar]
- 13.Lee SY, et al. Mutation spectrum in the Wnt/β-catenin signaling pathway in gastric fundic gland-associated neoplasms/polyps. Virchows Arch. 2015;467:27–38. doi: 10.1007/s00428-015-1753-4. [DOI] [PubMed] [Google Scholar]
- 14.Chiba T, et al. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings. Dig. Endosc. 2016;28:722–730. doi: 10.1111/den.12676. [DOI] [PubMed] [Google Scholar]
- 15.Chan K, Brown IS, Kyle T, Lauwers GY, Kumarasinghe MP. Chief cell-predominant gastric polyps: A series of 12 cases with literature review. Histopathology. 2016;68:825–833. doi: 10.1111/his.12859. [DOI] [PubMed] [Google Scholar]
- 16.Miyazawa M, et al. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features. World J. Gastroenterol. 2016;22:10523–10531. doi: 10.3748/wjg.v22.i48.10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato Y, et al. Twelve-year natural history of a gastric adenocarcinoma of fundic gland type. Clin. J. Gastroenterol. 2016;9:345–351. doi: 10.1007/s12328-016-0680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami T, et al. Epigenetic regulation of Wnt/β-catenin signal-associated genes in gastric neoplasia of the fundic gland (chief cell-predominant) type. Pathol. Int. 2017;67:147–155. doi: 10.1111/pin.12509. [DOI] [PubMed] [Google Scholar]
- 19.Imagawa A, Sano N. Gastric adenocarcinoma of the fundic gland (chief cell predominant type) with brownish pigmentation. Gastrointest. Endosc. 2018;87:1358–1359. doi: 10.1016/j.gie.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Yu YN, et al. Gastric adenocarcinoma of fundic gland type after Helicobacter pylori eradication: A case report. World J. Clin. Cases. 2019;7:1696–1702. doi: 10.12998/wjcc.v7.i13.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ushiku T, et al. Oxyntic gland neoplasm of the stomach: Expanding the spectrum and proposal of terminology. Mod. Pathol. 2020;33:206–216. doi: 10.1038/s41379-019-0338-1. [DOI] [PubMed] [Google Scholar]
- 22.Yao T, Vieth M. Oxyntic gland adenoma. Digestive system tumours. In: WHO Classification of Tumours Editorial Board, editor. World Health Organization Classification of Tumours. 5. International Agency for Research on Cancer, World Health Organization; 2019. pp. 83–84. [Google Scholar]
- 23.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 24.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: Revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi: 10.1007/s10120-011-0040-6. [DOI] [PubMed] [Google Scholar]
- 25.Benedict MA, Lauwers GY, Jain D. Gastric adenocarcinoma of the fundic gland type: Update and literature review. Am. J. Clin. Pathol. 2018;149:461–473. doi: 10.1093/ajcp/aqy019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.