Abstract
Scar formation as a result of corneal wound healing is a leading cause of blindness. It is a challenge to understand why scar formation is more likely to occur in the central part of the cornea as compared to the peripheral part. The purpose of this study was to unravel the underlying mechanisms. We applied RNA-seq to uncover the differences of expression profile in keratocytes in the central/peripheral part of the cornea. The relative quantity of mitochondrial RNA was measured by multiplex qPCR. The characterization of mitochondrial RNA in the cytoplasm was confirmed by immunofluoresence microscope and biochemical approach. Gene expression was analyzed by western blot and RT qPCR. We demonstrate that the occurrence of mitochondrial DNA common deletion is greater in keratocytes from the central cornea as compared to those of the peripheral part. The keratocytes with CD have elevated oxidative stress levels, which leads to the leakage of mitochondrial double-stranded RNA into the cytoplasm. The cytoplasmic mitochondrial double-stranded RNA is sensed by MDA5, which induces NF-κB activation. The NF-κB activation thereafter induces fibrosis-like extracellular matrix expressions and IL-8 mRNA transcription. These results provide a novel explanation of the different clinical outcome in different regions of the cornea during wound healing.
Subject terms: Cell biology, Immunology, Molecular biology
Introduction
Scar formation during corneal wound healing is a significant clinical problem. It is the fourth leading cause of blindness globally according to the World Health Organization (https://www.who.int/blindness/publications/globaldata/en/), accounting for about ~ 4% of all cases of avoidable vision loss. The only available curative treatment for corneal blindness at present, is transplantation surgery. However, since corneal transplants are not without complications and since there is sometimes a lack of donor grafts, it is important to increase the knowledge about the pathophysiological mechanisms of corneal wound healing to find ways to avoid scar formation. Among ophthalmologists specializing in the cornea, it is known that post-surgical scar formation in the periphery of the cornea—for instance after penetrating keratoplastic transplantation—is sparse, whereas the central part of the cornea is more prone to develop scars following trauma or infection. Furthermore, as the central part of the cornea is in the field of vision, scar formation in this area may result in a major visual problem for the patient.
It is known that the expression profiles of the collagen-synthesizing stromal cells (keratocytes) of the cornea are significantly different between keratocytes derived from the peripheral cornea as compared to those from the central cornea1. The expression of keratocyte markers (keratocan, lumican, CD34, and ALDH) are relatively higher in the central cornea while the cells in the peripheral cornea appear to be more characterized as stem cells2,3. The corneal stromal stem cells are important for corneal wound healing and have been successfully used to prevent fibrotic scar formation2,4. The extracellular matrix (ECM) production is crucial for wound healing and its disruption leads to scarring and opacities5. However, since no differences in ECM-related genes from keratocytes of different regions have been reported, this implies that other factors are of relevance.
The central part of the cornea is the region of the eye that is most exposed to UV radiation6. A UVA-induced transcriptomic and proteomic study of corneal stroma showed that cumulative UVA exposure causes changes in extracellular matrix7. Mitochondrial DNA (mtDNA) common deletion (CD) is a marker of chronic UV radiation in the skin8. The deletion is caused by the loss of a large fragment of mtDNA from nucleotide positions 8482-13459 bp. mtDNA CD is also found to be selectively concentrated in the central cornea and gradually decreases towards the periphery9. These findings suggest that ECM differences seen between central and peripheral cornea could be caused by UV radiation, which in turn affects mtDNA CD content.
mtDNA CD engulfs more than 1/3 of mtDNA, which can cause specific defects in complex I, IV and V of the electron transport chain (ETC). CD-induced ETC defects results in (1) increased oxidative stress and (2) mitochondrial reactive oxygen species (ROS) generation10–12. Oxidative stress can result in damage of the mtDNA, which subsequently results in an inflammatory response13. The immunostimulatory potential of mtDNA was first reported by Collins et al. in 200414. Since then, several studies have shown that mtDNA is also associated with induction of damage-associated molecular patterns (DAMPs) that even further enhance pro-inflammatory responses15–18. DAMPs are endogenous molecules that are released from damaged cells or organelles, including mitochondria. DAMPs, including protein, metabolites, DNA and RNA, can activate the innate immune system and are considered to have a pathogenic role in inflammatory diseases. Inflammation is a fundamental process in corneal wound healing, but excessive corneal inflammation can result in an increased profibrotic response with more corneal opacification19–21. DAMPs can cause persistent inflammation in cells22. The chronic inflammatory response may exacerbate tissue damage and eventually result in scar formation23.
Mitochondrial ROS generation can promote mtDNA release and induce DAMPs24,25. However, whether mitochondrial ROS also can cause mtRNA leakage is unknown. Dhir et al. first reported that mtRNA can activate the immune response driven by melanoma differentiation-associated protein 5 (MDA5) antiviral signaling pathway26. MDA5 belongs to the retinoic acid-inducible gene-I-like receptors receptor (RLR) family, which serves as a pattern recognition receptor to recognize double-strand RNA (dsRNA). Stimulation of MDA5 leads to the activation of NF-κB and type I inferferon response. It is also known that mtRNA helicase SUV3 and polynucleotide phosphorylase PNPase prevent the formation and release of mitochondrial double-stranded RNA into the cytoplasm. Interestingly, PNPase is also involved in reducing oxidative RNA damage and protecting against oxidative stress27,28. It is intriguing to study whether oxidative stress can promote mtRNA release to induce innate immune responses.
Inflammatory response plays an important role in corneal wound healing. Prolonged inflammation may not only compromise the wound healing, but also worsen the scar formation29,30. Proinflammatory cytokines released from keratocytes can recruit immune cells after corneal injury. Interleukin 8 (IL-8) is a key mediator in the migration of neutrophils to the sites of injury31. Uncontrolled release of various proteases by neutrophils may result in stromal degradation and ulceration, which might result in corneal opacity and neovascularization32.
We hypothesize that the central cornea suffers more from UV radiation, as indicated by the occurrence of mtDNA CD. The keratocytes with CD could suffer from elevated oxidative stress that leads to excessive mtDNA/RNA release into the cytoplasm. The released mtDNA/RNA can induce endogenous DAMPs and subsequent inflammation, which would ultimately exacerbate the scar formation pathogenesis in the cornea.
In the present study, we confirm that there are different levels of mtDNA CD in the central and peripheral cornea. We further confirm that IL-8 expression is exclusively regulated by NF-κB signaling, which is activated by released mtRNA via MDA5 signaling. Our results provide a potential explanation for the different outcomes of scar formation in the central and peripheral cornea. Moreover, the obtained results could also have a profound medical implication of corneal scar formation.
Results
Keratocytes with mitochondrial DNA common deletion have elevated oxidative stress and IL-8 expression
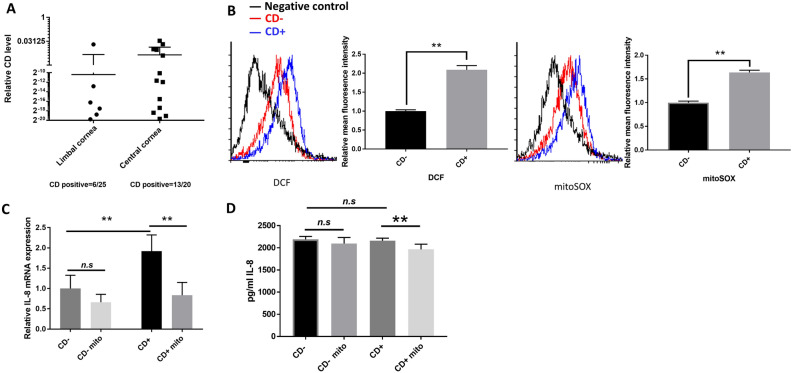
There was a higher occurrence of CD formation in the keratocytes from central cornea as compared to the limbal (i.e. peripheral) cornea (Fig. 1A). Here cells with CD were termed CD+ keratocytes, and cells without detectable CD were termed CD− keratocytes. Out of 25 samples of limbal cornea, 6 samples were CD+ , while 13 out of 20 samples of central cornea were CD+ . CD+ keratocytes showed an increased level of intracellular oxidative stress as compared to CD− keratocytes, as indicated by a ~ 2.0 fold difference in mean fluorescence intensity observed in DCF signal (Fig. 1B). Superoxide production (mitoSOX) in mitochondria in CD+ keratocytes was ~ 1.6 fold higher in mean fluorescence intensity than in CD− keratocytes (Fig. 1B). IL-8 is known to enhance keratocyte migration and recruit neutrophils, which are two necessary steps in the corneal wound healing33. Oxidative stress has been reported to upregulate IL-8 gene expression34. As expected, IL-8 expression, as measured at mRNA level, was higher in CD+ keratocytes as compared to in CD− keratocytes. There was no significant difference of IL-8 at extracellular secretion level measured by ELISA. Administration of mitoTEMPO significantly reduced the level of IL-8 expression in CD+ keratocytes (Fig. 1C-D), as well as the oxidative level in mitochondria (Additional Data Fig. 1), suggesting a positive correlation between mitochondrial ROS and IL-8 expression.
Figure 1.
Oxidative stress in CD+ keratocytes up-regulates the IL-8 expression. (A) CD/total mtDNA ratios in 45 human subjects quantified by multiplex-qPCR. 26 values were zero and are not included on the graph. (B) Flow cytometric detection of DCFH-DA and mitoSOX signal in CD+/− keratocytes (n = 3). (C) mRNA expression of IL-8 in CD+/− keratocytes. Cells either treated with 10 μM mitoTEMPO (Mito), or not, for 24 h (n = 3). (D) Quantification of secreted IL-8 level using ELISA. Cells treated, or not treated, with 10 μM mitoTEMPO for 24 h (n = 3). Values are means ± SD. n.s. (not significant); *P < 0.05, **P < 0.01.
MDA5 signaling pathway induces NF-kappaB activation in CD+ keratocytes
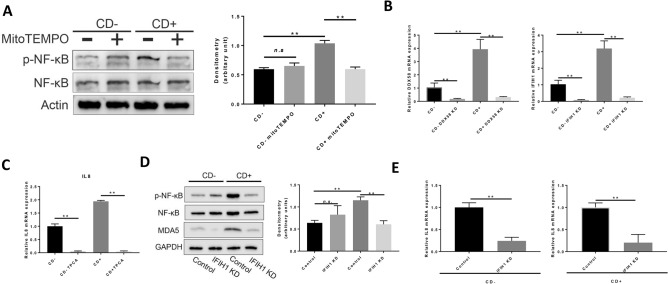
The RNA-sequencing analysis was performed to uncover the signaling pathway that might connect oxidative stress to IL-8 upregulation. There were 4485 genes that were differently expressed in CD+ keratocytes compared to in CD− keratocytes (Additional Data Fig. 2A). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis revealed several alternated pathways that were upregulated in CD+ keratocytes as compared to in CD− keratocytes. Interestingly, several enriched pathways were related to virus infection and immune response (Additional Data Fig. 2B). As we found increased IL-8 expression in CD+ keratocytes as compared to in CD− keratocytes, the KEGG enrichment pathway indicated that IL-8 upregulation could be regulated by the cytosolic RNA sensing pathway (Additional Data Fig. 2C). As shown in the diagram, RNA sensing mediated by RIG-I/MDA5 could transduce the signal to activate NF-κB, which in turn induce IL-8 up-regulation. To confirm this, we measured the phosphorylated NF-κB in CD+ keratocytes, combined with the treatment of mitoTEMO. Corresponding to the pattern of IL-8 expression, NF-κB phosphorylation was higher in CD+ keratocytes, and this was abolished by mitoTEMPO treatment (Fig. 2A), as was the case for IL-8. DDX58 and NFKBIA mRNA expression were consistent with the RNA-seq data (Fig. 2B, Additional Data Fig. 2D), while IFIH1 mRNA expression was higher in CD+ keratocytes as compared to in CD− keratocytes, which was not detected by RNA-seq data (Fig. 2B). TPCA1, an inhibitor of NF-κB nuclear localization, completely abrogated IL-8 mRNA expression (Fig. 2C), confirming a direct role of NF-κB in IL-8 regulation35–38.
Figure 2.
MDA5 signaling induces IL-8 upregulation via NF-κB phosphorylation in CD+ keratocytes. (A) Western blot of NF-κB phosphorylation in CD− and CD+ keratocytes with/without mitoTEMPO (n = 4). NF-κB and p-NF-κB were blotted and cropped from the same gel. p-NF-κB was blotted first and the membrane was stripped and washed for NF-κB blotting. Actin was blotted separately from a different gel using the corresponding samples. Exposures were adjusted automatically by the Odyssey Fc imaging system. (B) DDX58 and IFIH1 mRNA expression in CD+/− keratocytes 48 h after respective siRNA transfection (n = 3). (C) IL-8 mRNA expression in keratocytes 24 h after 20 nM TPCA treatment (n = 3). (D) NF-κB phosphorylation, analyzed by western blot, after IFIH1 knock-down in CD+/− keratocytes (n = 3). NF-κB and p-NF-κB were blotted and cropped from the same gel. p-NF-κB was blotted first and the membrane was stripped and washed for NF-κB blotting. GAPDH and MDA5 were blotted separately from a different gel using the corresponding samples. Exposures were adjusted automatically by the Odyssey Fc imaging system. (E) IL-8 mRNA expression in CD+/− keratocytes after IFIH1 knockdown by siRNA transfection (n = 3). Values are means ± SD. n.s. (not significant); *P < 0.05, **P < 0.01.
MDA5 expression was significantly reduced after siRNA transfection, as confirmed by qPCR (Fig. 2B) and western blot (Fig. 2D) in both CD+/− keratocytes. DDX58 mRNA expression was reduced 48 h after siRNA transfection (Fig. 2B) but the corresponding RIG-I expression was barely detectable by western blot using two antibodies (MA5-31715, Invitrogen; 3743S, Cell signaling) (data not shown). siRNA-mediated MDA5 depletion (Fig. 2D) resulted in a marked reduction of p-NF-κB in CD+ keratocytes, which was accompanied by a down-regulation of IL-8 mRNA expression (Fig. 2E). These results show that IL-8 expression in CD+ keratocytes was mediated by NF-κB via MDA5 signaling.
Mitochondria double-stranded RNA (mtdsRNA) escapes from mitochondria in CD+ keratocytes
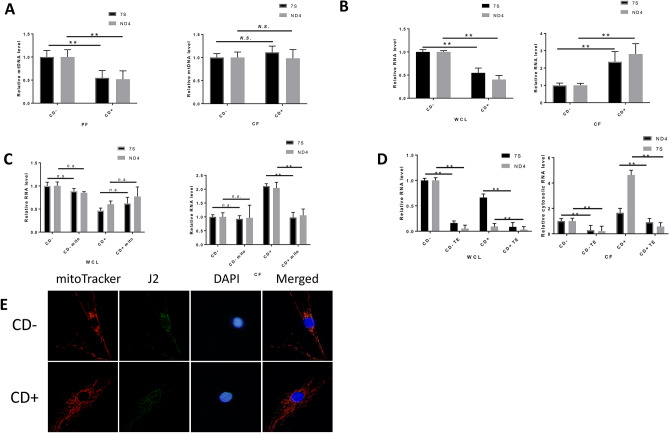
The stimulation of NF-κB can be induced by cytosolic DNA/RNA. Many studies have shown that cytosolic DNA induces NF-κB activation39–41. We first tried to determine whether cytosolic DNA was the source to induce NF-κB activation. Cytosolic nuclear DNA signal, as measured by qPCR using reference gene β2M and GAPDH probe, was close to background level (the negative control), indicating no leakage of nuclear DNA in the cytoplasm (data not shown). The source of DNA in the cytoplasm therefore could be from mitochondria. A multiplex PCR approach was applied to measure the cytosolic mtDNA content. Reduced mtDNA copy number in mitochondria was found in CD+ cells compared to in CD− keratocytes (Fig. 3A), whereas the cytosolic mtDNA content was at the same level in CD+/− keratocytes. These results contradict the possibility that cytosolic DNA triggered the immune response in CD+ keratocytes. Recent studies found that also mtRNA can induce innate immune response26,42. To determine whether mtRNA triggered an immune response in CD+ keratocytes, total mtRNA and cytosolic mtRNA were quantified using multiplex qPCR. The total mtRNA in CD− keratocytes was higher than that in CD+ keratocytes (Fig. 3B). This could be due to the lower mtDNA copy number in CD+ keratocytes compared to CD− keratocytes. Contrary, a significantly higher amount of mtRNA in the cytoplasm was found in CD+ keratocytes compared to in CD− keratocytes. Administration of mitoTEMPO markedly reduced the cytosolic cotent of mtRNA in CD+ keratocytes, while it has no significant effects on cytosolic mtRNA in CD− keratocytes, nor in the whole cell fraction (Fig. 3C). Dhir et al. found that mitochondria double-stranded RNA (mtdsRNA), which escaped from mitochondria into the cytosol, was a source to trigger antiviral signaling via MDA5 signaling26. Alternatively, RNA polymerase III (Pol III) can use cytosolic mtDNA as template to produce single-stranded mtRNA (mtssRNA) that can induce RIG-I-mediated signaling42. Terminator 5′-Phosphate-Dependent Exonuclease (TE) is an exonuclease that can digest RNA that have a 5′ monophosphate but cannot digest RNA having a 5′-triphosphate. RNA transcribed in mitochondria lacks 5′-cap protection from TE, while Pol III-transcribed RNA has a 5′-triphosphate cap. TE treatment can thus distinguish RNA transcribed in mitochondrial from RNA transcribed by Pol III in the cytoplasm. The level of mtRNA was significantly reduced after TE digestion both in the whole-cell and in the cytosolic fraction (Fig. 3D). RNA isolated from mitochondrial fraction can be completely digested by TE treatment, resulting in no detectable signal in qPCR (data not shown). Consistently, immunostaining of dsRNA with anti-dsRNA (J2) showed that J2 foci were mostly co-localized with mitochondria in both CD+/− keratocytes (Fig. 3E). Treatment of TE before J2 staining resulted in markedly reduction of J2 foci signal in keratocytes (Additional Data Fig. 3C). These results suggest that the majority of cytosolic mtRNA is from the mitochondrial matrix in CD+ keratocytes.
Figure 3.
Higher level of mtdsRNA in the cytoplasm of CD+ keratocytes compared to in that of CD− keratocytes. (A) The relative level of mtDNA in the pellet fraction (PF) or cytosolic fraction (CF) measured by multiplex qPCR (n = 3). (B) Relative mtRNA in whole-cell lysate (WCL) and CF of CD+/− keratocytes measured by multiplex qPCR (n = 3). (C) Relative mtRNA in CF of CD+ keratocytes 24 h after 10 μM mitoTEMPO treatment (n = 3). (D) Mitochondrial RNA level with/without Terminator 5′-phosphate-dependent exonuclease treatment (TE) in WCL and CF (n = 3). (E) Representative fluorescence image of dsRNA in CD+/− cells. mitoTracker (red), J2 foci (greed) and DAPI (blue) were merged in the right column (merged). Values are means ± SD. n.s. (not significant); *P < 0.05, **P < 0.01.
Altered extracellular matrix (ECM) expressions in CD+ myofibroblasts are linked to mitochondrial ROS and NF-kappaB signaling
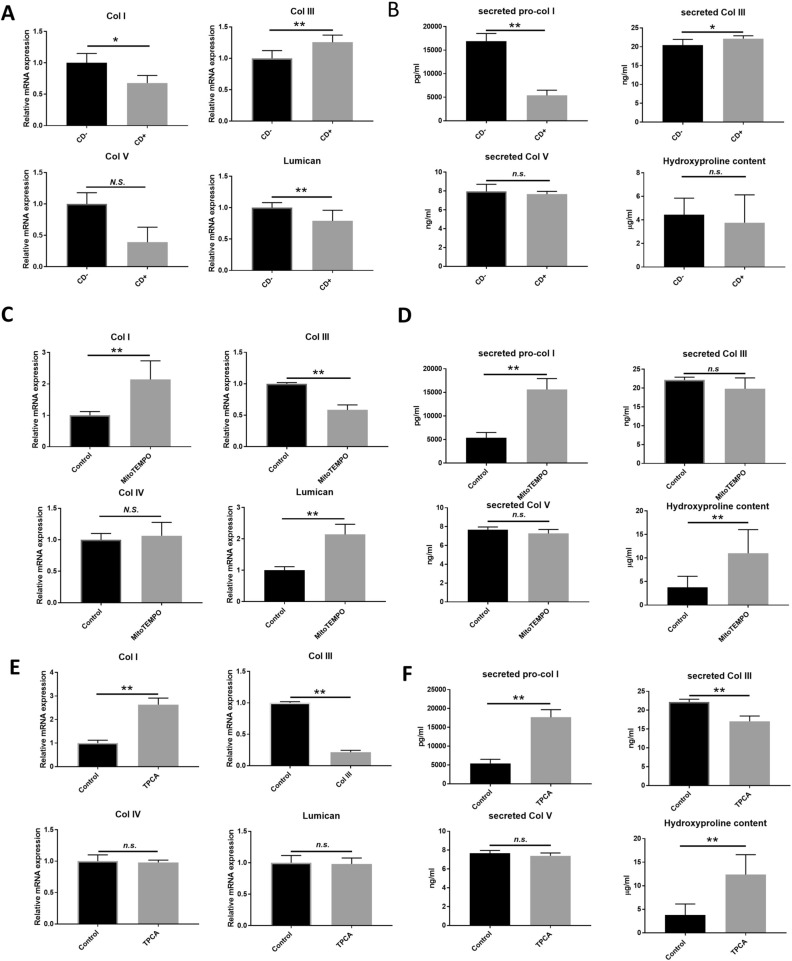
RNA-seq data showed that col3a1 expression was significantly up-regulated in CD+ cells (Additional Data Fig. 2A). This result prompted us to study whether there were differences in collagen expression in CD+/− keratocytes, since collagen expression and organization are vitally important for corneal wound healing and also key factors altered in scar formation. Keratocytes were transformed into myofibroblasts in an in vitro fibrosis model43, as identified by the expression of α-SMA (Additional Data Fig. 4). mRNA expression of COL1A1, COL5A1 and lumican were lower in CD+ myofibroblasts compared to CD− myofibroblast (Fig. 4A). Pro-col I secretion from CD+ myofibroblast was significantly reduced as compared to from CD− myofibroblast (Fig. 4b). Col III secretion and mRNA expression were higher in CD+ myofibroblasts as compared to CD− myofibroblasts (Fig. 4A, top right panel, and Fig. 4B, top right panel, respectively). ECM deposition, as measured by hydroxyproline assay, showed no significant difference between CD− and CD+ myofibroblasts (Fig. 4B). To study whether mitochondrial oxidative stress and NF-κB signaling are involved in the collagen expression patterns in CD+ myofibroblasts, mitoTEMPO or TPCA treatment was used. Both mitoTEMPO and TPCA treatment effectively reversed the mRNA expression and secretion of Col I and Col III, as well as hydroproline content (Fig. 4C-F). These results show that mitochondrial ROS and NF-κB signaling participate in the regulation of collagen expression and ECM depostion in CD+ myofibroblasts.
Figure 4.
Changes in various ECM components in corneal myofibroblasts are dependent on mitochondrial ROS and NF-κB signaling. (A) RT qPCR on the mRNA expression of COL1A1, COL3A1, COL5A1 and lumican in corneal myofibroblasts (n = 3). (B) Secretion of pro-collagen I, collagen III, collagen V, and hydroxyproline content in supernatant collected 2 d after fibrosis induction (n = 3). (C) RT qPCR of the mRNA expression of COL1A1, COL3A1, COL5A1 and lumican in CD+ corneal myofibroblasts. Cells were treated with 10 µM mitoTEMPO for 2 d (n = 3). (D) Secretion of pro-collagen I, collagen III, collagen V, and hydroxyproline content in supernatant collected 2 d after fibrosis induction in CD+ myofibroblasts simultaneously treated with 10 µM mitoTEMPO (n = 3). (E) RT qPCR of the mRNA expression of COL1A1, COL3A1, COL5A1 and lumican in CD+ corneal myofibroblasts treated with 20 nM TPCA for 48 h (n = 3). (F) Secretion of pro-collagen I, collagen III, collagen V, and hydroxyproline content in supernatant collected 2 days after fibrosis induction in CD+ myofibroblasts simultaneously treated with 20 nM TPCA (n = 3). Values are means ± SD. n.s. (not significant); *P < 0.05, **P < 0.01.
Discussion
CD (common deletion) has been reported to be concentrated in the central cornea as compared to in the limbal (peripheral) region9. Our results confirmed that a higher occurrence of CD is seen in central corneal tissue as compared to in limbal cornea. Keratocytes in the central cornea are more exposed to UV radiation compared to limbal keratocytes6, which means a higher risk of CD induction. As discussed by Gendron et al.9, the eyelid can cover most of the cornea in response to different light conditions but the central part is always the most exposed region of the eye6. Despite the observation of CD in the corneal tissue, the functional consequences of mtDNA deletion in keratocytes have not been reported. Our results showed an increase of mitochondrial superoxide, as well as a general elevation of oxidative stress, in CD+ keratocytes. However, it is not known whether the oxidative stress originated from mitochondria could affect the corneal wound healing process.
Previously we have found that IL-8 enhances keratocyte migration and neutrophil recruitment33, which are necessary steps during corneal wound healing. However, if the neutrophil infiltration in an injured cornea is not tightly controlled, the release of various proteases may lead to stromal degradation and ulceration, which in turn might result in corneal opacity and neovascularization32. It is interesting to note that ROS has been positively correlated to IL-8 expression44–46. Our results confirmed that mitochondria generated ROS upregulates IL-8 expression, at both mRNA level and in secretion, by specific mitochondrial superoxide scavenger. Approximately 90% of the cellular ROS are generated in the mitochondria through OXPHOS47. mtDNA CD ablates several mitochondrial genes encoding for ETC. The elevated mitoSOX signal in CD+ keratocytes is thus expected (Fig. 1B), since the deficiency of ETC leads to the increased generation of ROS48,49. However, the mechanism behind the overall increased oxidative stress in CD+ keratocytes needs further investigation.
IL-8 is encoded by the CXCL8 gene. Many factors are involved in the transcription regulation of CXCL8, such as NF-κB38, CAAT/enhancer-binding protein β (C/EBPβ)50, cAMP response element-binding protein (CREB)51, and activating protein 1 (AP-1)52. Among all the transcription factors in CXCL8 regulation, NF-κB is closely interacting with ROS (reviewed in53). ROS can directly oxidize the cysteine in the Rel-homology domain (RHD) of NF-κB to inhibit its DNA binding54–56. In contrast, ROS can also activate NF-κB through alternative IκBα phosphorylation57,58.
Besides the regulation of NF-κB by targeting related proteins directly, ROS also induces NF-κB activation indirectly by inducing DNA/RNA release into the cytoplasm. Mitochondrial ROS accumulation has been found to promote mtDNA release and initiate inflammation24,25. It is possible that mtRNA also escape from mitochondria in the same manner as mtDNA. Reports have shown that cytosolic RNA, from different sources, triggers the immune response. Suspene et al. proposed that cytoplasmic mtDNA can be transcribed by pol III and trigger RIG-I mediated IFN expression42. They found transfected mtDNA upregulated type I interferon expression in a human myeloid cell line. Dhir et al. found that mitochondrial dsRNA that had escaped into the cytoplasm in a PNPase-dependent manner triggers antiviral signaling26. Our RNA-seq data indicate the potential involvement of RIG-I-like receptor signaling pathway in NF-κB signaling in CD+ keratocytes (Additional Data Fig. 2). RIG-I-like receptor signaling can either be initiated by RIG-I or MDA5 RNA sensing. RIG-I specifically binds to 5′-pppRNA and short dsRNA, while MDA5 recognizes long dsRNA. Both receptors might be responsible for the induction of IL-8 in CD+ keratocytes. Both IFIH1 and DDX58 expression were higher in CD+ keratocytes as compared to that in CD− keratocytes (Fig. 2B). However, RIG-I protein was not detectable in any of the two types of keratocytes, whereas MDA5 protein level was more abundant in CD+ keratocytes (Fig. 2D), corresponding to its elevated level of IFIH1 mRNA. Notably, knockdown of IFIH1 led to reduced IL-8 mRNA in both CD+/− keratocytes (Fig. 2E), suggesting a regulatory role of MDA5 in IL-8 expression. Taken together, we confirmed that IL-8 upregulation requires the accumulation of ROS in the mitochondria and expression of MDA5, suggesting that dsRNA sensing via MDA5 accounts for the elevated IL-8 expression in CD+ keratocytes.
We found the presence of mtRNA in the cytoplasm in keratocytes. Similarly, background cytosolic dsRNA was also found in HeLa cells using J2 immunofluorescence staining26. Although we found quantitative differences of cytosolic mtRNA by qPCR in CD+/− keratocytes, the differences were not detectable by J2 staining. The method based on immunofluorescence staining might not be sensitive enough to detect subtle changes in cytosolic dsRNA content. The higher expression of mtRNA in the qPCR data, might also be explained by the mitochondria in CD+ keratocytes leaking more, since the RNA-seq data also showed that several genes involved in mPTP were upregulated (VDAC1, VDAC2, SLC25A4 and SLC25A5). Overall, the concomitant reduction of cytosolic mtdsRNA (Fig. 3E), IL-8 expression (Fig. 1C,D), and NF-κB phosphorylation (Fig. 2A) suggest that cytosolic mtdsRNA could activate NF-κB signaling in a mitochondrial ROS-dependent manner. It is interesting to note that IL-8 has been reported to induce NF-κB activation in a dose-dependent manner in different cell types59. Presumably, a positive feedback loop of IL-8–NF-κB might prolong the inflammatory state in CD+ cells in an autocrine manner.
Mitochondrial ROS generation has been found to promote mtDNA release24,25. Szczesny et al. found that low-level oxidative stress can also induce mtDNA oxidation and an related inflammatory response13. Notably, increased expression of cytoplasmic RNA sensors, such as DDX58 and IFIH1, were found in Tfam heterozygous knockout mouse cells60, which increase the innate immune response. These findings indicate that mtRNA escapes from mitochondria as mtDNA does. The liberation of mtRNA from mitochondria was recently reported26. Interestingly, they found that the release of mtdsRNA is dependent on Polynucleotide phosphorylase (PNPase). PNPase is an exoribonuclease primarily located in mitochondria61. It has been shown to remove oxidatively damaged RNA with high affinity27,28,62. There might be more mtdsRNA escape in cells under mitochondrial oxidative stress, if the capacity of PNPase is not increased correspondingly.
mtDNA mutation such as CD has been proposed as a molecular marker for photoaging of skin63,64 and cornea9. Photoaging is often referred to as a premature phenotype in skin caused by repeated exposure to ultraviolet (UV) radiation. Lower expression of type I pro-collagen has been found in the fibroblasts of photoaged skin compared to naturally aged skin65. Similarly, markedly reduced expression of collagen I (COL1A1) and collagen V (COL5A1 and A2) was observed in keratocytes treated by chronic UVA exposure as a model of photoaging7. Lumican, a protein that is responsible for the regulation of collagen assembly, is also downregulated in the in vitro photoaging model of corneal stroma keratocytes7. Collagen III is expressed weakly in the cornea under normal physiological conditions66. Its expression increases greatly during wound healing or inflammation and is markedly upregulated in corneal scars67. The elevated collagen III expression might be due to inflammation, which should be abolished—at least in later stages of wound healing—to prevent corneal scar formation. Taken together, the expression profile of CD+ cells resembles a photoaging phenotype. Considering the significant reduction of Col I expression and upregulation of Col III in photoaged keratocytes, corneal photoaging should be considered as a potential risk factor to the outcome of corneal wound healing and scar formation.
The different expression profiles of collagens in CD+/− cells suggest the potential role of NF-κB in corneal scar formation. NF-κB signaling has been reported to regulate collagen expression patterns. NF-κB activation inhibits expression of the COL1A168. Conversely, reduced NF-κB activity resulted in a significant increase in COL1A1 gene expression69. On the opposite, Col III expression is increased with NF-κB signaling is activated70,71. Our results revealed the negative regulation of collagen secretion by NF-κB and mitochondrial ROS (Fig. 4 C-F). However, there was no significant difference of the actual ECM deposition between CD− and CD+ myofibroblasts (Fig. 4 B, lower right panel), indicating there were other factors determining the total collagen synthesis in CD+ myofbibroblast. Overall, targeting mitochondrial redox status and/or preventing pathological NF-κB activation during corneal wound healing could be a potential approach to counter-act corneal scar formation.
Conclusion
It is well-known that the central part of the cornea is more likely to form a scar than peripheral parts. This is especially of concern since a central corneal scar can result in a major visual problem for the patient. To be able to prevent or reduce the incidence of scar formation in the central cornea is therefore of great clinical importance. Our findings provide a novel pathway to explain the onset of permanent corneal scarring/fibrosis post-surgery or trauma. Moreover, the obtained results could also have a profound medical impact beyond corneal tissue, as a new link between mtDNA deletions and scar formation is confirmed.
Materials and methods
Isolation and culture of human keratocytes
Isolation and culture of primary keratocytes were performed as previously described1. Healthy human corneal tissue was received for research purpose from the Tissue Establishment, Eye Bank Umeå, at the University Hospital of Umeå, Sweden. The tissue originated from deceased individuals who had chosen, when alive, to donate their corneas postmortem for transplantation and research, according to Swedish law, and grafts not used for transplantation, or the leftover tissue from healthy grafts used for transplant surgery, were delivered to the laboratory for research purpose. An overview of the donor information (mean age and age range; sex) is shown in Table 1. The Regional Ethical Review Board in Umeå reviewed the study and determined it to be exempt from the requirement for approval (2010‐373‐31 M). The study followed the principles of the Declaration of Helsinki.
Table 1.
Donor information.
| Cornea origin | N | Mean age | Age range | Male | Female |
|---|---|---|---|---|---|
| Central | 20 | 71.9 | 26–89 | n = 14 | n = 6 |
| Limbal | 25 | 70.0 | 26–89 | n = 20 | n = 5 |
Cell isolation and culture were performed as described previously1,33,72. Corneal samples were scraped using a sterile scalpel to remove any remaining epithelial or endothelial cells, before being washed in sterile Hanks' balanced salt solution (Invitrogen, Carlsbad, CA). The remaining stromal layer was cut into 1–2 mm2 pieces with a scalpel and then digested with 2 mg mL−1 collagenase (Sigma, St. Louis, MO) overnight at 37 °C. The suspension was centrifuged and the pellet was cultured in DMEM/F‐12 media (Gibco, Carlsbad, CA) supplemented with 2% fetal bovine serum (FBS; Gibco, Carlsbad, CA) and 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA) and placed in a humidified incubator at 37 °C with 5% CO2. Media was changed every third day until the cells reached confluence.
In vitro human corneal fibrosis model
The in vitro human corneal fibrosis model was adapted from Karamichos et al.73 and has been successfully established in our lab43. Corneal fibroblasts were plated on plastic culture dishes at desired densities in DMEM/F-12 10% FBS medium and stimulated by a stable vitamin C derivative L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate (VitC; Sigma-Aldrich, St. Louis, MO, USA, # A8960) at a concentration of 0.5 mM, and 0.25 ng/ml recombinant human TGF-β1 (R&D Systems, # 240-B).
Separation of cytosolic DNA
Confluent cells in 10 cm dish were harvested by centrifugation (2 min, 500 g). Cells were washed twice with ice cold PBS, before resuspended in 1 ml homogenization buffer (40 mM Tris–HCl, 25 mM NaCl, 5 mM MgCl2, 20 ug/ml digitonin) and incubated 5 min on ice. After the homogenization, 125 μL of equilibration buffer (400 mM Tris–HCl, 250 mM NaCl, 50 mM MgCl2) was added. The homogenate was centrifuged at 1200 g at 4 °C for 3 min to pellet the nuclear fraction (NF). The supernatant was collected and centrifuged at 20,000 g at 4 °C for 10 min to pellet the mitochondrial fraction (MF) and supernatant were used as cytosolic fraction (CF). NF and CF were combined and subjected to DNA extraction and subsequent multiplex qPCR for cytosolic mtDNA quantification.
DNA isolation
Total DNA was isolated by PureLink Genomic DNA kit (Invitrogen, Hilden, Germany) according to the manufacturer's protocol. For cytosolic DNA isolation, 20 μL Proteinase K and 20 μL RNase A were added to 200 μL of supernatant and incubated at 55 °C for 30 min and then heated at 95 °C for 5 min. The mixture was then cooled down at room temperature and mixed with same volume of 100% ethanol. The mixture was transferred to a PureLink Spin Column and centrifuged for 15 s at > 8000 g. The flow-though was discarded. These steps were repeated to increase the DNA concentration. Washing and elution were then performed according to the standard protocols.
Separation of cytosolic RNA
The cell homogenate was prepared using the protocol for cytosolic DNA separation. The homogenate was centrifuged at 20,000 g at 4 °C for 10 min and the cytosolic fraction (CF) were used for RNA extraction (c.f. RNA isolation) and subsequent multiplex qPCR. To test the purity of cytosolic RNA in the CF extraction, the proteins from whole cell lysate (WCL), CF and pellet fraction (PF) were blotted using GAPDH, Histone H3 and TFAM antibody. No nuclear and mitochondrial proteins were detected in CF, indicating that nuclear and mitochondria lysis did not occur, thus the CF extraction was successful. The purity can also be confirmed by ponceau S staining, as different pattern of protein band can be observed (Additional Data Fig. 3).
RNA isolation
Total RNA was isolated by RNeasy/miRNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. For cytosolic RNA isolation, 700 μl of supernatant were mixed with 100% ethanol and transferred to an RNeasy spin column placed in a 2 ml collection tube. The column was centrifuged for 15 s at > 8000 g. The flow-through was discarded. The step was repeated to increase the RNA concentration. After washing with 500 μl of Buffer RW1, the RNA is treated with DNase I while bound to the RNeasy membrane. The DNase I is removed by a second wash with Buffer RW1. Washing with Buffer RPE and elution of RNA were then performed according to the standard protocols.
Terminator 5′-phosphate-dependent exonuclease (TE) digestion
TE digestion was performed according to the manufacturer’s protocol. Briefly, 200–400 ng RNA were digested in 20 μL of reaction buffer containing 2 μL Terminator 10X reaction buffer A, 0.5 μL RiboGuard RNase Inhibitor and 1 μL Terminator Exonuclease. The mixture was incubated at 30 °C in a thermocycler for 60 min. The digested RNA was then purified and enriched by ethanol precipitation and RNeasy spin column as described above. TE digested treatment during immunofluorescence staining was performed after fixation. The slides with drop of digestion mixture were incubated in an oven at 30 °C for 60 min.
Western blot
Isolation and culture of primary keratocytes were performed as previously described1. Samples were lysed in Radioimmunoprecipitation Assay (RIPA) lysis buffer, supplemented with protease inhibitor (Sigma, St. Louis, MO) and diluted in Laemmli buffer (Bio‐Rad, Hercules, CA) supplemented with β‐mercaptoethanol. After boiling the samples, equal total proteins, were loaded into each well of a pre‐made gel of 12% (Mini‐PROTEAN TGX, Bio‐Rad, Hercules, CA) and ran at 120 V for ≈60 min. Subsequently, proteins were transferred to a polyvinylidene fluoride transfer membrane (PVDF; Santa Cruz, Dallas, TX) for 60 min at 100 V. Membranes were blocked for 60 min in room temperature before primary antibody was added and incubated at 4 °C overnight. After washing, the membranes were exposed to the secondary antibody (conjugated with horseradish peroxidase, HRP) for 60 min. After additional washing the membrane was exposed to the enhanced chemiluminescence solution (GE healthcare, Little Chalfont, UK) for 5 min in room temperature. The membranes were developed using Odyssey Fc imaging system (LI‐COR, Lincoln, NE). All antibodies used are summarized in Table 2.
Table 2.
Antibodies used for immunofluorescence staining and western blot.
| Antibody | Company | Code | Species |
|---|---|---|---|
| β‐actin | Cell signaling | 4967 | Rabbit |
| NF-κB p65 | Cell signaling | 8242 | Rabbit |
| phospho-NF-κB p65 (Ser536) | Cell signaling | 3033 | Rabbit |
| TFAM | Cell signaling | 8076S | Rabbit |
| Histone | Cell signaling | 7631 | Rabbit |
| MDA5 | Invitrogen | 700360 | Rabbit |
| RIG-I | Invitrogen | MA5-31715 | Rabbit |
| RIG-I | Cell signaling | 3743S | Rabbit |
| GAPDH | Cell signaling | 5174 | Rabbit |
| a-SMA | Abcam | 5694 | Rabbit |
| J2 | Scicons | Mouse | |
| Anti-rabbit IgG HRP‐linked | Cell signaling | 7074 | Rabbit |
| Anti-mouse IgG HRP‐linked | Cell signaling | 7076 | Mouse |
RT-qPCR
Isolation and culture of primary keratocytes were performed as previously described1. RNA was reverse transcribed into cDNA with High‐Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA). To determine the gene expression, TaqMan Gene Expression Assays (Applied Biosystems, Carlsbad, CA) were used. cDNA transcribed from 40 ng of RNA was run in duplicates with the ViiA7 Real‐Time PCR system and analyzed with its software (Applied Biosystems, Carlsbad, CA). Gene expression was measured by using TaqMan Gene Expression Assay (Applied Biosystems, Carlsbad, USA). To quantify mtRNA, the same multiplex PCR setting as for mtDNA quantification was used (c.f. Multiplex qPCR for mtDNA and CD quantification). Results are presented as target gene expression/β‐actin normalized to the control group. All probes used for real‐time PCR (Applied Biosystems, Carlsbad, CA) are summarized in Table 3.
Table 3.
Probes used for real‐time PCR from Applied Biosystems.
| Gene name | Assay ID |
|---|---|
| Lumican | Hs00929860_m1 |
| IL-8 | Hs0017103_m1 |
| NFKBIA | Hs00355671-g1 |
| DDX58 | Hs01061436_m1 |
| IFIH1 | Hs00223420_m1 |
| Keratocan | Hs00559941_m1 |
| Collagen I | Hs00164004_m1 |
| Collagen V | Hs00609133_m1 |
| β‐actin (reference gene) | 4352667 |
siRNA transfection
Silencing of genes of interest was performed using stealthRNA (Thermo Fisher Scientific) with Lipofectamine RNAiMAX (Thermo Fisher Scientific) in keratocytes according to the manufacturer’s instructions. The siRNA used were: HSS127414 and HSS127415 for IFIH1, HSS119010 and HSS177513 for DDX58, and 12935300 for the negative control. The stealthRNA oligonucleotides were used at a final concentration of 20 nM.
Multiplex qPCR for mtDNA and CD quantification
Multiplex qPCR protocol was adapted from Phillips et al.74. Real-time PCR was performed on the ViiA7 Real‐Time PCR system and analyzed with its software (Applied Biosystems, Carlsbad, CA). PCR was performed using the following thermal profile: 95 °C for 20 s; 40 cycles of 95 °C for 1 s, 60 °C for 20 s. The reaction components are as follows: 10 μL Taqman Fast advanced Master Mix, each primer at 50 nM, and each dual hybridization probe at 250 nM. The final reaction volume is 20 μL. For CD quantification, the ND4 primers and probe set was substituted by the CD primers and probe set. Other conditions were the same. Primer sequence information is summarized in Table 4.
Table 4.
Primers and probes used for multiplex qPCR real‐time PCR.
| Primer name | Sequence (5′–3′) |
|---|---|
| 7SF | CTAAATAGCCCACACGTTCCC |
| 7SR | AGAGCTCCCGTGAGTGGTTA |
| ND4F | CTGTTCCCCAACCTTTTCCT |
| ND4R | CCATGATTGTGAGGGGTAGG |
| Fβ2M | GCTGGGTAGCTCTAAACAATGTATTCA |
| Rβ2M CD quantification primers1 | CCATGTACTAACAAATGTCTAAAATGGT |
| HSSN8416 | CCTTACACTATTCCTCATCACC |
| HSAS8542 | TGTGGTCTTTGGAGTAGAAACC |
| Probe name | Sequence (5′–3′) |
|---|---|
| 7SP | 6FAM-CATCACGATGGATCACAGGT(NFQ) |
| ND4P | Texas Red-GACCCCCTAACAACCCCC(NFQ) |
| Pβ2MP (reference gene) | VIC-CAGCAGCCTATTCTGC(NFQ) |
| CD | Texas Red-TGGCAGCCTAGCATTAGCAGTT(NFQ)72 |
Immunofluorescence staining
Cells were incubated with MitoTracker Red CMXRos (50 nM) for 30 min at 37 °C. Cells were then washed twice with cold PBS before fixed and permeabilized with precolded methanol for 2 h at − 20 °C. After the fixation, cells were washed twice with PBS and blocked with 5% PBS for 60 min at room temperature. After that cells were incubated with the J2 antibody (1:200) 60 min at room temperature. After washing, cells were incubated with secondary donkey anti-mouse IgG conjugated with Alexa Fluor 488 at (1:300) concentration for 60 min. Finally cells were washed three times with PBS and mounted with Prolong Diamond antifade mountant with DAPI (Invitrogen). A Zeiss Axioskop 2 plus microscope equipped with epifluorescence and an Olympus DP70 digital camera was used for analysis.
ROS measurement
Cells cultured in six-well plate were labeled with DCFH-DA (5 µM) and MitoSOX (5 µM) in Serum-free DMEM/F‐12 media for 30 min. Subsequently, the cells were wash twice with PBS and harvested with trypsin and resuspended in 1 ml PBS. Flow Cytometry analysis was performed on FACSLSRII analysis machine and data was analyzed and illustrated by Flowing software (http://flowingsoftware.btk.fi/).
ELISA
ELISA was performed as described previously43. 0.3 × 104 corneal fibroblasts were seeded into 96-well plates in DMEM/F-12 supplemented with10% FBS for 24 h before treatment. Fibrosis was induced as described earlier. Desired wells were pretreated with either 10 μM mitoTEMPO or 20 nM TPCA. Supernatants were collected 2 days after treatments. Secretion of IL-8 was assessed with Human CXCL/IL-8 DuoSet (R&D Systems, #DY208), pro-collagen I was assessed with Human Pro-Collagen I alpha 1 DuoSet ELISA (R&D Systems, #DY6220), collagen III with Human Collagen, type III, alpha 1 (COL3A1) ELISA kit (Cusabio, Houston, TX, USA, # CSB-E13446h) and collagen V with Human Collagen, type V, alpha 1 (COL5A1) ELISA kit (Cusabio, # CSB-E13447h) according to the manufacturer protocol.
Hydroxyproline assay
Cells were treated the same as described in ELISA assay. Supernatants were collected 2 days after treatments. A hydroxyproline assay was used to determine the free hydroxyproline content in each group. This offered further insights on incorporation of free hydroxyproline in collagen synthesis. This assay was performed on hydrolyzed samples using a hydroxyproline assay kit (Abcam, Cambridge, UK). Briefly, supernatants were mixed with concentrated NaOH (10 N) in a tightened screw-capped polypropylene vial and then heated at 120 °C for 60 min. The alkaline lysate was cooled on ice, neutralized, and centrifuged to obtain supernatants. The hydrolysates were dried, oxidized, and finally reacted with 4-(dimethylamino) benzaldehyde. The resultant colored product was detected at 560 nm and found to be proportional to the hydroxyproline present in the sample.
RNA-seq
Total RNA was collected from cells from six unique donors (n = 6): Three donors with CD+ cells and three donors with CD− cells. From each biological donor, RNA was collected in three technical replicates. The total RNA from CD+/− cells were pooled together in each group, prior to sequencing. Three repeats were sequenced in each group (n = 3). Sequencing was performed at Novogene Co., Ltd. using the Illumina HiSeq 2500 instrument. RNA-Seq data was aligned to the reference genome (human assembly GRCh37/hg19) using Tophat2 (http://ccb.jhu.edu/software/tophat). HTSeq (http://www-huber.embl.de/HTSeq) was then applied on the aligned data set to determine differentially expressed genes with a “significant” status. The resulting P-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted P-value of < 0.05 were assigned as differentially expressed. The Gene Ontology and KEGG analyses of the differentially expressed genes were performed using DAVID (https://david.ncifcrf.gov/). The KEGG graph was rendered by Pathview75. Volcano plot was generated by BioJupies (http://biojupies.cloud.). Gene fold changes were transformed using log2 and displayed on the x-axis: P-values were corrected using the Benjamini–Hochberg method, transformed using –log10, and displayed on the y axis. Quality report and more detailed analysis can be downloaded from the following link: https://drive.google.com/drive/folders/1Kup4CKnaAotz2fuoF19rzSC7R7Sz1ha7?usp=sharing. The RNA-seq raw data is provided upon reguest.
Statistics
Statistical analysis was performed using Student's t‐test when comparing two groups. One‐way ANOVA with Tukey's multiple comparisons was performed when comparing between more than two groups. Differences were considered statistically significant at a P‐value of < 0.05. All experiments were repeated successfully at least three times (i.e., at least three separate experiments were performed with cells isolated from different donors). All experimental samples were prepared in triplicates.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Acknowledgements
The authors would like to thank Dr. Wanrooij for his advice when we set up the multiplex qPCR method in our laboratory, Dr. Wang for his suggestions on statistical analysis, and Dr. Marta Słoniecka, Dr. Juha Prittinen and Dr. Roine El-Habta for their scientific advice.
Author contributions
X.Z. and P.D. designed research; X.Z. performed research; L.B. contributed new reagents/analytic tools; X.Z. analyzed data; and X.Z., L.B. and P.D. wrote the paper.
Funding
Open access funding provided by Umea University. This work was supported by the national Swedish Research Council (Grant 2017-01138), the foundation Kronprinsessan Margaretas Arbetsnämnd för synskadade (KMA, Grant 2013/10), the foundation Ögonfonden, and via federal funds through a regional agreement (ALF) between Umeå University and Region Västerbotten (RV-930288).
Data availability
Data and materials related to this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xin Zhou, Email: xin.zhou@umu.se.
Patrik Danielson, Email: patrik.danielson@umu.se.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-86522-6.
References
- 1.Sloniecka M, et al. Expression profiles of neuropeptides, neurotransmitters, and their receptors in human keratocytes in vitro and in situ. PLoS ONE. 2015;10:e0134157. doi: 10.1371/journal.pone.0134157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funderburgh JL, Funderburgh ML, Du Y. Stem cells in the limbal stroma. Ocul Surf. 2016;14:113–120. doi: 10.1016/j.jtos.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branch MJ, et al. Mesenchymal stem cells in the human corneal limbal stroma. Invest. Ophthalmol. Vis. Sci. 2012;53:5109–5116. doi: 10.1167/iovs.11-8673. [DOI] [PubMed] [Google Scholar]
- 4.Basu S, et al. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 2014;6:266ra172. doi: 10.1126/scitranslmed.3009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson SL, El Haj AJ, Yang Y. Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering. J. Funct. Biomater. 2012;3:642–687. doi: 10.3390/jfb3030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sliney DH. Exposure geometry and spectral environment determine photobiological effects on the human eye. Photochem. Photobiol. 2005;81:483–489. doi: 10.1562/2005-02-14-RA-439.1. [DOI] [PubMed] [Google Scholar]
- 7.Gendron SP, Rochette PJ. Modifications in stromal extracellular matrix of aged corneas can be induced by ultraviolet A irradiation. Aging Cell. 2015;14:433–442. doi: 10.1111/acel.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birch-Machin MA, Swalwell H. How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis. 2010;25:101–107. doi: 10.1093/mutage/gep061. [DOI] [PubMed] [Google Scholar]
- 9.Gendron SP, Mallet JD, Bastien N, Rochette PJ. Mitochondrial DNA common deletion in the human eye: a relation with corneal aging. Mech. Ageing Dev. 2012;133:68–74. doi: 10.1016/j.mad.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Peng TI, et al. Visualizing common deletion of mitochondrial DNA-augmented mitochondrial reactive oxygen species generation and apoptosis upon oxidative stress. BBA-Mol. Basis Dis. 2006;1762:241–255. doi: 10.1016/j.bbadis.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Indo HP, et al. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Muftuoglu M, Mori MP, de Souza-Pinto NC. Formation and repair of oxidative damage in the mitochondrial DNA. Mitochondrion. 2014;17:164–181. doi: 10.1016/j.mito.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Szczesny B, et al. Mitochondrial DNA damage and subsequent activation of Z-DNA binding protein 1 links oxidative stress to inflammation in epithelial cells. Sci. Rep. 2018;8:914. doi: 10.1038/s41598-018-19216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- 15.Oka T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–U142. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreeva L, et al. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature. 2017;549:394–398. doi: 10.1038/nature23890. [DOI] [PubMed] [Google Scholar]
- 18.Shen YJ, et al. Genome-derived cytosolic DNA mediates type I interferon-dependent rejection of B cell lymphoma cells. Cell Rep. 2015;11:460–473. doi: 10.1016/j.celrep.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 19.Uchiyama M, et al. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol. Vis. 2013;19:2135–2150. [PMC free article] [PubMed] [Google Scholar]
- 20.Ellenberg D, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mobaraki M, et al. Corneal repair and regeneration: Current concepts and future directions. Front. Bioeng. Biotech. 2019;7:135. doi: 10.3389/fbioe.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venereau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front. Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bukowiecki A, Hos D, Cursiefen C, Eming SA. Wound-healing studies in cornea and skin: Parallels, differences and opportunities. Int. J. Mol. Sci. 2017;18:1257. doi: 10.3390/ijms18061257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol. Rev. 2015;265:35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhir A, et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature. 2018;560:238–242. doi: 10.1038/s41586-018-0363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, et al. Polynucleotide phosphorylase protects Escherichia coli against oxidative stress. Biochemistry. 2009;48:2012–2020. doi: 10.1021/bi801752p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Li Z. Human polynucleotide phosphorylase reduces oxidative RNA damage and protects HeLa cell against oxidative stress. Biochem. Biophys. Res. Commun. 2008;372:288–292. doi: 10.1016/j.bbrc.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon A, et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 2008;16:70–79. doi: 10.1111/j.1524-475X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 30.Li P, et al. Ski, a modulator of wound healing and scar formation in the rat skin and rabbit ear. J. Pathol. 2011;223:659–671. doi: 10.1002/path.2831. [DOI] [PubMed] [Google Scholar]
- 31.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines. Adv. Immunol. 1994;55:97–179. doi: 10.1016/S0065-2776(08)60509-X. [DOI] [PubMed] [Google Scholar]
- 32.Brown SI, Weller CA, Akiya S. Pathogenesis of ulcers of the alkali-burned cornea. Arch. Ophthalmol. 1970;83:205–208. doi: 10.1001/archopht.1970.00990030207014. [DOI] [PubMed] [Google Scholar]
- 33.Sloniecka M, Le Roux S, Zhou QJ, Danielson P. Substance P enhances keratocyte migration and neutrophil recruitment through interleukin-8. Mol. Pharmacol. 2016;89:215–225. doi: 10.1124/mol.115.101014. [DOI] [PubMed] [Google Scholar]
- 34.Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–1889. doi: 10.1182/blood.V94.6.1878. [DOI] [PubMed] [Google Scholar]
- 35.Jobin C, Haskill S, Sartor RB. Nf-Kb activation and Il-8 expression in stimulated colonic epithelial-cell lines. Gastroenterology. 1995;108:A844–A844. doi: 10.1016/0016-5085(95)27683-1. [DOI] [Google Scholar]
- 36.Mekhloufi A, et al. Bone marrow stromal cell-derived IL-8 upregulates PVR expression on multiple myeloma cells via NF-kB transcription factor. Cancers. 2020;12:440. doi: 10.3390/cancers12020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu SQ, Kirou KA, Crow MK. Pre-B-cell colony-enhancing factor (PBEF) induces NF-kB-mediated activation of IL-6 and IL-8. Faseb J. 2004;18:A1144–A1145. doi: 10.1096/fj.03-1065fje. [DOI] [Google Scholar]
- 38.Elliott CL, Allport VC, Loudon JAZ, Wu GD, Bennett PR. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol. Hum. Reprod. 2001;7:787–790. doi: 10.1093/molehr/7.8.787. [DOI] [PubMed] [Google Scholar]
- 39.Dunphy G, et al. Non-canonical activation of the DNA sensing adaptor STING by ATM and IFI16 mediates NF-kappa B signaling after nuclear DNA damage. Mol. Cell. 2018;71:745. doi: 10.1016/j.molcel.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth S, et al. Rad50-CARD9 interactions link cytosolic DNA sensing to IL-1 beta production. Nat. Immunol. 2014;15:538–545. doi: 10.1038/ni.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappa B activation through TBK1. J. Virol. 2014;88:5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suspene R, et al. Self-cytoplasmic DNA upregulates the mutator enzyme APOBEC3A leading to chromosomal DNA damage. Nucleic Acids Res. 2017;45:3231–3241. doi: 10.1093/nar/gkx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sloniecka M, Danielson P. Substance P induces fibrotic changes through activation of the RhoA/ROCK pathway in an in vitro human corneal fibrosis model. J. Mol. Med. 2019;97:1477–1489. doi: 10.1007/s00109-019-01827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ko JW, Lim SY, Chung KC, Lim JW, Kim H. Reactive oxygen species mediate IL-8 expression in Down syndrome candidate region-1-overexpressed cells. Int. J. Biochem. Cell Biol. 2014;55:164–170. doi: 10.1016/j.biocel.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Hwang YS, et al. Interleukin-1 beta stimulates IL-8 expression through MAP kinase and ROS signaling in human gastric carcinoma cells. Oncogene. 2004;23:6603–6611. doi: 10.1038/sj.onc.1207867. [DOI] [PubMed] [Google Scholar]
- 46.Narayanan PK, LaRue KE, Goodwin EH, Lehnert BE. Alpha particles induce the production of interleukin-8 by human cells. Radiat. Res. 1999;152:57–63. doi: 10.2307/3580049. [DOI] [PubMed] [Google Scholar]
- 47.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Sun Q, Zhong W, Zhang WL, Zhou ZX. Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: role of zinc deficiency. Am. J. Physiol.-Gastr. L. 2016;310:G205–G214. doi: 10.1152/ajpcell.00192.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria—Central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 50.Lin CH, Nai PL, Bien MY, Yu CC, Chen BC. Thrombin-induced CCAAT/enhancer-Binding protein beta activation and IL-8/CXCL8 expression via MEKK1, ERK, and p90 ribosomal S6 kinase 1 in lung epithelial cells. J. Immunol. 2014;192:338–348. doi: 10.4049/jimmunol.1203323. [DOI] [PubMed] [Google Scholar]
- 51.Mayer TZ, et al. The p38-MSK1 signaling cascade influences cytokine production through CREB and C/EBP factors in human neutrophils. J. Immunol. 2013;191:4299–4307. doi: 10.4049/jimmunol.1301117. [DOI] [PubMed] [Google Scholar]
- 52.de Oliveira S, Boudinot P, Calado A, Mulero V. Duox1-derived H2O2 modulates Cxcl8 expression and neutrophil recruitment via JNK/c-JUN/AP-1 signaling and chromatin modifications. J. Immunol. 2015;194:1523–1533. doi: 10.4049/jimmunol.1402386. [DOI] [PubMed] [Google Scholar]
- 53.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappa B signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toledano MB, Ghosh D, Trinh F, Leonard WJ. N-terminal DNA-binding domains contribute to differential DNA-binding specificities of Nf-kappa-B P50 and P65. Mol. Cell. Biol. 1993;13:852–860. doi: 10.1128/MCB.13.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matthews JR, Kaszubska W, Turcatti G, Wells TNC, Hay RT. Role of cysteine62 in DNA recognition by the P50 subunit of Nf-kappa-B. Nucleic Acids Res. 1993;21:1727–1734. doi: 10.1093/nar/21.8.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA-binding activity of Nf-Chi-B by reduction of a disulfide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoonbroodt S, et al. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J. Immunol. 2000;164:4292–4300. doi: 10.4049/jimmunol.164.8.4292. [DOI] [PubMed] [Google Scholar]
- 58.Takada Y, et al. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65—Evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 59.Manna SK, Ramesh GT. Interleukin-8 induces nuclear transcription factor-kappa B through a TRAF6-dependent pathway. J. Biol. Chem. 2005;280:7010–7021. doi: 10.1074/jbc.M410994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.West AP, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carpousis AJ, Vanhouwe G, Ehretsmann C, Krisch HM. Copurification of Escherichia coli RNAase-E and PNPase—Evidence for a specific association between 2 enzymes important in RNA processing and degradation. Cell. 1994;76:889–900. doi: 10.1016/0092-8674(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 62.Hayakawa H, Kuwano M, Sekiguchi M. Specific binding of 8-oxoguanine-containing RNA to polynucleotide phosphorylase protein. Biochemistry. 2001;40:9977–9982. doi: 10.1021/bi010595q. [DOI] [PubMed] [Google Scholar]
- 63.Birch-Machin M, Rees J. Mitochondrial DNA deletions in human skin reflect photo- rather than chronologic aging—Reply. J. Investig. Dermatol. 1998;111:710–710. doi: 10.1038/sj.jid.5602908. [DOI] [PubMed] [Google Scholar]
- 64.Kaya TI. Textbook of aging skin. Turk. Dermatol. Derg. 2016;10:170–170. [Google Scholar]
- 65.Chung JH, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Invest. Dermatol. 2001;117:1218–1224. doi: 10.1046/j.0022-202x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- 66.Michelacci YM. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003;36:1037–1046. doi: 10.1590/S0100-879X2003000800009. [DOI] [PubMed] [Google Scholar]
- 67.Galiacy SD, et al. Matrix metalloproteinase 14 overexpression reduces corneal scarring. Gene Ther. 2011;18:462–468. doi: 10.1038/gt.2010.159. [DOI] [PubMed] [Google Scholar]
- 68.Rippe RA, Schrum LW, Stefanovic B, Solis-Herruzo JA, Brenner DA. NF-kappaB inhibits expression of the alpha1(I) collagen gene. DNA Cell Biol. 1999;18:751–761. doi: 10.1089/104454999314890. [DOI] [PubMed] [Google Scholar]
- 69.Hozhabri NS, et al. Decreasing NF-kappaB expression enhances odontoblastic differentiation and collagen expression in dental pulp stem cells exposed to inflammatory cytokines. PLoS ONE. 2015;10:e0113334. doi: 10.1371/journal.pone.0113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang JB, Xu Y, Ding F, Wang XT. Expression of genes for collagen production and NF-kappaB gene activation of in vivo healing flexor tendons. J. Hand Surg. Am. 2004;29:564–570. doi: 10.1016/j.jhsa.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 71.Best KT, Lee FK, Knapp E, Awad HA, Loiselle AE. Deletion of NFKB1 enhances canonical NF-kappa B signaling and increases macrophage and myofibroblast content during tendon healing. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-47461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W, Chen JL, Backman LJ, Malm AD, Danielson P. Surface topography and mechanical strain promote keratocyte phenotype and extracellular matrix formation in a biomimetic 3D corneal model. Adv. Healthc. Mater. 2017;6:1601238. doi: 10.1002/adhm.201601238. [DOI] [PubMed] [Google Scholar]
- 73.Karamichos D, Guo XQ, Hutcheon AE, Zieske JD. Human corneal fibrosis: An in vitro model. Invest. Ophthalmol. Vis. Sci. 2010;51:1382–1388. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phillips NR, Sprouse ML, Roby RK. Simultaneous quantification of mitochondrial DNA copy number and deletion ratio: a multiplex real-time PCR assay. Sci. Rep. 2014;4:3887. doi: 10.1038/srep03887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo WJ, Brouwer C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29:1830–1831. doi: 10.1093/bioinformatics/btt285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials related to this study are available from the corresponding author on reasonable request.