Abstract
There are several studies that show that large amounts of acrylamide are detected in Jerusalem artichoke (Helianthus tuberosus L.) tea. This study examined acrylamide, inulin content and antioxidant properties of Jerusalem artichoke tea brewed in different conditions. Uniformly sliced Jerusalem artichokes were soaked in different salt and acidic solutions for 60 min at 20 °C and extracted with hot or cold water. The acrylamide content was analyzed by high-performance liquid chromatography–tandem mass spectrometry. The Inulin content and antioxidant activity were analyzed by spectrophotometer. Soaking significantly reduced acrylamide levels (p < 0.05) with the largest decrease observed for acetic acid, whereas the effects of all soaking treatments on inulin content were similar. Teas brewed using small-particle-size samples and hot water exhibited the highest acrylamide/inulin levels and antioxidant activity. Consequently, The most suitable treatment for Jerusalem Artichoke tea preparation was 1-h soaking in 1% acetic acid at 20 °C.
Keywords: Jerusalem artichoke tea, Soaking treatment, Acrylamide, Inulin, Antioxidant activity
Introduction
Tea, the most widely consumed beverage worldwide, has a particularly long-standing consumption history in East Asia (Venditti et al., 2010). According to the Korea Agro-Fisheries & Food Trade Corporation, the Korean tea production increased from 360,000 tons in 2015 to 400,000 tons in 2017, which is ascribed to the growing health awareness of Koreans and a shift away from coffee and carbonated beverages (Korea Agro-Fisheries & Food Trade Corporation, 2020). In addition, the tea market has experienced differentiation due to the release of teas with special ingredients such as dried Jerusalem artichoke (Helianthus tuberosus L.) (The DigitalTimes 2019).
Jerusalem artichoke, a perennial sunflower species first cultured by native Americans and resistant to frost, drought, and poor soil quality, is easily cultivated in the field and can be cooked, roasted, fried, or processed into tea, flour, chips, salads, and additives (Michalska-Ciechanowska et al., 2019). The tubers of this plant typically comprise 80 wt% water, 15 wt% carbohydrate, 1–2 wt% protein, but little or no starch and virtually no fat, thus having a relatively low calorific value (Baltacıoğlu, 2012).
In addition, Jerusalem artichoke is rich in phytochemicals such as (poly)phenols (Bach et al., 2015; Michalska-Ciechanowska et al., 2019). In plants, polyphenols are normally produced as secondary metabolites and are contained in polyhydroxy phenolic complexes (Bach et al. 2015; Michalska-Ciechanowska et al., 2019). Phenolics have diverse physiological functions, exhibiting antioxidant, antimutagenic, and antitumor activities (Bach et al., 2015). Jerusalem artichoke tubers are rich in phenolic acid antioxidants (16.6 wt% on a dry matter basis) (Michalska-Ciechanowska et al., 2019).
According to the Ministry of Food and Drug Safety, Jerusalem artichoke tea is rich in acrylamide (902–7331 µg/kg), which forms during the high-temperature (> 120 °C) cooking of certain starch-based foods such as bread, potato chips, and French fries primarily via the Maillard reaction of glucose and/or fructose with asparagine (Mottram et al., 2002; The Ministry of Food and Drug Safety, 2019). The International Agency for Research on Cancer (IARC) has classified acrylamide as potentially carcinogenic to humans (Group 2A) based on its carcinogenicity in rodents (International Agency for Research on Cancer, 1994).
Since the recognition of the importance of acrylamide in foods, methods of reducing acrylamide levels have been widely explored. Treatment with organic acids such as citric and acetic acids can also reduce acrylamide content by decreasing pH (Mestdagh et al., 2008). Moreover, monovalent and divalent cations such as Na+ or Ca2+ prevent acrylamide formation by interfering with the Maillard reaction of asparagine (Lindsay and Jang, 2005). However, the effect on the acrylamide content of Jerusalem artichoke tea due to soaking before roasting remain unknown.
In view of the above, the present study aimed to investigate the effects of various soaking treatments on the acrylamide and inulin contents and the antioxidant activity of teas prepared by hot and cold infusion of sliced and ground Jerusalem artichoke.
Materials and methods
Chemicals and reagents
Citric acid, formic acid (> 99%), D-fructose, Folin & Ciocalteu’s phenol reagent, gallic acid, 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, potassium persulfate, and ( ±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich, Inc. (St Louis, MO, USA). Sodium chloride, acetic acid, phenol, sulfuric acid (> 95%), and 3,5-dinitrosalicylic acid (DNS; 98.0%) were purchased from Samchun Pure Chemical Co., Ltd. (Pyeongtaek, Korea). Calcium chloride, sodium hydroxide, and potassium sodium tartrate were obtained from Duksan Pure Chemical (Ansan, Korea). Sodium carbonate was obtained from Daejung Chemical & Metals Co., Ltd. (Siheung, Korea).
The amino acid standard, amino acid supplement kit, borate buffer, as well as o-phthalaldehyde (OPA) and 9-fluorenylmethoxycarbonyl chloride (FMOC) were purchased from Agilent Technologies (Santa Clara, CA, USA). Acetonitrile was purchased from Merck (Darmstadt, Germany). Acrylamide (> 99%) and 13C3-acrylamide (> 99%) were supplied by Cambridge Isotope Laboratories (Andover, MA, USA). Oasis HLB (200 mg, 6 mL) and Bond Elut AccuCAT (200 mg, 3 mL) solid-phase extraction (SPE) cartridges were purchased from Waters Corp. (Milford, Massachusetts, USA) and Agilent Technologies (Santa Clara, CA, USA), respectively. Polyvinylidene fluoride (PVDF) syringe filters were supplied by Futecs Co. (Daejeon, Korea). Sodium disulfite and inulin were purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan) and MP Biomedicals (Irvine, CA, USA), respectively. High-performance liquid chromatography (HPLC)-grade water, methanol, and acetonitrile were purchased from Honeywell Burdick & Jackson (Ulsan, Korea).
Preparation of Jerusalem artichoke tea
Jerusalem artichokes purchased from a local market (Yeongam, Jeollanamdo, South Korea) were washed with tap water to eliminate soil and then, they were peeled. The peeled tubers were cut into pieces that were 2 cm in diameter and 0.5 cm in thickness, and samples weighing approximately 100 g were soaked in 1 kg of distilled water, 1% (w/v) NaCl, 1% (w/v) CaCl2, 1% (w/v) citric acid, or 1% (v/v) aqueous acetic acid solution for 60 min at 20 °C. The soaked samples were rinsed with distilled water for 10 s and gently wiped with tissue paper to remove the solution on the surface.
Prior to heating, 10 g of soaked Jerusalem artichoke was homogenized using a food mixer (BL482KRCO; Hai Xin Technology Co. Ltd., Shenzhen, China) for 20 s, and the pH of the homogenate was measured by a pH meter (S220k; Mettler Toledo, USA). The control sample did not undergo any pre-treatment before drying. The tubers were dried at 60 °C for 7 h using a hot air dryer (SFC 203; Shinsaeng, Korea) and then roasted at 180 °C for 30 min (CBR 101A; Genecafe Roaster, Ansan, Korea). According to particle size, the obtained samples were classified as sliced tea (non-ground group) and ground tea (10–12 mesh, 1.7–2.0 mm). Ground tea was prepared by grinding roasted tubers for 3 s using a food mixer (BL482KRCO; Hai Xin Technology Co., Ltd., Shenzhen, China) followed by sifting. Finally, samples weighing 1 g each were packed into tea bags.
Hot and cold tea infusions
Hot tea infusions were prepared by brewing 1 g of tea (sliced or ground) in 200 mL of distilled water at 100 ± 2 °C for 2 min, while cold tea infusions were prepared by brewing 1 g of tea (sliced or ground) in 200 mL of distilled water at 5 ± 2 °C for 4 min. The infused teas were filtered through Whatman No. 1 paper filters (Maidstone, England) and stored at − 20 °C in 50 mL tubes until further analysis.
Determination of free sugar contents in raw Jerusalem artichoke
The free sugar contents were determined using a modification of a previously reported HPLC method (Park et al., 2017). Freeze-dried samples (1–2 g) were dispersed in distilled water (50 mL) using a homogenizer (DE/MF-10; IKA, Germany), and the mixtures were centrifuged (Gyrozen 2236R, Gyrozen Inc., Dajeon, Korea) at 15,000 rpm for 10 min. The supernatants were filtered through 0.2 μm regenerated cellulose (RC) syringe filters (Phenomenex, USA) and transferred to vials. Analysis was performed on a Dionex Ultimate 3000 HPLC system (Thermo Dionex, USA) using a Sugar-pak column (Waters, 300 × 6.5 mm i.d., 10 µm particle size, USA) and distilled water was used as the mobile phase for sugar separation. The optimal flow rate equaled 0.5 mL/min and was employed in all experiments. A Shodex RI-101 detector (Shodex, Japan) was used for sugar detection, and the injection volume was set to 10 µL.
Determination of free amino acid contents in raw Jerusalem artichoke
Amino acid contents were determined using a modification of a previously reported HPLC method (Henderson et al., 2000). Freeze-dried samples (weighing 1–2 g) were mixed with distilled water (50 mL) using a homogenizer (DE/MF-10; IKA, Germany), and the homogenates were centrifuged (Gyrozen 2236R, Gyrozen Inc., Dajeon, Korea) at 15,000 rpm for 10 min. The supernatants were filtered through 0.2 μm RC syringe filters (Phenomenex, USA) and transferred to vials. The 17 amino acids standards (1 nmol/µL) and the amino acid supplement were dissolved in 0.1 N HCl. The standard solutions used for amino acid determination were diluted with distilled water to 10, 100, 500, and 1000 pmol/µL. The samples were analyzed on a Dionex Ultimate 3000 HPLC system (Thermo Dionex, USA) using an Agilent 1260 infinity fluorescence detector (Agilent, USA). The following were used for amino acid separation: an Inno C18 column (Youngjin Biochrom, 150 mm × 4.6 mm i.d., 5 μm particle size, Korea), 40 mM sodium phosphate solution as mobile phase A, and distilled water, acetonitrile, and methanol (10:45:45, v/v/v) as mobile phase B. Primary amino acids were derivatized with OPA, and secondary amino acids were derivatized with FMOC. The obtained derivatives were detected using a fluorescence detector at excitation/emission wavelengths of 340/450 nm for primary amino acids and at 266/305 nm for secondary amino acids.
Determination of acrylamide content
Acrylamide was quantified using a minor modification of the method previously reported by The Ministry of Food and Drug Safety (2018). Specifically, sliced Jerusalem artichoke (1 g) or tea infusion (1 mL) was added to water (9 mL) and a 13C3 acrylamide solution (1 mL, 200 ng/mL) in a 50 mL tube. The tube was shaken at 250 rpm for 20 min, centrifuged (COMBI-514R; Hanil Scientific Inc., South Korea) at 3,500 rpm for 5 min, and the filtrate was passed through a 0.45 μm PVDF syringe filter. An Oasis HLB cartridge was conditioned with methanol (3.5 mL) followed by water (3.5 mL). A 1.5 mL sample aliquot was introduced onto the cartridge, followed by water (0.5 mL), and the eluate was discarded. Then, further water (1.5 mL) was passed through the cartridge, and the eluate was collected. A Bond Elut AccuCAT SPE cartridge was conditioned using methanol (2.5 mL) followed by water (2.5 mL). Finally, a 0.5 mL aliquot of the eluate collected from the Oasis HLB cartridge was passed through the preconditioned Bond Elut AccuCAT SPE cartridge, and an aliquot (1 mL) of the eluate from Bond Elut AccuCAT SPE cartridge was introduced onto the Bond Elut AccuCAT SPE column and collected in 2 mL vials. High-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) analysis was performed using a Shimadzu 30A HPLC system coupled to a Shimadzu MS8040 MS/MS system (Shimadzu, Kyoto, Japan). The employed HPLC–MS/MS conditions are outlined in Table 1. The multiple reaction monitoring (MRM) transitions used for the quantitation of acrylamide and 13C3 acrylamide (internal standard) were m/z 72 → 55 and m/z 75 → 58, respectively.
Table 1.
Analytical conditions employed for acrylamide content analysis
| Items | Conditions |
|---|---|
| Chromatograph | Shimadzu 30A |
| Column | Kinetex polar C18 (150 mm × 2.1 mm i.d., 2.6 μm particle size, Phenomenex) |
| Flow rate | 0.3 mL/min |
| Oven temperature | 26 °C |
| Injection volume | 20 μL |
| Mobile phases | 0.5% methanol in distilled water and 0.1% acetic acid |
| Mass spectrometer | Shimadzu MS8040 |
| Ionization mode | Electrospray ionization, 5,000 V, positive mode |
| Detection mode | Multiple reaction monitoring (MRM) |
| Desolvation gas, collision gas | N2 |
| MRM ions | Acrylamide (m/z 72 → 55, 72 → 27), Internal standard (m/z 75 → 58, 72 → 29) |
A calibration curve for acrylamide was prepared in the range of 0.5–500 μg/L, and it showed a correlation coefficient of 0.995. Based on the calibration curve parameters, the limit of detection (LOD) and the limit of quantification (LOQ) were calculated as 1.86 and 5.62 μg/kg, respectively.
Determination of inulin content
The inulin content of Jerusalem artichoke tea was determined spectrophotometrically from the difference between total carbohydrate and reducing sugar contents (Lingyun et al., 2007). The total carbohydrate content was determined by the phenol–sulfuric acid method (Dubois et al., 1956). Fructose was used a standard solution (0–25 μg/ml). Briefly, 1 mL of the sample or distilled water (blank) was pipetted into a 15 mL test tube, and 1 mL of 5% phenol in distilled water was added. Then, concentrated sulfuric acid (5 mL, 95%) was added rapidly, with the acid stream directed against the liquid surface for better mixing. The tubes were left to stand for 10 min and then shaken and placed into a water bath held at 25–30 °C for 20 min. The sample absorbance was measured at 490 nm using an X-ma 1200 spectrophotometer (Human Corp, China).
The reducing sugar content was determined by the DNS method using D( −)-fructose as a standard. DNS (10 g), sodium hydroxide (10 g), phenol (2 g), and sodium sulfite (0.5 g) were dissolved in distilled water to a final volume of 1000 mL. Subsequently, 3 mL of the thus obtained DNS reagent and 3 mL of the sample or distilled water (blank) were added into a 15 mL test tube. The tube was held in a water bath (100 °C) for 15 min and charged with 1 mL of 40% sodium potassium tartrate (Rochelle salt) to stabilize color. The reaction mixture was cooled to room temperature (~20 °C), and absorbance was measured at 575 nm using the X-ma 1200 spectrophotometer (Human Corp., China).
Determination of total phenol content
The total phenol content of tea infusions was measured according to the method reported by Singleton and Rossi (Singleton and Rossi, 1965). Briefly, 2.5 mL of 1:10 (v/v) diluted Folin–Ciocalteu reagent was treated with 0.50 mL of the infusion and after 4 min, it was treated with 2 mL of saturated sodium carbonate solution (~75 g/L). The mixture was placed into a water bath under dark conditions at room temperature (~ 20 °C) for 2 h, and absorbance was measured at 760 nm using the X-ma 1200 spectrophotometer (Human Corp, China). Gallic acid solutions (20, 40, 60, and 80 mg/mL) were used as standards. The results were expressed as mg GAE (gallic acid equivalent) in 100 mL of tea infusion.
ABTS free radical anion scavenging activity
The antioxidant activity of Jerusalem artichoke tea infusions was characterized in terms of the ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt] radical scavenging ability according to the method of Re et al. (1999). Briefly, an ABTS+ stock solution was prepared by mixing a 14 mM aqueous ABTS solution with a 4.9 mM aqueous solution of potassium persulfate in a volume ratio of 1:1 followed by incubation in the dark at 25 °C for 16 h. The solution was then filtered through a 0.45 μm PVDF filter and diluted with distilled water to reach an absorbance of 0.70 ± 0.02 at 734 nm. Subsequently, 2.475 mL of the diluted ABTS+ solution was treated with 0.025 mL of tenfold diluted tea infusion or (in the blank experiment) 0.025 mL of water. The mixture was left for 6 min at room temperature (~ 20 °C), and absorbance at 734 nm was determined using the X-ma 1200 spectrophotometer (Human Corp., China). Trolox was used as a standard to construct a calibration curve, and the results were expressed in units of μmol Trolox equivalents per L.
Statistical analysis
Data were reported as means ± standard deviations and evaluated by analysis of variance. SPSS software (IBM Inc., Chicago, IL, USA) was employed to evaluate significant differences (p < 0.05) among the means of triplicate analysis using Duncan’s multiple range test. Differences between the means of steeping temperature or particle size experiments were estimated using the t-test for independent samples. The values were considered significant at p < 0.05.
Results and discussion
Sugar and amino acid contents of raw Jerusalem artichoke
Asparagine forms the backbone of acrylamide, while reducing sugars are essential for the formation of N-glycoside intermediates that are subsequently converted into acrylamide (De Wilde et al., 2005). The sugar and amino acid contents of raw Jerusalem artichoke are presented in Table 2.
Table 2.
Free sugar and amino acid contents of raw Jerusalem artichoke
| Matrix | Components | Type | Content |
|---|---|---|---|
| Raw Jerusalem artichoke | Sugars (g/100 g) | Fructose | 3.58 |
| Glucose | 2.3 | ||
| Sucrose | 19.64 | ||
| Amino acids (mg/100 g) | Arginine | 191.2 | |
| Asparagine | 166.86 | ||
| Glutamine | 155.84 | ||
| Aspartic acid | 123.74 | ||
| Glutamic acid | 61.5 | ||
| Alanine | 58.4 | ||
| Phenylalanine | 25.05 | ||
| Threonine | 19.73 | ||
| Histidine | 13.82 | ||
| Tryptophan | 13.02 | ||
| Serine | 11.4 | ||
| Leucine | 7.78 | ||
| Tyrosine | 6.51 | ||
| Lysine | 5.78 | ||
| Valine | 5.56 | ||
| Isoleucine | 4.94 | ||
| Proline | 4.29 | ||
| Glycine | 1.88 | ||
| Methionine | 0.4 |
The sugar contents of Jerusalem artichoke decreased in the order of sucrose > fructose > glucose. The contents of amino acids decreased in the order of arginine > asparagine > glutamine > aspartic acid, with isoleucine, proline, glycine, and methionine present at trace levels. Therefore, higher quantities of acrylamide can probably be formed in Jerusalem artichoke tea.
Effect of soaking treatments on acrylamide formation in roasted Jerusalem artichoke
Table 3 shows the effect of soaking treatments on acrylamide formation in roasted Jerusalem artichoke. The control sample had a pH of 6.34 ± 0.03 before drying and generated acrylamide up to an average contamination level of 4843.60 μg/g after roasting.
Table 3.
Effects of soaking treatments on acrylamide formation in roasted Jerusalem artichoke and Jerusalem artichoke tea
| Sample | Soaking treatment | Particle size | Acrylamide content (μg/kg) |
|---|---|---|---|
| Roasted Jerusalem artichokea | Control (without soaking) | 4843.60 ± 20.96a | |
| Distilled water | 2843.99 ± 20.90b | ||
| 1% NaCl | 1924.11 ± 27.93c | ||
| 1% CaCl2 | 1592.22 ± 18.33d | ||
| 1% Citric acid | 1788.33 ± 70.60c | ||
| 1% Acetic acid | 1287.96 ± 25.75e | ||
| Hot infused Jerusalem artichoke teab | Control (without soaking) | Sliced | 17.14 ± 0.48AY |
| Ground | 26.21 ± 2.91AX | ||
| Distilled water | Sliced | 12.23 ± 1.15BY | |
| Ground | 15.72 ± 0.89BX | ||
| 1% NaCl | Sliced | 11.62 ± 0.22BY | |
| Ground | 14.85 ± 0.73BX | ||
| 1% CaCl2 | Sliced | 5.19 ± 0.40CY | |
| Ground | 6.84 ± 0.13CX | ||
| 1% Citric acid | Sliced | 12.28 ± 0.88BX | |
| Ground | 13.32 ± 0.84BX | ||
| 1% Acetic acid | Sliced | 3.75 ± 0.43DY | |
| Ground | 4.85 ± 0.08DX | ||
| Cold infused Jerusalem artichoke teab | Control (without soaking) | Sliced | 11.79 ± 0.67AX |
| Ground | 17.47 ± 2.89AX | ||
| Distilled water | Sliced | 9.26 ± 0.81BY | |
| Ground | 12.92 ± 0.03BX | ||
| 1% NaCl | Sliced | 6.81 ± 0.75CY | |
| Ground | 9.71 ± 0.73CX | ||
| 1% CaCl2 | Sliced | 3.32 ± 0.32EY | |
| Ground | 4.95 ± 0.11DEX | ||
| 1% Citric acid | Sliced | 4.83 ± 0.42DY | |
| Ground | 5.94 ± 0.04DX | ||
| 1% Acetic acid | Sliced | n.d | |
| Ground | 3.00 ± 0.12EX |
aValues with different letter within a same column (a–e) are significantly different (p < 0.05) as measured by Duncan’s test
bLetters A–E indicate statistically significant differences (p < 0.05) between the acrylamide levels obtained for different soaking treatments but the same particle size and steeping temperature. Letters X and Y indicate statistically significant differences (p < 0.05) between the acrylamide levels obtained for different particle size but the same soaking treatment and steeping temperature
Compared to the control sample, soaking treatments significantly reduced (p < 0.05) acrylamide levels, which decreased in the order of distilled water > 1% NaCl > 1% citric acid > 1% CaCl2 > 1% acetic acid. Soaking in distilled water led to the leaching of acrylamide precursors such as glucose and asparagine and thus reduced acrylamide formation. Kita et al. (2004) reported that potato slice soaking in distilled water for 60 min at 20 °C resulted in the extraction of fructose (17%) and asparagine (40%). Moreover, soaking hindered acrylamide formation (by 38%) during potato slice heating at 170 °C. These results agree with those of Jung et al. (2003), who reported that 1-h dipping of potato strips into distilled water induced a 25% reduction of acrylamide formation during frying at 190 °C.
Monovalent and divalent cations such as Na+ or Ca2+ can interact with asparagine to prevent the formation of the Schiff base that is a key intermediate in the formation of acrylamide and the Maillard reaction (Lindsay and Jang, 2005). The inhibition of acrylamide formation during potato strip heating was mainly ascribed to the presence of monovalent or divalent cations in these strips after soaking treatment rather than to the reduced content of acrylamide precursors such as asparagine and reducing sugars (El-Saied et al., 2008). El-Saied et al. (2008) reported that potato strip soaking in 1% NaCl and 1% CaCl2 for 60 min resulted in a 69% and 92% decrease of acrylamide levels, respectively after frying at 180 °C. In addition, divalent cations such as Ca2+ and Mg2+ change the reaction pathway from the Maillard reaction to the dehydration of glucose and thus hinder the formation of acrylamide. Therefore, the divalent Ca2+ is more effective for the inhibition of acrylamide formation than the monovalent Na+.
Soaking in acetic acid solution was most effective for suppressing acrylamide formation, resulting in an acrylamide content decrease to 1287.96 μg/g, whereas soaking in citric acid resulted in a decrease to only 1788 μg/g. These results were ascribed to the decrease in pH upon soaking in acidic solutions. To be specific, the average pH of Jerusalem artichoke tuber soaked in distilled water, 1% NaCl, 1% CaCl2, 1% citric acid, and 1% acetic acid equaled 6.32 ± 0.01, 6.36 ± 0.02, 6.16 ± 0.01, 5.10 ± 0.02, and 4.61 ± 0.02, respectively. Citric acid has a lower pKa than acetic acid, but the corresponding solution had a higher pH value than the acetic acid solution, as the molar concentrations of 1% citric acid and 1% acetic acid solutions equaled 0.05 and 0.17 M, respectively. Kita et al. (2004) reported that when solutions of citric acid and acetic acid with the same concentration of acidic protons were used (0.05 M and 0.15 M, respectively), the latter solution was more effective for suppressing acrylamide formation. Food pH can influence acrylamide formation, which is most favored at pH values of 7–7.5 (Jung et al., 2003). Low pH probably induced the protonation of the reactive free α-NH2 group of asparagine to afford the nonreactive α -NH3+ form and promoted the acid-catalyzed partial hydrolysis of asparagine to aspartic acid and that of acrylamide to acrylic acid (Friedman and Levin, 2008). Furthermore, no Jerusalem artichoke tea prepared after 60 min soaking exhibited sour or salty taste. Jung et al. (2003) did not detect any taste differences between the control (no soaking treatment) and the 1% citric acid–soaked group. Kita et al. (2004) reported that taste differences were not detected when acetic acid was used. In addition, the sensory quality of strips fried after soaking in 1% CaCl2 was reported to be maintained (Khalaf et al., 2015). This result suggests that acetic acid treatment is the best way of inhibiting acrylamide formation during Jerusalem artichoke tea production.
Effect of particle size and steeping temperature on acrylamide content in Jerusalem artichoke tea
Table 3 show the acrylamide contents of sliced and ground Jerusalem artichoke tea infusions prepared under different steeping conditions. Regardless of particle size and steeping temperature, acrylamide content was lowest in infusions prepared using acetic acid pre-treatment, with the second lowest values observed for CaCl2 pre-treatment. The reason for this result is thought to be that it was formed less during the roasting process by the pretreatment of the solution. The contents of acrylamide in ground Jerusalem artichoke tea infusions (3.00–26.21 μg/g) exceeded those in sliced Jerusalem artichoke tea infusions (n.d.–17.14 μg/g). According to Liu et al. (2008), acrylamide content (ng/g) in tea products is n.d.–46 μg/g and our results were similar to that results. The particle size had a significant (p < 0.05) effect on the acrylamide level, as particle size reduction can increase surface area and allows solution uptake and/or extraction to occur more completely, uniformly, and rapidly (Dria et al., 2007). Hot tea infusions had significantly higher acrylamide contents (3.75–26.21 μg/g) than cold tea infusions (n.d.–17.47 μg/g), which was ascribed to the increase in mass transfer coefficients and rate constants at high temperature (Kaykhaii and Abdi, 2013).
Inulin contents of Jerusalem artichoke tea infusions
The inulin contents of hot and cold tea infusions prepared from Jerusalem artichoke subjected to different pre-treatments are listed in Table 4. The pre-treatment method did not strongly affect the inulin content of Jerusalem artichoke tea infusions. Still, the lowest inulin content was observed for citric acid pre-treatment, which was ascribed to the fact that inulinase, an enzyme catalyzing the breakdown of inulin, is most active at pH 5 (Jhon and Kim, 1988).
Table 4.
Inulin contents of different infusion method of Jerusalem artichoke tea
| Sample | Soaking treatment | Particle size | Inulin content (mg/100 mL) |
|---|---|---|---|
| Hot infused Jerusalem artichoke tea | Control (without soaking) | Sliced | 72.32 ± 2.87 aY |
| Ground | 122.90 ± 8.01 aX | ||
| Distilled water | Sliced | 65.73 ± 1.26 bY | |
| Ground | 94.49 ± 3.54 bX | ||
| 1% NaCl | Sliced | 62.02 ± 1.51 bY | |
| Ground | 91.72 ± 0.97 bX | ||
| 1% CaCl2 | Sliced | 61.23 ± 5.47 bY | |
| Ground | 91.71 ± 4.95 bX | ||
| 1% Citric acid | Sliced | 61.06 ± 0.97 bY | |
| Ground | 88.98 ± 2.80 bX | ||
| 1% Acetic acid | Sliced | 64.07 ± 3.05 bY | |
| Ground | 95.24 ± 1.89 bX | ||
| Cold infused Jerusalem artichoke tea | Control (without soaking) | Sliced | 40.33 ± 1.38 aY |
| Ground | 69.27 ± 2.86 aX | ||
| Distilled water | Sliced | 30.88 ± 0.92 bY | |
| Ground | 52.29 ± 3.48 bX | ||
| 1% NaCl | Sliced | 29.65 ± 2.36 bY | |
| Ground | 49.39 ± 1.39 bX | ||
| 1% CaCl2 | Sliced | 30.67 ± 1.79 bY | |
| Ground | 49.40 ± 2.99 bX | ||
| 1% Citric acid | Sliced | 28.99 ± 4.45 bY | |
| Ground | 48.75 ± 3.04 bX | ||
| 1% Acetic acid | Sliced | 31.59 ± 0.91 bY | |
| Ground | 50.70 ± 2.06 bX |
a,bEach value indicates statistically significant differences (p < 0.05) between the acrylamide levels obtained for different soaking treatments but the same particle size and steeping temperature
x,yIndicate statistically significant differences (p < 0.05) between the acrylamide levels obtained for different particle size but the same soaking treatment and steeping temperature
The inulin content of ground Jerusalem artichoke tea infusions (48.75–122.90 mg/100 mL) was significantly higher than that of sliced Jerusalem artichoke tea infusions (28.99–72.32 mg/100 mL) prepared under the same conditions, while the inulin content of hot tea infusions (61.06–122.90 mg/100 mL) significantly exceeded that of cold tea infusions (28.99–69.27 mg/100 mL). Notably, inulin solubility equals ~ 6 wt% at 25 °C, increasing to 35 wt% at 90 °C (Ahmed and Rashid, 2019). For this reason, most industrial extraction processes employ hot water diffusion systems (Paseephol et al., 2007).
Total phenol contents of Jerusalem artichoke tea infusions
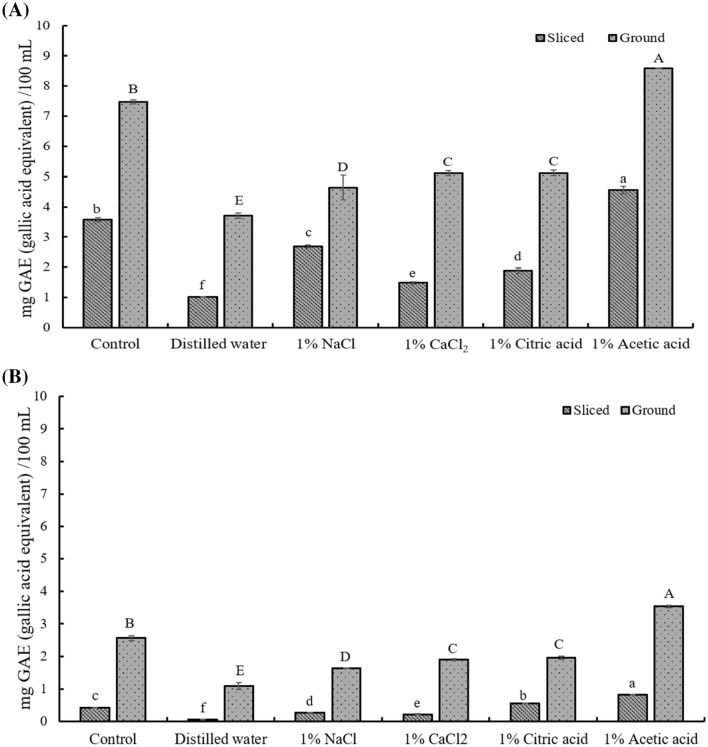
The total phenol contents of hot and cold tea infusions prepared using different pre-treatments are presented in Fig. 1. The decrease in total phenol content after soaking can be attributed to the leaching of phenols into the soaking solution (Afify et al., 2012). The infusion of Jerusalem artichoke tea subjected to acetic acid pre-treatment had the highest total phenol content. These results were ascribed to the effects of soaking solutions on the activity of polyphenol oxidase, the main enzyme responsible for the oxidative degradation of phenolics (Tan et al., 2015). This enzyme is most active at neutral pH, whereas the Jerusalem artichoke tuber soaked in acetic acid had a pH of 4.61 (ZİYAN and PEKYARDIMCI, 2003). Thus, the inhibitory effect of acetic acid was ascribed to maintaining pH well below that necessary for optimal polyphenol oxidase activity.
Fig. 1.
Total phenol contents (expressed as mg gallic acid equivalents (GAE) per 100 mL) of Jerusalem artichoke tea. (A) Hot infused Jerusalem artichoke tea. (B) Cold infused Jerusalem artichoke tea
In general, total phenol contents were significantly higher in ground tea infusions (1.09–8.6 mg GAE/100 mL) than in sliced tea infusions (0.05–4.55 mg GAE/100 mL). Thus, diffusion efficiency was higher for the former infusions, i.e., the tea particle size affected the extraction efficiency. These results were attributed to the fact that more time was required for complete surface wetting and bioactive compound extraction in the case of larger leaves (Castiglioni et al., 2015). And those results are agreed with those of Lee et al. (2014), who reported that the use of smaller particles improves the efficiency of phenol extraction from puffed Jerusalem artichoke tea.
Hot tea infusions had significantly higher total phenol contents (1.03–8.6 mg GAE/100 mL) than the corresponding cold tea infusions (0.05–3.53 mg GAE/100 mL). Similarly, Venditti et al. (2010) showed that the total phenol contents of green tea infusions were higher when extraction was performed with hot water for 7 min than in the case of 2-h extraction with cold water. This finding suggests that the type and amount of phenolic compounds as well as the extraction temperature and time corresponding to the highest antioxidant capacity depend on the tea type. Therefore, in the present study, the total phenol contents of hot and cold Jerusalem artichoke tea infusions reflected their phenolic compound speciation.
ABTS free radical anion scavenging activity
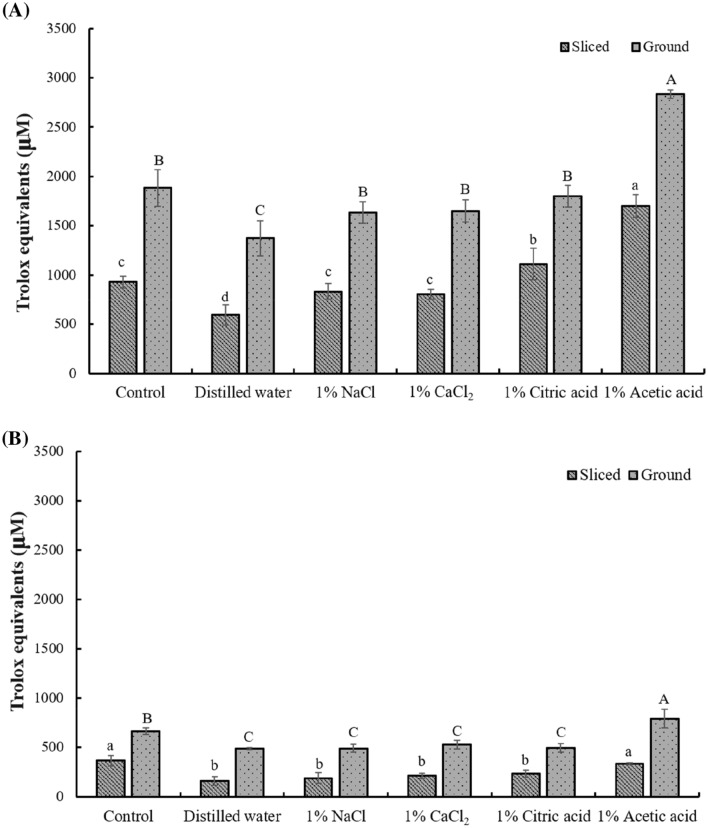
Figure 2 present the ABTS radical anion scavenging activities of hot and cold Jerusalem artichoke tea infusions, respectively. In the present study, although some small differences were observed among Jerusalem artichoke tea infusions, the general trend of antioxidant activity matched that of total phenol content. Infusions prepared using acetic acid pre-treatment had the highest activity, whereas the lowest activity was observed for distilled water pre-treatment. In general, total phenol content was significantly higher in ground tea infusions (161.67–1700.56 μM TEAC (Trolox equivalent antioxidant capacity)/L) than in sliced tea infusions (488.9–2833.89 μM TEAC/L). In addition, hot tea infusions had significantly higher total phenol contents (595–2833.89 μM TEAC/L) than the respective cold tea infusions (161.67–789.44 μM TEAC/L).
Fig. 2.
Antioxidant activity (expressed as μM Trolox equivalents) of Jerusalem artichoke tea (A) Hot infused Jerusalem artichoke tea. (B) Cold infused Jerusalem artichoke tea
This study reports the effects of soaking treatment on the acrylamide/inulin contents and antioxidant activity of hot and cold teas prepared from sliced and ground Jerusalem artichoke. The 1-h soaking (before roasting) of Jerusalem artichoke in distilled water or 1% NaCl, 1% CaCl2, 1% citric acid, and 1% acetic acid solutions significantly suppressed acrylamide formation, with the largest effect observed for acetic acid. The use of hot water and small tea particles increased acrylamide levels. Regarding inulin contents, no significant differences were observed among soaking treatments, and small particle size and high temperature were found to favor inulin extraction. The highest total phenol content and antioxidant activity were observed for the hot infusion of ground tea subjected to soaking in 1% acetic acid. Thus, considering the levels of acrylamide/inulin and antioxidant activity, the best tea preparation conditions were identified as the hot infusion of ground tea pre-soaked in 1% acetic acid. The obtained results are expected to facilitate the further optimization of Jerusalem artichoke tea processing in terms of acrylamide reduction. In the future, more research will be needed on methods of reducing acrylamide in Jerusalem artichoke because it is still selling as health food in Korea.
Acknowledgements
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2019.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jeong-Min Jo, Email: wjdals3083@daum.net.
Jong-Sun Lee, Email: gpqls3873@naver.com.
Munyhung Jung, Email: munjung@woosuk.ac.kr.
Myung-Sub Chung, Email: chungms@cau.ac.kr.
References
- Afify AE-MM, El-Beltagi HS, Abd El-Salam SM, Omran AA. Biochemical changes in phenols, flavonoids, tannins, vitamin E, β–carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pacific Journal of Tropical Biomedicine. 2012;2:203–209. doi: 10.1016/S2221-1691(12)60042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W, Rashid S. Functional and therapeutic potential of inulin: A comprehensive review. Critical Reviews in Food Science and Nutrition. 2019;59:1–13. doi: 10.1080/10408398.2017.1355775. [DOI] [PubMed] [Google Scholar]
- Bach, V., Clausen, M. R., & Edelenbos, M. Production of Jerusalem artichoke (Helianthus tuberosus L.) and impact on inulin and phenolic compounds. Processing and Impact on Active Components in Food 97-102 (2015)
- Baltacıoğlu, C. Production of chips and crisp from jerusalem artichoke. PhD thesis. The graduate school of natural and applied sciences of middle east technical university, Çankaya/Ankara, Türkiye (2012)
- Castiglioni S, Damiani E, Astolfi P, Carloni P. Influence of steeping conditions (time, temperature, and particle size) on antioxidant properties and sensory attributes of some white and green teas. International Journal of Food Sciences and Nutrition. 2015;66:491–497. doi: 10.3109/09637486.2015.1042842. [DOI] [PubMed] [Google Scholar]
- De Wilde T, De Meulenaer B, Mestdagh F, Govaert Y, Vandeburie S, Ooghe W, Fraselle S, Demeulemeester K, Peteghem K, Calus A, Degroodt J, Verhé R. Influence of storage practices on acrylamide formation during potato frying. Journal of Agricultural and Food Chemistry. 2005;53:6550–6557. doi: 10.1021/jf050650s. [DOI] [PubMed] [Google Scholar]
- Dria, G., Zyzak, D., Gutwein, R., Villagran, F., Young, H., Bunke, P., Lin, P. Howie, J., Schafermeyer, R. Method for reduction of acrylamide in roasted coffee beans, roasted coffee beans having reduced levels of acrylamide, and article of commerce. In: Google Patents. (2007)
- Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- El-Saied M, Sharaf A, Abul-Fad M, El-Badry N. Reduction of acrylamide formation in fried potato strips by different pre-frying treatments. World Journal of Dairy & Food Sciences. 2008;3:17–24. [Google Scholar]
- Friedman M, Levin CE. Review of methods for the reduction of dietary content and toxicity of acrylamide. Journal of agricultural and food chemistry. 2008;56:6113–6140. doi: 10.1021/jf0730486. [DOI] [PubMed] [Google Scholar]
- Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C. Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Amino Acid Anal. Using Zorbax Eclipse AAA Columns Agil. 2000;1100:1–10. [Google Scholar]
- International Agency for Research on Cancer . Some industrial chemicals: IARC monographs on the evaluation of carcinogenesis risks to humans. Geneva: World Health Organization Press; 1994. [Google Scholar]
- Jhon D-Y, Kim M-H. Studies on inulase from Jerusalem artichoke. Journal of the Korean Society of Food Science and Nutrition. 1988;17:205–210. [Google Scholar]
- Jung M, Choi D, Ju J. A novel technique for limitation of acrylamide formation in fried and baked corn chips and in French fries. Journal of Food Science. 2003;68:1287–1290. doi: 10.1111/j.1365-2621.2003.tb09641.x. [DOI] [Google Scholar]
- Kaykhaii M, Abdi A. Rapid and sensitive determination of acrylamide in potato crisps using reversed-phase direct immersion single drop microextraction-gas chromatography. Analytical Methods. 2013;5:1289–1293. doi: 10.1039/c2ay26560e. [DOI] [Google Scholar]
- Khalaf H, Sharoba A, El-Desouky A, El-Bassiony K, Afifi S. Effect of some pre-treatments on acrylamide concentration in potato chips. Annals of Agricultural Sciences. 2015;3:211–220. [Google Scholar]
- Kim, A.-L., Jerusalem artichoke… Tea must also be unique. The DigitalTimes. Available online: http://www.dt.co.kr/contents.html?article no=2019112002109932060002&ref=naver (accessed on 23 June 2020)
- Kita A, Bråthen E, Knutsen SH, Wicklund T. Effective ways of decreasing acrylamide content in potato crisps during processing. Journal of Agricultural and Food Chemistry. 2004;52:7011–7016. doi: 10.1021/jf049269i. [DOI] [PubMed] [Google Scholar]
- Korea Agro-Fisheries & Food Trade Corporation. Processed Food Segment Market Report-Tea Market. Available online: https://www.atfis.or.kr/article/M001050000/view.do?articleId=3093 (accessed on 15 May 2020)
- Lee Y-J, Lee M-G, Yu S-Y, Yoon W-B, Lee O-H. Changes in physicochemical characteristics and antioxidant activities of Jerusalem artichoke tea infusions resulting from different production processes. Food Science and Biotechnology. 2014;23:1885–1892. doi: 10.1007/s10068-014-0257-3. [DOI] [Google Scholar]
- Lindsay, R. C., & Jang, S. Chemical intervention strategies for substantial suppression of acrylamide formation in fried potato products. Chemistry and Safety of Acrylamide in Food, 393-404 (2005) [DOI] [PubMed]
- Lingyun, W., Jianhua, W., Xiaodong, Z., Da, T., Yalin, Y., Chenggang, C., Tianhua F., Fan, Z. Studies on the extracting technical conditions of inulin from Jerusalem artichoke tubers. Journal of Food Engineering 79: 1087-1093 (2007)
- Liu J, Zhao G, Yuan Y, Chen F, Hu X. Quantitative analysis of acrylamide in tea by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Food Chemistry. 2008;108:760–767. doi: 10.1016/j.foodchem.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Mestdagh F, Maertens J, Cucu T, Delporte K, Van Peteghem C, De Meulenaer B. Impact of additives to lower the formation of acrylamide in a potato model system through pH reduction and other mechanisms. Food Chemistry. 2008;107:26–31. doi: 10.1016/j.foodchem.2007.07.013. [DOI] [Google Scholar]
- Michalska-Ciechanowska, A., Wojdyło, A., Bogucka, B., & Dubis, B. Moderation of inulin and polyphenolics contents in three cultivars of Helianthus tuberosus L. by potassium fertilization. Agronomy 9: 884 (2019)
- Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- Park S-H, Do Y-S, Kim Y-S, Kim N-Y, Lee J-H, Kim J-H, Yoon MH. Determination of Phenolic Contents in Rooibos (Asphalthus linearis) Tea Depending on the Steeping Temperature and Time. Journal of Food Hygiene and Safety. 2017;32:389–395. doi: 10.13103/JFHS.2017.32.5.389. [DOI] [Google Scholar]
- Paseephol T, Small D, Sherkat F. Process optimisation for fractionating Jerusalem artichoke fructans with ethanol using response surface methodology. Food Chemistry. 2007;104:73–80. doi: 10.1016/j.foodchem.2006.10.078. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16:144–158. [Google Scholar]
- Tan JJY, Lim YY, Siow LF, Tan JBL. Effects of drying on polyphenol oxidase and antioxidant activity of Morus alba leaves. Journal of Food Processing and Preservation. 2015;39:2811–2819. doi: 10.1111/jfpp.12532. [DOI] [Google Scholar]
- The Ministry of Food and Drug Safety. Test method for substances that have not established standard standards among foods, etc. Available online: http://www.law.go.kr/LSW/admRulInfoP.do?admRulSeq=2000000012210 (accessed on 20 May 2020).
- The Ministry of Food and Drug Safety. Year book of imported food inspection (in 2018). Available online: https://www.mfds.go.kr/brd/m_99/view.do?seq=43665 (accessed on 10 May 2020)
- Venditti, E., Bacchetti, T., Tiano, L., Carloni, P., Greci, L., & Damiani, E. Hot vs. cold water steeping of different teas: do they affect antioxidant activity? Food Chemistry 119: 1597-1604 (2010)
- Ziyan, E., & Pekyardimci, Ş. Characterization of polyphenol oxidase from Jerusalem artichoke (Helianthus tuberosus). Turkish Journal of Chemistry 27: 217-226 (2003)