Abstract
In this study, cholesterol (CH), β-sitosterol (SI), and stigmasterol (ST) were explored to improve the stability of retinol in the liposome bilayer. Retinol was incorporated into liposomes composed of soybean-derived L-α-phosphatidylcholine (PC) and 10% sterol (w/w), which were prepared as multilamellar vesicles. Under all conditions, the efficiency of retinol incorporation into liposomes was higher than 99%, and the average particle size of liposomes was similar to that of PC alone. Liposomes were stored at 4 and 25 °C, with and without light, respectively, for 10 days. It was found that during the storage, CH and SI were effective in stabilizing the retinol in liposomes. These results indicate that an appropriate phytosterol could improve the stability of retinol in liposomes.
Keywords: Retinol, Liposome, Phytosterol, Stability
Introduction
Retinol or vitamin A is necessary for human growth including bone development, skin improvement, and visual, reproductive, and immune functions (Kafi et al., 2007; WHO/FAO, 2005). Generally, fat-soluble vitamins such as retinol are sensitive to oxygen, heat, metal ions, and ultraviolet rays, and can be easily destroyed (Combs 2007). Retinol is an isoprenoid which has conjugated double bonds; thus, it reacts easily with oxygen. This oxidation is catalyzed by excitation with light.
Liposomes are an effective tool for the protection of retinol against oxidation by light, and the addition of sterols into liposomes could delay oxidation. Many studies have been conducted on microencapsulation for improving the stability of retinol (Gonçalves et al., 2016). The size of microcapsules varies from several nanometers to millimeters; due to their ability to protect internal substances and control the rate of release, they are used in the development of functional foods, cosmetics, and medicines (Moumita et al., 2018; Whelan, 2001).
There are many methods for the microencapsulation of retinols, which include liposome conversion, cochleate formation, spray-drying, spray-cooling, coacervation, solid lipid nanoparticle development, emulsion, and inclusion complexation (Gonçalves et al., 2016). The structure of a liposome contains a phospholipid bilayer; hydrophilic heads are directed inwards and outwards, while the space between the phospholipid bilayer is composed of hydrophobic fatty acids that can incorporate retinol inside (Singh and Das, 1998). The liposome has been reported to effectively protect incorporated retinol against light (Lee et al., 2002).
Cholesterol (CH) is known to contribute to the stability of the phospholipid double membranes. CH in the phospholipid bilayer induces close presence of fatty acyl chain and causes liquid ordered state (Veatch and Keller, 2005). However, since CH is generally known to pose a risk of developing cardiovascular disease, the effect of liposomes supplemented with phytosterol, a molecule structurally similar to CH, has been studied for retinol stability (Lee et al., 2005a; 2020).
β-Sitosterol (SI) is a phytosterol that has an additional ethylene group at the C-24 position of CH, and it reduces blood cholesterol and prevents the onset of arteriosclerosis (Brufau et al., 2008). Stigmasterol (ST) is also a phytosterol in which a double bond is added to the position C-22 of the SI (Fig. 1). ST acts as a precursor during the biosynthesis of hormones such as progesterone, estrogen, and vitamin (Kaur et al., 2011). These phytosterols are not only structurally similar to CH but also have beneficial physiological functions in human body. Although the effect of CH (Lee et al., 2005b) and SI (Lee et al., 2005a) on the stability of incorporated retinol in liposomes have been reported, there has been no direct comparison of the effect of phytosterol and CH at same condition. In the present study, therefore, we tried to compare the effect of two phytosterols (SI and ST) and CH on the stability of incorporated retinol in liposomes.
Fig. 1.
Structure of sterols used in this study
Materials and methods
Reagents
L-α-Phosphatidylcholine [from soybean, ≥ 30% phosphatidyl choline (PC) basis], CH, SI, ST, and retinol were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals and reagents were of analytical grade or purer and procured from Daejung Chemicals & Metals Co. (Kyonggi do, Siheung, KOR) and Sigma-Aldrich Co. (St. Louis, MO, USA).
Preparation of liposomes
Liposomes in this study were prepared by the method of Lee et al. (2002) with a slight modification. The sterols (CH, SI, or ST) were added to PC at ratios of 100:0 and 90:10 (w/w, PC:sterol). Retinol was added at a ratio of 0.01:1 (w/w) for PC and sterol. In the 90:10 (w/w, PC:sterol) group, 30 mg of each sterol was added to a 100 mL round bottom flask containing 270 mg of PC; 3 mg of retinol was then added. PC was added instead of sterol in the 100:0 (w/w, PC:sterol) group. The mixture was then dissolved in 30 mL of chloroform/methanol solvent mixture (2:1, v/v). The solution was evaporated at 30 °C using a rotary evaporator, forming a dry lipid film on the flask wall. The solvent that remained in the flask was removed by treating nitrogen gas for 5 min at 4 °C. Then, 30 mL of 10 mM glycine buffer (pH 9.0, containing 0.115 M NaCl) was added for suspending the lipid film, and 1.5 g of glass beads were further added to assist the desorption of the film. The lipid film was suspended in a buffer to form multilamellar vesicles (MLVs) by rotating the flask in a rotary evaporator without vacuum. To remove free retinol, the liposome suspension was centrifuged at 80,000 × g (Optima XE-100 Ultracentrifuge, Beckman Coulter Inc., Brea, CA, USA) for 1 h, and the supernatant was decanted. The pellet was re-suspended in 30 mL of 10 mM potassium phosphate buffer (pH 7.0) and was used as a sample.
Liposome size measurement
The size of prepared liposome was measured using a particle size analyzer (Mastersizer 3000, Malvern Instruments, Malvern, Worcestershire, UK). The mean value was obtained by the diameter calculated from the volume of the particle (D4,3), whereas the median was the size value corresponding to 50% of the measured volume of particles in order (Dv50).
Stability of incorporated retinol into liposomes during storage
Liposomes containing retinol were sufficiently homogenized and saturated with oxygen by placing 1 mL aliquots into 8 mL glass vials and equilibrating to the atmosphere for 2 h in the dark. The vials were stored with ambient and fluorescence lights at 4 and 25 °C, respectively. Vials were wrapped in aluminum foil for storage in the dark. Retinol was measured at 0, 2, 4, 6, 8, and 10 days of storage.
Analytical method
Retinol in the liposomes was analyzed using colorimetric analysis (Subramanyam and Parrish, 1976). Liposome suspension (0.2 mL) containing retinol was mixed with 0.6 mL of chloroform/methanol solvent mixture (2:1, v/v). The mixture was centrifuged at 4200 × g for 3 min. Subsequently, 0.1 mL of the organic solvent layer was taken and reacted with 1 mL of SbCl3 reagent (20 g of SbCl3 in 100 mL of chloroform with 3 g of acetic anhydride), and immediately measured for color at 620 nm by using a UV–VIS spectrophotometer (X-ma 1200 V, Human Corporation, Seoul, Korea). The concentration of retinol was determined by a standard curve that was prepared using pure retinol dissolved in chloroform.
Statistical analysis
All measurements were done in triplicate, and data were presented as mean ± standard deviations. IBM SPSS software 23.0 (Armonk, NY, USA) was used for statistical analysis, and significant differences in mean values were compared with Duncan’s multi-range test (p < 0.05). The residual amount of retinol was plotted as an average value and an error range.
Results and discussion
Incorporation efficacy of retinol into liposome
After preparation of MLV liposomes with a ratio of 0.01:1 (retinol:PC with or without sterol), the incorporation efficacy of retinol into liposome was determined by analyzing retinol in the supernatant and precipitated liposome after centrifugation. Retinol was incorporated into liposome in the range of 99.14 ± 0.08 to 99.47 ± 0.11% (Table 1). In the composition of liposomes, 10% of sterol did not show a significant reduction effect on the incorporation of retinol in a 0.01:1 ratio (retinol:PC and sterol, w/w). Lee et al. (2005b) reported that the retinol incorporated into liposomes containing PC and CH (0-50%, w/w) at a ratio of 0.01:1 (retinol: lipid, w/w) was over 94.52%. It was also reported that the addition of SI in PC liposome did not affect the incorporation of retinol (Lee et al., 2005a). Liposomes in MLV have enough space for incorporating hydrophobic retinol as they have a high lipid content and a low capacity for water-soluble matter (Reineccius, 1995).
Table 1.
The effect of sterol content of liposomes on retinol incorporation efficiency and liposome size
| Liposome | Ratio1 | Incorporation efficiency (%)2 | Average size of liposome (µm)2 | |
|---|---|---|---|---|
| Mean | Median | |||
| PCL | 100:0 | 99.47 ± 0.11a | 10.6 ± 0.2b | 9.8 ± 0.8a |
| CHL | 90:10 | 99.41 ± 0.11a | 12.3 ± 3.1ab | 11.0 ± 2.7a |
| SIL | 90:10 | 99.14 ± 0.08b | 10.1 ± 0.6b | 9.8 ± 0.4a |
| STL | 90:10 | 99.23 ± 0.03b | 13.7 ± 0.2a | 10.1 ± 0.6a |
Retinol: (PC + sterol) = 0.01:1 (w/w)
PCL PC liposomes, CHL liposomes containing CH, SIL liposomes containing SI, STL liposomes containing ST
Different letters (a through b) indicate significant difference (p < 0.05); n = 3
1Weight ratio of phosphatidylcholine to sterol
2Mean ± standard deviation of triplicate measurements
Size of liposomes
The particle size of liposomes affects various properties such as encapsulation efficiency, stability, drug release, and cell absorption, and thus becomes one of the very important factors when applied to food and pharmaceutical fields. For example, useful ingredients such as vitamins and micronutrients, including Retinol, can negatively affect sensory properties such as color, taste, and aroma when added to food, but this can be overcome by including them inside the liposome. In this case, since the liposome has transparency at the nano-scale, it is possible to minimize undesirable sensory properties while including various useful ingredients without impairing the color of the beverage (Danaei et al., 2018). As shown at Table 1, the mean and median sizes of PCL (PC liposomes), CHL (liposomes containing CH), SIL (liposomes containing SI), and STL (liposomes containing ST) were 10.6 ± 0.2 and 9.8 ± 0.8, 12.3 ± 3.1 and 11.0 ± 2.7, 10.1 ± 0.6 and 9.8 ± 0.4, and 13.7 ± 0.2 and 10.1 ± 0.6 μm, respectively. Lee et al. (2005b) indicated that at pH 7.0, the mean size of CHL decreased slightly from 4.54 to 4.49 μm at 0–10% sterol concentration and increased by 85.04 μm when increased to 10–50%. On the other hand, at pH 5.0 and 9.0, the size of liposomes increased with increasing sterol concentration from 0 to 50%. Zhao et al. (2015) reported that the mean size of SIL decreased when the molar concentration of SI increased from 0 to 10%, and increased from 146.9 ± 8.72 to 245.5 ± 7.14 nm when it increased from 10 to 50%. Lee et al. (2020) measured the average size of the STL as 7.28, 6.00, and 10.86 μm at 100:0, 90:10, and 70:30 (PC:ST) ratios, respectively. Sterols are inserted between the acyl chain tails close to the phospholipid head group. At low concentrations, it separates the hydrophobic acyl tail, increases the fluidity inside the membrane and promotes the formation of small vesicles. Above a certain concentration, it limits the movement of the hydrophobic acyl tail and reduces its fluidity, resulting in a larger vesicle size (Zhao et al., 2015). The liposome prepared in this experiment has a sterol content of about 17.2 mol%, and these results show that the liposome containing 10% (w/w) sterol has a size larger or smaller than PCL depending on various environmental conditions. At higher concentrations, it indicates an increase in liposome size as the concentration of sterol increases.
Stability of incorporated retinol into liposome
Retinol was incorporated into the liposomes containing CH, SI, or ST, and the liposomes were then suspended in a 10 mM potassium phosphate buffer (pH 7.0). The solutions of liposomes with retinol or free retinol solutions in distilled water containing 1% ethanol were stored at 4 and 25 °C, either in the dark or exposed to ambient room light with fluorescent light. The retinol degradation was monitored for 10 days, and the results were plotted as a percentage of the remaining retinol versus time (Fig. 2). The retinol incorporated in the liposomes prepared in this study was found to be more stable than free retinol. In most cases, the addition of CH, SI, and ST into liposomes increased the stability of the incorporated retinol.
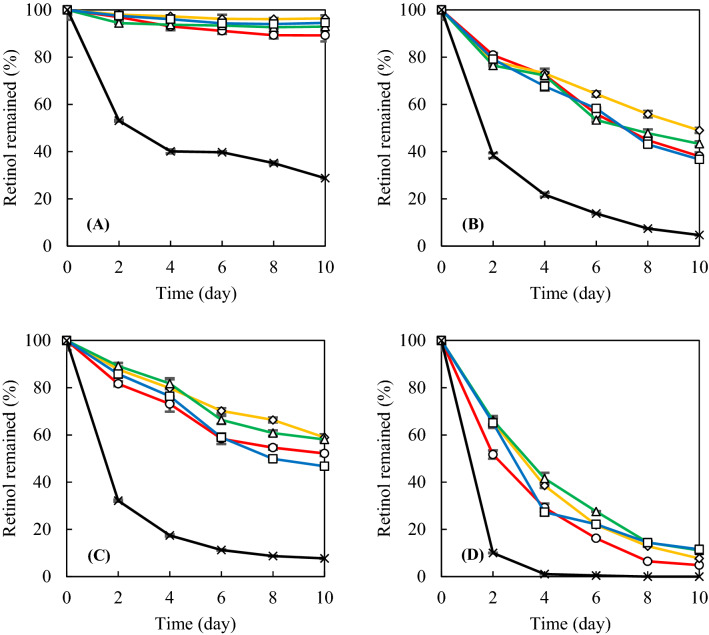
Fig. 2.
Effect of sterol in liposomes on the stability of incorporated retinol: multilamellar liposomes containing retinol were suspended in 10 mM phosphate buffer (pH 7.0) and stored at 4 °C A without light and B with light; at 25 °C C without light and D with light. Liposomes were composed of PC 100% (○), PC:CH (90:10, ♢), PC:SI (90:10, △), PC:ST (90:10, □). Retinol was dissolved in distilled water containing 1% ethanol as a control (×)
At 4 °C in dark conditions (Fig. 2A), the incorporated retinol degraded very slowly during 10 days of storage, with 89.2, 96.4, 92.9, and 94.5% remaining at PCL, CHL, SIL, and STL, respectively. Free retinol in distilled water that contained 1% ethanol rapidly degraded with only 28.7% remaining after 10 days of storage in the same conditions. As shown at Fig. 2B, light increased the kinetics of retinol degradation at 4 °C; however, liposomes also increased the stability of incorporated retinol. After 10 days of storage, the retinol incorporated into PCL, CHL, SIL, and STL remained at 38.0, 49.0, 43.3, and 36.7%, respectively.
At 25 °C in dark conditions (Fig. 2C), liposomes also effectively protected incorporated retinol. After 6 days of storage, the retinol in PCL, CHL, SIL, and STL remained only 58.4, 70.2, 66.3, and 58.9%, respectively. At 25 °C in light (the most severe conditions in this study), the degradation of retinol was dramatically fast. After 6 days of storage, retinol in PCL, CHL, SIL, and STL remained only at 16.2, 21.9, 27.6, and 22.2%, respectively (Fig. 2D), while free retinol remained at 0.5% in the same conditions. Temperature is a very important factor in the physical state and structure of liposomes. The acyl chain of phospholipids in the liposome can escape from the gel and sol state to a disordered and fluid state. The transition temperature of soybean PC was reported as 26.5 °C (Brody, 1982). PC in liposomes are primarily in the solid gel state at 4 °C, and oxygen and water permeability might be lower than at 25 °C. Therefore, increasing temperature increased the degradation of incorporated retinol.
In Fig. 2B and C, the addition of ST was not very effective for the protecting incorporated retinol. There are two possible explanations for the relatively lower protective effects of ST in liposomes. Firstly, ST has a lower solubility in phospholipids than in CH and SI. Schuler et al. (1990) reported that the solubility limit of CH, SI, and ST into soybean PC liposome was 23, 30, and 15 mol%, respectively, and that the relatively lower solubility of ST was mainly due to the introduction of a double bond in the side chain of C-22. In this experiment, the 10% (w/w) content of CH, SI, and ST can be converted as 17.89, 16.88, and 16.95 mol%, respectively. This means that the ST content in this study exceeded the limit of solubility of PC. Secondly, ST shows a lower inhibition on water permeability in liposomes than in CH and SI. CH and SI in liposomes effectively lead to a decrease in water permeability, however, ST exhibited no ability in regulating membrane permeability even at 15 mol% (Schuler et al., 1991). Retinol, a hydrophobic compound, can easily be isomerized and photo-oxidized in a hydrophilic environment, resulting in loss of activity or decomposition (Semenova et al., 2002). Therefore, CH and SI are effective in improving the stability of incorporated retinol into the liposome bilayer, while ST is relatively less effective.
In the aspect on molecular structure, SI and ST have an additional ethyl group at the C-24 position of CH. ST has double bond at C-22 and C-23, while CH and SI did not have. The presence of additional ethyl group reduces the flexibility and bulk of the overall packing, and double bonds in ST lead to repulsion against additional adjacent hydrocarbon chains compared to SI, resulting in less effective PC/sterol interactions (Hac-Wydro et al., 2007; Hodzic et al., 2008). Our results suggested that double bond on ST is more critical on relatively inferior effect on the protection of retinol than CH.
It is well-known that CH stabilizes the phospholipid bilayer in liposomes, which is effective in stabilizing incorporated and/or encapsulated bioactive materials in them. However, the negative impact of CH on the human body has limited its use in liposomes. In this study, SI and ST, typical phytosterols with positive physiological activities, showed similar protective effects on the stabilization of incorporated retinol in liposomes, which might support the broad application of phytosterols in liposomes.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (NRF-2018R1D1A1B07041141).
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dong-Uk Lee, Email: azwq@naver.com.
Hye-Won Park, Email: baechu1997@naver.com.
Seung-Cheol Lee, Email: sclee@kyungnam.ac.kr.
References
- Brody SS. Absorption and picosecond fluorescence characteristics of chlorophyll vesicles as a function of temperature. Zeitschrift fur Naturforschung. 1982;37:260–267. doi: 10.1515/znc-1982-3-419. [DOI] [Google Scholar]
- Brufau G, Canela MA, Rafecas M. Phytosterols: physiologic and metabolic aspects related to cholesterol-lowering properties. Nutrition Research. 2008;28:217–225. doi: 10.1016/j.nutres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Combs GF., Jr . The Vitamins: Fundamental Aspects in Nutrition and Health. 3. Cambridge, MA, USA: Academic Press; 2007. pp. 42–62. [Google Scholar]
- Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A, Estevinho BN, Rocha F. Microencapsulation of vitamin A: a review. Trends in Food Science and Technology. 2016;51:76–87. doi: 10.1016/j.tifs.2016.03.001. [DOI] [Google Scholar]
- Hąc-Wydro K, Wydro P, Jagoda A, Kapusta J. The study on the interaction between phytosterols and phospholipids in model membranes. Chemistry and Physics of Lipids. 2007;150:22–34. doi: 10.1016/j.chemphyslip.2007.06.211. [DOI] [PubMed] [Google Scholar]
- Hodzic A, Rappolt M, Amenitsch H, Laggner P, Pabst G. Differential modulation of membrane structure and fluctuations by plant sterols and cholesterol. Biophysical Journal. 2008;94:3935–3944. doi: 10.1529/biophysj.107.123224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafi R, Kwak HSR, Schumacher WE, Cho S, Hanft VN, Hamilton TA, King AL, Neal JD, Varani JV, Fisher GJ, Voorhees JJ, Kang S. Improvement of naturally aged skin with vitamin A (retinol) Archives of Dermatology. 2007;143:606–612. doi: 10.1001/archderm.143.5.606. [DOI] [PubMed] [Google Scholar]
- Kaur N, Chaudhary J, Jain A, Kishore L. Stigmasterol: a comprehensive review. International Journal of Pharmaceutical Sciences and Research. 2011;2:2259–2265. [Google Scholar]
- Lee SC, Yuk HG, Lee DH, Lee KE, Hwang YI, Ludescher RD. Stabilization of retinol through incorporation into liposomes. Journal of Biochemistry and Molecular Biology. 2002;35:358–363. [PubMed] [Google Scholar]
- Lee SC, Kim JJ, Lee KE. Effect of β-sitosterol in liposome bilayer on the stabilization of incorporated retinol. Food Science and Biotechnology. 2005;14:604–607. [Google Scholar]
- Lee SC, Lee KE, Kim JJ, Lim SH. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. Journal of Liposome Research. 2005;15:157–166. doi: 10.1080/08982100500364131. [DOI] [PubMed] [Google Scholar]
- Lee DU, Park HW, Lee SC. Effect of stigmasterol in liposome bilayer on the stabilization of encapsulated ascorbic acid. Korean Journal of Food Science and Technology. 2020;52:200–203. [Google Scholar]
- Moumita S, Das B, Hasan U, Jayabalan R. Effect of long-term storage on viability and acceptability of lyophilized and spray-dried synbiotic microcapsules in dry functional food formulations. LWT - Food Science and Technology. 2018;96:127–132. doi: 10.1016/j.lwt.2018.05.030. [DOI] [Google Scholar]
- Reineccius GA. Liposomes for controlled release in the food industry. In: Encapsulation and Controlled Release of Food Ingredients. Reineccius GA (ed). American Chemical Society, Washington DC, USA. pp. 113-131 (1995)
- Schuler I, Duportail G, Glasser N, Benveniste P, Hartmann MA. Soybean phosphatidylcholine vesicles containing plant sterols: a fluorescence anisotropy study. Biochimica et Biophysica Acta - Biomembranes. 1990;1028:82–88. doi: 10.1016/0005-2736(90)90268-S. [DOI] [PubMed] [Google Scholar]
- Schuler I, Milon A, Nakatani Y, Ourisson G, Albrecht AM, Benveniste P, Hartman MA. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:6926–6930. doi: 10.1073/pnas.88.16.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova EM, Cooper A, Wilson CG, Converse CA. Stabilization of all-trans-retinol by cyclodextrins: a comparative study using HPLC and fluorescence spectroscopy. Journal of Inclusion Phenomena and Macrocyclic Chemistry. 2002;44:155–158. doi: 10.1023/A:1023042612880. [DOI] [Google Scholar]
- Singh AK, Das J. Liposome encapsulated vitamin A compounds exhibit greater stability and diminished toxicity. Biophysical Chemistry. 1998;73:155–162. doi: 10.1016/S0301-4622(98)00158-6. [DOI] [PubMed] [Google Scholar]
- Subramanyam GB, Parrish DB. Colorimetric reagents for determining vitamin A in feeds and foods. Journal - Association of Official Analytical Chemists. 1976;59:1125–1130. [PubMed] [Google Scholar]
- Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. Biochimica et Biophysica Acta - Molecular Cell Research. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Whelan J. Nanocapsules for controlled drug delivery. Drug Discovery Today. 2001;6:1183–1184. doi: 10.1016/S1359-6446(01)02055-4. [DOI] [PubMed] [Google Scholar]
- WHO/FAO (World Health Organization, Food and Agriculture Organization). Vitamin and Mineral Requirements in Human Nutrition. 2nd ed. World Health Organization, Bangkok, Thailand. pp. 17-37 (2005)
- Zhao L, Temelli F, Curtis JM, Chen L. Preparation of liposomes using supercritical carbon dioxide technology: effects of phospholipids and sterols. Food Research International. 2015;77:63–72. doi: 10.1016/j.foodres.2015.07.006. [DOI] [Google Scholar]