Abstract
Since researchers began studying the mechanism of flavonoids’ anticancer activity, little attention has been focused on the modulation of redox state in cells as a potential chemotherapeutic strategy. However, recent studies have begun identifying that the anticancer effect of flavonoids occurs both in their antioxidative activity which scavenges ROS and their prooxidative activity which generates ROS. Against this backdrop, this study attempts to achieve a comprehensive analysis of the individual and separate study findings regarding flavonoids’ modulation of redox state in cancer cells. It focuses on the mechanism behind the anticancer effect, and mostly on the modulation of redox potential by flavonoids such as quercetin, hesperetin, apigenin, genistein, epigallocatechin-3-gallate (EGCG), luteolin and kaempferol in both in vitro and animal models. In addition, the clinical applications of and bioavailability of flavonoids were reviewed to help build a treatment strategy based on flavonoids’ prooxidative potential.
Keywords: Flavonoid, Redox state modulation, Anticancer, Chemotherapeutic strategy
Introduction
Cancer is still the second biggest cause of death worldwide and a considerable number of patients have not been completely cured once they had it. Scientists have developed many chemotherapeutic agents to treat and prevent cancer, but those agents left us with problems of side effects and drug resistance. Fortunately, however, it takes several years for cancer cells to transform into a full malignancy via multistep process and we have several points during cancer progression that can be used to prevent cancer cells from proliferating or progressing further. In this regard, many investigators have focused on polyphenolic flavonoids, which are bioactive food components within a number of natural dietary products. Dietary flavonoids (hereinafter “flavonoids”) have been reported to have anticancer effects by scavenging free radicals, regulating inflammatory responses, regulating enzymatic activities, arresting mitotic cycle, stimulating immune system, inhibiting angiogenesis, altering gene expression, or inducing apoptosis via several different signal pathways (Antosiak et al., 2017; Choi et al., 2002; Kaushik et al., 2019; Kim et al., 2014b; Klauser et al., 2014; Martinez et al., 2003; Souza et al., 2017). These research reports have suggested that dietary flavonoids may serve as promising chemotherapeutic agents that inhibit the progression of cancers (Fantini et al., 2015).
Among many bioactive properties of flavonoids, antioxidative activity which helps scavenging free radicals is one of the most significant mechanisms in their anticancer effects and is known to be a primary step to prevent disease in normal cells, which is why it is still a focus of many recent studies. However, it was also reported that flavonoids’ anticancer activity upset the redox balance in cancer cells and was accompanied by self-oxidization and prooxidization that induced ROS generation (Elbling et al., 2005; Hou et al., 2005; Suh et al., 2010). Since then, many studies have started focusing on the prooxidative activity but reported mixed results depending on whether the study subject was normal cells or cancer cells and the type of flavonoid that was studied. In particular, there were reports on dual activity of flavonoids in terms of their (anti)oxidation or (anti)cancer effect, depending on the type of cancer and the concentration of flavonoids used to treat the cancer cells (Choi et al., 2018; Wätjen et al., 2005; Whitehouse et al., 2016; Wu et al., 2018b).
For example, EGCG (2–30 μM) in keratinocytes, cerebellar granule neurons or umbilical vein endotherial cells generated a low level of ROS or even scavenged ROS, thus inhibiting apoptosis (Elbling et al., 2010; Kim et al., 2014c), while EGCG (10 μM and 30 μM) promoted ROS generation and induced apoptosis in lung and breast cancer cells respectively (Farhan et al., 2016; Li et al., 2010). In other words, even similar concentrations of flavonoids resulted in different redox potentials and thus became either anti-apoptotic or pro-apoptotic depending on whether cells were normal or cancerous. As seen in the findings by Farhan et al. (2016) and Li et al. (2010), a growing body of evidence suggests that flavonoids’ anticancer activity in cancer cells was accompanied by prooxidative potential, and such findings were more apparent in the case of hesperetin, genistein, EGCG and apigenin (Abotaleb et al., 2019).
A number of recent studies have still reported that antioxidative activity was a major mechanism of flavonoids’ anticancer effect. In most of the cases, anticancer activity prevented cancer initiation in normal cells by scavenging ROS that was increased by external factors. However, a growing number of in vitro studies have reported that flavonoids’ anticancer activity in cancer cells was accompanied by prooxidative potential. Whether the anticancer activity of flavonoids in cancer cells involves antioxidative potential which has long been known or prooxidative potential which has not been widely known is a controversial question. Because the research findings on this issue were a result of separate and individual studies, this review attempts to provide a comprehensive analysis of related findings. This paper examined reports on anticancer activity of several flavonoids such as quercetin, hesperetin, apigenin, genistein, EGCG, luteolin and kaempferol which exhibit strong anticancer effects in in vitro and animal models, with a focus on their redox potential (Table 1). In particular, the paper focused on identifying an effective concentration level, cell types, and cellular mechanisms of anticancer activity accompanied by prooxidative potential. In addition, clinical applications and bioavailability of flavonoids were studied to help build a treatment strategy.
Table 1.
In vitro cytotoxic activity and animal study of flavonoids accompanied by pro-oxidative potential and mechanism
| Compound (Source) | Mechanism | Doses | Human cancer cell(type)/Animal | Possible mechanisms | References |
|---|---|---|---|---|---|
| Quercetin (Onion, elderberry, pear,apple) | Apoptosis Cycle arrest | 42.5 μM, 48 h (IC50) | Colon cancer (HT29) | ROS↑,caspase-3↑, cytochrome C↑, COX↑, pAkt↓, cyclin D1↓ | Raja et al. (2017) |
| Apoptosis | 153 μM, 24 h (IC50) | Cervix cancer (SKOV-3) | ROS↑, XIAP↓, Bcl-2↓, Bcl-xL↓, cFLIP ↓, ER-protin(eIF2α, IRE1α, CHOP, DR5) | Yi et al. (2014) | |
| Antitumor | 2 mg/kg (30 days, i.g.) | SKOV-3 xenograft mice | tumor volume↓, caspase-3↑, CHOP↑ | ||
| Apoptosis | 110 μM, 18 h (IC50) | Cervix cancer (HeLa) | ROS↑, cytochrome c↑, p53↑, Bax↑, pAkt↓, Bcl-2↓ | Bishayee et al. (2013) | |
| Apoptosis | 23.1 μM, 48 h (IC50) | Breast cancer (MCF-7) | ROS↑, G2/M↓, cycle arreat | Wu et al. (2018b) | |
| Apoptosis | 160 μM, 48 h (IC55) | Gastric cancer (AGS) | ROS↑MMP↓, Bad↑, Bax↑, Bid↑, TP53INP11↑,Bcl-2↓, TNFRSF10D↑ | Shang et al. (2018) | |
| Apoptosis | 75 μM, 48 h (IC50) | Leukemia cell (K562) | ROS↑, catalase activity↓ | Majumder et al. (2017) | |
| Antitumor | 25 mg/kg (14wks, i.g.) | DMBA induced hepatoma Hamster | tumor mass↓,SOD↓, catalase↓, CYP1A1↓ | Priyadarsini and Nagini (2012) | |
| Hesperetin (citrus.lemon) | Apoptosis | 240 μM, 24 h (IC50) | Lung cancer (A549) | ROS↑, migration↓, UGTS↓ | Wang et al. (2019) |
| Antitumor | 40 mg/kg (every 3 days, 40 days, i.g.) | LLC xenograft mice | tumor volume↓ | ||
| Apoptosis | 200 μM,48 h (IC50) | Gastric cancer (SGC-7901) | ROS↑, Bcl-2/Bax↓, Apaf-l↑, cytoch-rome C↑, caspase-3,-9↑ | Zhang et al. (2015d) | |
| Antitumor Apoptosis | 40 mg/kg (thrice/wk, 36 days, i.p) | SGC-7901 xenograft mice | tumor mass↓, tumor cell apoptosis | ||
| Apoptosis | 40 μM, 48 h (IC50) | Breast cancer (MCF-7) | ROS↑, Bax/Bcl-2↑, cytochrome C↑, ASK1↑, JNK↑, caspase-3,9↑ MMP↓ | Palit et al. (2015) | |
| Apoptosis | 250 μM, 60 h (IC50) | Hepatocarcinoma (HepG2) | ROS↑, Apaf-l↑, cytochrome C↑, caspase-3↑, Bax/Bcl-2↑, caspase-9↑ | Zhang et al. (2015c) | |
| Antitumor | 20 ~ 40 mg/kg (thrice /week, 36 days, i.g.) | HepG2 xenograft mice | tumor mass↓, tumor cell apoptosis | ||
| Apoptosis | 200 μM, 72 h (IC50) | Esophageal cancer (Eca109) | ROS↑, Apaf-l↑, cytochrome C↑, caspase-3,-9↑, Bax/Bcl-2↑ | Wu et al. (2016) | |
| Antitumor | 90 mg/kg (every 3 days,30 days, i.p.) | Eca109 xenograft mice | tumor mass↓, tumor cell apoptosis | ||
| Apigenin (parsley, onion, rosemary, celery, chamomile) | Apoptosis | 75 μM, 48 h (IC50) | Osteoblastoma (U-2 OS) | ROS↑, caspare-3,-8,-9↑, Bax↑ | Lin et al. (2012) |
| Antitumor | 2 mg/kg (every 3 days, 30 days, i.p.) | U-2 OS xenocraft mice | tumor weight↓ | ||
| Apoptosis | 20 μM, 48 h (IC50) | DEN induced HCC of rat | ROS↑, cytochrome C↑ caspase-3↑ | Seydi et al. (2016) | |
| Apoptosis Autophage | 50 μM, 24 h (IC60) | Thyroid cancer (BCPAP) | ROS↑, DNA impairment↑, G2/M↓, LC3-II↑, Beclin-1 ↑, AVO↑ | Zhang et al. (2015a) | |
|
Apoptosis Antitumor |
34.31 μM,72 h (IC60) 20 mg/Kg (once/wk, 6 mon, i.p.) |
Mesothelioma (H-Meso-1), H-Meso-1 xenograft animal |
ROS↑, pAkt pERK1/2 ↑, pJNK↑, Tumor growth↓, life time↑ |
Masuelli et al. (2017) | |
| Apoptosis | 60 μM, 48 h (IC70) | Colon cancer (HCT-116) |
ROS↑, caspase-3,-8,-9↑, CHOP ↑, DR5↑ Bid ↑, Bax ↑, cytochrome C↑, MMP↓ |
Wang and Zhao (2017) | |
|
Apoptosis Anti invasion |
10 μM, 48 h (IC50) 72 μM, 48 h (IC50) |
Cervix cancer (HeLa) Cervix cancer (SiHa) |
ROS↑, MMP↓, cell migration↓, invasion↓, lipid peroxdation↑ | Souza et al. (2017) | |
|
Antiproliferation Autophage |
24.8 μM, 24 h (IC50) 9.8 μM, 24 h (IC50) |
Pancreatic cancer (PaCa44) Pancreatic cancer (Panc 1) |
ROS↑, m TOR-Hsp90↓, mutp53↓, SOD↓, catalase↓ |
Gilardini et al. (2019) | |
|
Apoptosis Cycle arrest |
75 μM, 48 h (IC50) 8 μM, 48 h (IC50) |
Cervix cancer (OVCAR-3) Cervix cancer (SKOV-3) |
ROS↑, G2/M↓, caspase-3 ↑, caspase 9 ↓, invasion↓ | Tavsan and Kayali (2019) | |
| Genistein (soy beans, red bean) |
Apoptosis Cycle arrest |
50 μM, 48 h (IC50) | Breast cancer (MCF-7, MDA-MB-231) | ROS↑, G2/M↓, PI3K/Akt↓ | Kaushik et al. (2019) |
| Antitumor | 200 mg/kg (thrice/ week, 3 weeks, i.g.) | 4T1 xenograft mice | tumor mass↓ | ||
|
Apoptosis Cycle arrest |
25 μM, 48 h (IC50) | Hepatocarcinoma (HepG2) |
ROS↑, G2/M↓,caspase-3,-9↑ Bax/Bcl-2↑, cytochrome C↑ |
Zhang et al. (2019) | |
| Apoptosis | ≧100 μM, 48 h | Colon cancer (HT-29) |
ROS↑, DNA break↑, p53↑, topoisome- rase 2 cleavage↑ |
Schroeter et al. (2019) | |
|
Apoptosis Cycle arrest |
42.5 μM, 48 h(IC50) | Leukemia (HL-60) |
ROS↑, G2/M↓, MMP↓, ER-protein (IRE-1α, calpain 1, GRP78, GADD153↑ |
Hsiao et al., (2019) | |
| Antitumor |
0.4 mg/kg (every 2 days, 28 days, i.p.) |
HL-60 xenograft mice |
tumor weight ↓, ATF-6α↑, Bax↑, GRP78↑, Bak↑, Bid↑ |
||
| Apoptosis | 60 μM, 48 h (IC60) | Cervix cancer (SK-OV-3) | ROS↑, cytochrome C↑ | Antosiak et al., (2017) | |
| Apoptosis | 20 μM, 48 h (IC70) | Leukemia (HL-60) | ROS↑, GSH/GSSG↓, ICDH↓ | Kim et al., (2014b) | |
|
Apoptosis Cycle arrest |
25 μM, 48 h (IC50) |
Pancreatic cancer (Mia-Paca2, PANC-l) |
ROS↑,Bax/Bcl-2↑, MMP-2,9↓, caspase-3,-9↑, cytochrome C↑, G2/M↓ |
Bi et al., (2018) | |
|
Apoptosis Cycle arrest |
160 μM, 48 h (IC50) | Bladder cancer (T24) |
ROS↑, G2/M↓,caspase-3,-6,-9↑, Bax/Bcl-2↑, cytochrome C↑, PI3K/Akt↓ |
Park et al., (2019) | |
|
Epigallocatechin-3-gallate (tea, green tea tree) |
Apoptosis | 20 μM, 24 h (IC50) | Lung cancer (H1299) | ROS↑, MMP↓, caspase3↑, γ-H2AXγ↑ | Li et al. (2010) |
| Anti-tumor | 30 mg/kg (45 days, i.p.) | H1299 xenograft mice | tumor weight↓, tumor cell apoptosis | ||
|
Apoptosis Cycle arrest |
300 μM, 72 h (IC50) | Hepatocarcinoma (HepG2) | ROS↑, Bcl-2↓, MMP↓, G2/M↓ | Khiewkamrop et al. (2018) | |
| Apoptosis | 100 μM, 24 h (IC50) | Lung cancer (A549) | ROS↑, PARP 1↑, Nrf-HO-1↑ | Yu et al. (2017) | |
| Anti-tumor | 50 mg/Kg, (every 2 days, 11 times, i.p.) | A549 xenograft mice | Nrf2/HO-1↑, tumor weight ↓ | ||
| Apoptosis Autophage | 50 μg/mL, 48 h (IC90) | Primary effusion lymphoma (BCBL-1) | ROS↑, p53↑, Bax↑, caspase-3↑, LC3-II↑, Beclin 1↑, AVO↑, | Tsai et al. (2017) | |
| Apoptosis | 162 μM, 48 h (IC50) | Esophageal cancer (Te-l) | ROS↑, caspase3↑, VEGF↑ | Liu et al. (2015) | |
| Anti-tumor | 10 mg/kg (2 weeks, i.p.) | Eca 109 xenograft mice | tumor weight↓, mitosis↓ | ||
| Apoptosis | 30 μM, 48 h (IC50) | Breast cancer (MDA-MB-231) | ROS↑, mobilization of copper ion | Farhan et al. (2016) | |
| Apoptosis Autophage |
42 μM, 24 h (IC50) 128 μM, 24 h (IC50) |
Mesothelioma (ACC Meso) Mesothelioma (Y-meso) |
ROS↑, p-p38↑, PARP ↑, p-p53↑, PARP ↑, p-JNK↑, caspase-3↑, LC3-II↑ | Satoh et al. (2013) | |
| Anti-proliferation | 50 μM, 72 h (IC84) | Pancreatic cancer (Panc-1) | ROS↑, MMP↓ glycolytic enzyme activity↓ | Wei et al. (2019) | |
| Anti-tumor | 10 mg/kg (16 days, i.p.) | KPC xenograft mice | tumor mass↓ | ||
| Luteolin (pear, celery, parsley) | Apoptosis | 25 μM, 24 h (IC50) | Cholangiocarcinoma (KKu-100) | ROS↑, MMP↓, Bcl-2↓,caspase-3,-9↑ | Kittiratphattana et al. (2016) |
| Apoptosis | 25 μM, 72 h (IC50) | Lung cancer (NCI-H460, NCI-H1299) | ROS↑, caspase-3,-8,-9↑, Bcl-2↓, Bcl-XL↓ | Cho et al. (2015) | |
| Anti-tumor | 10 mg/Kg (every 5 days, 7 times, s.c.) | NCI-H460 xenograft mice | tumor volume↓, tumor cell apoptosis | ||
| Apoptosis |
40 μM, 48 h ((IC80) 40 μM, 48 h ((IC70) |
Glioblastoma (U251 MG) Glioblastoma (U87 MG) |
ROS↑, Bax↑, caspase-3↑, ER-protein (PERK, eIF2α, ATF4, CHOP, caspase 12)↑ | Wang et al. (2017) | |
|
Antitumor Apoptosis |
10 mg/kg (thrice/week, 5 weeks, i.p.) | U87 MG xenograft mice |
caspase-3↑, ER-protein (ATF4, CHOP, caspase 12)↑, tumor cell apoptosis |
||
| Antiproliferation | 12 μM, 48 h (IC50) | HCC of rat | ROS↑ | Seydi et al. (2018) | |
|
Cycle arrest Autophage |
Luteoside (60 μM,36 h) (IC50) | Lung cancer (A549, H292) |
ROS↑, G0/G1↓, cyclin D1 ↓, CDK4↓, Akt/m TOR/p7056K pathway↓ |
Zhou et al. (2017) | |
| Apoptosis | 35 μg/mL, 36 h (IC50) | Melanoma (A2058) |
ROS↑, ER-protein(eIF2α↑, ATF-6α ↑, CHOP↑, caspase 12)↑ |
Kim et al., (2016) | |
|
Apoptosis Cycle arrest |
40 μM, 48 h (IC50) | Esophageal carcinoma (EC1) |
cPARP ↑, p53↑, G2/M↓, p21↑, ROS↑, caspase-3↑, cytochrome C↑ |
Chen et al. (2017b) | |
| Antitumor | 50 mg/Kg(i.p.) | EC1 xenograft mice | Tumor weight↓ | ||
| Kaempferol (grape, apple, kale broccoli) |
Apoptosis Cycle arrest |
46 μM, 48 h (IC50) 87 μM, 48 h (IC50) |
Colon cancer (HCT116) Colon cancer (HCT15) |
ROS↑, p53↑, PARP ↑, G2/M↓, p38 MAPK↑, caspase-3,-8,-9↑ |
Choi et al. (2018) |
| Antiproliferation | 30 μM, 48 h (IC50) | HCC of rat | ROS↑, caspase-3↑ | Seydi et al. (2018) | |
| Apoptosis | 40 μM, 48 h (IC50) | Breast cancer (VM7Luc4E2) | ROS↑, Bcl-2↓, Bax↑ | Lee et al. (2018) | |
|
Apoptosis Cycle arrest |
50 μM, 48 h (IC50) | Melanoma (A375 SM) |
ROS↑, Bax↑, Bcl-2 ↓, p53↑, p-p38↑, p21↑, cyclin B↓, cyclin E↓, G2/M↓, p-eIF2α↑, CHOP↑ |
Heo et al., (2018) | |
| Apoptosis | 80 μM, 48 h (IC50) | Hepatocarcinoma (HepG2) | ROS↑, Bax/Bcl-2↑, ERK↑, caspase-3,- 9↑ | Zhang et al. (2015b) | |
| Apoptosis | 100 μM, 48 h (IC35) | Glioma (A172) | ROS↑, caspase-3↑, ERK↓, Akt↓, XIAP↓ | Jeong et al. (2009b) |
All cancer cells above are from human except hepatocellular carcinoma cell (HCC) is from rat. IC50 (inhibitory concentration to 50%) is from original paper, but some IC50 was adjusted from the original data. CYP: cytochrome P450, Nrf2/HO-1: NF-E2-related factor 2/heme oxygenase-1, PI3K/Akt:: phosphoinositide 3-kinase/protein kinase B, COX: cyclo-oxygenase, TNFRSF10D: tumor necrosis factor receptor superfamily member 10D, MMP-2,9: matrix metalloproteinase-2,9, TP53INP1: tumor suppressor protein p53 inducible nuclear protein 1, ASK1: apoptosis signal-regulating kinase 1, UGTS: UDP-glucuronosyl-transferases, γ-H2AXγ: histone 2A variant, PARP: Poly (ADP-ribose) polymerase, VEGF: vascular endotherial growth factor, PERK: RNA-dependent protein kinase (PKR)-like ER kinase, JNK: c-Jun N-terminal kinase, MAPK: mitogen-activated protein kinase, ERK: extracellular signal-regulated kinases, CDK4: cyclin dependent kinase 4
ROS scavenging effects of flavonoids in normal cells
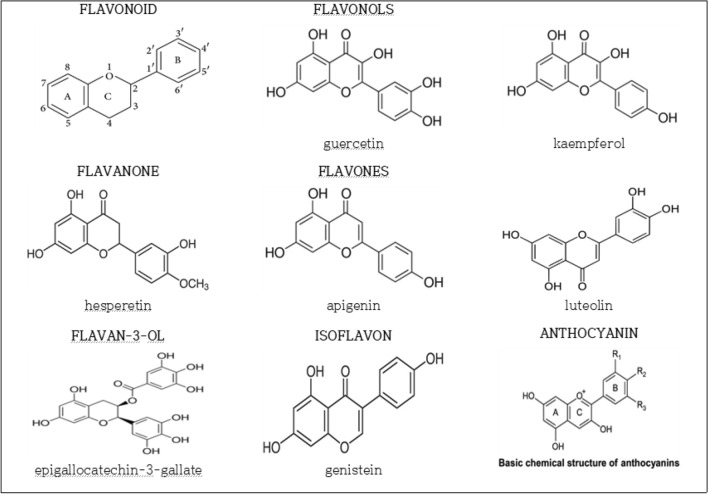
Flavonoids are composed of 15 carbon atoms in their basic structure, which we have abbreviated to C6-C3-C6. The first C6 represents aromatic carbon ring A and the latter C6 represents aromatic carbon ring B, A and B rings are linked by a unit of three carbon atoms that is the C-ring. Depending on variations of the C-ring, flavonoids are subclassed into flavone, flavanone, flavonol, flavan-3-ol, isoflavone or anthocyanidin (Beecher et al., 2003) (Fig. 1).
Fig. 1.
Structure of flavonoids
Flavonols (e.g. quercetin, kaempferol) are present in plants in glycosylated form, with glucose or rhamnose on the 3-position of the C-ring. Flavanones (e.g. hesperetin) and flavones (e.g. apigenin, luteolin) are also in plant glycated form, mainly as 7-0-glycosides. Isoflavones (e.g. genistein) exist as aglycones or glycosides in foods. Flavan-3-ols (e.g. EGCG) are not glycosylated in foods as are the other classes of flavonoids.
ROS or RONS (reactive oxygen and nitrogen species) are highly reactive and exist in various types such as ROO:, O2−, NO·, and ONOO−. They are generated by NADPH oxidase and inducible nitric oxide synthase (iNOS) in mitochondria as a defense reaction against pathogens such as microorganism, allergen, pollutant, and radioactivity (Reuter et al., 2010; Violi et al, 2015). If an external factor promotes ROS generation in normal cells, antioxidant enzymes help maintain a redox balance or antioxidative material removes ROS by reacting with them. If the normal cells or tissues keep exposed to ROS, structural changes or cell damages occur, inducing gene expressions that will induce the transformations of proteins and DNA (Mahalingaiah and Singh, 2014; Schieber and Chandel, 2014). In short, the long-term exposure of normal cells to ROS causes their structural changes and subsequently induces the formation and proliferation of cancer. Therefore, antioxidative potential that scavenges ROS is one of the major mechanisms that inhibit the formation of cancer.
Chemical structural features of flavonoids exhibit ROS scavenging and antioxidant activities. The most related chemical structures are an OH group of the C-ring at position 3 and OH groups of the B-ring. The OH groups can scavenge and reduce ROS, thus exhibiting antioxidant activity (Belinha et al., 2007). It has been known that their ROS scavenging activity becomes stronger according to the number of OH groups of the B-ring (Chen et al., 2002). For example, quercetin shows relatively stronger ROS scavenging activity than other flavonoids, which is attributed to its structure in which the number of OH groups at the B-ring of quercetin is more than that of other flavonoids (Fig. 1). In addition to the hydroxyl groups of the B-ring, EGCG has another hydroxyl groups in the D-ring (Fig. 1.), so EGCG effectively scavenges free oxygen radical (Severino et al., 2009).
As evidence of a potent ROS-scavenging activity by flavonoids, quercetin and kaempferol scavenged nitrogen free radical, with the IC50 values of 4.53 μg/ml and 3.97 μg/ml respectively (Szewczyk et al., 2014), and hesperetin (1–10 uM, 18 h) also scavenged superoxide anion and 2,2-diphenyl-β-picrylhydrazyl (DPPH) radical in RAW 264.7 macrophage cells (Yang et al., 2012). As evidence of a protective effect based on the ROS scavenging activity of flavonoids, pretreatment of cervical cancer cells (HeLa) with quercetin (15 μg/mL) reduced ROS formation and cell damage caused by H2O2 (Pawlikowska-Pawlęga et al., 2014). In another study, an intragastric (i.g.) administration of quercetin (10 mg/kg/day, 12 weeks) inhibited the increase of ROS levels in brain cells caused by an i.g. administration of aluminum in mice (Sharma et al., 2016). Flavonoids’ potent ROS-scavenging activity has also contributed to carcinogenesis prevention. For example, EGCG (2 and 5 μM) scavenged ROS radicals in neuronal cells (BV 2) and protected them from neurodegeneration and neuroblastoma (Kim et al., 2009); and kaempferol (20 μM) decreased the ROS activity in H2O2 treated APRE-19 cells (human retinal pigment epithelial cells), and thus protected them from H2O2-induced oxidative cell damage and apoptosis (Du et al, 2018).
In sum, these studies show that flavonoids function as antioxidative agents with potent ROS-scavenging capabilities. In most cases, a low concentration of flavonoids protected normal cells from future damage (Pawlikowska-Pawlęga et al., 2014; Sharma et al. 2016) or prevented cancer initiation in normal cells (Du et al., 2018; Kim et al., 2009) by scavenging ROS that was increased as a consequence of external factors.
Dual functions of flavonoids in cancer cells
Dual functions of flavonoids in cancer cell line depending on their concentration have been reported since the mid-2000s. Quercetin (10–25 μM) showed protective effects against H2O2-induced cytotoxicity in hepatoma cell (H4IIE cell), but different concentration level (50–250 μM) showed DNA strand breaks, caspases activation and apoptosis (Wätjen et al, 2005). It was also reported that EGCG (100 μM) induced cell death while low concentration (10 μM) did not in most mesothelioma cell lines (Satoh et al., 2013). As breast cancer cells, in particular, were sensitive to an extremely low concentration of flavonoids, the growth of the cancer cell was found in in vitro study. Treatment with apigenin (1 μM) against breast cancer cells (MCF-7, T47D) resulted in the proliferation of the cancer cells (Seo et al., 2006). Treatment with genistein (1.0 μM, 3.125 μM, 5 μM each) against breast cancer cells (MCF-7, MDA-MB-468) caused a little proliferation of the cancer cells (Klein and King, 2007; Nadal-Serrano et al., 2013; Wei et al., 2015) for each genistein concentration, while genistein (50 μM) inhibited the cell growth by 50% and induced apoptosis in MCF-7 cell (Kaushik et al., 2019). A low concentration of luteolin (10 μM) resulted in a little proliferation of breast cancer cells (MCF-7), but higher concentrations (50 μM, 150 μM) in the same cells inhibited the cell growth by 40% and 90% each (Sato et al., 2015). The promotion of the growth of breast cancer cells by an extremely low concentration of flavonoids was more clearly found in the case of genistein.
Prooxidative activity of flavonoids as anticancer agents in cancer cells
The biological response of flavonoids’ prooxidative potential has been reported since the mid-2000s. EGCG showed anticancer activity in esophageal cancer cells via auto-oxidation (Hou et al., 2005), and EGCG (20 μM) inhibited cell growth, increased ROS generation, decreased mitochondrial membrane potential (MMP), and induced apoptosis in lung cancer cells (H1299) (Li et al., 2010). A total of about 60 studies have reported on the cytotoxic activities of flavonoids which exhibited prooxidative potential through ROS generation in cancer cells, of which we analyzed 50 cases (Table 1).
Overall, flavonoids at a concentration of 10 μM or higher induced cytotoxic activity accompanied by prooxidative potential in various cancer cells (Table 1). This paper also found that flavonoids induced cytotoxic activity at relatively low concentrations of between 10 and 50 μM, and these dosages were used in 27 cases out of the total of 50. The remaining cases showed cytotoxic activity accompanied by prooxidative potential at concentrations in the range of 51–200 μM. Flavonoids’ cytotoxic activity at lower concentrations (10–50 μM) should contribute to their evaluation as an excellent anticancer agent according to the NCI standard (Hay et al,. 1994) which acknowledges a substance as anticancer therapeutics if its IC50 value is 230 μg/ml or lower.
Several suggestions were made on the anticancer (cytotoxic) activity accompanied by prooxidative potential, i.e. ROS generation. In normal cells, even if ROS level was increased by various factors, such level is low enough to be scavenged by flavonoids as antioxidative agents (Glasauer et al., 2014). In cancer cells, however, a high amount of ATP is needed to fuel abnormal proliferation of cancer cells. At this level of energy production, ROS would have reached high and sensitizing levels (Gorrini et al., 2013), which are thought to be difficult to be thoroughly scavenged by flavonoids. In addition, it is known that ROS generation occurs when mitochondrial membranes within cells are disrupted or MMP is reduced (Raj et al., 2011; Shaw et al., 2011). Therefore, it is considered that the mitochondrial membrane disruption and MMP reduction caused by flavonoids are accompanied by a prooxidative response.
This study analyzed the reasons that flavonoids increased ROS levels in cancer cells and found that decrease in antioxidative enzymes or substances was the primary reason in only three cases while decrease in MMP was the reason in more than 10 cases (Table 1). Treatment with quercetin (75 μM) induced apoptosis, inhibited catalase activity, and then increased ROS generation in gastric cancer cells (Majumder et al., 2017). Quercetin (25 mg/Kg/day, 14 weeks, i.g.) injected into hamsters inhibited the volume of hepatocarcinomas and the expression of superoxide dismutase(SOD), catalase, cytochrome P-450(CYP) 1A1 and 1B1 (Priyadarsini and Nagini, 2012). Genistein (20 μM) induced ROS generation and apoptosis in blood cancer cells (HL-60), reduced glutathione/glutathione disulfide (GSH/GSSG) ratio, and inhibited the expression of NADP-dependent isocitrate dehydrogenase (ICDH) which controls oxidation–reduction potential (Kim et al., 2014b).
MMP decrease was reported in many cases (Hsiao et al., 2019; Khiewkamrop et al., 2018; Kittiratphattana et al., 2016; Li et al., 2010; Palit et al., 2015; Shang et al., 2018; Souza et al., 2017; Wang and Zhao, 2017). Flavonoids were found to have increased ROS generation via mitochondrial membrane disruption in cancer cells. This finding is in line with a report that when ROS level was increased by altered ROS homeostasis caused by internal/external changes of cancer cells, or the depolarization of the mitochondrial membrane, the cancer cell was changed from a pro-survival to a pro-apoptotic state and experienced programmed cell death (Gorrini et al., 2013). It was also reported that during drug-mediated anticancer activity, cancer cells were more sensitive to the system that activated ROS generation and accumulation in the cytoplasm of cancer cells (Kim et al., 2014a; Piao et al., 2012).
As a result of this research, this paper concludes that treatment with flavonoids at a concentration of 10 μM or higher is accompanied by a prooxidative potential and converts cancer cells into a pro-apoptotic state through various mechanisms.
Quercetin
Treatment of quercetin at concentrations ranged from 23.1 to 160 μM (IC50) inhibited cancer cell proliferation and induced apoptosis with the generation of ROS in colon cancer, cervix cancer, breast cancer, gastric cancer and leukemia in in vitro studies (Table 1). Most of these apoptosis were induced via intrinsic apoptotic pathway in which mitochondrial membrane disruption and ROS increase induced cytochrome C release, activation of caspase-3 proteins, and increase of Bax/Bcl-2 expression ratio. Treatment with quercetin (153 μM, 24 h) in cervix cancer cells (SKOV-3) reduced expressions of X-linked inhibitor of apoptosis protein (XIAP), Bcl-2, Bcl-xL, and cellular FLICE (FADD-like IL-1β-converting enzyme) inhibitory protein (cFLIP) and dramatically increased expressions of endoplasmic reticulum (ER)-stress associated proteins, such as eukaryotic initiation factor 2 (eIF2α), inositol requiring kinase enzyme 1 alpha (IRE1α), CCAAT-enhancer-binding protein homologous protein (CHOP), and death receptor5 (DR5). Apoptosis was accompanied by cell cycle arrest in colon cancer cells (HT29) (Raja et al., 2017). In xenograft animal model, quercetin (2 mg/kg/day, 30 days, i.g.) decreased the volume of tumor in SKOV-3 cell-implanted tumor mice, while increasing expressions of caspase-3, CHOP, and DR5 proteins in the tumor tissues of mice (Yi et al., 2014). Quercetin (25 mg/kg/day, 14 weeks, i.g.) into hamsters inhibited the volume of hepatocarcinoma caused by dimethylbenzanthracene (DMBA) by 70% (Priyadarsini and Nagini, 2012). As seen above, quercetin at concentrations of 23.1 μM or higher increased ROS generation in various cancer cells and quercetin induced prooxidative potential mediated apoptosis through intrinsic mitochondrial activation (either ER-stress mediated or not) and cell cycle arrest.
Hesperetin
Treatment with hesperetin at concentrations ranged from 40 to 240 μM (IC50) inhibited cancer cell proliferation and induced apoptosis with the generation of ROS in lung cancer, gastric cancer, breast cancer, hepatocarcinoma and laryngeal cancer in in vitro studies (Table 1). Most of these apoptosis were accompanied by increased expressions of apoptotic peptidase activating factor 1 (Apaf-1), cytochrome C, caspase-3, -9, and Bax proteins via intrinsic mitochondrial pathway triggered by ROS level. Intraperitoneal (i.p.) injection of hesperetin (40 mg/kg/day, thrice/week, 4 weeks) into SGC-7901 cell-implanted tumor mice reduced tumor mass by 72% compared to the control group and induced apoptosis in tumor tissue cells (Zhang et al., 2015d). Hesperetin (20–40 mg/kg/day, thrice/week, 36 days, i.g.) reduced the volume and mass of tumor and induced apoptosis in tumor tissue cells of HepG2-implanted tumor mice. Hesperetin (90 mg/kg/day, every 3 days, 30 days, i.p.) in Eca 109 cell-implanted tumor mice inhibited tumor growth by 74% compared to the control group and induced apoptosis in tumor tissue cells (Wu et al., 2016).
Apigenin
Treatment with apigenin at concentrations ranged from 10 to 75 μM (IC50) inhibited cancer cell proliferation and induced apoptosis with the generation of ROS in osteoblastoma, diethylnitrosamine(DEN) induced hepatocellular carcinoma (HCC), thyroid cancer, mesothelioma, colon cancer, cervix cancer, and pancreatic cancer in in vitro studies. In thyroid cancer cells (BCPAP), the pretreatment with apigenin (50 μM, 24 h) inhibited cancer cell growth by 60% and induced apoptosis. Under the same conditions, it resulted in ROS generation, DNA damage, G2/M phase arrest and increased expression of LC3-II, Beclin 1, acidic vescular organelles (AVO) proteins which are the standard marker of autophagosome or autophage, thus leading to autophagy (Zhang et al., 2015a). Apigenin (20 μM, 24 h) increased ROS generation by 1.3 times and resulted in G2/M phase arrest, invasion inhibition, caspase-3 activation and caspase-9 inactivation in OVCAR-3 and SKOV-3 cells. The inactivation of caspase-9 suggested the activation of extrinsic pathway and cell cycle arrest was accompanied (Tavsan and Kayali, 2019). Apigenin (2 mg/Kg, every 3 days, 30 days, i.p.) in U-2 OS cell-implanted tumor mice reduced tumor weight by 75% compared to the control group (Lin et al., 2012). Apigenin (20 mg/Kg, once/week, 6 months) inhibited tumor growth to 50% and prolonged life time in mesothelioma cell (H-Meso-1) implanted mice (Masuelli et al., 2017).
Genistein
Treatment with genistein at concentrations ranged from 20 to 160 μM (IC50) inhibited cancer cell proliferation and induced apoptosis with the generation of ROS in breast cancer, hepatocarcinoma, colon cancer, leukemia, cervix cancer, pancreatic cancer and bladder cancer (Table 1). Even at a low concentration, genistein (20 μM, 48 h) inhibited cancer cell growth by 70% and induced ROS generation and apoptosis in blood cancer cells (HL-60). It reduced GSH/GSSG ratio and inhibited the expression of NADP-dependent ICDH which controls oxidation–reduction potential (Kim et al., 2014b). In this case, it is concluded that the administration of flavonoids inhibited antioxidative enzyme and substrate accompanied by ROS generation. In addition, genistein (42.5 μM, 48 h) inhibited leukemia (HL-60) cell growth by 50% and induced G2/M phase arrest, ROS generation, and apoptosis and caused the increased expression of caspase-3, -9, and Bax, poly ADP-ribose polymerase (PARP) cleavage, and the decreased expression of Bcl-2 (Hsiao et al., 2019). It increased the expression of ER stress-associated proteins such as IRE-1α, calpain 1, 78-kDa glucose-regulated protein (GRP78), growth arrest and DNA damage protein 153 (GADD153), caspase-4, -7, and activated transcription factor (ATF-6α). Genistein (0.4 mg/kg/day, every 2 days, 28 days, i.p.) also increased the expression of ATF-6α, GRP78, Bax, Bad, and Bak in HL-60 cell-implanted tumor mice. In conclusion, genistein decreased the number of cancer cells by inducing G2 /M phase arrest and apoptosis via ER stress- and mitochondria-dependent pathways in HL-60 cells and suppressed tumor properties in vivo (Hsiao et al., 2019). Genistein (200 mg/kg/day, thrice/week, 3 weeks, i.g.) also suppressed tumor mass growth in breast cancer (4T1) cell-implanted tumor mice (Kaushik et al., 2019).
EGCG
Treatment with EGCG at concentrations ranged from 20 to 162 μM (IC50) inhibited cancer cell proliferation and induced apoptosis with the generation of ROS in lung cancer, lymphoma, breast cancer, mesothelioma, and pancreatic cancer in in vitro studies. Abnormally, EGCG in hepatocarcinoma cell (HepG2) induced apoptosis at the concentration of 300 μM (IC50) (Table 1). Apoptosis was accompanied by cell cycle arrest in hepatocarcinoma cell (khiewkamrop et al., 2018) and by autophagy in methothelioma cells (Satoh et al., 2013). EGCG (50 mg/Kg/day, every 2 days, 11 times, i.p.) into A549-implanted tumor mice inhibited tumor progression (Yu et al., 2017), and EGCG (10 mg/kg/day, 16 days, i.p.) into KPC cell-implanted mice inhibited tumor growth by 60% (Wei et al., 2019). EGCG (10 mg/kg/day, 2 weeks, i.p.) into Eca 109 cell-implanted tumor mice markedly reduced tumor weight, inhibited mitosis in tissue cells (Liu et al., 2015). An i.p. administration (30 mg/kg/day, 45 days) of EGCG into H1299-implanted tumor mice inhibited tumor growth by 70% and induced apoptosis in the tumor cells (Li et al., 2010). As seen above, it is suggested that EGCG (10–50 mg/Kg, ≧10 times, i.p.) reduced tumor weight and inhibited tumor progression.
Luteolin
Treatment with luteolin at concentrations ranged from 12 to 40 μM (IC50) inhibited cancer cell proliferation and induced apoptosis with the generation of ROS in cholangiocarcinoma, lung cancer, glioblastoma, hepatocellular carcinoma (HCC), melanoma and esophageal carcinoma (Table 1). In glioblastoma cells (U251 MG, U87 MG), luteolin (40 μM, 48 h) inhibited cell growth by 80% and 70% each, induced apoptosis, ROS generation, and the increased expression of Bax, caspase-3, and ER-stress proteins (PERK, eIF2α, ATF4, CHOP, and caspase-12) (Wang et al., 2017). Luteolin (10 mg/Kg, thrice a week, 5 weeks, i.p) into U87 MG cell-implanted tumor mice inhibited tumor growth and induced apoptosis and the activation of ER-stress associated proteins (caspase-3, -12, ATF4, and CHOP). Therefore, it is considered that luteolin induced ROS-mediated apoptosis in glioblastoma cells via ER-stress associated intrinsic pathway (Wang et al., 2017). In addition, luteolin (35 μg/mL, 36 h) inhibited cell growth by 50% in melanoma cells (A2058), induced apoptosis, and increased the expression of ER-stress associated proteins (caspase-12, eIF2α, ATF6, and CHOP). Luteolin (35 μg/mL, 1 h) increased ROS level and the mitochondrial pathway causing ER-stress was also found in melanoma cell (Kim et al., 2016). In NCI-H460 cell-implanted tumor mice, subcutaneous injection (s.c) of luteolin (10 mg/Kg, every 5 days, 7 times) reduced tumor volume markedly and induced apoptosis in the tissue cell (Cho et al., 2015). Luteolin (50 mg/Kg, i.p.) reduced tumor weight in EC1 cell-implanted tumor mice (Chen et al., 2017b).
Kaempferol
Treatment with kaempferol at concentrations ranged from 30 to 100 μM (IC50) inhibited cancer cell proliferation and induced apoptosis with the generation of ROS in colon cancer, HCC, breast cancer, melanoma, hepatocarcinoma and glioma (Table 1). Most of these apoptosis by kaempferol were induced via intrinsic apoptotic pathway in which ROS increase induced cytochrome C release, the activation of caspase-3, -9 proteins, and the increase of Bax expression or decrease of Bcl-2 expression. Kaempferol (50 μM, 48 h) inhibited cancer cell growth by 50% in melanoma cells (A375 SM), and increased ROS generation and the level of phosphorylated p38, p53 (which is downstream of p38) and p21. The expression of both cyclin B and cyclin E was decreased, which implies cell cycle arrest in the G2/M phase. It was also reported that ER-stress mediated intrinsic apoptosis was activated by the increased expression of p-eIF2α and CHOP proteins (Heo et al., 2018).
Anticancer mechanism of programmed cell death by flavonoids accompanied by prooxidative potential
ROS level increases in cancer cells via the following two pathways: external factor itself or its disruption of antioxidative system which has already existed in cancer cells. When electron transport system in mitochondria is disrupted, redox system is also disrupted or polarized, which results in the generation of ROS and damage of the cells (Chen et al., 2017a; Zhang et al., 2015b; Zorov et al., 2014). This pathway has been utilized in the anticancer activities of chemotherapeutics such as alkaloids and cisplatin (He et al., 2016; Yokoyama et al., 2017). Another way of disrupting antioxidative system is suppressing antioxidant enzymes in cytoplasm and mitochondria and thus promoting ROS generation. Superoxide dismutase (SOD) is a typical antioxidant enzyme, and glutathion peroxidase and glutathion reductase are also one of antioxidant enzymes. The ROS generation by flavonoids in this study was mostly caused by the disruption of electron transport system in mitochondria (Hsiao et al., 2019; Khiewkamrop et al., 2018; Kittiratphattana et al., 2016; Li et al., 2010; Palit et al., 2015; Raja et al., 2017; Souza et al., 2017; Wang and Zhao, 2017).
As this study aims to highlight anticancer activities of flavonoids accompanied by prooxidative potential, it is important to identify the relation between ROS level and the mechanisms of cancer cell death such as apoptosis, inhibition of metastasis and cell cycle, cell necrosis, and autophagy. The extrinsic pathway of apoptosis involves binding by death-inducing ligands, such as Fas ligand (FasL) to death receptors (i.e. Fas), activation of adaptor proteins which are Fas-associated death domain (FADD), activation of procaspases, and expression of caspase-3 and -8 mostly, and this interaction is competed by c-FLIP (Goldar et al., 2015; Hassan et al.; 2014). It was reported that the increased ROS level suppressed the expression of c-FLIP proteins and induced apoptosis in the extrinsic pathway (Raja et al., 2017; Wilkie-Grantham et al., 2013). The intrinsic pathway of apoptosis involves mitochondrial disruption, increase of Bax/Bcl-2 expression ratio and cytochrome C release, activation of apoptotic peptidase activating factor 1 (Apaf-1), and then increased expression of caspase-3 and -9 mostly. It has been known that Bcl-2, a major anti-apoptotic protein, and Bax, a pro-apoptotic protein, regulate apoptosis in the intrinsic pathway. Most of the anticancer agents that inhibit the growth of cancer cells by increasing ROS generation were reported to activate this intrinsic pathway (Perillo et al., 2020; Sabharwal and Schumacker, 2014; Yang et al., 2016).
Cell necrosis is an unregulated digestion and loss of cell components by external stimuli such as toxins or trauma. It was reported that increase of energy-metabolism and resulting increase in ROS level in cancer cells facilitated cell necrosis (Dixon and Stockwell, 2014; Vandenabeele et al., 2010). The activation of p53 protein, which is known as a tumor suppression protein, was found to play an important role in cell necrosis induced by ROS level increase (Vandenabeele et al., 2010).
Autophagy is a process that removes the cellular components that were digested and degraded in lysosome in vacuolar form. Some suggested that autophagy promoted cancer cell growth by providing recycled cellular components to the cell again while others suggested that it enhanced anticancer activity through the interplay with apoptosis (Saha et al., 2018; Yoshii and Mizushima, 2017; Yu and Tooze, 2018). There are two pathways involved in autophagy and cell death. The first pathway consists of activation of AMP-activated protein kinase (AMPK) by ROS, inhibition of mammalian target of rapamycin (mTOR) activation, and promotion of autophagosome formation, while the second pathway consists of activation of nuclear factor erythroid 2-related factor 2 (Nrf2) by ROS, degradation of kelch-like ECH-associated protein 1(KHAP1), release of Bcl-2, and promotion of autophagosome formation (Poillet-Perez et al., 2015). In normal cells, a low ROS concentration caused autophagy and apoptosis, but in transformed or tumor cells, autophagy and apoptosis were induced only when ROS concentration reached the level that would induce oxidative stress (Kaminsky and Zhivotovsky 2014; Um and Yun, 2017; Zhao, 2016).
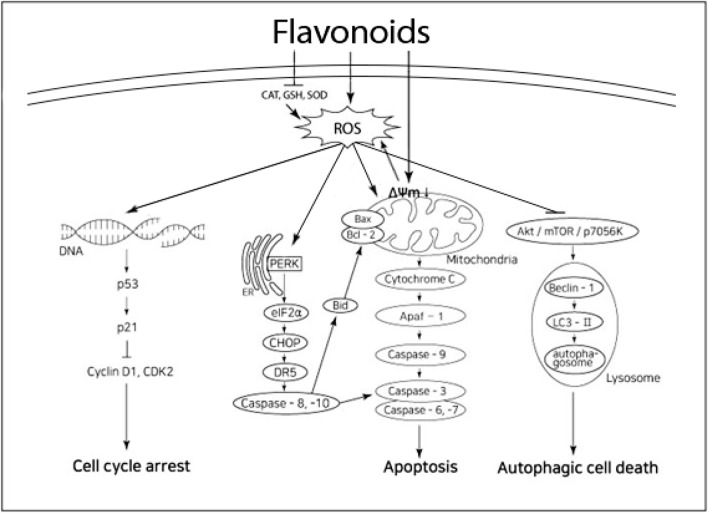
In about 80% of the mechanisms where flavonoids’ anticancer activity was accompanied by ROS generation, intrinsic apoptotic pathway (ER stress was either related or not) was activated, while the rest included autophagy, cell cycle arrest or enzymatic modulation (Table 1, Fig. 2). In the intrinsic apoptotic pathway, mitochondrial membrane disruption and ROS increase induced cytochrome C release, activation of caspase-3, -6, -9 proteins, and increase of Bax/Bcl-2 expression ratio. Depending on the type of cancer cells, quercetin, hesperetin, apigenin, and luteolin activated the intrinsic apoptotic pathway accompanied by the extrinsic pathway (Cho et al., 2015; Raja et al., 2017; Wang and Zhao, 2017). Apoptosis accompanied by activation of ER-stress pathway was clearly found in quercetin (in SKOV-3), genistein (in HL-60), luteolin (in U 87 and A2058), and kaempferol (in A375 SM) (Heo et al., 2018; Hsiao et al., 2019; Kim et al., 2016; Wang et al, 2017; Yi et al, 2014). ROS increase by flavonoids induced DNA impairment, p53 activation, decrease of cyclin D1 and CDK2, thus causing cell cycle arrest in a few cancer cells. But the cell cycle arrest induced by ROS generation did not work alone, but with intrinsic apoptosis or autophagy to result in cell death (Table 1). In addition, apigenin, EGCG, and luteolin induced autophagic cell death through increase of ROS, inhibition of protein kinase B(Akt)/mTOR/p7056K proteins, and activation of Beclin-2 and LC3-II (Tsai et al., 2017; Zhang et al., 2015a; Zhou et al., 2017). In cases of ROS-mediated autophagic cell death by flavonoids, 50% of the cell deaths were not the result of autophagy alone but the result accompanied by intrinsic apoptosis and cell cycle arrest.
Fig. 2.
Prosed schema for flavonoids-induced cancer cell death with ROS. ΔΨm; mitochondrial membrane potential
Antitumor effect of flavonoids in animal model
The researcher who observed that flavonoids were found to have anticancer effect accompanied by prooxidative potential in in vitro study also found that flavonoids had antitumor effect in animal experiments (Table 1). 10–100 mg/kg of flavonoids injected more than 3 times per week for 3 weeks, though the period and frequency of oral administration varied, induced antitumor effect in xenograft animal model without toxicity. Even a level of less than 10 mg/kg of intravenously (i.v.) administered flavonoids caused antitumor effect, but blood concentration of flavonoids in animal model has not been reported yet. Various other study findings reported that quercetin (Gupta et al., 2011; Hashemzaei et al., 2017; Khan et al., 2016), luteolin (Manju et al., 2007; Samy et al., 2006), genistein (Chodon et al., 2007; Gu et al., 2005, 2009) and apigenin (Chunhua et al., 2013; Meng et al., 2017; Shukla et al., 2015) inhibited the generation and proliferation of tumor and expressed anticancer activity in xenograft animal model, thus extending the survival time of mice by more than 160% (Hashemzaei et al., 2017; Masuelli et al., 2017).
Epidemiological studies
There have been many epidemiological studies on the anticancer activity of flavonoids in the form of either case–control or cohort studies. These were mostly focused on prevention of cancer risk rather than their chemotherapeutic activity. This paper reviewed case–control studies which included accurate information on the amount of flavonoids administered via food frequency questionnaire (FFQ) (Table 2). Among these case–control studies, only results with more than one year of administration are summarized in Table 2. The amounts of daily administration of total flavonoids was reported to be 80.1–106.4 mg/day (Cho et al., 2017; Woo et al., 2014). The administered amounts of flavonol (i.e. quercetin, and kaempferol), known to be the most highly consumed flavonoids, were 15.0–29.7 mg/day (Bobe et al., 2010; Cho et al., 2017) while for flavone (i.e. apigenin, and luteolin), known as the least consumed, it was 1.0–3.88 mg/day (Cho et al., 2017; Storelli et al., 2019; Theodoratouet et al., 2007). Quercetin, a highly consumed flavonoid contained in apple and onion, and EGCG, contained in green tea, were reported to reduce the incidence of colon cancer (Theodoratouet et al., 2007), lung cancer (Cui et al., 2008) or gastric cancer (Ekstrőm et al., 2010).
Table 2.
Association of flavonoids intake and cancer risk
| Study | Country | Populations | Age (M) | Flavonoids | Daily consumption of flavonoids in control and case (mg/day) | Results | |
|---|---|---|---|---|---|---|---|
| Theodoratou et al. (2007) | Scotland | Cororetal cancer (1456) | 16–79 | Flavonols | 28.0 | 26.8 | 30% reduction in colorectal cancer risk |
| Control(1453) | 16–79 | ||||||
| Quercetin | 18.1 | 17.3 | 30% reduction in colorectal cancer risk | ||||
| EGCG | 24.5 | 23.7 | 30% reduction in colorectal cancer risk | ||||
| Flavones | 1.0 | 1.1 | No significant effect | ||||
| Flavanones | 20.6, | 20.2 | No significant effect | ||||
| Hesperetin | 10.6 | 10.5 | No significant effect | ||||
| Cui et al. (2008) | USA | Lung cancer (558) | 50–52 | Hesperetin | 80 (M) | Positively inverse relation to cancer risk | |
| Control (837 | 50–52 | ||||||
| EGCG | 10 (M) | Stronger inversely related to cancer risk | |||||
| Quercetin | 9 (M) | Stronger inversely related to cancer risk | |||||
| Kaempferol | 2 (M) | Stronger inversely related to cancer risk | |||||
| Bobe et al. (2010) | USA | Participants who had at least one confirmed corolectal cancer (872) | 48–62 | Flavonols | 29.7 ≥ (M) | Greatest reduction in high risk adenoma recurrence, and serum IL6↓ | |
| Ekstrőm et al. (2010) | Sweden | Gastric cancer (505) | 67(M) | Quercetin | 0.16–11.89 (according to grade) | Stronger inversely related to gastric carcinoma risk than lowest intake( ≦4 mg), especially for smoking women | |
| Control (1116) | 66(M) | ||||||
| Woo et al. (2014) | Korea | Gastric cancer (334) | 51(M) | Total flavonoids | 106.4 | 105.2 | Reduced cacer risk in women but not in men, except for isoflavones |
| Control (334) | 51(M) | Flavone | 1.4 | 1.3 | Significant inverse relation with cancer risk | ||
| Cho et al. (2017) | Korea | Colorectal cancer (923) | 56(M) | Total flavonoids | 98.6 | 80.1 | No relation |
| Controls (1846) | 56(M) | ||||||
| Flavonols | 19.2 | 15.0 | Strongest association with a reduced cancer risk | ||||
| Flavones | 1.1 | 1.0 | No relation | ||||
| Flavan-3-ols | 13.1 | 8.8 | Strongest association with a reduced cancer risk | ||||
| Isoflavones | 26.7 | 24.1 | No relation | ||||
| Flavanones | 3.7 | 3.6 | No relation | ||||
| Shin et al. (2015) | Korea | Coloretal cancer (901) | 49–62 | Isoflavone | 13.0 | 9.7–20.89 | Reduced risk of distal colon cancer in men and rectal cancer in Women |
| Controls (2669) | 49–62 | ||||||
| Storelli et al. (2019) | Spain | Gastric cancer (329) | 63(M) | Total flavonoids | 371.25 | 358.5 | Reduced gastric cancer risk to 24–40% |
| Control (2700) | 65(M) | Flavan-3-ols | 26.94(M) | Reduced gastric cancer risk | |||
| Flavanones | 43.81(M) | Reduced gastric cancer risk | |||||
| Flavones | 3.88(M) | No effect on gastric cancer risk | |||||
| Flavonols | 23.75(M) | No effect on gastric cancer risk | |||||
| Isoflavones | 1.35(M) | Increased risk of gastric cancer | |||||
These are all from case–control studies. The flavonoid intake was calculated using the food consumption data from FFQ: The daily amount of flavonoids ingested had no significant differences between cases and controls. No evidence of any interaction effect. The period of consumption is at least more than 1 year until the time of analysis. M: mean
Most flavonoids contributed to the reduction of cancer incidence while isoflavone suggested mixed results. It was reported that Korean women who consumed isoflavone (20.89 mg/day) showed a decreased incidence of colon cancer (Shin et al., 2015) while Spaniards who consumed isoflavone at 1.35 mg/day showed an increased incidence of gastric cancer (Storelli et al., 2019). When more than 29.7 mg/day of flavonol was administered to patients who had recovered from colon cancer, a dramatic reduction of relapses was reported (Bobe et al., 2010), but the amount was almost the maximum level that could be consumed in daily life.
Bioavailability
When flavonoids are administered by various routes such as oral ingestion or intravascular injection, they arrive at their target tissue through blood, and then become biologically active. Their bioavailability is measured as the maximum concentration (Cmax) of bioactive compounds measured in plasma. Flavonoids that were found to be potent anticancer agents from in vitro studies but did not result in similar effects in vivo, were found to have low bioavailability (Manach et al., 2004; Spagnuolo et al., 2015). Flavonoids except for flavane-3-ol exist in a glycosylated form (glycoside) in the food. When glycoside is hydrolyzed to aglycone, the aglycone is absorbed in the intestine due to its lipophilicity (Fantini et al., 2015; Manach et al., 2004). Glycosylated flavonoids are usually absorbed more slowly than aglycone flavonoids (Fantini et al., 2015; Manach et al., 2004; Scalbert and Williamson, 2000). Through methylation or sulfation conjugation in the intestine and the liver, these conjugated flavonoids reach target tissues via circulating plasma (Fantini et al., 2015; Manach et al., 2004; Scalbert and Williamson, 2000). The anticancer effect of flavonoids could be attributed largely to their metabolites (Ávila-Gálvez et al., 2018; Jaramillo et al., 2010; Sak 2014). It is known that flavonoids are stable at high temperatures, with various cooking methods having little impact on their bioavailability.
The average daily consumption of flavonoids by Americans has been reported to be 20 mg–1 g. A report on U.S. daily consumption of flavonoids in 2011 found that daily amounts of flavan-3-ols (i.e. EGCG) were 12.0–189.2 mg/day, flavanones (i.e. hesperetin) were 14.4 mg/day, flavonols (i.e. quercetin, kaempferol) were 12.9 mg/day, flavones (i.e. apigenin, luteolin) were 0.3–1.6 mg/day, and isoflavone (i.e. genistein) was 0.1–1.2 mg/day (Fantini et al., 2015). Bioavailability was, from higher to lower in the order of genistein, apigenin, luteolin, EGCG and quercetin glycoside (Manach et al, 2004). When each flavonoids with 50 mg aglycone equivalent were consumed in normal diet, plasma concentration of the active compound was reported to be 0–4 μM (Manach et al., 2005), and physiological concentrations of each of the flavonoids in human plasma from a normal diet was 0–5.1 μM (Manach et al., 2004; Manach et al., 2005; Thilakaratha and Rupasinghe, 2013; Zhen et al., 2012). The half lives of quercetin and EGCG are 10–28 h (Manach et al., 2004), and it implicated that Cmax of human eating diet rich in this flavanol or flavan-3-ol could be around 10 μM (Chu et al., 2017; Jeong et al., 2009a; O'Prey et al., 2003).
In the above analysis, a physiologically active plasma concentration of each flavonoids for people on normal diets, which was 5 μM or lower, was expected to have a chemopreventive effect that reduces cancer risk (Table 2). However, in several in vitro studies that suggested the potential for flavonoids’ to act chemotherapeutically, cytotoxic activity (IC50) was also induced with supraphysiological concentrations even in flavonoids’ aglycone concentration. While the concentration of flavonoids needed to induce chemopreventive activity dependent on their antioxidative potential was very low for normal cells, in in vitro results (Cai et al., 1997; Du et al., 2018; Szewczyka et al., 2014), our literature survey (Table 1) suggested that the concentration of flavonoids needed to induce anticancer and chemotherapeutic activity was 10–200 μM for cancer cells. In more than 50% of the studies (27 of 50) which investigated a low concentration range of flavonoids (10–50 μM), which is a physiologically achievable concentration, flavonoids exerted excellent anticancer activity. In order to take advantage of the chemotherapeutic activity of flavonoids in clinical applications, we therefore need to have more in-depth studies aimed at enhancing the bioavailability of flavonoids.
Perspectives and preclinical strategies
As seen above, flavonoids have shown promising anticancer activity as non-toxic natural substances (Table 1). However, their low water solubility and bioavailability limit clinical applications and many researchers have tried to enhance their anticancer efficacy. Earlier efforts took advantage of the non-toxic quality of flavonoids and encapsulated flavonoids in the form of glycosides or aglycones. Later, nanotechnology was used to enhance the delivery of flavonoids.
For people consuming soy isoflavone diets of which more than 90% was genistein (2 mg/Kg, 4 mg/Kg, 8 mg/Kg each), his or her Cmax ranged between 4.3–16.3 μm for total genistein, concentration levels that could have anti-metastatic and anticancer effects in vitro (Takimoto et al., 2003). In addition, an oral digestion of the dietary supplement capsule, PTI G-2535 (containing approximately 600 mg genistein/day), for 10 times during 84 days resulted in the overall mean Cmax of genistein at 12 μM without toxicity (Pop et al., 2008), and it was also concluded that doses of 900 mg per day was safe.
Ingestion of 150 mg quercetin-3-glucoside capsule resulted in Cmax value as 5 μM (Olthof et al., 2000), and ingestion of capsule including quercetin-4′-glucoside equivalent to 100 mg quercetin resulted in Cmax value as 7 μm (Graefe et al., 2001). From the data on quercetin pharmacokinetics until 2004, Wätjen et al.(2005) suggested that dietary supplement of 1–2 g of quercetin might result in Cmax value of 10–50 μM if the quercetin was ingested properly. Ingestion of chews (RealFX™ Q‐Plus™) including 500 mg of quercetin resulted in Cmax value of 1051.9 ± 393.1 μg/L (3.5 μM) in blood (Kaushik et al., 2012). The Cmax value after ingestion of 135 mg of aglycone hesperetin capsules was 825.8 ng/mL (about 2.7 μM) (Kanaze et al., 2007), and the Cmax value of a person who ingested 150 mg of EGCG capsule twice a day with meals for five days was 95.1–577.0 μg/L (0.2–1.3 μM) (Scholl et al., 2018). Encapsulated quercetin and EGCG have been used for dietary supplementation, but the variations in Cmax were large, and their clinical efficacy chemotherapeutically has not yet been reported. As an indirect measure, administration of encapsulated EGCG (400 mg, three times daily) for eight weeks in breast cancer patients receiving radiotherapy, reduced the serum levels of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), which are associated with cancer progression (Zhang et al., 2012). Clinical applications of encapsulated flavonoids for chemotherapeutic purposes have not been tried because not enough information is available on the stability of capsule and metabolic interactions with other medications.
Since 2009, flavonoid delivery systems have been developed through nanoparticalization or liposomalization (Table 3). Quercetin-nanoparticles (NP) led to the inhibition of cancer cell growth at a lower concentration than free quercetin; 10 μM (IC50) and 1.4 μM (IC50) in cervical and breast cancer cells respectively (Cirillo et al., 2013; Chang et al., 2018). EGCG-NP and luteolin-NP showed dosage advantages by inhibiting cancer cell growth in various cancer cell lines at a low concentration level of 1.4–20 μM (IC50) (Chang et al., 2018; de Pace et al., 2013; Hsieh et al., 2011; Khan et al., 2014; Majumdar et al., 2014; Sanna et al., 2017; Siddigui et al., 2009; Siddigui et al., 2014; Wu et al., 2018a). Siddiqui et al. (2009) suggested that EGCG-NP showed a tenfold dosage advantage compared to free EGCG. In xenograft animal models, EGCG-NP and luteolin-NP were also reported to inhibit the generation and growth of tumors at lower administrations than free EGCG and free luteolin (Chang et., 2018; Khan et al., 2014; Majumdar et al., 2014; Sanna et al., 2017; Siddigui et al., 2009; Siddigui et al., 2014; Wu et al., 2018a). One study aimed to increase the plasma concentration of flavonoids based on NP. A single administration of lecithin-stabilized polymeric micelles (LsbPMs) of quercetin (100 mg/kg, i.v.) in rats increased Cmax, to 1.52 μg/mL (vs 0.53 μg/mL for free quercetin), and a single administration of water(w) /w emulsion form of quercetin(100 mg/Kg, i.v.) also increased Cmax, value to 7.4 μg/mL (vs 0.34 μg /mL for for free quercetin) (Chang et al., 2018; Pangeni et al., 2018).
Table 3.
Characteristics of nanotechnology based flavonoids as anticancer agents
| Flavonoids | Material & Technology | Size (nm) | Target | Dose | Activity | References |
|---|---|---|---|---|---|---|
| Genistein | GenLip HSPC main | < 407 | Murine breast cancer (4T1), human ovarian cancer(OVCAR-3), prostate cancer(PC-3) | 22.7–34.1 µM (IC50) | DNA laddering and apoptosis with 1/3 concentration of free G | Phan et al., (2013) |
| Quercetin | PMAA-NP | – | Cervix cancer (HeLa) | 100 µM | 87% reduction of viability vs free Q, and 95% suppression of viability vs control | |
| CNT-PMAA-NP | 80 | Cervix cancer (HeLa) | 10 µM (IC50) | Inhibit cell proliferation and DNA condensation strongerly | ||
| LsbPMS | 92.2 | Breast cancer (MCF-7) | 1.4 µM (IC50) | Inhibit cell proliferation vs free Q (IC50: > 30 µM) | ||
| Breast cancer (SKBR-3) | 4.63 µM (IC50) | Inhibit cell proliferation vs free Q (IC50: > 30 µM) | ||||
| CT-26 Xenograft mice |
50 mg/Kg, i.v. 7 days |
No significant reduction of tumor volume vs free Q |
Pouci et al., (2012) Cirillo et al., (2013) Chang et al., (2018) |
|||
| EGCG | PLA-PEG-NP | 260 |
Prostate cancer (PC-3) PC-3 xenograft mice |
3.74 µM (IC50) 100 µg/mice/d i.p., thrice/wk, 3 wks |
Inhibit cell proliferation and prolong half life Reduction of tumor volume almost same as free EGCG(1 mg/mice/d)group |
Siddigui et al., (2009) |
|
PNG-EGCG Chitosan-NP |
– 180 |
Bladder cancer (MBT-2) MBT-2 xenograft mice Melanoma(Mel 928) Mel 928 xenograft mice |
4.3 ppm (IC50) 2 mg EGCG + 1.5 ppm PNG/mice 7 µM (IC50) 100 µg/mice i.g. 5 times/wk, 3 wks |
Inhibit cell proliferation vs free E (IC50:28.4 µM) 70% reduction of tumor volume vs free E (2 mg) Inhibit cell proliferation vs free E (IC50:53 µM) 28% reduction of tumor volume vs free E (1 mg/mice), PSA↓ |
Heish et al., (2011) Siddigui et al., (2014) |
|
| CSLIPO | 85 | Breast cancer(MCF-7) |
10 µM (IC40) 100 µM |
50% reduction of viability vs free E Increase cellular E contents 34 times vs free E |
de Pace et al., (2013) | |
| Chit-NP | 200 < | PCa xenograft mice | 6 mg/Kg i.g. 3 wks | 50% reduction of tumor volume vs free E (40 mg/Kg) | Khan et al., (2014) | |
| PLGA-PEG-NP | 251 |
Prostate cancer (LNCaP, PC-3, DU-145) 22 Rv 1 xenograft mice |
20 µM 100 µg/mice/d i.v 5 times/wk, 3 wks |
40% reduction of viability vs free E 40% reduction of tumor volume vs free E (1 mg/mice/d) |
Sanna et al., (2017) | |
| Luteolin | PLA-PEG-OMe-NP | 115 |
Head and neck cancer (Tu212) Tu212 xenograft mice |
4.13 µM (IC50) 3.3 mg/Kg i.p. every 2 days, 30 days |
Slight reduction of viability vs free L (IC50:6.96 µM) 25% reduction of tumor volume vs free L |
Majumdar et al., (2014) |
| Lipo-Lut | 105 | Prostate cancer (CT-26) |
20 µM (IC50) 50 mg/Kg, i.v. 5 times, every 2 days |
Inhibit cell proliferation vs free L (IC50: > 60 µM) 70% reduction of tumor volume vs free L, Inhibit tumor vascularization, prolong plasma concentration |
Wu et al., (2018a) | |
| Hesperetin | CFH-NP | 450 | Colon cancer (HCT-15) | 28 µM (IC50) | Inhibit cell proliferation vs free H (IC50:190 µM) and inhibit apoptotic gene | Lazer et al., (2018) |
The undescribed amounts of doses of free G, free Q, free E and free L are the same with the amounts of doses of genistein-NP, quercetin-NP, EGCG-NP and luteolin-NP, respectively. Some reduction percentages are adjusted from the original data. Lip or Lipo: liposome, HSPC: fully-hydrogenated soy phosphatidylcholine, NP: nanopanticle, PMAA: polymetha acrylic acid, CNT: carbon nanotube, LsbPMS: lecithin-stabilized polymeric micelles, PNG: nanogold particle, PSA: prostate cancer specific antigen, CSLIPO: chitosan-coated nanoliposome, Chit: chitosan, PLGA-PEG: poly-(lactide-co-glyco syl)-carboxylic acid- polyethyleneglycol, PLA-PEG: polylactic acid-polyethyleneglycol, OMe: methanol, CFH: chitosan folate hesperetin, EGCG: epigallocatechin-3-gallate, Q: quercetin, L: luteolin, G: genistein, H: hesperetin, d:day, wk: week
As a result, NP-delivery systems are promising in that they facilitate chemotherapeutic plasma concentrations, improve bioavailability (Table 3), and furthermore increase biological half-life and cellular uptake in an innovative way (Naksuriya et al., 2014; Wu et al., 2018a). However they also have downsides in terms of low encapsulation efficiency and instability (Tyagi et al., 2017), and thus far systematic and large scale toxicity tests have not yet been conducted yet. Therefore currently, no data has been reported on the clinical applications of flavonoid-NPs.
To increase the anticancer efficacy and bioavailability of flavonoids, combinatorial theraphy has also been suggested as a way to take advantage of flavonoids’ non-toxic qualities and to target synergistic pathways (Fantini et al., 2015; Niedzwiecki et al., 2016). Comprehensive preclinical studies are required on the various metabolites of flavonoids (conjugated or not) to establish the chemotherapeutic role of flavonoids that have not been uncovered thus far in in vivo studies.
Acknowledgements
This work was supported by Dongseo University Research Fund of 2020.
Declarations
Conflict of interest
None of the authors of this study has any financial interest or conflict with industries or parties.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abotaleb M, Samuel SM , Varghese E, Varghese S, Kubatka P, Liskova A, Büsselberg D. Flavonoids in cancer and apoptosis. Cancers (Basel). 11: 28-64 (2019) [DOI] [PMC free article] [PubMed]
- Antosiak A, Milowska K, Maczynska K. Rozalska S, Gabryelak T. Cytotoxic activity of genistein-8-C-glucoside form Lupinus luteus L. and genistein against human SK-OV-3 ovarian carcinoma cell line. Medicinal Chemistry Research. 26: 64-73 (2017) [DOI] [PMC free article] [PubMed]
- Ávila-Gálvez MÁ, Espín JC, González-Sarrías A, Ávila-Gálvez MÁ. Physiological relevance of the antiproliferative and estrogenic effects of dietary polyphenol aglycones versus their phase-II metabolites on breast cancer cells: A call of caution. Agricultural Food Chemistry. 66: 8547-8555 (2018) [DOI] [PubMed]
- Beecher GR. Overview of dietary flavonoids: Nomenclature, occurrence and intake. Nutrition. 133: 3248S-3254S (2003) [DOI] [PubMed]
- Belinha I, Amorim MA, Rodrigues P, de Freitas V, Moradas-Ferreira P. Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae. Agricultural Food Chemistry. 55: 2446-2451 (2007) [DOI] [PubMed]
- Bi YL, Min M, Shen W, Liu Y. Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1 cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine. 39: 10-16 (2018) [DOI] [PubMed]
- Bishayee K, Ghosh S, Mukherjee A, Sadhukhan R, Mondal J, Khuda-Bukhsh AR. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug-DNA interaction. Cell Proliferation. 46: 153-63 (2013) [DOI] [PMC free article] [PubMed]
- Bobe G, Albert PS, Sansbury LB, Lanza E, Schatzkin A, Colburn NH, Cross AJ. Interleukin-6 as a potential indicator for prevention of high-risk adenoma recurrence by dietary flavonols in the polyp prevention trial. Cancer Prevention Research (Phila). 3: 764-75 (2010) [DOI] [PMC free article] [PubMed]
- Cai Q, Rahn RO, Zhang R. Dietary flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Letter. 119: 99-107 (1997) [DOI] [PubMed]
- Chang CE, Hsieh CM, Huang SC, Su CY, Sheu MT, Ho HO. Lecithin-stabilized polymeric micelles (L(sb)PMs) for delivering quercetin: Pharmacokinetic studies and therapeutic effects of quercetin alone and in combination with doxorubicin. Scientific Reports 8: 17640-17649 (2018) [DOI] [PMC free article] [PubMed]
- Chen YF, Liu H, Luo XJ, Zhao Z, Zou ZY, Li J. The roles of reactive oxygen species(ROS) and autophagy in the survival and death of leukemia cells. Critical Reviews in Oncology and Hematology. 112: 21-30 (2017a) [DOI] [PubMed]
- Chen P, Zhang JY, Sha BB, Ma YE, Hu T, Ma YC, Sun H, Shi JX, Dong ZM, Li P. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget. 8: 27471-27480 (2017b) [DOI] [PMC free article] [PubMed]
- Chen JW, Zhu ZQ, Hu TX, Zhu DY. Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects. Acta Pharmacologica Sinica. 23: 667-672. (2002) [PubMed]
- Cho HJ, Ahn KC, Choi JY, Hwang SG, Kim WJ, Um HD, Park JK. Luteolin acts as a radiosensitizer in non-small cell lung cancer cells by enhancing apoptotic cell death through activation of a p38/ROS/caspase cascade. International Journal of Oncology. 46: 1149-1158 (2015) [DOI] [PubMed]
- Cho YA, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, Kim J. Dietary flavonoids, CYP1A1 genetic variants, and the risk of colorectal cancer in a Korean population. Scientific reports. 7: 128-139 (2017) [DOI] [PMC free article] [PubMed]
- Chodon D, Banu SM, Padmavathi R, Sakthisekaran D. Inhibition of cell proliferation and induction of apoptosis by genistein in experimental hepatocellular carcinoma. Molecular Cellular Biochemistry. 297: 73-80 (2007) [DOI] [PubMed]
- Choi JS, Chung HY, Kang SS, Jung MJ, Kim JW. The structure activity relationship of flavonoids as scavengers of peroxynitrite. Phytotherapy Research. 16:232-235 (2002) [DOI] [PubMed]
- Choi JB, Kim JH, Lee H, Pak JN, Shim BS, Kim SH. Reactive oxygen species and p53 mediated activation of p38 and caspases is critically involved in kaempferol induced apoptosis in colorectal cancer cells. . Agricultural Food Chemistry. 66: 9960-9967 (2018) [DOI] [PubMed]
- Chu C, Deng J, Man Y, Qu Y. Green tea extracts epigallocatechin-3-gallate for different treatments. BioMed Research International. 2017: 5615647-5615655 (2017) [DOI] [PMC free article] [PubMed]
- Chunhua L, Donglan L, Xiuqiong F, Lihua Z, Qin F, Yawei L, Liang Z, Ge W, Linlin J, Ping Z, Kun L, Xuegang S. Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. Nutritional Biochemistry. 24:1766-1775 (2013) [DOI] [PubMed]
- Cirillo G, Vittorio O, Hampel S, Iemma F, Parchi P, Cecchini M, Puoci F, Picci N. Quercetin nanocomposite as novel anticancer therapeutic: improved efficiency and reduced toxicity. European Journal of Pharmcological Sciences. 49: 359-65 (2013) [DOI] [PubMed]
- Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao JT. Dietary flavonoid intake and lung cancer - A population-based case-control study. Cancer. 112: 2241-2248 (2008) [DOI] [PMC free article] [PubMed]
- de Pace CR, Liu X, Sun M, Nie S, Zhang J, Cai Q, Gao W, Pan X, Fan Z, Wang S. Anticancer activities of (-)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 23: 187-196 (2013) [DOI] [PubMed]
- Dixon SJ and Stockwell BR. The role of iron and reactive oxygen species in cell death. Nature Chemical Biology. 10: 9-17 (2014) [DOI] [PubMed]
- Du W, An Y, He X, Zhang D, He W. Protection of kaempferol on oxidative stress-Induced retinal pigment epithelial cell damage. Oxidative Medicine and Cellular Longevity. Article ID: 1610751 (2018) [DOI] [PMC free article] [PubMed]
- Ekstrőm AM, Serafini M, Nyren O, Wolk A, Bosetti C, Bellocco R. Dietary quercetin intake and risk of gastric cancer: Results from a population-based study in Sweden. Annals of Oncology. 22: 438-443 (2011) [DOI] [PMC free article] [PubMed]
- Elbling L, Herbacek I, Weiss RM, Jantschitsch C, Micksche M, Gerner C, Pangratz H. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free Radical Biology and Medicine. 49: 1444-1452 (2010) [DOI] [PubMed]
- Elbling L, Weiss RM, Teufelhofer O. Green tea extract and (–)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. Federation of American Societies for Experimental Biology. 19: 807-809 (2005) [DOI] [PubMed]
- Fantini M, Benvenuto M, Masuelli L, Vanni Frajese GV, Tresoldi G. Modesti A. Bei R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. International Journal of Molecular Science. 16: 9236-9282 (2015) [DOI] [PMC free article] [PubMed]
- Farhan M, Khan HY, Oves M, Al-Harrasi A, Rehmani N, Arif H, Hadi SM, Ahmad A. Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins (Basel) 8: 37-51 (2016) [DOI] [PMC free article] [PubMed]
- Gilardini Montani MS, Cecere N, Granato M, Romeo MA, Falciarelli L, Ciciarelli U, Faggioni A. Mutant p53, stabilized by its Interplay with HSP90, activates a positive feed-back loop between NRF2 and p62 that Induces chemo-resistance to apigenin in pancreatic cancer. Cancers (Basel) 11: 703 (2019) [DOI] [PMC free article] [PubMed]
- Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochemical Pharmacology. 92: 90-102 (2014) [DOI] [PubMed]
- Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pacific Journal of Cancer Prevention. 16: 2129-2144 (2015) [DOI] [PubMed]
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nature Reviews Drug Discovery. 12: 931-945 (2013) [DOI] [PubMed]
- Graefe EU, Wittig J. Mueller S. Riethling AK, Uehleke B, Drewelow B. Pforte H, Jacobasch G, Derendorf H, Veit M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. Clinical Pharmacology. 41: 492-499 (2001) [DOI] [PubMed]
- Gu Y, Zhu CF, Dai YL, Zhong Q, Sun B. Inhibitory effects of genistein on metastasis of human hepatocellular carcinoma. World Journal of Gastroenterology. 15:4952-4957 (2009) [DOI] [PMC free article] [PubMed]
- Gu Y, Zhu CF, Iwamoto H, Chen JS. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World Journal of Gastroenterology. 11: 6512-6517 (2005) [DOI] [PMC free article] [PubMed]
- Gupta C, Tripathi DN, Vikram A, Ramarao P, Jena GB. Quercetin inhibits diethylnitrosoamine-induced hepatic preneoplastic lesions in rats. Nutrition and Cancer. 63: 234-241(2011) [DOI] [PubMed]
- Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K, Kouretas D, Tzanakakis G, Nikitovic D, Anisimov NY, Spandidos DA, Tsatsakis AM, Rezaee R. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncology Reports. 38: 819-828 (2017) [DOI] [PMC free article] [PubMed]
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed Research International. Article ID: 150845 (2014) [DOI] [PMC free article] [PubMed] [Retracted]
- Hay RJ, Park JG, Goldar A. Atlas of human tumor cell lines. Academic press, San Diego, USA. pp 34-40 (1994)
- He G, He G, Zhou R, Pi Z, Zhu T, Jiang L, Xie Y. Enhancement of cisplatin-induced colon cancer cells apoptosis by shikonin, a natural inducer of ROS in vitro and in vivo. Biochemical Biophysical Research Communications. 469: 1075-1082 (2016) [DOI] [PubMed]
- Heo JR, Lee GA, Kim GS, Hwang KA, Choi KC. Phytochemical-induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis and differentiation in malignant melanoma cells. Phytomedicine. 39: 100-110 (2018) [DOI] [PubMed]
- Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of action of (-)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Research. 65: 8049-8056 (2005) [DOI] [PubMed]
- Hsiao YC, Peng SF, Lai KC, Liao CL, Huang YP, Lin CC, Lin ML, Liu KC, Tsai CC, Ma YS, Chung JG. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environmental Toxicology. 34: 443-456 (2019) [DOI] [PubMed]
- Hsieh DS, Wang H, Tan SW, Huang YH, Tsai CY, Yeh MK, Wu CJ. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials. 32: 7633-7640 (2011) [DOI] [PubMed]
- Jaramillo S, Lopez S, Varela LM, Rodriguez-Arcos R, Jimenez A. The flavonol isorhamnetin exhibits cytotoxic effects on human colon cancer cells. Agricultural Food Chemistry. 58: 10869-10875 (2010) [DOI] [PubMed]
- Jeong JH, Ahn JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. Cellular Biochemistry. 106: 73-82 (2009a) [DOI] [PMC free article] [PubMed]
- Jeong JC, Kim MS, Kim TH, Kim YK. Kaempferol induces cell death through ERK and Akt-dependent down-regulation of XIAP and survivin in human glioma cells. Neurochemical Research. 34: 991-1001 (2009b) [DOI] [PubMed]
- Kaminsky VO, Zhivotovsky B. Free radicals in cross talk between autophagy and apoptosis. Antioxidants and Redox Signaling 21: 86-102 (2014) [DOI] [PubMed]
- Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. European Journal of Clinical Nutrition. 61: 472-477 (2007) [DOI] [PubMed]
- Kaushik D, Kevin O'Fallon, Clarkson PM, Dunne CP, Conca KR, Michniak-Kohn B. Comparison of quercetin pharmacokinetics following oral supplementation in humans. Food Science. 77: H231-8 (2012) [DOI] [PubMed]
- Kaushik S, Shyam H, Agarwal S, Sharma R, Nag TC, Dwivedi AK, Balapure AK. Genistein potentiates centchroman induced antineoplasticity in breast cancer via PI3K/Akt deactivation and ROS dependent induction of apoptosis. Life Science. 239: 117073-117085 (2019) [DOI] [PubMed]
- Khan N, Bharali DJ, Adhami VM, Siddiqui IA, Cui H, Shabana SM. Oral administration of naturally occurring chitosan based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 35: 415-423 (2014) [DOI] [PMC free article] [PubMed]
- Khan F, Niaz K, Maqbool F, Hassan FI, Abdollahi M, Venkata KCN, Nabavi SM, Bishayee A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients. 8: 529-548 (2016) [DOI] [PMC free article] [PubMed]
- Khiewkamrop P, Phunsomboon P, Richert L, Pekthong D, Srisawang P. Epistructured catechins, EGCG and EC facilitate apoptosis induction through targeting de novo lipogenesis pathway in HepG2 cells. Cancer Cell International. 18: 46-58 (2018) [DOI] [PMC free article] [PubMed]
- Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 123: 625-631 (2014a) [DOI] [PMC free article] [PubMed]
- Kim CY , Chan Lee, Park GH, Jang JH. Neuroprotective effect of epigallocatechin-3-gallate against beta-amyloid-induced oxidative and nitrosative cell death via augmentation of antioxidant defense capacity. Archives of Pharmacal Research. 32: 869-881 (2009) [DOI] [PubMed]
- Kim JK, Kang KA, Ryu YS, Piao MJ, Han X, Oh MC, Boo SJ, Jeong SU, Jeong YJ, Chae S, Na SY, Hyun JW. Induction of endoplasmic reticulum stress via reactive oxygen species mediated by luteolin in melanoma cells. Anticancer Research 36: 2281-2289 (2016) [PubMed]
- Kim IG, Kim JS, Lee JH, Cho EW. Genistein decreases cellular redox potential, partially suppresses cell growth in HL-60 leukemia cells and sensitizes cells to γ-radiation-induced cell death. Molecular Medicine Reports. 10: 2786-2792 (2014b) [DOI] [PMC free article] [PubMed]
- Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biology. 2: 187-195 (2014c) [DOI] [PMC free article] [PubMed]
- Kittiratphatthana N, Kukongviriyapan V, Prawan A, Senggunprai L. Luteolin induces cholangiocarcinoma cell apoptosis through the mitochondrial-dependent pathway mediated by reactive oxygen species. Pharmacy and Pharmacology. 68: 1184-1192 (2016) [DOI] [PubMed]
- Klauser E, Gulden M, Master E, Sabine S, Seibert H. Additivity, antagonism, and synergy in arsenic trioxide-induced growth inhibition of C6 glioma cells: effects of genistein, quercetin and buthionine sulfoximine. Food and Chemical Toxicology. 67: 212-221 (2014) [DOI] [PubMed]
- Klein CB, King AA. Genistein genotoxicity: critical considerations of in vitro exposure dose. Toxicology and Applied Pharmacology. 224: 1-11 (2007) [DOI] [PubMed]
- Lazer LM, Sadhasivam B , Palaniyandi K, Muthuswamy T , Ramachandran L, Balakrishnan A. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. International Journal of Biological Macromolecules. 107(Pt B): 1988-1998 (2018) [DOI] [PubMed]
- Lee GA, Choi KC, Hwang KA. Treatment with phytoestrogens reversed triclosan and bisphenol A-induced anti-apoptosis in breast cancer cells. Biomolecules and Therapeutics (Seoul). 26: 503-511 (2018) [DOI] [PMC free article] [PubMed]
- Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, Lee MJ, Liu B, Guan F, Yang Z, Yu A, Yang CS. Prooxidative activities and dose-response relationship of (-)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 31: 902-910 (2010) [DOI] [PMC free article] [PubMed]
- Lin CC, Chuang YJ, Yu CC, Yang JS, Lu CC, Chiang JH, Lin JP, Tang NY, Huang AC, Chung JG. Apigenin induces apoptosis through mitochondrial dysfunction in U-2 OS human osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth in vivo. Agricultural Food Chemistry. 60: 11395-11402 (2012) [DOI] [PubMed]
- Liu L, Hou L, Gu S, Zuo X, Meng D, Luo M, Zhang X, Huang S, Zhao X. Molecular mechanism of epigallocatechin-3-gallate in human esophageal squamous cell carcinoma in vitro and in vivo. Oncology Reports. 33: 297-303 (2015) [DOI] [PubMed]
- Mahalingaiah PK, Singh KP. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PloS One. 9: e87371-e87380 (2014) [DOI] [PMC free article] [PubMed]
- Majumder D, Das A, Saha C. Catalase inhibition an anti cancer property of flavonoids: A kinetic and structural evaluation. International Journal of Biological Macromolecules. 104: 929-935 (2017) [DOI] [PubMed]
- Majumdar D, Jung KH, Zhang H, Nannapaneni S, Wang X, Amin AR, Chen Z, Chen ZG, Shin DM. Luteolin nanoparticle in chemoprevention: in vitro and in vivo anticancer activity. Cancer Prevntion Research (Phila). 7: 65-73 (2014) [DOI] [PMC free article] [PubMed]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: Food sources and bioavailability. American Journal of Clinical Nutrition. 79: 727-747 (2004) [DOI] [PubMed]
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. American Journal of Clinical Nutrition. 81: 230S-242S (2005) [DOI] [PubMed]
- Manju V, Nalini N. Protective role of luteolin in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Cell Biochemisry and Function. 25: 189-194 (2007) [DOI] [PubMed]
- Martinez C, Yanez J, Vicente V, Alcaraz M, Benavente-Garcia O. Effects of several polyhydroxylated flavonoids on the growth of B16F10 melanoma and Melan-a melanocyte cell lines: influence of the sequential oxidation state of the flavonoid skeleton. Melanoma Reearch. 13: 3-9 (2003) [DOI] [PubMed]
- Masuelli L, Benvenuto M, Mattera R, Di Stefano E, Zago E, Taffera G, Tresoldi I, Giganti MG, Frajese GV. In Vitro and In Vivo anti-tumoral effects of the flavonoid apigenin in malignant mesothelioma. Frontiers in Pharmacology. 8: 373-387 (2017) [DOI] [PMC free article] [PubMed]
- Meng S, Zhu Y, Li JF, Wang X, Liang Z, Li SQ, Xu X, Chen H, Liu B, Zheng XY, Xie LP. Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget. 8: 19834-19842 (2017) [DOI] [PMC free article] [PubMed]
- Nadal-Serrano M, Pons DG, Sastre-Serra J, Blanquer-Rosselló Mdel M, Roca P, Oliver J. Genistein modulates oxidative stress in breast cancer cell lines according to ERα/ERβ ratio: effects on mitochondrial functionality, sirtuins, uncoupling protein 2 and antioxidant enzymes. International Journal of Biochemisry and Cell Biology. 45: 2045-2051 (2013) [DOI] [PubMed]
- Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 35: 3365-3383 (2014) [DOI] [PubMed]
- Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 8: 552-574 (2016) [DOI] [PMC free article] [PubMed]
- Olthof MR, Hollman PC, Vree TB, Katan MB. Bioavailabilities of quercetin-3-glucoside and quercetin-4-glucoside do not differ in humans. Nutrition. 130: 1200-1203 (2000) [DOI] [PubMed]
- O'Prey J, Brown J, Fleming J, Harrison PR. O'Prey J. Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochemical Pharmacology. 66: 2075-2088 (2003) [DOI] [PubMed]
- Palit S, Kar S, Sharma G, Das PK. Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ROS and activation of ASK1/JNK pathway. Cellular Physiology. 230: 1729-1739 (2015) [DOI] [PubMed]
- Pangeni R, Panthi V, Yoon I, Park JW. Preparation, characterization, and in vivo evaluation of an oral multiple nanoemulsive system for co-delivery of pemetrexed and quercetin. Pharmaceutics. 10: 158-170 (2018) [DOI] [PMC free article] [PubMed]
- Park C, Cha HJ, Lee H, Hwang-Bo H, Ji SY, Kim MY, Hong SH, Jeong JW, Han MH, Choi SH, Jin CY, Kim GY, Choi YH. Induction of G2/M cell cycle arrest and apoptosis by genistein in human bladder cancer T24 cells through inhibition of the ROS-dependent PI3k/Akt signal transduction pathway. Antioxidants (Basel). 8: 327-339 (2019) [DOI] [PMC free article] [PubMed]
- Pawlikowska-Pawlęga B, Dziubińska H, Król E, Trębacz K, Jarosz-Wilkołazka A, Paduch R. Characteristics of quercetin interactions with liposomal and vacuolar membranes. Biochimica et Biophysica Acta. 1838: 254-265 (2014) [DOI] [PubMed]
- Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G. ROS in cancer therapy: the bright side of the moon. Experimental and Molecular Medicine. 52: 192-203 (2020) [DOI] [PMC free article] [PubMed]
- Phan V, Walters J, Brownlow B, Elbayoumi T. Enhanced cytotoxicity of optimized liposomal genistein via specific induction of apoptosis in breast, ovarian and prostate carcinomas. Drug Target. 21: 1001-1011 (2013) [DOI] [PubMed]
- Piao MJ, Hyun YJ, Cho SJ, Kang HK, Yoo ES, Koh YS, Lee NH, Ko MH, Hyun JW. An ethanol extract derived from Bonnemaisonia hamifera scavenges ultraviolet B (UVB) radiation-induced reactive oxygen species and attenuates UVB-induced cell damage in human keratinocytes. Marine Drugs. 10: 2826-2845 (2012) [DOI] [PMC free article] [PubMed]
- Poillet-Perez L, Despouy G, Mourroux RD, Guittaut MB. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biology. 4: 184-192 (2015) [DOI] [PMC free article] [PubMed]
- Pop EA, Fischer LM, Coan AD, Gitzinger M, Nakamura J, Zeisel SH. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis and estrogenic outcomes in healthy, postmenopausal women - a Phase I clinical trial. Menopause. 15(4 Pt 1): 684-692 (2008) [DOI] [PMC free article] [PubMed]
- Priyadarsini RV, Nagini S. Quercetin suppresses cytochrome P450 mediated ROS generation and NFκB activation to inhibit the development of 7,12-dimethylbenz[a] anthracene (DMBA) induced hamster buccal pouch carcinomas. Free Radical Research. 46: 41-49 (2012) [DOI] [PubMed]
- Puoci F, Morelli C, Cirillo G, Curcio M, Parisi OI, Maris P, Sisci D, Picci N. Anticancer activity of a quercetin-based polymer towards HeLa cancer cells. Anticancer Research. 32: 2843-2847 (2012) [PubMed]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 475: 231-234 (2011) [DOI] [PMC free article] [PubMed] [Retracted]
- Raja SB, Rajendiran V, Kasinathan NK, Amrithalakshmi P, Venkatabalasubramanian S, Murali MR, Devaraj H. Differential cytotoxic activity of quercetin on colonic cancer cells depends on ROS generation through COX-2 expression. Food and Chemical Toxicology. 106: 92-106 (2017) [DOI] [PubMed]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation and cancer: how are they linked? Free Radical Biology and Medicine. 49: 1603-1616 (2010) [DOI] [PMC free article] [PubMed]
- Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nature Reviews Cancer. 14: 709-721 (2014) [DOI] [PMC free article] [PubMed]
- Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: A comprehensive review. Biomedicine and Pharmacotherapy. 104: 485-495 (2018) [DOI] [PubMed]
- Sak K. Site-Specific Anticancer effects of dietary flavonoid quercetin. Nutrition and Cancer 66: 177-193 (2014) [DOI] [PubMed]
- Samy RP, Gopalakrishnakone P, Ignacimuthu S. Anti-tumor promoting potential of luteolin agains 7,12-dimethylbenz(a) anthracene-induced mammary tumors in rats. Chemico-Biologlcal Interactions. 164: 1-14 (2006) [DOI] [PubMed]
- Sanna V, Singh CK, Jashari R, Adhami VM, Chamcheu JC, Rady I, Sechi M, Mukhtar H, Siddiqui IA. Targeted nanoparticles encapsulating (-)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Science Reports. 7: 41573-41582 (2017) [DOI] [PMC free article] [PubMed]
- Sato Y, Sasaki N, Saito M, Endo N, Kugawa F, Ueno A. Luteolin attenuates doxorubicin-induced cytotoxicity to MCF-7 human breast cancer cells. Biological and Pharmaceutical Bulletin. 38: 703-709 (2015) [DOI] [PubMed]
- Satoh M, Takemura Y, Hamada H, Sekido Y, Kubota S. EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cell International. 13: 19-31 (2013) [DOI] [PMC free article] [PubMed]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. Nutrition. 130: 2073S-2085S (2000) [DOI] [PubMed]
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Current Biology. 24: R453-R462 (2014) [DOI] [PMC free article] [PubMed]
- Scholl C, Lepper A, Lehr T, Hanke N, Schneider KL, Brockmo¨ller J. Population nutrikinetics of green tea extract. PLoS ONE 13: e0193074 (2018) [DOI] [PMC free article] [PubMed]
- Schroeter A, Aichinger G, Stornig K, Marko D. Impact of oxidative metabolism on the cytotoxic and genotoxic potential of genistein in human colon cancer cells. Molecular Nutrition and Food Research. 63: e1800635. (2019) [DOI] [PubMed]
- Seo HS, DeNardo DG, Jacquot Y, Laïos I, Vidal DS, Zambrana CR, Brown PH. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Research and Treatment. 99: 121-134 (2006) [DOI] [PubMed]
- Severino JF, Goodman BA, Kay CW, Stolze K, Tunega D, Reichenauer TG. Free radicals generated during oxidation of green tea polyphenols: electron paramagnetic resonance spectroscopy combined with density functional theory calculations. Free Radical Biology Medicine. 46:1076-1088 (2009) [DOI] [PubMed]
- Seydi E, Rasekh HR, Salimi A, Mohsenifar Z, Pourahmad J. Selective toxicity of apigenin on cancerous hepatocytes by directly targeting their mitochondria. Anticancer Agents in Medicinal Chemistry. 16: 1576-1586 (2016) [DOI] [PubMed]
- Seydi E, Salimi A, Rasekh HR, Mohsenifar Z, Pourahmad J. Selective cytotoxicity of luteolin and kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: involvement of ROS-mediated mitochondrial targeting. Nutrition and Cancer. 70: 594-604 (2018) [DOI] [PubMed]
- Shang HS, Lu HF, Lee CH, Chiang HS, Chu YL, Chen A, Lin YF, Chung JG. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environmental Toxicology. 33: 1168-1181 (2018) [DOI] [PubMed]
- Sharma DR, Wani WY, Sunkaria A, Kandimalla RJ, Sharma RK, Verma D. Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience 324: 163-176 (2016) [DOI] [PubMed]
- Shaw AT, Winslow MM, Magendantz M, Ouyang C, Dowdle J, Subramanian A. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 108: 8773-8778 (2011) [DOI] [PMC free article] [PubMed]
- Shin A, Lee J, Lee J , Park MS , Park JW , Park SC, Oh JH , Kim JS. Isoflavone and soyfood intake and colorectal cancer risk: A case-control study in Korea. Plos one 10: e0143228 (2015) [DOI] [PMC free article] [PubMed]
- Shukla S, Kanwal R, Shankar E, Datt M, Chance MR, Fu P, MacLennan GT, Gupta S. Apigenin blocks IKKα activation and suppresses prostate cancer progression. Oncotarget. 6: 31216-31232 (2015) [DOI] [PMC free article] [PubMed]
- Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 69:1712-1716 (2009) [DOI] [PMC free article] [PubMed]
- Siddiqui IA, Bharali DJ, Nihal M, Adhami VM, Khan N, Chamcheu JC, Khan MI, Shabana S, Mousa SA, Mukhtar H. Excellent anti-proliferative and pro-apoptotic effects of (-)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine. 10: 1619-1626 (2014) [DOI] [PubMed]
- Souza RP, Bonfim-Mendonça PS, Gimenes F, Ratti BA, Kaplum V, Bruschi ML, Nakamura CV, Silva SO, Maria-Engler SS, Consolaro ME. Oxidative stress triggered by apigenin induces apoptosis in a comprehensive panel of human cervical cancer-derived cell lines. Oxidative Medicine and Cellular Longevity. 2017: 1512745-1512752 (2017) [DOI] [PMC free article] [PubMed]
- Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP, Loizzo MR, Tundis R, Nabavi SM. Genistein and cancer: current status, challenges, and future directions. Advances in Nutrition. 6: 408-419 (2015) [DOI] [PMC free article] [PubMed]
- Aragonés N, Obón-Santacana M, Guevara M, Gómez-Acebo I, Fernández-Tardón G, Molina-Barceló A. Flavonoids and the risk of gastric cancer: An exploratory case-control study in the MCC-Spain study. Nutrients. 11: 967-982 (2019) [DOI] [PMC free article] [PubMed]
- Suh KS, Chon S, Oh S, Kim SW, Kim JW, Kim YS, Woo JT. Prooxidative effects of green tea polyphenol (-)-epigallocatechin-3-gallate on the HIT-T15 pancreatic beta cell line. Cell Biology and Toxicology. 26: 189-199 (2010) [DOI] [PubMed]
- Szewczyka K, Krzaczekab T, Łopatyn´skic T, Gawlik-Dzikid U, Zidorn C. Flavonoids from Jovibarba globifera (Crassulaceae) rosette leaves and their antioxidant activity. Natural Product Research. 28: 1655-1658 (2014) [DOI] [PubMed]
- Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiology, Biomarkers and Prevention. 12: 1213-1221 (2003) [PubMed]
- Tavsan Z, Kayali HA. Flavonoids showed anticancer effects on the ovarian cancer cells: involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomedicine and Pharmacotherapy. 116: 109004-109018 (2019) [DOI] [PubMed]
- Theodoratou E, Kyle J, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiology Biomarkers and Prevention. 16: 684-693 (2007) [DOI] [PubMed]
- Thilakarathna SH, Rupasinghe HP. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 5: 336733-87 (2013) [DOI] [PMC free article] [PubMed]
- Tsai CY, Chen CY, Chiou YH, Shyu HW, Lin KH, Chou MC, Huang MH, Wang YF. Epigallocatechin-3-gallate suppresses human herpesvirus 8 replication and induces ROS leading to apoptosis and autophagy in primary effusion lymphoma cells. International Journal of Molecular Science. 19: 16-32 (2017) [DOI] [PMC free article] [PubMed]
- Tyagi N, Dr R, Begun J, Popat A . Cancer therapeutics with epigallocatechin-3-gallate encapsulated in biopolymeric nanoparticles. International Journal of Pharmacology. 518: 220-227 (2017) [DOI] [PubMed]
- Um JH, Yun J. Emerging role of mitophagy in human diseases and physiology. BMB Reports. 50: 299-307 (2017) [DOI] [PMC free article] [PubMed]
- Vandenabeele P, Galluzzim L, Vandenm Berghem T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Reviews Molecular Cell Biology. 11: 700-714 (2010) [DOI] [PubMed]
- Violi F, Pastori D, Carnevale R, Pignatelli P. Nutritional and therapeutic approaches to modulate NADPH oxidase-derived ROS signaling in platelets. Current Pharmaceutical Design. 21: 5945-5950 (2015) [DOI] [PubMed]
- Wang Y, Liu S, Dong W, Qu X, Huang C, Yan T1, Du J. Combination of hesperetin and platinum enhances anticancer effect on lung adenocarcinoma. Biomedicine and Pharmacotherapy. 113: 108779-108788 (2019) [DOI] [PubMed]
- Wang Q, Wang H, Jia Y, Pan H, Ding H. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemotherapy and Pharmacology. 79: 1031-1041 (2017) [DOI] [PubMed]
- Wang B, Zhao XH. Apigenin induces both intrinsic and extrinsic pathways of apoptosis in human colon carcinoma HCT-116 cells. Oncology Reports. 37: 1132-1140 (2017) [DOI] [PubMed]
- Wätjen W, Michels G, Steffan B, Niering P, Chovolou Y. Low concentrations of flavonoids are protective in rat H4IIE cells whereas high concentrations cause DNA damage and apoptosis. Nutrition. 135: 525-531 (2005) [DOI] [PubMed]
- Wei R, Hackman RM, Wang Y, Mackenzie GG. Targeting glycolysis with epigallocatechin-3-gallate enhances the efficacy of chemotherapeutics in pancreatic cancer cells and xenografts. Cancers (Basel) 11: 1496-1515 (2019) [DOI] [PMC free article] [PubMed]
- Wei YK, Gamra I, Davenport A, Lester R, Zhao L, Wei Y. Genistein induces cytochrome P450 1B1 gene expression and cell proliferation in human breast cancer MCF-7 Cells. Environmental Pathology, Toxicology and Oncology. 34: 153-159 (2015) [DOI] [PubMed]
- Whitehouse S, Chen PL, Greenshields AL, Nightingale M, Hoskin DW, Bedard K. Resveratrol, piperine and apigenin differ in their NADPH-oxidase inhibitory and reactive oxygen species-scavenging properties. Phytomedicine. 23: 1494-1503 (2016) [DOI] [PubMed]
- Wilkie-Grantham RP, Matsuzawa S, Reed JC. Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-FLIP protein. Biological Chemistry. 288: 12777–12790 (2013) [DOI] [PMC free article] [PubMed]
- Woo HD, Lee J, Choi IJ, Kim CG, Lee JY, Kwon O, Kim J. Dietary flavonoids and gastric cancer risk in a Korean population. Nutrient. 6: 4961-4973 (2014) [DOI] [PMC free article] [PubMed]
- Wu G, Li J, Yue J, Zhang S, Yunusi K. Liposome encapsulated luteolin showed enhanced antitumor efficacy to colorectal carcinoma. Molecular Medicine Reports. 17: 2456-2464 (2018a) [DOI] [PMC free article] [PubMed]
- Wu Q, Needs PW, Lu Y, Kroon PA, Ren D, Yang X. Different antitumor effects of quercetin, quercetin-3'-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food and Function. 9: 1736-1746 (2018b) [DOI] [PubMed]
- Wu D, Zhang J, Wang J, Li J, Liao F, Dong W. Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species. Tumor Biology. 37: 3451-349 (2016) [DOI] [PubMed]
- Yang HL, Chen SC, Senthil Kumar KJ, Yu KN, Lee Chao PD, Tsai SY, Hou YC, Hseu YC. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: an ex vivo approach. Agricultural Food Chemistry. 60: 522-532 (2012) [DOI] [PubMed]
- Yang Y, Karakhanova S, Hartwig W, D'Haese JG, Philippov PP, Werner J. Mitochondria and mitochondrial ROS in cancer: Novel targets for anticancer therapy. Cellular Physiology. 231: 2570-2581 (2016) [DOI] [PubMed]
- Yi L, Zongyuan Y, Cheng G, Lingyun Z, Guilian Y, Wei G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Science. 105: 520-527 (2014) [DOI] [PMC free article] [PubMed]
- Yokoyama C. Sueyoshi Y. Ema M. Mori Y. Takaishi K. Hisatomi H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncology Letters. 14: 6066–6070 (2017) [DOI] [PMC free article] [PubMed]
- Yoshii SR, Mizushima N. Monitoring and measuring autophagy. International Journal of Molecular Science. 18: 1865-1877 (2017) [DOI] [PMC free article] [PubMed]
- Yu L, Chen Y. Tooze SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 14: 207-215 (2018) [DOI] [PMC free article] [PubMed]
- Yu C, Jiao Y, Xue J, Zhang Q, Yang H, Xing L, Chen G, Wu J, Zhang S, Zhu W, Cao J. Metformin sensitizes non-small cell lung cancer cells to an epigallocatechin-3-gallate (EGCG) treatment by suppressing the Nrf2/HO-1 signaling pathway. International Journal of Biological Science. 13: 1560-1569 (2017) [DOI] [PMC free article] [PubMed]
- Zhang Q, Bao J, Yang J. Genistein-triggered anticancer activity against liver cancer cell line HepG2 involves ROS generation, mitochondrial apoptosis, G2/M cell cycle arrest and inhibition of cell migration. Archives of Medical Science. 15: 1001-1009 (2019) [DOI] [PMC free article] [PubMed]
- Zhang Li, Cheng X, Gao Y, Zheng J, Xu Q, Sun Y, Guan H, Yu H, Sun Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma BCPAP cells. Food and Function. 6: 3464–3472 (2015a) [DOI] [PubMed]
- Zhang Q, Cheng G, Qiu H, Zhu L, Ren Z, Zhao W, Zhang T, Liu L. The p53-inducible gene 3 involved in flavonoid-induced cytotoxicity through the reactive oxygen species-mediated mitochondrial apoptotic pathway in human hepatoma cells. Food and Function. 6: 1518-1525 (2015b) [DOI] [PubMed]
- Zhang J, Song J, Wu D, Wang J, Dong W. Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium. Medical Oncology. 32: 101-114 (2015c) [DOI] [PubMed]
- Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu K, Wang F, Liu K, Liu Q, Yang C, Yu P, Huang Y, Wang S, Jiang P, Qu Z, Luan J, Duan H. Anticancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Current Molecular Medicine. 12: 163-76 (2012) [DOI] [PMC free article] [PubMed]
- Zhang J, Wu D, Vikash, Song J, Wang J, Yi J, Dong W. Hesperetin induces the apoptosis of gastric cancer cells via activating mitochondrial pathway by increasing reactive oxygen species. Digestive Diseases and Sciences. 60: 2985–2995 (2015d) [DOI] [PubMed]
- Zhao Y, Qu T, Wang P, Li X, Qiang J, Xia Z. Unravelling the relationship between macroautophagy and mitochondrial ROS in cancer therapy. Apoptosis. 21: 517-531 (2016) [DOI] [PubMed]
- Zhen Y, Kaustubh K, Wei Z, Ming H. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anticancer Agents in Medicinal Chemistry. 12: 1264-1280 (2012) [DOI] [PMC free article] [PubMed]
- Zhou M, Shen S, Zhao X, Gong X. Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines. Biochemical and Biophysical Research Communications. 494: 263-269 (2017) [DOI] [PubMed]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews. 94: 909-950 (2014) [DOI] [PMC free article] [PubMed]