Abstract
Background
The aim of this study was to evaluate regional postoperative preserved pulmonary function (PPPF) and three‐dimensional (3D) volumetric changes according to the number of resected subsegments and investigate the factors that most affected pre‐/post PPPF.
Methods
Patients who underwent thoracoscopic lobectomy (n = 73), and segmentectomy (n = 87) were eligible for inclusion in the study. They were classified according to the number of resected subsegments which ranged from 1 to 10. The percentage of pre‐/postoperative forced expiratory volume in 1 s (FEV1) was used for comparison. Furthermore, lung volumetric changes were calculated using 3D computed tomography (CT) volumetry.
Results
The percentage of pre‐/postoperative EFV1 between 4 and 5–7 and between 5–7 and 10 were significant (p = 0.03 and p < 0.01, respectively), but not between 1–2 to 4 (p = 0.99). The difference between volumetric changes in the left lower lobe of patients with a number of resected subsegments was significant (p < 0.01). On univariate and multivariate analyses, chronic inflammation was significant for decrease in recovery percentages. When the PPPF was compared among resected subsegments, it gradually decreased with an increase in the number of patients without a postoperative procrastination of inflammation (p < 0.01).
Conclusions
Segmentectomy is feasible and useful for PPPF. Even a relatively large‐volume resection procedure where 5–7 subsegments are resected can preserve pulmonary function. Chronic inflammation was statistically identified as a risk factor for postoperative preserved pulmonary function.
Key points
Keywords: forced expiratory volume, left upper lobe, lobectomy, segmentectomy, thoracoscopic surgery
Segmentectomy is feasible and useful for postoperative preserved pulmonary function. However, chronic inflammation has been statistically identified as a risk factor for postoperative preserved pulmonary function.
INTRODUCTION
As postoperative preserved pulmonary function (PPPF) after segmentectomy remains controversial when selecting the most appropriate surgical procedure, the accurate estimation of the volume ratio of lung segments to the volume of the corresponding lobe is clinically required. With the development of modern radiological modalities, including single photon emission computed tomography (SPECT) and thin‐slice computed tomography (TSCT) for three‐dimensional (3D) volumetry, the identification of lung segments has become possible. Several studies have previously suggested that PPPF can be used to compare the effectiveness of segmentectomy with that of lobectomy. 1 , 2 Most of these results have been derived from thoracotomy studies, and a few have postulated that an increased number of resected segments have been found to be associated with decreased pulmonary function. 3 Irrespective of this, thoracoscopic surgery is a general technique that has been linked to improved postoperative lung function. 4
Postoperative pulmonary complications such as pneumonia, atelectasis, and pleuritis, with or without effusion, can impair lung recruitment and worsen PPPF. Several authors have previously reported that intraoperative pleural adhesions are likely to be associated with postoperative complications. 5 There have been few reports on the examination of regional PPPF in a lobe subjected to thoracoscopic segmentectomies. The aim of this study was to evaluate the percentages of postoperative pulmonary function in relation to preoperative function (measured by forced expiratory volume 1 s [FEV1] [pre‐/post] and 3D volumetry) in patients who underwent left upper thoracoscopic pulmonary resections (LUPR) with a number of resected subsegments. In conjunction, we investigated the factors that most affected pre‐/post PPPF.
METHODS
Patients
This retrospective study was approved by the Ethics Committee of Aichi Cancer Center Hospital (2017‐1‐298). Between January 2013 and December 2018, 208 serial patients underwent thoracoscopic segmentectomy (n = 87 [54.4%]) and thoracoscopic lobectomy (n = 73 [45.6%]). Patients who underwent concomitant chest wall resection, or resection except the left upper lobe (LUL) and had postoperative diaphragmatic paralysis or no examination of respiratory function during follow‐up, were excluded since this procedure probably strongly affects PPPF. In all cases, the lung segmental boundaries were identified by a previously reported intravenous indocyanine green injection technique, and a powered linear cutter instead of electrocautery was usually used. 6 This study was conducted in accordance with the Declaration of Helsinki. The anonymity of individual patients was ensured. The informed consent was waived because of the retrospective nature of this study.
The following variables were investigated for effect on changes in PPPF: patient characteristics (age, gender, smoking status, body mass index, and prognostic nutritional index), preoperative radiological findings (chronic obstructive pulmonary disease, and interstitial pneumonia), respiratory function (vital capacity [VC]; F18‐fluorodeoxyglucose positron emission tomography [FEV1]; diffusing capacity of the lungs for carbon monoxide [DLCO]); 3D volumetry (the measurement of pre‐/post lung volumes for contralateral right whole lung [CRWL], left upper lobe [LUL], and left lower lobe [LLL]); intraoperative findings (with or without adhesions, lymph node dissection, time and bleeding); and perioperative information (duration of drainage, histology, and pathological stage). Postoperative chronic inflammation was defined as C‐reactive protein >1 at more than three months on follow‐up duration.
Our institutional indication of intensive segmentectomy is essentially based on the Japanese oncological trials for non‐small cell lung cancer (NSCLC) in patients without compromised reasons such as low cardiorespiratory function or multiple nodules. 7 , 8 Moreover, we established our original criteria according to our previous reports 9 , 10 as follows: (i) the proportion of consolidation‐to‐tumor size was less than 0.5; (ii) tumor size on TSCT was less than 20 mm because of high sensitivity and specificity of being diagnosed as pathological minimally invasive cancer; and (iii) indication of left upper lobectomy is suitable for NSCLC patients with high sensitivity and specificity of no vascular, lymphatic, and pleural invasion. 11 , 12
Classification according to the number of resected subsegments
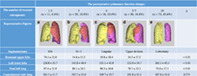
Lobectomy and segmentectomies were classified into five categories according to the number of subsegments as follows (Table 1): (i) one to 2 subsegments (n = 11): this category corresponded to less than a 1‐segment resection; (ii) Three subsegments (n = 30): this category corresponded to a 1‐segment resection or combination of subsegments; (iii) Four subsegments, n = 16: this category corresponded to a lingual segmentectomy or 1‐segment in the left upper division (LUD) plus 1 subsegment; (iv) Five to 7 subsegments, n = 30: this category corresponded to an LUD or lingual segmentectomy plus 1–3 subsegments in LUD; and (v) Ten subsegments n = 73: this category corresponded to a lobectomy.
TABLE 1.
Distribution of the subsegments based on the number of resected subsegments in the left upper lobes
| Number of resected subsegments | Location | Number | Total (%) |
|---|---|---|---|
| 1 | S1 + 2a | 1 | 11 |
| S1 + 2c | 2 | ||
| S3b | 1 | ||
| S3c | 1 | ||
| 2 | S1 + 2a + b | 2 | |
| S1 + 2a + S3a | 1 | ||
| S1 + 2a + S3c | 1 | ||
| S4 | 1 | ||
| S5 | 1 | ||
| 3 | S1 + 2 | 27 | 30 |
| S1 + 2c + S4 | 1 | ||
| S3 | 2 | ||
| 4 | Lingular | 9 | 17 |
| S1 + 2 + S3a | 2 | ||
| S1 + 2 + 3c | 2 | ||
| S1 + 2a + S3 | 1 | ||
| S3 + S4b | 2 | ||
| 5 | Lingular + S3b | 1 | |
| 6 | Upper division | 28 | 29 |
| 7 | S3 + lingular | 1 |
3D volumetry
3D lung constructs were reconfigured from TSCT data using a commercial workstation (Synapse Vincent, Fuji Film Co. Ltd). Two thoracic specialists designated segmental boundaries and lobe fissures manually on TSCT images. Individual 3D lung volumes in each lobe could then be obtained by the distribution of pulmonary artery immediately (Figure 1).
FIGURE 1.
The volumetric changes according to the number of resected subsegments and representative volumetric figures in each category of resection. (a) After S3b subsegmentectomy by 1 subsegmental resection. (b) After S1 + 2 segmentectomy by 3 subsegmental resection. (c) After lingular segmentectomy by 4 subsegmental resection. (d) After left upper division segmentectomy by 6 segmental resection. (e) After lobectomy by 10 subsegmental resection; yellow, contralateral right whole lung; red, the remnant left upper lobe; purple, left lower lobe
Predictive pulmonary volume ratio:
(total volume of whole lung − the volume of resected subsegments) ÷ total volume of whole lung
The percentage of postoperative 3D volume divided by the percentage of preoperative 3D volume:
Postoperative ÷ preoperative 3D volume × 100
Calculation of actual pulmonary function in FEV1
Each patient underwent TSCT before LUPR, at six and 12 months after surgery, as well as spirometry before LUPR, at three, six, and 12 months after surgery. A significant difference was found between the three‐ and six‐month FEV1 after segmentectomy and lobectomy (p < 0.01 and p < 0.01, respectively), but not between six and 12 months (p = 0.28 and p = 0.69, respectively). Therefore, we used the data of PPPF at six months.
In addition, thoracoscopy and thoracotomy for pre‐/post FEV1 after lobectomy at six months were compared. Although the difference between the two groups was not significant, thoracoscopy was greater than thoracotomy (82.9 ± 10.6% and 79.7 ± 11.6%, respectively; p = 0.31). Therefore, we focused on thoracoscopy only.
We used the following formulas:
The percentage of postoperative function divided by the percentage of preoperative function: (pre‐/post FEV1)
Postoperative ÷ preoperative FEV1 × 100
Predictive pulmonary function:
Predictive pulmonary volume ratio × preoperative FEV1
Attained percentage:
Actual postoperative function ÷ predictive pulmonary function × 100
Statistical analysis
All data were analyzed using SPSS software, version 25.0 (SPSS Institute Incorporated). Differences between two groups were assessed by the Mann–Whitney test, and comparisons between more than three groups were assessed by the Kruskal‐Wallis test. Multivariate logistic regression analysis was used to calculate odds ratios with 95% confidence intervals and to estimate probabilities for the worsening of PPPF. A p‐value <0.05 was considered statistically significant.
RESULTS
Patient characteristics
The patient characteristics are summarized in Table 2. More male than female and patients younger in age underwent lobectomy. The operative time was shorter, and there was less bleeding in patients who underwent segmentectomy than lobectomy. Systemic lymph node dissection, advanced disease (II–IIIA) and postoperative chronic inflammation were more frequent in lobectomy patients. Days of drainage was shorter in lobectomy than in segmentectomy patients. The median surgical margin was 20 mm (range: 5–62 mm), and lymphatic, vascular and pleural invasion were diagnosed in 15 (17.2%), seven (8.0%), and two (23.0%) patients, respectively.
TABLE 2.
Characteristics of patients undergoing left upper resections
| Segmentectomy | Lobectomy | ||
|---|---|---|---|
| Variables | (n = 87) | (n = 73) | p‐value |
| Patient | |||
| Age mean (range) | 67 (32–86) | 64 (41–80) | 0.01* |
| Gender (male/%) | 33 (45.8%) | 47 (53.4%) | 0.05* |
| Smoking (pack‐year) | 20.9 ± 27.3 | 23.6 ± 29.6 | 0.22 |
| Body mass index | 22.3 ± 3.1 | 22.3 ± 3.0 | 0.95 |
| PNI | 51.1 ± 7.5 | 51.9 ± 9.5 | 0.52 |
| Respiratory function | |||
| VC (%) | 3066.9 ± 782.5 | 3235.3 ± 730.7 | 0.04* |
| FEV1 (ml) | 2322.8 ± 585.3 | 2502.4 ± 580.3 | 0.10 |
| DLCO (%) | 112.4 ± 24.6 | 114.9 ± 21.7 | 0.36 |
| Lung volume (3D) | |||
| Resected volume | 519.7 ± 255.2 | 949.1 ± 441.9 | <0.01 |
| Left upper | 1058.4 ± 295.1 | 1071.0 ± 309.1 | 0.46 |
| Disease (%) | 0.66 | ||
| Lung cancer | 60 (83.3%) | 83 (94.3%) | |
| Metastatic | 6 (8.3%) | 4 (4.5%) | |
| Benign | 6 (8.3%) | 1 (1.1%) | |
| Computed tomography | |||
| COPD | 15 (20.8%) | 14 (15.9%) | 0.31 |
| Interstitial pneumonia | 3 (4.2%) | 3 (3.4%) | 0.43 |
| Intraoperative adhesions (half to whole) (yes, %) | 4 (5.6%) | 6 (6.8%) | 0.30 |
| Operative time | 190.9 ± 43.5 | 244.9 ± 54.7 | <0.01* |
| Operative bleeding | 18.1 ± 57.9 | 22.7 ± 31.8 | <0.01* |
| Systemic lymph node dissection (%) | 28 (38.9%) | 71 (80.7%) | <0.01* |
| Postoperative chronic inflammation | 0.49 ± 0.63 | 0.36 ± 0.74 | <0.01* |
| Postoperative complications (yes, %) | 4 (5.6%) | 8 (9.1%) | 0.06 |
| Pathological stage | <0.01* | ||
| I | 59 (98.3%) | 64 (77.1%) | |
| II–IIIA | 1 (1.7%) | 19 (22.9%) | |
| Days of drainage | 1.03 ± 1.63 | 0.86 ± 1.23 | <0.01* |
Abbreviations: 3D, 3‐dimensional; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 s; VC, vital capacity.
A p‐value <0.05 was considered statistically significant.
Postoperative respiratory functions and lung volumes at six months after left upper resections on thoracoscopy
Lobectomy versus segmentectomy
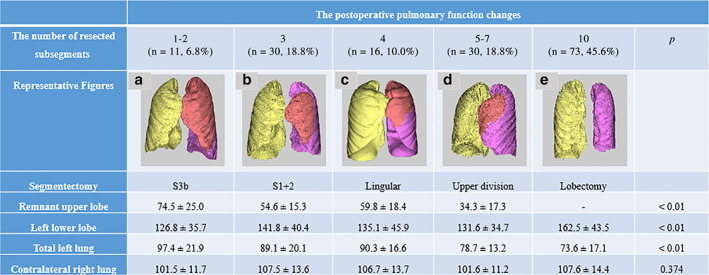
As shown in Figure 2a, the pre‐/post FEV1 in 1–2, 3, 4, 5–7, and 10 were 97.0 ± 5.3%, 96.9 ± 8.0%, 97.3 ± 10.3%, 90.3 ± 8.2%, and 83.9 ± 10.6%, respectively. The differences in the pre‐/post FEV1 between 4 and 5–7 and between 5–7 and 10 were significant (p = 0.03 and p < 0.01, respectively), but not between 1–2 to 4 (p = 0.99).
FIGURE 2.
The percentage of pre‐/postoperative forced expiratory volume in 1 s (FEV1) according to the number of resected subsegments in the left upper lobe. (a) In all patients; and (b) in patients without a postoperative chronic inflammation. Black: segmentectomy and gray: lobectomy. *a p‐value <0.05 was considered significant
As shown in Figure 1, the difference between volumetric changes of total left lung was significant in patients with the number of resected subsegments. The difference between volumetric changes of remnant LUL in patients with the number of resected subsegments was significant (p < 0.01), but between those with 1–2, 3, and 4–5 was not (p = 0.06). The difference between volumetric changes of LLL in patients with number of resected subsegments was significant (p < 0.01), but between those from 1–2 to 4, and those 4 and 5–7 was not (p = 0.43 and p = 0.58, respectively). No significant differences in CRWL were found.
Differences between predicted FEV1 and actual FEV1, and results of multivariate regression analysis of factors that might impede recovery of pulmonary function
When we investigated whether actual FEV1 attained predicted FEV1 by the resected 3D volume method, the percentages were 72.7% in 1–2, 86.7% in 3, 81.3% in 4, 86.7% in 5–7 and 86.3% in 10, respectively. The difference was not significant (p = 0.81).
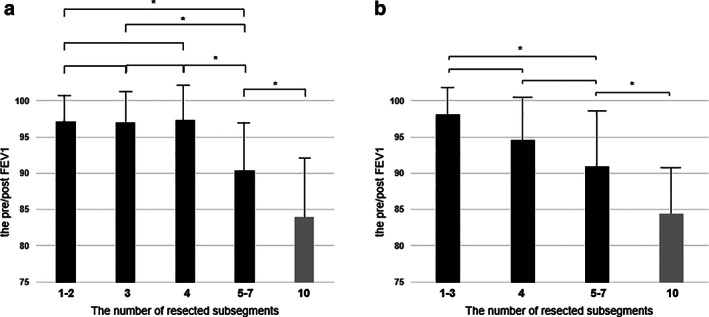
The univariate and multivariate analyses revealed that chronic inflammation was only significant for decrease in attained percentages in LUPR (Table 3). When avoiding the influence from chronic inflammation, the pre‐/post FEV1 in the pre‐/post FEV1 in 1–3 (n = 37), 4 (n = 14), 5–7 (n = 25), and 10 (n = 68) were 98.1 ± 6.5%, 94.5 ± 7.5%, 90.9 ± 8.7%, and 84.4 ± 8.9%, respectively (Figure 2b). When the PPPF was compared among the number of resected subsegments, it was gradually decreased with an increase of the numbers in patients without a postoperative procrastination of inflammation (p < 0.01). By individual comparison, the differences in the pre‐/post FEV1 between 1–3 and 5–7 and between 5–7 and 10 were only significant (p < 0.01 and p < 0.01, respectively) (Figure 2b). A significant difference was found in VC and FEV1 between the three‐ and six‐month spirometry values by chronic inflammation (p = 0.81 and p = 0.82, respectively) (Figure 3). Therefore, chronic inflammatory circumstances might impede the short‐ and long‐term respiratory improvement.
TABLE 3.
Univariate and multivariate analysis of clinicopathological factors possibly impeding pulmonary recovery
| Variables | Univariate | Multivariate | |
|---|---|---|---|
| p‐value | HR (95% CI) | p‐value | |
| Patient characteristics | |||
| Age (>75) | 0.55 | ||
| Gender male (vs. female) | 0.66 | ||
| Smoking (vs. never) | 0.26 | 1.18 (0.45–3.13) | 0.74 |
| Body mass index | 0.67 | ||
| PNI (>50) | 0.63 | ||
| %FEV1 (<70%) | 0.30 | ||
| %DLCO (<80%) | 0.32 | ||
| Preoperative radiological findings | |||
| LLL occupation ratio to left lung (<0.5) | 0.45 | ||
| Intraoperative findings | |||
| Adhesions | 0.65 | ||
| Intraoperative information | |||
| Time (>240 min) | 0.08 | 2.23 (0.83–5.99) | 0.11 |
| Bleeding (>100 ml) | 0.22 | 1.75 (0.27–11.4) | 0.56 |
| Perioperative information | |||
| Drainage days | 0.34 | ||
| Complications | 0.87 | ||
| Laboratory data | |||
| Chronic inflammation (CRP) (one month, >1 CRP) | <0.01* | 6.03 (1.90–19.1) | <0.01* |
| Systemic lymph node dissection | 0.60 | ||
Abbreviations: CRP, C‐reactive protein; LLL, left lower lobe.
A p‐value <0.05 was considered statistically significant.
FIGURE 3.
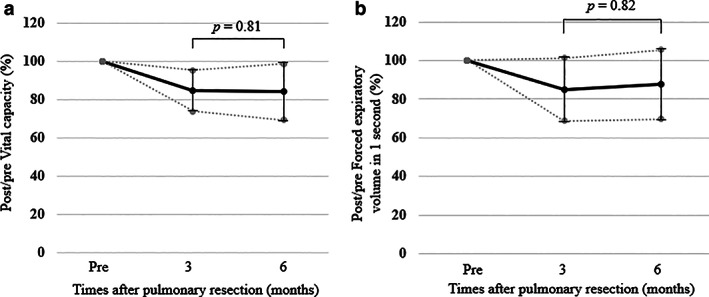
Pre‐/postoperative changes in vital capacity. (a) and forced expiratory volume in 1 s (FEV1) (b) in the patients with a postoperative procrastination of inflammation preoperatively, and at three and six months
DISCUSSION
In this study, we retrospectively investigated to what extent the number of resected subsegments contributed to regional PPPF. The results of the study are summarized as follows: (i) with regard to 4 subsegmental resection, the pre‐/post FEV1 and 3D volumetric changes were not significant; (ii) we determined that 5–7 subsegmental resection was superior to 10 subsegmental resection (lobectomy) in both PPPF and volumetric changes of the total left lung; (iii) however, the difference in volumetric changes of LLL after lobectomy was highest. These adverse data indicate that excessive expansion of LLL in patients undergoing lobectomy does not contribute to PPPF. In other words, the remnant upper lobe after segmentectomy played a role in weight and prevented the excessive expansion of the LLL; (iv) the multivariate analyses revealed that chronic inflammation statistically had a negative impact on PPPF; and (v) the difference between ideal respiratory functional changes was significant in patients without a postoperative procrastination of inflammation with number of resected subsegments. The results indicate that regional segmentectomies are promising procedures for PPPF.
The results of pulmonary function and 3D volumetry were significantly improved at six months after LUPR over those at three months and were equivalent at 12 months. Although there is evidence that thoracoscopic lobectomy does not provide long‐term PPPF superior to lobectomy by means of thoracotomy, 13 the value of PPPF after segmentectomy remains controversial. 14 , 15 , 16 Likewise, the long‐term outcomes including PPPF and patient prognosis associated with thoracoscopic segmentectomy remain undetermined. There are few reports documenting the significant relationship between the PPPF and number of resected segments, 3 which is consistent with the results of our study.
Several investigators have reported that 83.3% to 86.9% of FEV1 was preserved after LUL lobectomy, 17 , 18 whereas various reports determined that segmentectomy preserved 87.1% to 95.4%. 15 , 18 , 19 , 20 Notably, functional recovery after thoracoscopic segmentectomies was generally better than that of patients in previously reported studies, because the number of resected subsegments was smaller in our patients than in previous studies, which might reflect improved surgical techniques, and the procedure being completely performed by thoracoscopy. Another plausible explanation for the difference in functional recovery might be that our patients were not as adversely affected by postoperative pain and began walking earlier during postoperative recovery. 21
Microscopic and radiological evaluations of lung morphology have provided reliable evidence that the compensatory adaptation of the remaining lung after a lung resection or lobectomy is not simply a consequence of hyperinflation of the pre‐existing alveolar septal tissue, but is also a result of an increase in functional lung tissue. 22 The remnant lungs parenchyma are naturally forced to intensity to expand and to fill the vacant space. The more tissue is surgically removed the more the lung compliance is reduced, which means that in order to re‐expand the lung to its original volume and fill the whole chest volume, a greater pressure is required. 19 Suzuki et al. commented that the expansion of residual lung might contribute more to improved postoperative residual lung function after lobectomy than after segmentectomy. 23 This study showed a significant difference in the expansion volumes of LLLs between segmentectomies and lobectomy but not in those of CRWLs. Furthermore, although lobectomy led to greater expansion of LLL volume than segmentectomy, that result did not lead to recovery of actual FEV1.
In this study, we wanted to investigate the factors that led to worsened PPPF, and chronic inflammation corresponded statistically on multivariate analysis (Table 3). In fact, the volumetric changes of LLL were also higher in lobectomy (n = 5, 150.4% ± 68.0%) than in segmentectomy (n = 11, 118.4% ± 26.3%), but no significant difference was obtained (p = 0.58). Over distension is one of the most feared complications of lung resection as it may not only cause injury to the lung, but it could also cause three major complications: air leak, hydrothorax and lung edema. 24 Tissue injury to the lung alveolar derived from chronic inflammation could lead to the loss of the alveolar epithelial cells, damage or loss of the basement membrane and endothelial cells, and at the most severe end of the spectrum a complete collapse of the collagen and elastic support framework of the alveoli. 25 The efficacy of the elastic characteristics of the lung parenchyma could hold a sufficient intrathoracic pressure and result in a sufficient recovery of postoperative pulmonary function. Possibly because an advantage of thoracoscopic surgery is that it is minimally invasive thereby contributing to a reduction in complications might be utilized if surgeons can minimize postoperative complications caused by an operation maneuverer.
Some limitations of this study are obvious. First, a relatively smaller proportion of patients underwent LUD than underwent S1 + 2 segmentectomy. We previously found that not only the histopathological appearance of invasiveness, but also the sizes of consolidation on CT scan and mediastinal diameter (MD) might also be associated with malignant potential or outcome. 26 , 27 Therefore, our preference is to perform a 1 to 3 subsegment resection rather than an LUD segmentectomy, except in patients with poor respiratory function. Second, we could not analyze the quality of parenchyma regarding compensatory restoration of the remnant lobe. Wu et al. proposed that quantitative CT with perfusion scintigraphy could be performed to assess functional lung volume, which could be calculated using a specific cutoff value of −910 HU. 28 However, in our study, there were few eligible patients with poor preoperative pulmonary function. Therefore, differences between measurement modalities might not have affected the results. Third, we used the modality of estimating the volumes of resected subsegments to calculate predicted function. Hemodynamics may play a key role in pulmonary function preservation. Ideally, perfusion scintigraphy is essential for the work‐up of patients, but we considered that frequent filming could be a burden on them and thus perfusion scintigraphy was not performed. Therefore, we measured the segmental volume using the distribution of the pulmonary artery and the regional segment should reduce the environmental bias. The final limitations are the single‐institutional design of the study and the small number of patients. Additional prospective, large‐scale, multi‐institutional studies are warranted to confirm our results. However, we believe that our clinical study data is of value because it is a regional comparative study of the LUL only. Previous studies referring to PPPF after segmentectomy only provided data on other pulmonary lobes.
In conclusion, thoracoscopic segmentectomy is useful for postoperative pulmonary preservation. Even a relatively large‐volume resection procedure that resects many subsegments, such as a resection of the left upper division or a resection of the lingula plus anterior segments, can preserve pulmonary function. However, in crisis prevention for PPPF, we should pay further consideration to the postoperative course avoiding complications such as chronic inflammation.
CONFLICT OF INTEREST
All authors declare there are no competing interests and funding.
Kuroda H, Sakata S, Takahashi Y, et al. Subsegmental resection preserves regional pulmonary function: A focus on thoracoscopy. Thorac Cancer. 2021;12:1033–1040. 10.1111/1759-7714.13841
REFERENCES
- 1. Fan J, Wang L, Jiang GN, Gao W. Sublobectomy versus lobectomy for stage I non‐small‐cell lung cancer, a meta‐analysis of published studies. Ann Surg Oncol. 2012;19(2):661–8. [DOI] [PubMed] [Google Scholar]
- 2. Bao F, Ye P, Yang Y, Wang L, Zhang C, Lv X, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta‐analysis. Eur J Cardiothorac Surg. 2014;46(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3. Nomori H, Shiraishi A, Cong Y, Sugimura H, Mishima S. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg. 2017;53:640–7. 10.1093/ejcts/ezx357. [DOI] [PubMed] [Google Scholar]
- 4. Kaseda S, Aoki T, Hangai N, Shimizu K. Better pulmonary function and prognosis with video‐assisted thoracic surgery than with thoracotomy. Ann Thorac Surg. 2000;70(5):1644–6. [DOI] [PubMed] [Google Scholar]
- 5. Kouritas VK, Kefaloyannis E, Tcherveniakov P, Milton R, Chaudhuri N, Brunelli A, et al. Do pleural adhesions influence the outcome of patients undergoing major lung resection? Interact Cardiovasc Thorac Surg. 2017;25(4):613–9. [DOI] [PubMed] [Google Scholar]
- 6. Ikeda N, Yoshimura A, Hagiwara M, Akata S, Saji H. Three‐dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg. 2013;19(1):1–5. [DOI] [PubMed] [Google Scholar]
- 7. Mimae T, Morito O. Are segmentectomy and lobectomy comparable in terms of curative intent for early stage non‐small cell lung cancer? Gen Thorac Cardiovasc Surg. 2020;68(7):703–6. 10.1007/s11748-019-01219-y. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki K, Watanabe S, Mizusawa J, Moriya Y, Yoshino I, Tsuboi M, et al. Predictors of non‐neoplastic lesions in lung tumours showing ground‐glass opacity on thin‐section computed tomography based on a multi‐institutional prospective studydagger. Interact Cardiovasc Thorac Surg. 2015;21:218–23. [DOI] [PubMed] [Google Scholar]
- 9. Kuroda H, Mori S, Tanaka H, Yoshida T, Mizuno T, Sakakura N, et al. Prognostic significance of combined radiologic imaging modalities for prognosis of clinical IA adenocarcinomas. Oncotarget. 2017;9:10745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakakura N, Inaba Y, Yatabe Y, Mizuno T, Kuroda H, Yoshimura K, et al. Estimation of the pathological invasive size of pulmonary adenocarcinoma using high‐resolution computed tomography of the chest: a consideration based on lung and mediastinal window settings. Lung Cancer. 2016;95:51–6. 10.1016/j.lungcan.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 11. Sakao Y, Kuroda H, Mun M, Uehara H, Motoi N, Ishikawa Y, et al. Prognostic significance of tumor size of small lung adenocarcinomas evaluated with mediastinal window settings on computed tomography. PLoS One. 2014;9(11):e110305. 10.1371/journal.pone.0110305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuroda H, Sakao Y, Mun M, Uehara H, Nakao M, Matsuura Y, et al. Lymph node metastases and prognosis in left upper division non‐small cell lung cancers: the impact of interlobar lymph node metastasis. PLoS One. 2015;10(8):e0134674. 10.1371/journal.pone.0134674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolliger CT, Jordan P, Solèr M, Stulz P, Tamm M, Wyser C, et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J. 1996;9(3):415–21. [DOI] [PubMed] [Google Scholar]
- 14. Deng B, Cassivi SD, de Andrade M, Nichols FC, Trastek VF, Wang Y, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg. 2014;148(4):1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kashiwabara K, Sasaki J, Mori T, Nomori H, Fujii K, Kohrogi H. Relationship between functional preservation after segmentectomy and volume‐reduction effects after lobectomy in stage I non‐small cell lung cancer patients with emphysema. J Thorac Oncol. 2009;4(9):1111–6. [DOI] [PubMed] [Google Scholar]
- 16. Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg. 2005;80(6):2041–5. [DOI] [PubMed] [Google Scholar]
- 17. Kim SJ, Lee YJ, Park JS, Cho YJ, Cho S, Yoon HI, et al. Changes in pulmonary function in lung cancer patients after video‐assisted thoracic surgery. Ann Thorac Surg. 2015;99(1):210–7. [DOI] [PubMed] [Google Scholar]
- 18. Nomori H, Cong Y, Sugimura H. Systemic and regional pulmonary function after segmentectomy. J Thorac Cardiovasc Surg. 2016;152(3):747–53. [DOI] [PubMed] [Google Scholar]
- 19. Keenan RJ, Landreneau RJ, Maley RH Jr, Singh D, Macherey R, Bartley S, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78(1):228–33; discussion 228‐33. [DOI] [PubMed] [Google Scholar]
- 20. Yoshimoto K, Nomori H, Mori T, Kobayashi H, Ohba Y, Shibata H, et al. Prediction of pulmonary function after lung lobectomy by subsegments counting, computed tomography, single photon emission computed tomography and computed tomography: a comparative study. Eur J Cardiothorac Surg. 2009;35(3):408–13. [DOI] [PubMed] [Google Scholar]
- 21. Kuroda H, Mizuno H, Dejima H, Watanabe K, Yoshida T, Naito Y, et al. A retrospective study on analgesic requirements for thoracoscopic surgery postoperative pain. J Pain Res. 2017;10:2643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Thoracic Society . American Thoracic Society Workshop Document. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170(3):319–43. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki H, Morimoto J, Mizobuchi T, Fujiwara T, Nagato K, Nakajima T, et al. Does segmentectomy really preserve the pulmonary function better than lobectomy for patients with early‐stage lung cancer? Surg Today. 2017;47(4):463–9. [DOI] [PubMed] [Google Scholar]
- 24. Paone G, Rose GD, Giudice C, Cappelli S. Physiology of pleural space after pulmonary resection. J Xiangya Med. 2018;3:10. 10.21037/jxym.2018.03.01. [DOI] [Google Scholar]
- 25. Wallace WAH, Fitch PM, Simpson AJ, Howie SEM. Inflammation‐associated remodelling and fibrosis in the lung ‐ a process and an end point. Int J Exp Pathol. 2007;88(2):103–10. 10.1111/j.1365-2613.2006.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakao Y, Nakazono T, Sakuragi T, Natsuaki M, Itoh T. Predictive factors for survival in surgically resected clinical IA peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2004;77:1157–62. [DOI] [PubMed] [Google Scholar]
- 27. Kuroda H, Nakada T, Oya Y, Takahashi Y, Shirai S, Matsui T, et al. Computed tomography and positron emission tomography‐staged cN0 non‐small cell lung cancer. Video‐assist Thorac Surg. 2020;5:14. 10.21037/vats.2020.04.01. [DOI] [Google Scholar]
- 28. Wu MT, Pan HB, Chiang AA, Hsu HK, Chang HC, Peng NJ, et al. Prediction of postoperative lung function in patients with lung cancer: comparison of quantitative CT with perfusion scintigraphy. AJR Am J Roentgenol. 2002;178(3):667–72. [DOI] [PubMed] [Google Scholar]


