Abstract
Background
The aim of this study was to investigate the long‐term outcome of superior vena cava (SVC) replacement after chemotherapy or chemoradiotherapy for advanced thymoma.
Methods
The medical information of patients with advanced thymoma who underwent thymoma resection and SVC replacement in Beijing Tongren Hospital from 2002 to 2017 were reviewed. We compared surgical outcomes, postoperative complications and long‐term prognosis in the chemoradiotherapy + surgery group (CRT + surgery group, 19 cases) and the surgery group (26 cases).
Results
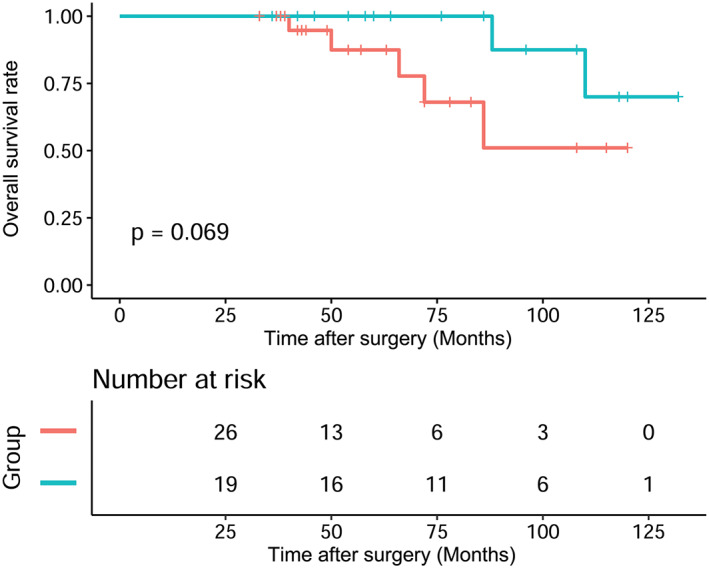
The operation time (486.05 ± 148.01 vs. 370.77 ± 124.32 min; p = 0.007) and intraoperative blood loss (1400 ml [IQR 1125–2105 ml] vs. 855 ml [IQR 555–1682.5 ml], p = 0.036), poor wound healing (three cases [15.79%] vs. zero cases [0.0%], p = 0.036) in the CRT + surgery group were significantly higher than those of the surgery group. There was no significant difference between the CRT + surgery group and the surgery group in postoperative chest tube drainage time, hospitalization time, postoperative arrhythmia and incidence of pneumonia. Kaplan Meier analysis showed that the recurrence‐free survival (RFS) curves of the CRT + surgery group patients were better than those of the surgery group (p = 0.031). However, overall survival (OS) between the two groups was not significantly different (p = 0.069).
Conclusions
Thymoma resection and SVC replacement is feasible for patients undergoing preoperative induction chemotherapy or chemoradiotherapy for advanced thymoma. Although patients in the CRT + surgery group had a longer operation time and increased intraoperative bleeding, the RFS rate seemed to be better than that in the surgery group.
Keywords: advanced thymoma, chemoradiotherapy, chemotherapy, superior vena cava replacement
Thymoma resection and SVC replacement is feasible for patients undergoing preoperative induction chemotherapy or chemoradiotherapy for advanced thymoma
INTRODUCTION
Thymic epithelial tumors are the most common neoplasms in the anterior mediastinal region. 1 Symptoms in more than 80% of patients with advanced thymic tumors may be due to compression or direct invasion of contiguous structures, or may be associated with paraneoplastic syndrome. 2 Invasion to mediastinal structures is one of the clinical features of advanced thymic tumors. Because of its aggressive behavior, thymic tumors may invade adjacent organs or large vessels, such as the superior vena cava (SVC), innominate veins and pericardium, etc. 3 In recent years, several reports have described surgical resection with SVC reconstruction for the treatment of advanced thymic tumors. 4 , 5 However, the prognosis of the initial surgery for advanced thymic tumors invading the SVC is still not satisfactory. 6 , 7 Meanwhile, if surgery is not indicated, the outcomes of multidisciplinary treatment including definitive chemoradiotherapy (CRT) also remain suboptimal. 8 , 9 , 10 , 11 Therefore, the optimal treatment of patients with advanced thymic tumors invading the SVC remains controversial, and new therapeutic strategies are urgently required.
Radiotherapy is expected to prevent cancer cell microresidues at local sites, whereas chemotherapy has the potential to eliminate micrometastases, thus facilitating complete resection. 12 , 13 , 14 Therefore, salvage surgery after definitive chemoradiotherapy is sometimes performed to control local disease by experienced surgical institutions. 3 , 15 It is for this reason that surgical cases after neoadjuvant chemoradiotherapy treatment for advanced thymic tumors may not be rare. 16 , 17 However, preoperative chemoradiotherapy may cause immune impairment and prolonged wound healing. 13 , 18 , 19 , 20 Thus, this retrospective study investigated details of the clinical course of patients with advanced thymic tumors undergoing SVC replacement surgery after neoadjuvant chemoradiotherapy, together with long‐term prognosis.
METHODS
Patients
From January 2002 to December 2017, a total of 767 consecutive patients underwent surgical resection for thymic epithelial tumors at Beijing Tongren Hospital. A total of 49 patients with thymoma infiltrating the SVC underwent thymoma resection, 45 of which were reconstructed with Polytetrafluoroethylene (PTFE) interposition graft and four were reconstructed with autologous pericardial patch. The inclusion criteria were as follows: (i) pathological confirmation of thymoma obtained from a biopsy sample, (ii) preoperative imaging showed tumor invasion of SVC, (iii) no surgery contraindications, and (iv) complete basic information available. Eligible patients for the study were classified into two groups according to the initial treatment: surgery group (patients who underwent primary tumor resection) and CRT + surgery group (patients who received chemotherapy alone or chemoradiotherapy followed by surgery). The stage of the disease for each patient was determined by at least two pathologists according to the TNM classification (eighth edition) and the Masaoka staging system for thymic epithelial tumors. This retrospective cohort study was reviewed and approved by the Ethics Committee of Beijing Tongren Hospital of Capital Medical University. Data on patient demographics, tumor size, invasion status, pathological results, surgical data, treatment protocols, graft size, postoperative graft patency and method of SVC reconstruction were recorded using the hospitals patient database system. Tumor extension and stage of disease was evaluated using contrast‐enhanced thoracic computed tomography (CT) scans (layer thick 0.625 mm), brain magnetic resonance imaging (MRI) or CT, radionuclide bone scan, and in more recent patients, 18‐fluoro‐2‐deoxyglucose (18‐FDG) positron emission tomography (PET). Routine preoperative assessments of cardiopulmonary function include pulmonary function tests, arterial blood gas analyses, electrocardiography and echocardiography. Patients with suspected tumor infiltration into the trachea or possibly involving the bronchial tree typically undergo fiberoptic bronchoscopy (FB). CT‐guided fine‐needle aspiration biopsy (FNAB) or thoracoscopic biopsy are generally used to obtain the preoperative histological diagnosis of tumors.
Chemoradiotherapy, surgery, and adjuvant treatment
Patients in the CRT + surgery group underwent two‐dimensional (2D) chest radiotherapy treatment, and the tumor and tumor bed were irradiated. Generally, the total radiation dose was 40 Gy. Preoperative chemotherapy based on cisplatin or carboplatin was two cycles, and the final number of chemotherapy cycles was determined by the attending surgeon according to the response and adverse effects. After chemotherapy alone or chemoradiotherapy, the response of patients to the treatment was routinely evaluated based on CT scans. Patients without progressive disease underwent surgery within two months after the end of the induction treatment.
All cases underwent median sternotomy incision surgery with double‐lumen endotracheal intubation. Central venous pressure (CVP) was monitored via catheter placed in the femoral vein after induction. The type of resection and vascular reconstructive was based on the localization and depth of tumor invasion during the operation. When the tumor invaded the SVC and less than 25% of the vascular circumference was involved, tangential placement of a partial occlusion clamp was performed, part of the SVC was removed, and then Prolene sutures were used for continuous anastomosis. An autologous pericardial patch repair was routinely used if tumor invasion was less than 50% of the circumference of the SVC. Tumors infiltrating and encompassing more than 50% of the SVC needed to be transplanted with a ringed polytetrafluoroethylene (PTFE) graft after circumferential resection of the SVC. Reconstruction methods include grafting from the right innominate vein to the SVC, from the left innominate vein to the SVC, from the distal SVC to the proximal SVC, and from each innominate vein to the SVC with a Y‐shaped graft (Figure 1). The cross‐clamping technique was used to reconstruct the vascular conduit during the operation without cardiopulmonary bypass. Heparin sodium (0.5 mg/kg) was injected intravenously before clamping. Low molecular weight heparin was administrated on day 2 postoperatively and then transferred to long‐term oral anticoagulation therapy (more than 12 months) upon discharge. Prothrombin time/international normalized ratio (INR) should be within a range of 1.8 to 2.5. The intraoperative blood loss, operation time, duration of hospitalization, incidence of recurrences were compared between the two groups.
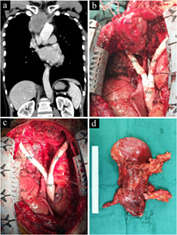
FIGURE 1.
Intraoperative view of a prosthetic superior vena cava (SVC) reconstruction with thymoma resection through median sternotomy. (a) Preoperative computed tomography scan showed the thymoma had invaded the left innominate vein, right innominate vein and SVC. (b) The Y‐shaped graft was used to reconstruct the SVC and both the innominate veins before thymoma resection to avoid complete blockage of the SVC during the operation. (c) The thymoma was resected en bloc. (d) Intact excised specimen shows both upper poles of the thymus. SVC, superior vena cava; LIV, left innominate vein; RIV, right innominate vein; RAA, right atrial appendage; RUL, right upper lobe; RLL, right lower lobe; PC, pericardium
Follow‐up and statistical analysis
Patients were followed up and examined by chest CT at one month after operation and every six months thereafter. Follow‐up time started at the date of surgery and ended at onset of tumor recurrence, complete graft blockage, death, or December 31, 2019, whichever occurred first. Recurrence after tumor resection was defined as locoregional recurrence, pleural dissemination, and distant metastases. The survival statuses of patients were checked during the outpatient review and telephone interview. Patients who have not been contacted by phone for at least one year after the last examination were considered lost to follow‐up. Overall survival (OS) refers to the time interval from the date of surgery to the date of death or the last visit. Recurrence‐free survival (RFS) refers to the time from surgery to local or distant recurrence of the tumor.
Mean values and standard deviations (SD) were reported for normally distributed data and numeric continuous data were expressed as the median, with interquartile range (IQR) (25th–75th percentile). Frequencies and proportions were reported for categorical data. Student's t‐test or Wilcoxon rank sum test was used to compare continuous variables between the groups, and Fisher's exact test or chi‐square test was used to compare categorical data. OS and RFS curves were estimated by Kaplan–Meier analysis. Statistical calculations were performed and verified by a professional statistician, data were analyzed with the use of the statistical packages R (The R Foundation, version 3.4.3) and Empower (R) (www.empowerstats.com, X&Y solutions). All the statistical tests were two‐sided, and the differences were considered to be statistically significant when the p‐value was <0.05.
RESULTS
Baseline characteristics
After applying the inclusion criteria, 45 patients with advanced thymoma invading the SVC were included in this study. There were 22 men (48.89%) and 23 women (51.11%). The median patient age was 55 years (range, 28–72 years). A total of 26 patients who underwent thymoma resection and SVC replacement were defined as the surgery group, and 19 patients who received chemotherapy or chemoradiotherapy before surgery were defined as the CRT + surgery group. The patient characteristics are summarized in Table 1. There was no significant difference in clinical features and pathological features between the two groups. Patients in the CRT + surgery group received preoperative chemotherapy alone (chemotherapy regimens given were cisplatin + doxorubicin + cyclophosphamide in three patients, cisplatin + epirubicin + ifosfamide in two patients, carboplatin + epirubicin + cyclophosphamide in one patients and carboplatin + epirubicin + etoposide in one patients), or chemoradiotherapy therapy (chemotherapy regimens given were cisplatin + doxorubicin + cyclophosphamide in six patients, cisplatin + epirubicin + ifosfamide in three patients, carboplatin + epirubicin + cyclophosphamide in three patients, and the radiation dose was 40 Gy in seven patients, 46 Gy in three patients, 54 Gy in one patient, and 60 Gy in one patient).
TABLE 1.
Demographic characteristics of the patients
| Characteristics | Total (n = 45) | Surgery (n = 26) | CRT + surgery (n = 19) | p‐value |
|---|---|---|---|---|
| Age (years) | 55.00 (47.00–60.00) | 53.00 (46.25–59.25) | 56.00 (48.50–60.00) | 0.578 |
| Gender | 0.167 | |||
| Male | 22 (48.89%) | 15 (57.69%) | 7 (36.84%) | |
| Female | 23 (51.11%) | 11 (42.31%) | 12 (63.16%) | |
| BMI (kg/m2) | 24.15 ± 3.22 | 24.85 ± 2.42 | 23.21 ± 3.94 | 0.091 |
| FEV1 (%) | 86.33 ± 7.11 | 87.13 ± 6.75 | 85.24 ± 7.63 | 0.385 |
| %FEV1 | 0.193 | |||
| <80 | 6 (13.33%) | 2 (7.69%) | 4 (21.05%) | |
| ≥80 | 39 (86.67%) | 24 (92.31%) | 15 (78.95%) | |
| Smoke | 0.363 | |||
| Never | 35 (77.78%) | 21 (80.77%) | 14 (73.68%) | |
| Current smokers | 4 (8.89%) | 3 (11.54%) | 1 (5.26%) | |
| Abstained for at least one year | 6 (13.33%) | 2 (7.69%) | 4 (21.05%) | |
| ASA grade | 0.091 | |||
| 1 | 27 (60.00%) | 13 (50.00%) | 14 (73.68%) | |
| 2 | 15 (33.33%) | 12 (46.15%) | 3 (15.79%) | |
| 3 | 3 (6.67%) | 1 (3.85%) | 2 (10.53%) | |
| Thymoma‐related syndrome | 0.290 | |||
| None | 33 (73.33%) | 18 (69.23%) | 15 (78.95%) | |
| Ocular myasthenia gravis | 6 (13.33%) | 5 (19.23%) | 1 (5.26%) | |
| Generalized myasthenia gravis | 4 (8.89%) | 1 (3.85%) | 3 (15.79%) | |
| Hypogammaglobulinemia (Good's syndrome) | 1 (2.22%) | 1 (3.85%) | 0 (0.00%) | |
| Pure red cell aplasia (PRCA) | 1 (2.22%) | 1 (3.85%) | 0 (0.00%) | |
| Duration of myasthenia (months) | 2.00 (0.00–6.00) | 3.00 (1.00–10.50) | 2.00 (0.00–6.00) | 0.648 |
| Tumor size based on preoperative radiological examination (cm) | 0.394 | |||
| <5 | 9 (20.00%) | 7 (26.92%) | 2 (10.53%) | |
| 5–10 | 25 (55.56%) | 13 (50.00%) | 12 (63.16%) | |
| >10 | 11 (24.44%) | 6 (23.08%) | 5 (26.32%) | |
| Main tumor location | 0.154 | |||
| Midline | 24 (53.33%) | 13 (50.00%) | 11 (57.89%) | |
| Left | 8 (17.78%) | 7 (26.92%) | 1 (5.26%) | |
| Right | 13 (28.89%) | 6 (23.08%) | 7 (36.84%) | |
| Preoperative Masaoka stage | 0.974 | |||
| IIIB | 24 (53.33%) | 14 (53.85%) | 10 (52.63%) | |
| IVA | 19 (42.22%) | 11 (42.31%) | 8 (42.11%) | |
| IVB | 2 (4.44%) | 1 (3.85%) | 1 (5.26%) | |
| Preoperative TNM stage | 0.367 | |||
| IIIA | 15 (33.33%) | 6 (23.08%) | 9 (47.37%) | |
| IIIB | 6 (13.33%) | 4 (15.38%) | 2 (10.53%) | |
| IVA | 22 (48.89%) | 15 (57.69%) | 7 (36.84%) | |
| IVB | 2 (4.44%) | 1 (3.85%) | 1 (5.26%) | |
| Site of tumor infiltration based on preoperative radiological examination | 0.250 | |||
| SVC + lung | 20 (44.44%) | 9 (34.62%) | 11 (57.89%) | |
| SVC + pulmonary artery trunk | 4 (8.89%) | 2 (7.69%) | 2 (10.53%) | |
| SVC + pericardium | 5 (11.11%) | 5 (19.23%) | 0 (0.00%) | |
| SVC + pleural dissemination | 14 (31.11%) | 9 (34.62%) | 5 (26.32%) | |
| SVC + pulmonary metastasis | 2 (4.44%) | 1 (3.85%) | 1 (5.26%) | |
| Radiation dose (Gy) | ‐ | |||
| ≤46 | ‐ | ‐ | 10 (22.22%) | |
| >46 | ‐ | ‐ | 2 (4.44%) | |
| Chemotherapy regimens | ‐ | |||
| cisplatin + doxorubicin + cyclophosphamide | 9 (20.00%) | |||
| cisplatin + epirubicin + ifosfamide | 5 (11.11%) | |||
| carboplatin + epirubicin + cyclophosphamide | 4 (8.89%) | |||
| carboplatin + epirubicin + etoposide | 1 (2.22%) |
Categoric data are expressed as number (%) and continuous data as mean ± SD or median (interquartile range). Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; FEV1, forced expiratory volume in 1 second; SVC, superior vena cava.
Surgical outcomes and pathological stage
The surgical outcomes are shown in Table 2. Although the median longest diameter of the tumor in CRT + surgery group after preoperative chemotherapy or chemoradiotherapy seemed to be smaller than that in surgery group (5.5 vs. 7 cm), there were no significant differences in tumor size and postoperative pathological staging between the two groups. The operation time (486.05 ± 148.01 vs. 370.77 ± 124.32 min; p = 0.007) and intraoperative blood loss (1400 ml [IQR 1125–2105 ml] vs. 855 ml [IQR 555–1682.5 ml], p = 0.036) in the CRT + surgery group were significantly higher than those of the surgery group. Among the 26 patients in the surgery group, four patients had incomplete tumor resection, and two patients had pleural dissemination. One of the 19 patients in the CRT + surgery group had incomplete tumor resection, the surgical margin was positive on microscopic examination, and the residual tumor site was the pulmonary artery trunk. There was no significant difference in complete resection rate between the two groups (p = 0.286).
TABLE 2.
Surgical outcomes and pathological stage
| Variables | Surgery (n = 26) | CRT + surgery (n = 19) | p‐value |
|---|---|---|---|
| Tumor size | |||
| Longest diameter primary lesion (cm) | 7.00 (4.62–9.38) | 5.50 (5.00–8.40) | 0.347 |
| Second dimension primary lesion (cm) | 6.00 (3.50–7.85) | 4.50 (3.40–6.25) | 0.266 |
| Third dimension primary lesion (cm) | 4.05 (2.50–5.88) | 3.50 (2.85–5.00) | 0.526 |
| Masaoka‐Koga stage | 0.830 | ||
| IIIB | 16 (61.54%) | 13 (68.42%) | |
| IVA | 9 (34.62%) | 5 (26.32%) | |
| IVB | 1 (3.85%) | 1 (5.26%) | |
| Primary tumor | 0.216 | ||
| T3 | 24 (92.31%) | 19 (100.00%) | |
| T4 | 2 (7.69%) | 0 (0.00%) | |
| Regional lymph nodes | 0.073 | ||
| N0 | 22 (84.62%) | 19 (100.00%) | |
| N1 | 4 (15.38%) | 0 (0.00%) | |
| Distant metastasis | 0.384 | ||
| M0 | 10 (38.46%) | 11 (57.89%) | |
| M1a | 15 (57.69%) | 7 (36.84%) | |
| M1b | 1 (3.85%) | 1 (5.26%) | |
| TNM stage | 0.317 | ||
| IIIA | 9 (34.62%) | 11 (57.89%) | |
| IIIB | 2 (7.69%) | 0 (0.00%) | |
| IVA | 14 (53.85%) | 7 (36.84%) | |
| IVB | 1 (3.85%) | 1 (5.26%) | |
| WHO classification | 0.669 | ||
| AB | 3 (11.54%) | 1 (5.26%) | |
| B1 | 4 (15.38%) | 1 (5.26%) | |
| B2 | 6 (23.08%) | 4 (21.05%) | |
| B3 | 9 (34.62%) | 10 (52.63%) | |
| Combined thymoma | 4 (15.38%) | 3 (15.79%) | |
| Site of combined resection | 0.318 | ||
| Lobectomy | 3 (11.54%) | 2 (10.53%) | |
| Wedge resection | 6 (23.08%) | 10 (52.63%) | |
| Segmentectomy | 3 (11.54%) | 2 (10.53%) | |
| Partial pericardiectomy | 5 (19.23%) | 2 (10.53%) | |
| Resection of dissemination | 9 (34.62%) | 3 (15.79%) | |
| Mode of SVC reconstruction | 0.320 | ||
| Innominate – SVC (Y graft) | 13 (50.00%) | 11 (57.89%) | |
| Left innominate – SVC | 7 (26.92%) | 1 (5.26%) | |
| Right innominate – SVC | 2 (7.69%) | 4 (21.05%) | |
| Proximal SVC – distal SVC | 2 (7.69%) | 1 (5.26%) | |
| Autologous pericardial patch repair | 2 (7.69%) | 2 (10.53%) | |
| Operation time (min) | 370.77 ± 124.32 | 486.05 ± 148.01 | 0.007 |
| Intraoperative blood loss (ml) | 855.00 (555.00–1682.50) | 1400.00 (1125.00–2105.00) | 0.036 |
Categoric data are expressed as number (%) and continuous data as mean ± SD or median (interquartile range). Abbreviation: SVC, superior vena cava.
Postoperative complications
No perioperative mortality occurred. The characteristics and postoperative complications of the two groups are shown in Table 3. There was no significant difference between the CRT + surgery group and the surgery group in postoperative chest tube drainage time (8 days [IQR 5.5–11.5 days] vs. 7 days [IQR 4–8.75 days], p = 0.072) and hospitalization time (22 days [IQR 16.5–33 days] vs. 19.5 days [IQR 15–26.5 days], p = 0.101). Arrhythmia and pulmonary complications were the most common complications in the two groups. The incidence of pneumonia was similar in the two groups (five cases [26.32%] vs. four cases [15.38%], p = 0.461). Radiation pneumonitis occurred in four patients in the CRT + surgery group, all of which improved after steroid treatment. There were 13 cases (28.89%) of postoperative arrhythmia in the two groups, including six cases in the surgery group (three cases of atrial fibrillation, two cases of supraventricular tachycardia, one case of atrioventricular block) and seven cases in the CRT + surgery group (five cases of atrial fibrillation, one case of supraventricular tachycardia, one case of atrioventricular block). There was no significant difference between the two groups (seven cases [36.84%] vs. six cases [23.08%], p = 0.314). There were three cases of poor wound healing in the CRT + surgery group, which was significantly higher than that in the surgery group (three cases [15.79%] vs. zero cases [0.0%], p = 0.036). These three patients healed in 30 days, 32 days and 38 days respectively after local debridement and dressing change (Figure 2).
TABLE 3.
Postoperative course and complications
| Variables | Surgery (n = 26) | CRT + surgery (n = 19) | p‐value |
|---|---|---|---|
| Postoperative period of chest tube drainage (days) | 7.00 (4.00–8.75) | 8.00 (5.50–11.50) | 0.072 |
| Postoperative hospital stays (days) | 19.50 (15.00–26.50) | 22.00 (16.50–33.00) | 0.101 |
| Arrhythmia | 0.314 | ||
| No | 20 (76.92%) | 12 (63.16%) | |
| Yes | 6 (23.08%) | 7 (36.84%) | |
| Pneumonia | 0.461 | ||
| No | 22 (84.62%) | 14 (73.68%) | |
| Yes | 4 (15.38%) | 5 (26.32%) | |
| Surgical site infection | 0.036 | ||
| No | 26 (100.00%) | 16 (84.21%) | |
| Yes | 0 (0.00%) | 3 (15.79%) | |
| Radiation pneumonitis | 0.014 | ||
| No | 26 (100.00%) | 15 (78.95%) | |
| Yes | 0 (0.00%) | 4 (21.05%) | |
| Hoarseness | 0.636 | ||
| No | 22 (84.62%) | 17 (89.47%) | |
| Yes | 4 (15.38%) | 2 (10.53%) | |
| Myasthenic crisis | 0.820 | ||
| No | 25 (96.15%) | 18 (94.74%) | |
| Yes | 1 (3.85%) | 1 (5.26%) | |
| Postoperative bleeding | 0.375 | ||
| No | 25 (96.15%) | 17 (89.47%) | |
| Yes | 1 (3.85%) | 2 (10.53%) | |
| Empyema | 0.164 | ||
| No | 25 (96.15%) | 16 (84.21%) | |
| Yes | 1 (3.85%) | 3 (15.79%) | |
| Pulmonary thromboembolism | 0.820 | ||
| No | 25 (96.15%) | 18 (94.74%) | |
| Yes | 1 (3.85%) | 1 (5.26%) |
Categoric data are expressed as number (%) and continuous data as median (interquartile range).
FIGURE 2.
A 50‐year‐old male patient received a 46 Gy dose of radiotherapy prior to surgery. Poor wound healing occurred after the operation, and the incision healed after local debridement and dressing change. The patient's incision on the (a) 9th, (b) 15th, and (c) 32nd day after the operation
Recurrence
When the final data analysis was conducted in February 2018, the median follow‐up period for the surviving patients was 68 months, and ranged from 33 to 132 months. Disease recurrence included local recurrence, mediastinal lymph node metastasis, and pleural dissemination. Recurrence occurred in 12 patients. In the surgery group, there were two cases of local recurrence, six cases of pleural dissemination, and one case of mediastinal lymph node metastasis. In the CRT + surgery group, there was one case of local recurrence and two cases of pleural dissemination. The three‐, five‐, and 10‐year OS of all patients were 100%, 93.94%, and 60.81%, respectively, and the three‐, five‐, and 10‐year RFS rates were 90.94%, 81.31%, and 52.3%, respectively. Kaplan Meier analysis showed that the RFS (p = 0.031) survival curves of the CRT + surgery group patients were better than those of the surgery group (Figure 3). However, OS (p = 0.069) between the two groups was not significantly different (Figure 4).
FIGURE 3.
The Kaplan–Meier survival curve of overall survival (OS) rates for patients between the two groups ( ) Surgery, (
) Surgery, ( ) CRT + surgery
) CRT + surgery
FIGURE 4.
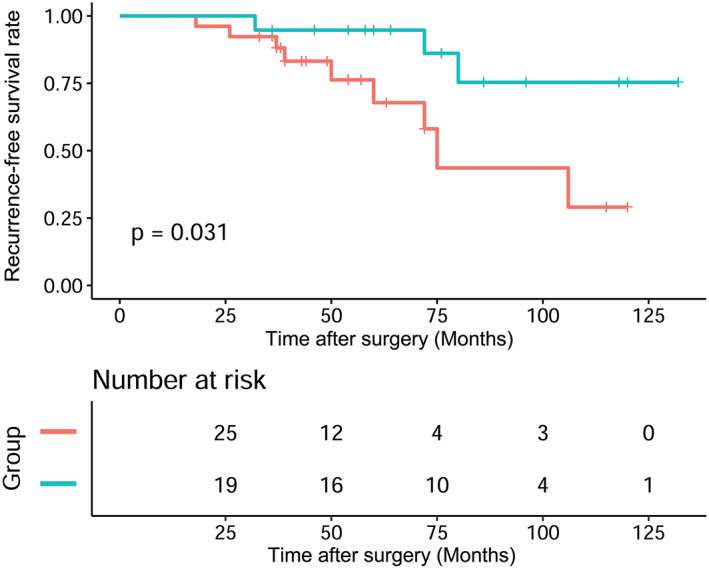
The Kaplan–Meier survival curve of recurrence‐free survival (RFS) rates for patients between the two groups ( ) Surgery, (
) Surgery, ( ) CRT + surgery
) CRT + surgery
DISCUSSION
Currently, complete surgical resection is the main treatment for patients with stage I, II and IIIA thymoma, 15 , 21 but the treatment of patients with advanced thymoma that invades the mediastinal large vessels, pericardium, or pleural dissemination is more complicated, and is still controversial. 22 Some studies have shown that patients with advanced thymoma, in whom preoperative examination confirms that resection of their tumor is not achievable, should be treated with induction chemoradiotherapy to improve the feasibility of complete surgical resection. 7 , 9 , 14 , 18 , 20 However, studies on the therapeutic effect of preoperative induction chemoradiotherapy for patients with advanced thymoma invading the SVC is still limited, 3 , 6 , 21 , 23 and whether preoperative induction therapy will affect the patency of vascular grafts is a concern for many clinicians. 16 , 24 , 25 , 26 Extensive resection of malignant thymic tumors combined with reconstruction of SVC and left innominate vein have been reported to be beneficial, 16 , 25 , 26 but there are few reports on induction chemoradiotherapy before thymoma resection and SVC replacement and reconstruction surgery. To our knowledge, this is the first study of the efficacy and prognosis of preoperative induction chemoradiotherapy for SVC replacement in advanced thymoma.
The results of the present study show that for patients with thymoma with large blood vessel invasion, surgery can be safely performed after chemoradiotherapy. Most of the characteristics of the surgery group and the CRT + surgery group were similar, although Kaplan Meier analysis showed that the CRT + surgery group had a higher RFS rate than that in the surgery group. Maurizi et al. reported that the SVC resection and large vessel reconstruction are feasible and safe and even after neoadjuvant chemotherapy, radical resection of locally advanced thymic tumors can be performed. 16 Although there was no significant statistical difference (p = 0.318), the number of patients in the surgery group (nine cases, 34.62%) who underwent resection of disseminations in this study appeared to be more than the CRT + surgery group (three cases, 15.79%). Generally, if pleural metastasis is found before surgery, induction chemotherapy is often performed first. In fact, some small pleural metastasis nodules are often detected intraoperatively. It is difficult to assess whether there are micrometastases on the pleura through chest CT scan before surgery, especially when patients do not undergo a PET‐CT scan in the early stage of this study. The five‐ and 10‐year overall survival rates of the two groups in this study were 93.94% and 60.81%, respectively and the long‐term prognosis was good. We found that the complete resection rate was 88.89%. This result is better than some previous reports for several reasons. 27 First, our study only focused on thymoma and did not include patients with thymic cancer. There is a huge difference between these two pathological entities. Thymoma is a more indolent, solid tumor than other malignancies, and patients with thymoma tend to live with long‐term disease‐free survival. 28 Second, radiotherapy and chemotherapy can reduce tumor microinvasion and improve the surgical resection rate. 1 , 15 Our results showed that the number of T4, N1, and M1a in the CRT + surgery group seemed to be lower than that in surgery group. These factors may influence the prognosis of the patients. We speculate that this may be due to the fact that patients have received chemotherapy or chemoradiotherapy prior to surgery, and the micrometastases formed through blood flow or pleural dissemination can be treated at an early stage, which may lead to a decline in tumor stage. It has been previously reported that in patients with thymoma undergoing surgery after chemotherapy or chemoradiotherapy, a necrotic pathological response has been observed in patients who received chemotherapy, with a fibrotic response seen in patients receiving chemoradiotherapy, 13 which confirms this viewpoint to some extent. Third, in experienced surgical centers, the invasion of large blood vessels by tumors will increase the difficulty of surgical resection of advanced thymoma, but it is not the primary factor limiting complete tumor resection (artificial blood vessel replacement technology is mature and can be considered). The reports in the literature suggest that when mediastinal masses infiltrate SVC pathologically, the necessity of SVC reconstruction should not be regarded as a contraindication to surgical resection, which is consistent with our point of view. 28 , 29 Yamada et al. reported that tumor invasion of the great vessels is not an unfavorable prognostic factor in thymoma patients, and invasion of the chest wall is the only adverse independent risk factor for disease‐free survival, and suggests that postoperative radiotherapy is an effective treatment strategy for thymoma patients who cannot be completely resected or have aggressive disease. 28 The complete tumor resection rates of patients in the CRT + surgery group and surgery group were 94.74% and 84.62%, respectively. There was no significant statistical difference in the complete tumor resection rates between the two groups, which may have been because the sample size was not large enough. However, it seems to indicate that preoperative induction radiotherapy and chemotherapy can minimize the volume of solid tumors and increase the resectable rate, which is consistent with previous reports. 13 , 16 , 28 , 29 , 30 , 31
The operative time and intraoperative blood loss in the CRT + surgery group were significantly longer than that in the surgery group, which may be due to the formation of connective tissue around the thymoma by chemoradiotherapy, making it more difficult to separate the tumors. 6 Apart from the higher incidence of poor wound healing in the CRT + surgery group than in the surgery group, there was no significant difference in postoperative complications between the two groups. As is widely known, chemoradiotherapy, especially radiotherapy, has a negative impact effect on wound healing. It has been previously reported that surgery in the radioactive tissue area increases the incidence of postoperative wound complications. 32 Sternotomy in patients with sternal irradiation may impair wound healing, leading to wound infection and postoperative mediastinitis, 32 and is consistent with our findings. The reason for the high incidence of arrhythmia in both groups may be related to the extensive dissociation of the perivascular tissue (including cardiac plexus) and routine pericardiotomy during the surgical reconstruction of SVC.
Due to the retrospective nature of this study, the preoperative radiotherapy and chemotherapy regimens are not uniform, and the effect of different chemotherapy drugs with different doses, radiotherapy doses and the duration of the radiotherapy and chemotherapy on the prognosis of thymoma patients has not been discussed. Further studies are needed to explore the optimal procedure for preoperative chemoradiotherapy. Moreover, since this was a retrospective study of an experience in a single institution, patient and treatment selection biases could not be ruled out. Second, the number of patients was limited with a slight marginal imbalance between the two groups. A multicenter study should be conducted to clarify the importance of preoperative treatment for advanced thymoma. Third, some patients may receive postoperative chemoradiotherapy according to intraoperative conditions and postoperative pathology.
In conclusion, in this study with regard to surgical outcome, postoperative complications, recurrence rate and patient survival rate, we provide evidence that for advanced thymoma with SVC invasion, thymoma resection and SVC replacement can still be performed after induction chemotherapy or chemoradiotherapy. Although patients in the CRT + surgery group in our study had longer operation time and increased intraoperative bleeding, the recurrence‐free survival rate appeared to be better than that in the surgery group.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
ACKNOWLEDGMENTS
This work was financially supported by the Clinical Medicine Development of Special Funding Support from Beijing Municipal Administration of Hospitals (XMLX201839). The study sponsors were not involved in the study design, collection, management, analysis, interpretation of data, the writing of the manuscript, and in the decision to submit it for publication.
Yu Z, Yu L, Yu T, Yang X‐g, Zhang B‐x, Du X. Surgical feasibility and long‐term outcome of superior vena cava replacement for advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy. Thorac Cancer. 2021;12:1074–1083. 10.1111/1759-7714.13872
Funding information Beijing Municipal Administration of Hospitals, Grant/Award Number: XMLX201839
REFERENCES
- 1. Lim YJ, Kim HJ, Wu H. Role of postoperative radiotherapy in nonlocalized thymoma. J Thorac Oncol. 2015;10:1357–63. [DOI] [PubMed] [Google Scholar]
- 2. D'Andrilli A, Venuta F, Rendina EA. Surgical approaches for invasive tumors of the anterior mediastinum. Thorac Surg Clin. 2010;20:265–84. [DOI] [PubMed] [Google Scholar]
- 3. Maurizi G, D Andrilli A, Vanni C, Ciccone AM, Ibrahim M, Andreeti C, et al. Salvage resection of advanced mediastinal tumors. J Thorac Dis. 2019;11:S1653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang S, Tan D, Wu W, He B, Jing T, Tang M, et al. Extracorporeal membrane oxygenation (ECMO) assisted mediastinal tumor resection and superior vena cava replacement are safe and feasible. Thorac Cancer. 2019;10:1846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen W, Cao Y, Wang X, Zhang P, Zhou Q. Invasive thymoma with intravascular growth into the great veins and right atrium: a case report. Thorac Cancer. 2020;11:1326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandri A, Cusumano G, Lococo F, Alifano M, Granone P, Margaritora S, et al. Long‐term results after treatment for recurrent thymoma: a multicenter analysis. J Thorac Oncol. 2014;9:1796–804. [DOI] [PubMed] [Google Scholar]
- 7. Kim S, Bull DA, Hsu C, Hsu CC. The role of adjuvant therapy in advanced thymic carcinoma: a National Cancer Database Analysis. Ann Thorac Surg. 2020;109:1095–103. [DOI] [PubMed] [Google Scholar]
- 8. Rimner A, Gomez DR, Wu AJ, Shi W, Yorke ED, Moreira AL, et al. Failure patterns relative to radiation treatment fields for stage II–IV thymoma. J Thorac Oncol. 2014;9:403–9. [DOI] [PubMed] [Google Scholar]
- 9. Merveilleux Du Vignaux C, Dansin E, Mhanna L, Greillier L, Pichon E, Kerjouan M, et al. Systemic therapy in advanced thymic epithelial tumors: insights from the RYTHMIC prospective cohort. J Thorac Oncol. 2018;13:1762–70. [DOI] [PubMed] [Google Scholar]
- 10. Boothe D, Orton A, Thorpe C, Kokeny K, Hitchcock YJ. Postoperative radiotherapy in locally invasive malignancies of the thymus: patterns of care and survival. J Thorac Oncol. 2016;11:2218–26. [DOI] [PubMed] [Google Scholar]
- 11. Yu L, Zhang BX, Du X, Yu Z, Yang XG, Jiang YX. Evaluating the effectiveness of chemotherapy for thymic epithelial tumors using the CD‐DST method. Thorac Cancer. 2020;11:1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang X, Zhuo M, Shi A, Yang S, Wang Z, Wu M, et al. Optimal first‐line treatment for advanced thymic carcinoma. Thorac Cancer. 2019;10:2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chu RF, Hussien A, Li QK, Wang J, Friedes C, Ferro A, et al. Radiologic response of chemotherapy alone versus radiation and chemotherapy in the treatment of locally‐advanced or advanced thymic epithelial tumors. Thorac Cancer. 2020;11:2924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wright CD. Extended resections for thymic malignancies. J Thorac Oncol. 2010;5:S344–7. [DOI] [PubMed] [Google Scholar]
- 15. Kelly RJ, Petrini I, Rajan A, Wang Y, Giaccone G. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol. 2011;29:4820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maurizi G, Poggi C, D'Andrilli A, Vanni C, Ciccone AM, Ibrahim M, et al. Superior vena cava replacement for thymic malignancies. Ann Thorac Surg. 2019;107:386–92. [DOI] [PubMed] [Google Scholar]
- 17. Shintani Y, Funaki S, Ose N, Kanou T, Fukui E, Kimura K, et al. Surgical management of thymic epithelial tumors. Surg Today. 2020. 10.1007/s00595-020-02070-y. [DOI] [PubMed] [Google Scholar]
- 18. Fukuda M, Funaki S, Yamazaki T, Sato S, Mukae H, Takenoyama M, et al. S‐1 plus cisplatin with concurrent radiotherapy for locally advanced thymic carcinoma: study protocol of LOGIK1605/JART‐1501. Thorac Cancer. 2020;11:693–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okuma Y, Ko R, Shukuya T, Tateishi K, Imai H, Iwasawa S, et al. Prognostic factors for patients with metastatic or recurrent thymic carcinoma receiving palliative‐intent chemotherapy. Lung Cancer. 2020;148:122–8. [DOI] [PubMed] [Google Scholar]
- 20. Berghmans T, Durieux V, Holbrechts S, Jungels C, Lafitte JJ, Meert AP, et al. Systemic treatments for thymoma and thymic carcinoma: a systematic review. Lung Cancer. 2018;126:25–31. [DOI] [PubMed] [Google Scholar]
- 21. Ruffini E, Detterbeck F, Van Raemdonck D, Rocco G, Thomas P, Weder W, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol. 2014;9:541–8. [DOI] [PubMed] [Google Scholar]
- 22. Shepherd A, Riely G, Detterbeck F, Simone CB II, Ahmad U, Huang J, et al. Thymic carcinoma management patterns among international thymic malignancy interest group (ITMIG) physicians with consensus from the thymic carcinoma working group. J Thorac Oncol. 2017;12:745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu H, Gu Z, Qiu B, Detterbeck FC, Roden AC, Ruffini E, et al. A recurrence predictive model for thymic tumors and its implication for postoperative management: a Chinese Alliance for research in thymomas database study. J Thorac Oncol. 2020;15:448–56. [DOI] [PubMed] [Google Scholar]
- 24. Shintani Y, Ohta M, Minami M, Shiono H, Hirabayashi H, Inoue M, et al. Long‐term graft patency after replacement of the brachiocephalic veins combined with resection of mediastinal tumors. J Thorac Cardiovasc Surg. 2005;129:809–12. [DOI] [PubMed] [Google Scholar]
- 25. Picquet J, Blin V, Dussaussoy C, Jousset Y, Papon X, Enon B. Surgical reconstruction of the superior vena cava system: indications and results. Surgery. 2009;145:93–9. [DOI] [PubMed] [Google Scholar]
- 26. Lanuti M, De Delva PE, Gaissert HA, Wright CD, Wain JC, Allan JS, et al. Review of superior vena cava resection in the management of benign disease and pulmonary or mediastinal malignancies. Ann Thorac Surg. 2009;88:392–7. [DOI] [PubMed] [Google Scholar]
- 27. Okumura M, Miyoshi S, Takeuchi Y, Yoon HE, Minami M, Takeda SI, et al. Results of surgical treatment of thymomas with special reference to the involved organs. J Thorac Cardiovasc Surg. 1999;117:605–13. [DOI] [PubMed] [Google Scholar]
- 28. Yamada Y, Yoshino I, Nakajima J, Miyoshi S, Ohnuki T, Suzuki M, et al. Surgical outcomes of patients with stage III thymoma in the Japanese Nationwide database. Ann Thorac Surg. 2015;100:961–7. [DOI] [PubMed] [Google Scholar]
- 29. Khorfan R, Bharat A, Odell DD. Management and long‐term outcomes of advanced stage thymoma in the United States. Ann Thorac Surg. 2020;111:223–30. 10.1016/j.athoracsur.2020.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bretti S, Berruti A, Loddo C, Sperone P, Casadio C, Tessa M, et al. Multimodal management of stages III–IVa malignant thymoma. Lung Cancer. 2004;44:69–77. [DOI] [PubMed] [Google Scholar]
- 31. Kanzaki R, Kanou T, Ose N, Funaki S, Shintani Y, Minami M, et al. Long‐term outcomes of advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy followed by surgery: a 20‐year experience. Interact Cardiovasc Thorac Surg. 2019;28:360–7. [DOI] [PubMed] [Google Scholar]
- 32. Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–36. [DOI] [PubMed] [Google Scholar]


