Abstract
Background
Differences in the resistance mechanisms of epidermal growth factor receptor tyrosine kinase inhibitors in patients with non‐small cell lung cancer (NSCLC) harboring epidermal growth factor receptor mutations are unknown. This meta‐analysis aimed to clarify the differences in resistance mechanisms after treatment with various epidermal growth factor receptor tyrosine kinase inhibitors.
Methods
We systematically searched PubMed, Cochrane, and Web of Science on July 29, 2020, for relevant studies on acquired resistance mechanisms against epidermal growth factor receptor tyrosine kinase inhibitors. The primary outcome measure was differences in the resistance mechanism between individual or generations of epidermal growth factor receptor tyrosine kinase inhibitors.
Results
In total, 33 trials involving 2418 individuals were included and analyzed. T790M was significantly less frequent after afatinib treatment (40.2%, 95% confidence interval [CI]: 31.7%–48.7%) than after gefitinib and erlotinib treatments (52.5%, 95% CI: 48.7%–56.3%, p = 0.005). There were no significant differences between Asian and non‐Asian patients in the incidence of T790M after gefitinib, erlotinib, and afatinib treatments. Regarding epidermal growth factor receptor pathway‐independent resistant mechanisms, the incidences of small cell lung cancer transformation (osimertinib: 7.9%, 95% CI: 3.6%–12.2%, others: 2.3%, 95% CI: 0.8%–3.8%) and Kirsten rat sarcoma (KRAS) viral oncogene homolog mutation (osimertinib: 4.6%, 95% CI: 1.5%–7.7%, others: 0.2%, 95% CI: 0.0%–1.7%) were significantly higher following osimertinib treatment than with others.
Conclusions
Significant differences in the incidence of resistance mechanisms among epidermal growth factor receptor tyrosine kinase inhibitors exist, which should be taken into consideration when choosing the treatment strategy.
Keywords: epidermal growth factor receptor tyrosine kinase inhibitors, NSCLC, resistance mechanisms, T790M
This meta‐analysis was conducted to clarify the differences in resistance mechanisms after treatment with various EGFR‐TKIs. A total of 2418 patients from 33 trials were included in our meta‐analysis. Our study revealed significant differences in the incidence of resistance mechanisms among EGFR‐TKIs.

INTRODUCTION
Epidermal growth factor receptor (EGFR) mutation is the most prevalent driver oncogene mutation in lung carcinoma and is detected in almost half of all untreated non‐small cell lung cancer (NSCLC) patients in Asia. 1 EGFR tyrosine kinase inhibitors (TKIs) have been established as standard first‐line therapy for EGFR‐mutant NSCLC patients owing to their superiority over conventional cytotoxic chemotherapy. 2 , 3 , 4 Osimertinib is a third‐generation EGFR‐TKI that inhibits both major EGFR‐activating and Thyr790Met (T790M) resistance mutations. 5 Based on the AURA3 phase III clinical trial results, osimertinib has been approved for the treatment of NSCLC patients with T790M resistance mutations upon disease progression after previous EGFR‐TKI therapy. This clinical trial showed that osimertinib was superior to standard cytotoxic chemotherapy with respect to the objective response rate, progression‐free survival (PFS), and tolerability in patients with T790M‐mediated acquired resistance. 6 Furthermore, osimertinib has also been approved as a first‐line treatment for NSCLC patients harboring EGFR exon 19 deletions or L858R mutations based on clinical evidence of a direct comparison with first‐generation EGFR‐TKIs. 7 When comparing first‐ and second‐generation TKIs, the second‐generation TKI, dacomitinib, yields better PFS and overall survival (OS) than the first‐generation TKI, gefitinib. 8 However, no clinical trial to date has compared second‐generation TKIs with osimertinib in the first‐line setting.
Despite their survival benefit, most patients treated with EGFR‐TKIs develop acquired resistance within two years. EGFR T790M‐acquired mutations are the most frequent resistance mechanism after treatment with first‐ or second‐generation EGFR‐TKIs. 9 , 10 However, increasing evidence supports that there are several acquired resistance mechanisms after treatment with Osimertinib. 11 Unfortunately, there is limited information regarding the difference in resistance mechanisms with regard to EGFR‐TKI treatment. Clarifying this difference may influence the treatment strategy for patients with activating EGFR mutations, possibly leading to the selection of better treatment options. Thus, this meta‐analysis aimed to clarify the difference in resistance mechanisms among EGFR‐TKIs and compare these differences between Asian and non‐Asian populations.
METHODS
Ethics
The need for institutional review board approval and patient consent for this study was waived because it was a review. The systematic review and meta‐analysis was performed according to the Meta‐analysis of Observational Studies in Epidemiology guidelines (Table S1). 12 The study protocol was included on the University Hospital Medical Information Network Clinical Trials Registry (UMIN000040759). 13
Study overview and search strategy
We systematically searched PubMed, the Cochrane Database, and Web of Science on July 29, 2020, for relevant studies on acquired resistance mechanisms against EGFR‐TKIs. The search strategies are presented in Table S2. Gefitinib and erlotinib, afatinib and dacomitinib, and osimertinib were defined as first‐, second‐, and third‐generation EGFR‐TKIs, respectively. Two investigators independently screened the titles and abstracts and scrutinized the full text. The reference list of all included articles was also manually checked to further identify other relevant studies. Papers that involved repeated participation of the same patient in multiple and/or duplicated studies were excluded. If conflicts arose between the review authors during the selection process, the inconsistencies were discussed, and a consensus was reached.
Study selection
Design
The inclusion criteria were as follows: (i) availability of data for the number of acquired resistance mechanisms against EGFR‐TKIs: EGFR T790M mutation, EGFR C797S mutation, MET amplification, Kirsten rat sarcoma (KRAS) viral oncogene homolog mutation, phosphatidylinositol‐4,5‐bisphosphate 3‐kinase catalytic subunit alpha (PIK3CA) mutation, and transformation to small cell lung cancer (SCLC); and (ii) written in English as a full article or a brief report regardless of its primary endpoint.
Patients
Patients with a pathologically or cytologically confirmed NSCLC diagnosis who relapsed after EGFR‐TKI therapy and were examined for resistance mechanisms were included. There was no restriction based on age, sex, smoking history, clinical staging, performance status, and NSCLC pathological subtype. Patients treated with one of the following EGFR‐TKIs were eligible: gefitinib, erlotinib, afatinib, dacomitinib, or osimertinib. Patients treated with combination chemotherapy of two or more EGFR‐TKIs, EGFR‐TKI, and any cytotoxic agent, EGFR‐TKI and antivascular endothelial growth factor receptor agents, or EGFR‐TKI and immune checkpoint inhibitors were excluded because such treatments may have influenced the resistance mechanism.
Data extraction
Data regarding the study characteristics, type of EGFR‐TKI used for the patient, incidences of detected resistant mechanisms, and risk of bias were independently extracted by two review investigators. Inconsistencies were discussed to reach a consensus.
Assessment of study quality
The Newcastle–Ottawa scale was used to evaluate the study quality. The scores ranged from 0–9, with 9 points suggesting the best study quality. The included studies were assessed according to their methodological quality for patient selection, comparability, and outcome. Quality evaluation was independently conducted by two investigators. Disagreements among them were resolved through discussion to reach a consensus.
Outcome measures
The outcomes of this meta‐analysis were the incidence of acquired resistance mechanisms, EGFR T790M mutations, EGFR C797S mutations, MET amplification, transformation to SCLC, PIK3CA mutations, and KRAS mutations. The primary outcome measure was the difference in resistance mechanisms among EGFR‐TKIs or generations of EGFR‐TKIs. The secondary outcome measure was the difference in the resistance mechanism between Asian and non‐Asian patients.
Statistical analysis
We used the random‐model generic inverse variance method. 14 Preceding the meta‐analysis, the standard error was estimated using the Agrestia‐Coull method, as we could not obtain standard error for outcomes with a prevalence of 0% through the commonly used method (standard error = standard deviation/square root of n). 15 Heterogeneity among studies was quantified using the I2 statistic, with an I2 value of 0% set to indicate no heterogeneity and higher values signifying increasing heterogeneity. In particular, heterogeneity was interpreted as follows: I2 = 0% to 40%: may not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity. 16 Random‐effect model meta‐analysis was performed using Reviewing Manager software, ver. 5.4 (Cochrane Collaboration). 17
RESULTS
Study characteristics
Among the 6746 articles initially reviewed, 33 studies that revealed resistance mechanisms after EGFR‐TKI treatment in patients with advanced lung cancer were selected for full review (Figure 1, Table S3). Among the 33 studies, 17 were reported from Asian countries. The list of studies included in this meta‐analysis is shown in Table S1. Most of the included studies were reported after 2016. In total, 13, 12, nine, and eight studies investigated acquired resistance mechanisms after treatment with erlotinib, gefitinib, afatinib, and osimertinib, respectively. The median Newcastle–Ottawa scale score of the 33 studies was 8 points (range, 5–9), indicating good quality (Table S4).
FIGURE 1.
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flowchart showing the selection process of published articles
Incidence of EGFR T790M mutations
T790M was significantly less frequent with afatinib treatment (40.2%, 95% confidence interval [CI]: 31.7%–48.7%, I2 = 66%) than with gefitinib and erlotinib treatments (52.5%, 95% CI: 48.7%–56.3%, I2 = 51%, p for heterogeneity = 0.005) (Figure 2(a)). However, T790M was significantly less frequent with osimertinib treatment (41.6%, 95% CI: 34.5%–48.7%, I2 = 66%) than with gefitinib and erlotinib treatments (Figure 2(b)). There was no significant difference in resistance mechanisms between afatinib and osimertinib treatments (Figure S1A) or between gefitinib and erlotinib treatments (Figure S1B). All cases were confirmed to have T790M before osimertinib treatment. C757S, a major secondary EGFR mutation resistant to osimertinib, was seen in 21.5% (95% CI: 17.2%–25.9%, I2 = 0%) of patients who developed progressive disease after osimertinib treatment (Figure 3). Heterogeneity analysis using the I2 statistic indicated that the highest variation was observed with afatinib and osimertinib treatments.
FIGURE 2.
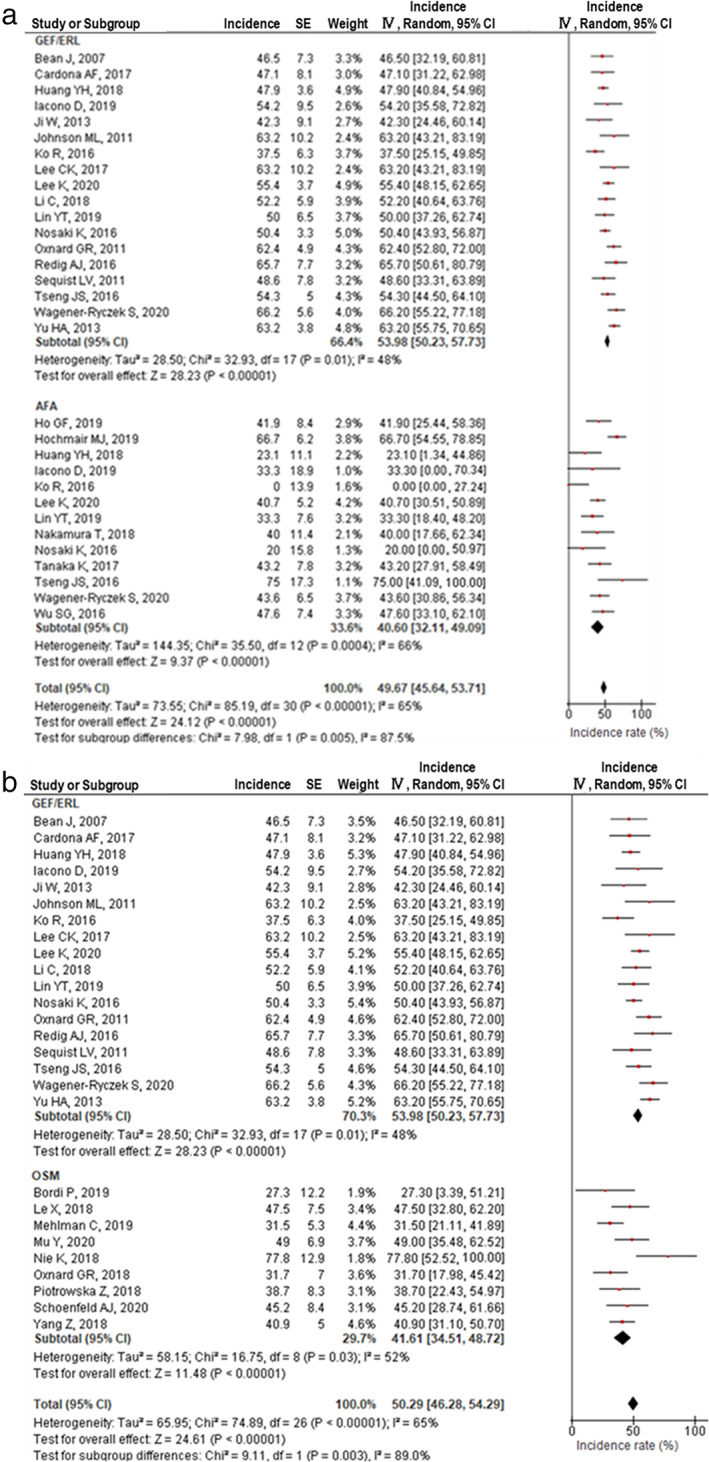
EGFR T790M mutation incidence among patients treated with EGFR‐TKIs. (a) First‐generation EGFR‐TKIs (gefitinib and erlotinib) versus afatinib. (b) First‐generation EGFR‐TKIs (gefitinib and erlotinib) vs. osimertinib
FIGURE 3.
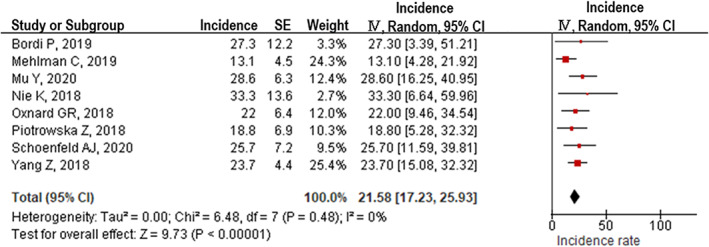
EGFR C797S mutation detected in patients who developed acquired resistance to osimertinib
Differences in the incidence of T790M acquired mutations between Asian and non‐Asian populations
The included studies were divided into two groups based on patient information. The incidence of T790M mutations after gefitinib or erlotinib treatment was 50.7% (95% CI: 47.3%–54.1%, I2 = 11%) among Asian and 52.8% (95% CI: 45.4%–60.2%, I2 = 55%) among non‐Asian patients (Figure 4(a)). However, the incidence of T790M mutations after afatinib was 35.4% (95% CI: 24.5%–46.2%, I2 = 60%) among Asian and 39.3% (95% CI: 27.4%–51.2%, I2 = 49%) among non‐Asian patients (Figure 4(b)). There were no significant differences between Asian and non‐Asian patients in the incidence of T790M mutation after gefitinib, erlotinib, and afatinib treatments.
FIGURE 4.
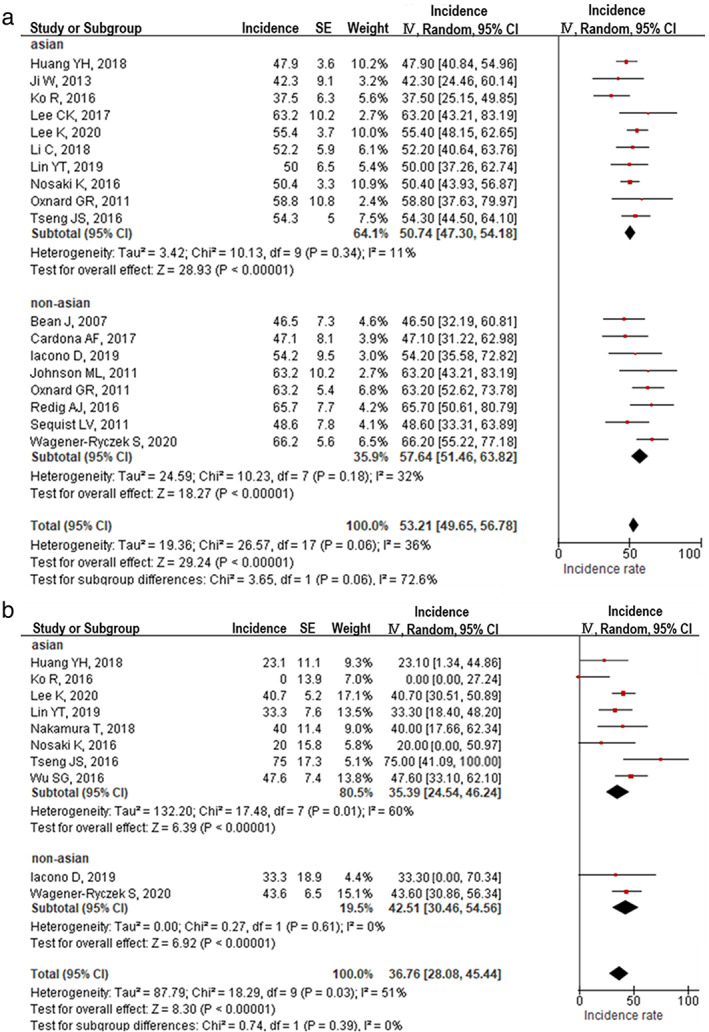
EGFR T790M mutation incidence in Asian and non‐Asian patients who developed acquired resistance to EGFR‐TKIs. (a) T790M incidence after first‐generation EGFR‐TKI treatment (gefitinib or erlotinib). (b) T790M incidence after afatinib treatment
Other resistance mechanisms against EGFR‐TKIs through EGFR‐independent signaling pathways
The incidence of other resistant mechanisms through EGFR‐independent signaling pathways was compared between patients treated with osimertinib and those treated with first‐ or second‐generation EGFR‐TKIs (gefitinib, erlotinib, or afatinib) (Figure 5). SCLC transformation was significantly more frequent with osimertinib treatment (7.9%, 95% CI: 3.6%–12.2%) than with the other TKIs (2.3%, 95% CI: 0.8%–3.8%, p = 0.02, Figure 5(a)). KRAS mutations were also significantly more frequent with osimertinib treatment (4.6%, 95% CI: 1.5%–7.7%) than with the other TKIs (0.2%, 95% CI: 0.0%–1.7%, p = 0.01, Figure 5(b)). However, there were no significant differences in the incidence of MET amplification (Figure 5(c)) and PIK3CA mutations (Figure 5(d)) among the EGFR‐TKIs.
FIGURE 5.
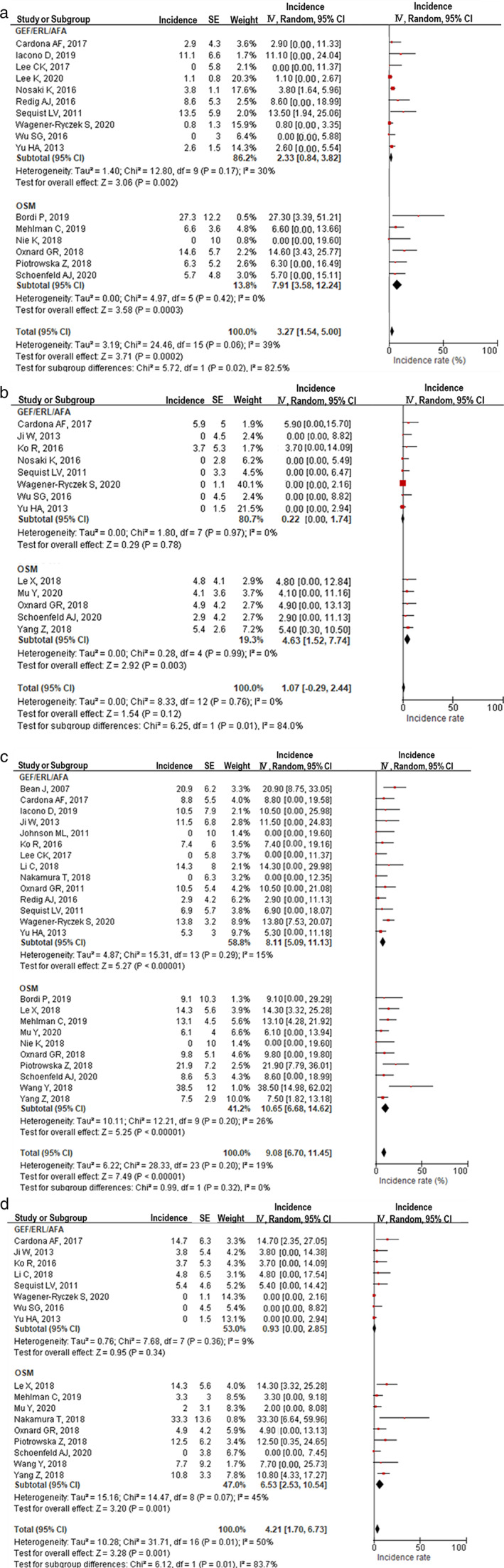
EGFR pathway‐independent resistance mechanisms in patients who developed acquired resistance to EGFR‐TKIs. (a) Incidence of small cell lung cancer transformation between gefitinib/erlotinib/afatinib and osimertinib. (b) KRAS mutation incidence between gefitinib/erlotinib/afatinib and osimertinib. (c) MET amplification incidence between gefitinib/erlotinib/afatinib and osimertinib. (d) PIK3A mutation incidence between gefitinib/erlotinib/afatinib and osimertinib
DISCUSSION
Evidence on the difference in resistance mechanisms with regard to EGFR‐TKI treatment is scarce. In this study, T790M mutations were significantly less frequent in patients who had disease progression after treatment with afatinib than in those treated with first‐generation EGFR‐TKIs. After osimertinib treatment, T790M disappeared in 58.4% of patients (Figure 2(b)), whereas C757S was detected in 20.8% of patients. There was no significant difference between Asian and non‐Asian patients in the incidence of T790M after treatment with first‐ or second‐generation TKI. SCLC transformations and KRAS mutations were more frequent after treatment with osimertinib than after treatment with other TKIs. To the best of our knowledge, this is the first meta‐analysis on the resistance mechanisms involved in various generations of EGFR‐TKI treatments among patients with EGFR mutations.
No phase 3 trial has directly compared second‐generation EGFR‐TKIs with osimertinib treatment. Therefore, we investigated the optimal EGFR‐TKI for first‐line treatment that would result in longer OS. Osimertinib as first‐line treatment was reportedly superior to the first‐generation EGFR‐TKIs with respect to PFS (osimertinib: 18 months, first‐generation: 10 months) and OS (osimertinib: 38.6 months, first‐generation: 31.8 months). 7 However, the second‐generation EGFR‐TKI, dacomitinib, as first‐line treatment yielded a longer PFS of 14.7 months 8 than osimertinib as second‐line treatment (10.7 months). 18 Therefore, treatment with second‐generation EGFR‐TKIs as the first‐line treatment, followed by osimertinib as the second‐line treatment after a successful detection of T790M, could confer better PFS than first‐line osimertinib treatment. To prove this concept, a retrospective observational study clarified the utility of afatinib as a first‐line treatment, followed by osimertinib. 19 The combined PFS was 28.7 months, as expected. However, this was only observed in patients proven to have a T790M mutation in the EGFR gene, using specimens obtained during progression after treatment with second‐generation TKIs. In contrast, our data revealed that T790M was significantly less frequent after afatinib treatment than after treatment using first‐generation TKIs (Figure 2(a)). This implies that fewer patients may obtain this ideal PFS associated with the sequential therapy of afatinib followed by osimertinib.
Conventional chemotherapy is currently commonly adopted after first‐line osimertinib treatment, which is expected to have a limited effect on extending survival. 20 Our data revealed that T790M disappeared in almost half of the patients treated with osimertinib (Figure 2(b)). Moreover, C757S, an acquired mutation to osimertinib that was observed in one of five patients, could be treated with first‐ or second‐generation TKIs. These data suggest that first‐line osimertinib treatment followed by previous‐generation EGFR‐TKIs could also be effective for specific patients. Several clinical trials exploring the efficacy of first‐ or second‐generation EGFR‐TKIs after osimertinib treatment have already been conducted. 21 , 22 Clinical practice may have already changed based on the results of these trials.
In the FLAURA trial, a randomized clinical trial that compared osimertinib and first‐generation EGFR‐TKIs as first‐line treatment, the OS was similar in both treatment arms of the Asian population. 7 Our meta‐analysis results indicate that there was no significant difference in the incidence of T790M between the Asian and non‐Asian populations. C757S was another possible mechanism that could explain the lesser efficacy of osimertinib in Asian patients. Unfortunately, we could not examine the difference in the incidence of C757S among various racial groups because of the limited number of studies on this topic. We speculate that the C757S mutation could be more frequent in Asian patients than in non‐Asian patients and could, therefore, limit the efficacy of osimertinib.
Our data also provide novel insights into the mechanism of osimertinib treatment. The incidence of T790M after osimertinib treatment was only 41.6%. Oxnard et al. revealed that alterations in the EGFR‐independent signaling pathway, such as an acquired abnormality in other EGFR genes including KRAS, MET amplification, and PIK3CA mutation, or the transformation to SCLC, were more frequent in patients whose T790M mutations disappeared. 11 In our comparison of the incidence of the EGFR‐independent mechanisms between osimertinib and other TKIs, KRAS mutations and SCLC transformations were significantly more frequent with osimertinib treatment than with treatment using first‐ and second‐generation EGFR‐TKIs. Moreover, the incidence of KRAS mutations was almost 10‐fold higher with osimertinib treatment than with other TKIs. Although there were no significant differences in the incidence of MET amplification and PIK3CA mutations among TKIs, both mutations were more frequent after osimertinib treatment than after treatment with other TKIs. Therefore, identifying acquired resistance mechanisms is more beneficial for patients treated with osimertinib. This will allow for a more specific and effective treatment, such as KRAS inhibitors (e.g., AMG510, and MRTX489) for patients with KRAS mutations, 23 combination therapy with osimertinib plus savolitinib 24 or crizotinib 25 for patients with MET amplification, and pictilisib or PX‐866 for patients with PIK3CA mutations. 26 , 27 Our data support that the fact that resistance mechanisms in patients who acquire resistance to osimertinib should be examined to improve prognosis through specific treatment.
The major reasons for the differences in resistance mechanisms among TKIs remain unknown. Regarding the incidence of T790M after first‐generation TKI or afatinib, Byung et al. revealed that afatinib could inhibit the growth of gefitinib‐resistant cancer cells with low T790M allele frequencies. 28 This effect of afatinib is the possible reason for the lower incidence of T790M after resistance than in first‐generation TKIs. Afatinib is unique in its multi‐inhibitory activity targeting the pan‐HER family, including EGFR, HER2, ErbB3, and ErbB4 compared to other TKIs. 29 This difference might affect other differences in resistance mechanisms.
Our study has some limitations. First, the detection methods for analyzing the various resistance mechanisms varied. The difference may have influenced the results of the incidence of resistance mechanisms. Second, while analyzing the differences between the Asian and non‐Asian populations, some reports could not be included because of the lack of information on race. Finally, the resistance mechanism against osimertinib in the first‐line setting is unknown because there are no reports in this setting to date.
In conclusion, there are significant differences in the incidence of resistance mechanisms among EGFR‐TKIs. These findings provide new insights into the difference in resistance mechanisms among EGFR‐TKIs, as well as the influence of the therapeutic sequence to be chosen. Our data suggest that resistance mechanisms should be identified to pursue a more specific treatment for patients with acquired resistance to osimertinib.
CONFLICT OF INTERST
The authors have no conflicts of interest to declare.
Supporting information
Table S1 MOOSE checklist
Table S2: Search strategies
Table S3: Studies included in the meta‐analysis
Table S4: Assessment of the study quality
Figure S1: Incidence of EGFR T790M mutations detected in patients who developed acquired resistance to gefitinib, erlotinib, afatinib, or osimertinib.
ACKNOWLEDGMENTS
We would like to thank Editage (http://www.editage.com) for English language editing.
Kobayashi N, Katakura S, Kamimaki C, et al. Resistance mechanisms of epidermal growth factor receptor tyrosine kinase inhibitors in non‐small cell lung cancer patients: A meta‐analysis. Thorac Cancer. 2021;12:1096–1105. 10.1111/1759-7714.13878
REFERENCES
- 1. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai C‐M, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- 3. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- 4. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- 5. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376:1993–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- 8. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first‐line treatment for patients with EGFR‐mutation‐positive non‐small‐cell lung cancer (ARCHER 1050): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2017;18:1454–66. [DOI] [PubMed] [Google Scholar]
- 9. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res. 2013;19:2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee K, Kim Y, Jung HA, Lee SH, Ahn JS, Ahn MJ, et al. Repeat biopsy procedures and T790M rates after afatinib, gefitinib, or erlotinib therapy in patients with lung cancer. Lung Cancer. 2019;130:87–92. [DOI] [PubMed] [Google Scholar]
- 11. Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M‐positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4:1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 13. UMIN . https://www.umin.ac.jp/. Accessed 29 July 2020.
- 14. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training . https://training.cochrane.org/handbook/current. Accessed 29 July 2020.
- 15. Agresti A, Coull BA. Approximate is better than "exact" for interval estimation of binomial proportions. Am Stat. 1998;52:119–26. [Google Scholar]
- 16.Chapter 10: Analysing data and undertaking meta‐analyses | Cochrane Training. https://training.cochrane.org/handbook/current/chapter-10#section-10-10. Accessed 29 July 2020
- 17. RevMan|Cochrane Training . https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. Accessed 29 July 2020.
- 18. Mok TS, Wu Y‐L, Ahn M‐J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum‐Pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JCH, et al. Sequential afatinib and osimertinib in patients with EGFR mutation‐positive non‐small‐cell lung cancer: updated analysis of the observational GioTag study. Future Oncol. 2019;15:2905–14. [DOI] [PubMed] [Google Scholar]
- 20. Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. NCCN guidelines insights: non‐small cell lung cancer, version 1.2020. J Natl Compr Canc Netw. 2019;17:1464–72. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi N, Hashimoto H, Kamimaki C, Nagasawa R, Tanaka K, Kubo S, et al. Afatinib + bevacizumab combination therapy in EGFR‐mutant NSCLC patients with osimertinib resistance: protocol of an open‐label, phase II, multicenter, single‐arm trial. Thorac Cancer. 2020;11:2125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hata A, Katakami N, Kaji R, Yokoyama T, Kaneda T, Tamiya M, et al. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant non‐small cell lung cancer: multicenter, single‐arm, phase 2 trial (ABC study). Cancer. 2018;124:3830–8. [DOI] [PubMed] [Google Scholar]
- 23. Bar‐Sagi D, Knelson EH, Sequist LV. A bright future for KRAS inhibitors. Nat Cancer. 2020;1:25–7. [DOI] [PubMed] [Google Scholar]
- 24. Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H, Kim SW, et al. Osimertinib plus savolitinib in patients with EGFR mutation‐positive, MET‐amplified, non‐small‐cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open‐label, phase 1b study. Lancet Oncol. 2020;21:373–86. [DOI] [PubMed] [Google Scholar]
- 25. Katakura S, Kobayashi N, Somekawa K, Masumoto N, Kudo M, Kaneko T. Non‐small cell lung cancer with mesenchymal‐epithelial transition gene exon 14 skipping mutation treated with crizotinib. Respirol Case Rep. 2019;7:e00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soria JC, Adjei AA, Bahleda R, Besse B, Ferte C, Planchard D, et al. A phase IB dose‐escalation study of the safety and pharmacokinetics of pictilisib in combination with either paclitaxel and carboplatin (with or without bevacizumab) or pemetrexed and cisplatin (with or without bevacizumab) in patients with advanced non‐small cell lung cancer. Eur J Cancer. 2017;86:186–96. [DOI] [PubMed] [Google Scholar]
- 27. Levy B, Spira A, Becker D, Evans T, Schnadig I, Camidge DR, et al. A randomized, phase 2 trial of docetaxel with or without PX‐866, an irreversible oral phosphatidylinositol 3‐kinase inhibitor, in patients with relapsed or metastatic non‐small‐cell lung cancer. J Thorac Oncol. 2014;9:1031–5. [DOI] [PubMed] [Google Scholar]
- 28. Yoon BW, Kim JH, Lee SH, Choi CM, Rho JK, Yoon S, et al. Comparison of T790M acquisition between patients treated with afatinib and gefitinib as first‐line therapy: retrospective propensity score matching analysis. Transl Oncol. 2019;12:852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 MOOSE checklist
Table S2: Search strategies
Table S3: Studies included in the meta‐analysis
Table S4: Assessment of the study quality
Figure S1: Incidence of EGFR T790M mutations detected in patients who developed acquired resistance to gefitinib, erlotinib, afatinib, or osimertinib.


