Abstract
Background
ALK rearrangement is a very rare subset of squamous cell carcinoma (SCC) and one of the clinical features in patients is lack of data. Here, we report eight patients diagnosed with SCC of the lung harboring ALK rearrangement.
Methods
We collected primary NSCLC samples at the Beijing Chest Hospital between January 2012 and December 2018 for Ventana (D5F3) immunohistochemical detection. Among the 148 patients was diagnosed ALK‐rearranged non small cell lung cancer (NSCLC), only eight cases was SCC. We collected patients information from electronic patent records (EPRs).
Results
The eight cases of SCC were diagnosed by immunohistochemistry (IHC). Two were given crizotinib as second‐line therapy. One patient had stable disease (SD) and progression‐free survival (PFS) of six months. The other patient had progressive disease (PD) but PFS was only one month. The side effects were tolerable. This report identified 31 cases of ALK rearrangement in SCC patients from a literature search (including the eight patients in this study). These fusion genes are often seen in a younger age group (mean age: 55.6 years) and non‐smokers (18/31, 58.1%). A total of 20 cases received an ALK inhibitor as first‐ or second‐line treatment which included 11 with a partial response (PR), four with SD, and five with PD. The DCR and ORR was 75.0% (15/20) and 55.0% (11/20), respectively. The median duration time of therapy was 6.4 ± 4.4 months.
Conclusions
Patients with ALK‐rearranged SCC obtained clinical benefit from ALK‐inhibitor therapy, especially those who were non‐smokers and whose tumors had been identified by IHC+/FISH+.
Keywords: ALK inhibitor drug, ALK rearrangement, immunotherapy, PDL1, squamous cell carcinoma
Clinical benefit was obtained from ALK‐inhibitor treatment in ALK‐rearranged SCC especially in non‐smokers and patients whose tumors had been identified by IHC+/FISH+.
INTRODUCTION
Lung cancer has among the highest morbidity and mortality of all cancer types, and is responsible for the highest rate of cancer‐related mortality worldwide. 1 Primary lung cancer is mainly divided into two pathological types: small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC), which mainly include adenocarcinoma (ADC), squamous cell cancer (SCC) and other subtypes. 2 Treatment methods for lung cancer mainly include surgical resection, chemotherapy and molecular targeted therapy. 3
Numerous oncogenic driver mutations have been identified in patients with NSCLC. Echinoderm microtubule‐associated protein‐like 4 and anaplastic lymphoma kinase rearrangement fusion genes (EML4‐ALK) have been previously identified in approximately 5% of NSCLC patients, a population that consists mostly of adenocarcinoma patients. 4 These fusion genes are often seen in a younger age group of never or light ex‐smokers. Patients with SCC who harbor the ALK rearrangement are extremely rare (only 1%). 5
Targeted treatment of metastatic ALK‐rearranged non‐small cell lung cancer with ALK inhibitors leads to higher response rates and improves progression‐free survival (PFS) relative to conventional chemotherapy regimens. 6
However, only limited data on ALK rearrangement in SCC are available and we seldom have the opportunity to see those patients in daily clinical practice. Here, in this study, we report eight cases with ALK‐positive SCC together with a review of the literature.
METHODS
Clinical data collection
We collected primary NSCLC samples at the Beijing Chest Hospital between January 2012 and December 2018 for Ventana (D5F3) immunohistochemical detection. Among the 148 patients with pathologically diagnosed ALK‐rearranged NSCLC, overexpression of ALK protein occurred in eight patients with SCC, as confirmed by immunohistochemistry (IHC).
Treatment response evaluation and follow‐up
Patient treatment information was obtained from electronic patient records (EPRs). The tumor response in patients who received standard chemotherapy was assessed every two cycles. In the patients who received an ALK inhibitor, the tumor response in this group was assessed after the first cycle (every 28‐day cycle) of treatment and subsequently after every two cycles. Tumor responses were assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 7 During follow‐up, CT scans of the thorax and enhanced MRI of the brain were used to assess the disease. Responses to treatment were reported as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).
Regular IHC and Ventana IHC analysis
Immunohistochemistry analysis
There were five surgically resected samples and eight biopsy samples assessed by immunohistochemistry analysis including P40, P63, cytokeratin 5/6, CK7, thyroid transcription factor 1(TTF‐1), and napsin‐A. IHC revealed that the tumor cells showed diffuse staining and were positive for P40, P63, CK5/6, CK7, but were completely negative for thyroid transcription factor‐1 (TTF‐1) and napsin‐A. Although few cells (less than 5% of all) showed adenomatous differentiation, most had typical characteristics of SCC. Based on these findings, the results confirmed the histological type was SCC.
ALK immunohistochemistry analysis
Sections of formalin‐fixed and paraffin‐embedded (FFPE) tissue 4 μm thick were stained with the Ventana ALK (D5F3) rabbit monoclonal primary antibody, together with rabbit monoclonal negative control immunoglobulin, Optiview DAB IHC detection kit, and an Optiview amplification kit on a Ventana BenchMark ULTRA stainer (Ventana Medical Systems). This procedure was performed according to the manufacturer's instructions. 8 , 9
Immunoreactivity was evaluated as positive when the tumor (any percentage of positive tumor cells) showed intense granular cytoplasmic staining. The test results were evaluated using light microscopy. A binary method of interpretation was used as follows: strong granular cytoplasmic staining (any percentage) in the tumor cells was scored EML4‐ALK (+); otherwise, and they were scored EML4‐ALK (−).
Statistical analysis
The association between ALK rearrangement status and clinicopathological data was assessed using SPSS software, version 17.0 (SPSS, Chicago, IL, USA), and survival analysis was performed using Fisher's exact test and the Kaplan–Meier test. A two‐sided p‐value < 0.05 was considered statistically significant.
RESULTS
Clinicopathological features of ALK‐rearranged SCC (TABLE 1)
TABLE 1.
Clinical details of patients
| Age (y) | Sex | Method of diagnosis and/type of tissue sample | ALK detection | Stage | Smoking history pack‐year | Prior treatment | ALK inhibitor | Efficacy | PFS (m) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 54 | F | Bronchial biopsy | IHC | IV | Non‐smoker | PDC | Crizotinib | SD | 6.0 |
| Case 2 | 76 | M | Lung biopsy | IHC | IV | Smoker | PDC | None | ||
| Case 3 | 53 | M | Lung biopsy operation | IHC, | IIIa | Smoker | Adjunctive PDC | None | ||
| Case 4 | 35 | F | Lung biopsy cervical lymph node | IHC, | IV | Non‐smoker | PDC | Crizotinib | PD | 1.0(Dead) |
| Case 5 | 64 | M | Bronchial biopsy/surgery | IHC, | II | Smoker | None | None | ||
| Case 6 | 64 | M | Bronchial biopsy/surgery | IHC | IIIa | Smoker | Adjunctive PDC | None | ||
| Case 7 | 63 | M | Bronchial biopsy/surgery | IHC | I | Non‐smoker | PDC | None | ||
| Case 8 | 64 | M | Bronchial biopsy /surgery | IHC | IIIa | Smoker | Adjunctive PDC | None |
Abbreviations: F, female; FISH, fluorescence in situ hybridization; M, male; m, months; PD, progressive; PDC, platinum‐doublet chemotherapy; PFS, progression‐free survival; PR, partial response; RT‐PCR, reverse transcription polymerase chain reaction; SD, stable disease; y, year.
Two of the eight cases in this study were female and six were male. Five of eight patients with ALK‐rearranged SCC were ex‐ or current smokers. Pathological staging was I–IV and included one patient (stage I), one patient (stage II), three patients (stage III) and three patients (stage IV), respectively.
SCC was confirmed by typical histopathological features. All eight cases were positive for CK5/6, CK7, p63 and/or p40 expression, negative for TTF‐1 and napsin A. Ventana IHC (D5F3) confirmed the presence of ALK in all cases.
Five patients received surgery. Three of five cases (Cases 3, 6 and 8) received adjunctive chemotherapy and no recurrence was observed after regular follow‐up. Intrapulmonary metastasis recurred 14 months after surgery in Case 5 and the patient abandoned further treatment. There was a recurrence in Case 7 with local metastasis 25 months after surgery who subsequently received radiotherapy. Then, 10 months later, the patient suffered from intrapulmonary and bone metastasis and received first‐line chemotherapy with a combination of four cycles of gemcitabine and carboplatin to which there was no treatment response. The patient refused a further course of treatment for economic reasons.
Three patients had advanced NSCLC. Case 2 was a male aged 76 years. He was unable to afford the high cost of targeted therapy, and only accepted one cycle of chemotherapy before refusing subsequent treatment. The other two patients with ALK‐rearranged SCC had previously undergone standard chemotherapy. Two patients were treated with crizotinib and then switched regimens when they were diagnosed with PD during chemotherapy. One received a SD response; unfortunately, the second patient underwent crizotinib for only one month's duration and then died as a result of cancer progression.
Case 1: An ALK inhibitor benefit
A 54‐year‐old woman with no previous history of smoking was clinically diagnosed with NSCLC (cT2N2M1b, stage IV). A histopathological analysis of biopsied tumor tissue revealed SCC. IHC confirmed positivity for p40 and p63, negativity for TTF‐1 and napsin‐A (FIGURE 1); ALK protein was overexpressed which was confirmed via IHC ALK rearrangement. The patient received first‐line chemotherapy with four cycles of a combination of gemcitabine and carboplatin which did not suppress rapid tumor growth. The chemotherapy regimen was interrupted and replaced; the patient accepted crizotinib as second‐line treatment and showed SD but relapsed after six months.
FIGURE 1.
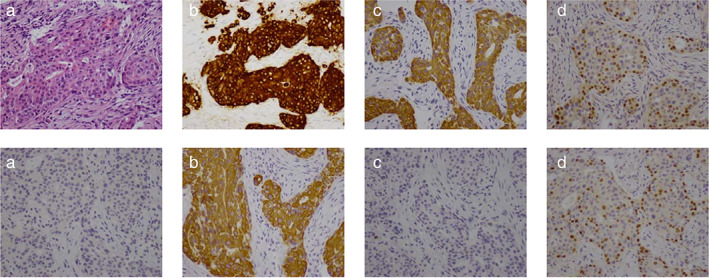
Immunobiological characteristics of case 1, Immunohistochemical analysis of lung cancer tissue. (a) Hematoxylin and eosin staining (200×); (b) ALK Ventana (DF53, 200×), (c) positive staining for CK56 (200×), (d) positive staining for P40(200×), (e) negative staining for TTF‐1 (200×), (f) positive staining for CK7(200×), (g) Naspin‐A (200×), (h) positive staining for P63 (200×)
Case 2: An ALK inhibitor nonresponder
Case 2 was a 35‐year‐old woman who was a non‐smoker. She was clinically diagnosed with NSCLC (cT4N3M1b, stage IV). IHC analysis of biopsied tumor tissue revealed positive CK7 and negative TTF‐1 and napsin‐A immunoreactivity, confirming a diagnosis of SCC (FIGURE 2). Carboplatin plus taxol was administered as first‐line chemotherapy. However, this regimen was discontinued following disease progression. Rebiopsy was performed and confirmed a diagnosis of SCC and IHC ALK rearrangement. The patient accepted crizotinib as second‐line treatment but there was no response and she subsequently died of disease after one month.
FIGURE 2.
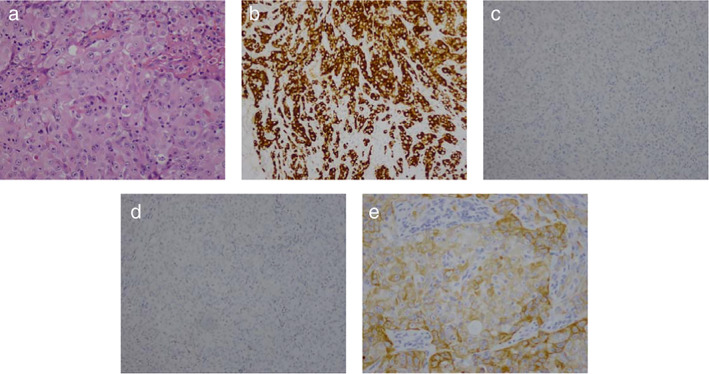
Immunohistological characteristics of case 2, Immunohistochemical analysis of lung cancer tissue. (a) Hematoxylin and eosin staining (200×); (b) ALK ventana (DF53, 200×), (c) negative staining for TTF‐1 (200×), (d) Naspin‐A (200×); (e) positive staining for CK56 (200×)
Descriptive analysis and qualitative synthesis
We identified 31 cases of ALK rearrangement in SCC patients from a literature search (TABLE 2). In this study, a total of 31 cases were included which includes the eight patients. There was a prevalence of female (15 females, 16 male), and never smoker patients (18 never smokers, 12 smokers). The average age of patients was 55.6 ± 13.3. The mean age of female patients was 49.6, younger than 61.1 of male. The pathological staging was I + II, III and IV in three (9.6%), 10 (32.3%; IIIA eight, IIIB two) and 18 (58.1%) patients, respectively. There were eight surgically resected samples and 31 biopsies. A total of 13 (41.9%) cases were recorded as focal positive and 18 (58.1%) cases with advanced disease. In 31 cases of SCC, 10 were identified as ALK‐positive only by Ventana IHC (D5F3), one case was by NGS, 20 cases were diagnosed as ALK‐positive by Ventana IHC (D5F3) and FISH, two cases were IHC (−)/FISH (+), two cases were IHC (+)/FISH (−), and 16 cases were IHC (+)/FISH (+).
TABLE 2.
Literature review of all clinical cases to date
| Author | Age (years) | Sex | Method of diagnosis and/or type of tissue sampled | ALK detection | Stage | Smoking history (pack‐year) | Prior treatment | ALK inhibitor | Efficacy | Duration of treatment (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Kimh et al. 2013 10 | 36 | F | Bronchial biopsy of the cervical lymph node | IHC+, FISH+ | IV | Non‐smoker | None | None | ||
| 2. Ochi et al. 2013 11 | 45 | F | Bronchial biopsy of cervical lymph node | IHC −, FISH + | IV | Smoker | None | None | ||
| 3. Alrifai et al. 2013 12 | 69 | M | Bronchial biopsy of cervical lymph node | IHC+, FISH+ | IIIa | Smoker | Radiation | None | None | |
| 4. Wang et al. 2014 13 | 55 | F | Bronchial biopsy of primary lesion | IHC+, FISH+ | IV | Non‐smoker | PDC | Crizotinib | PR | 5.8 |
| 5. Mikes et al. 2015 14 | 36 | M | Bronchial biopsy of primary lesion | IHC+, FISH+, RT‐PCR | IV | Non‐smoker | None | Crizotinib | PR | 3.0 (PR maintain |
| 6. Zhang et al. 2015 15 | 55 | F | Bronchial biopsy of primary lesion | IHC+ | IV | Non‐smoker | PDC | Crizotinib | PR | 6 |
| 7. Takanashi et al. 2015 16 | 60 | M | Bronchial biopsy of primary lesion | IHC+, FISH+, RT‐PCR+ | II | Smoker | Adjuvant | None | ||
| 8. Vergne et al. 2016 17 | 58 | F | Bronchial biopsy of primary lesion | IHC+, FISH+ | IV | Non‐smoker | None | Crizotinib | PR | 7.1 |
| 9. Tamiya et al. 2015 18 | 78 | M | Bronchial biopsy of primary lesion | IHC+, FISH+ | IV | Smoker | None | Alectinib | PD | 1.5 |
| 10. Wang et al. 2016 19 | 37 | F | Bronchial biopsy of primary lesion | IHC+ | IIIb | Non‐smoker | Radiation, PDC | Crizotinib | PR | 9 |
| 11. Yamamoto et al. 2016 20 | 76 | M | Bronchial biopsy of primary lesion | IHC−, FISH+ | IIIa | Non‐smoker | Radiation | None | ||
| 12. Mamesaya et al. 2017 21 | 52 | F | Bronchial biopsy of primary lesion | IHC+, FISH+ | IV | Non‐smoker | PDC | Alectinib | PR | 11 |
| 13. Bolzacchini et al. 2017 22 | 51 | M | Biopsy of primary lesion, surgery | IHC+, FISH+ | IIIa | Non‐smoker | PDC, | Crizotinib, Alectinib | PR | 14 |
| 14. Li et al. 2017 23 | 45 | F | Biopsy of primary lesion | IHC+, FISH+, NGS | IV | Non‐smoker | None | Crizotinib, | PR | 9 |
| 15. Sagawa et al. 2018 24 | 73 | M | Bronchial biopsy | IHC+, FISH+ | IV | Non‐smoker | None | Alectinib | PR | >9 |
| 16. Huang et al. 2018 25 | 50 | F | Lung biopsy | NGS+ | IV | Smoker | PDC | Crizotinib, Alectinib | PR/PR (QTprolong) | >4 |
| 17. Watanabe et al. 2018 26 Case 1 | 65 | F | Bronchial biopsy | IHC−; FIFH+ | IV | Smoker | PDC | Crizotinib, Alectinib | PD | 2/1 |
| Case 2 | 36 | M | Bronchial biopsy | IHC+, FISH+ | IIIb | Smoker | PDC | Crizotinib, Alectinib | PR | 12/5 |
| Case 3 | 62 | M | Bronchial biopsy | IHC−, FISH+ | IV | Smoker | PDC | Crizotinib | PD | 1 |
| 18. Wang et al. 2019 27 Case 1 | 63 | M | Bronchial biopsy | IHC+, FISH−, NGS+ | IV | Smoker | None | Crizotinib, | SD | 3 |
| Case 2 | 32 | F | Bronchial biopsy | IHC+, FISH−, NGS+ | IV | Non‐smoker | None | Crizotinib | PD | 4 |
| Case 3 | 53 | F | Bronchial biopsy surgery | IHC+, FISH+, NGS+ | IIIA | Non‐smoker | PDC | Crizotinib | SD | 9 |
| Case 4 | 73 | F | Bronchial biopsy | IHC+, FISH+, NGS+ | IV | Smoker | PDC | Crizotinib | SD | 10 |
Abbreviations: F, female; FISH, fluorescence in situ hybridization; M, male; PD, progressive disease; PDC, platinum‐doublet chemotherapy; PR, partial response; RT‐PCR, reverse transcription polymerase chain reaction; SD, stable disease.
Eight patients underwent tumor resection. Five received postoperative adjunctive chemotherapy and attended regular check‐ups for postoperative recurrence. After regular follow‐up appointments, two patients were found to have recurrent disease. One accepted crizotinib, resulting in SD of nine months duration, the other patient accepted alectinib resulting in PR for 14 months. 22 , 27
Three patients 12 , 19 , 20 diagnosed with stage III SCC underwent radiation therapy. After regular check‐ups, one patient was confirmed to have recurrent disease, and underwent treatment with crizotinib. This patient was considered to have obtained a PR with nine months.
This report includes all patients who were treated with at least one type of ALK‐TKI therapy and some patients had more than one treatment with different kinds of TKI. All the treatments administered for each lines of therapy were analyzed. A total of 20 patients received at least one ALK‐TKI (20 cases evaluable lines of treatment). There were 13 patients with ALK‐rearranged SCC who had previously undergone standard chemotherapy for SCC prior to treatment with crizotinib and switched regimens when ALK rearrangement was detected, when they had been diagnosed with progressive disease (PD) during chemotherapy (three cases received more than one kind of ALK‐TKI). Seven patients were treated as first‐line administration (one case was PR for three months without further information) (Table 3).
TABLE 3.
Qualitative synthesis of reported best response
| Npts | NPR | NSD | NPD | ORR (CR + PR%) | DCR (CR + PR + SD%) | Duration time of treatment | |
|---|---|---|---|---|---|---|---|
| Mean (month) | |||||||
| Total patients | 20 | 11 | 4 | 5 | 55.0% | 75.0% | 6.4 ± 4.4 |
| First‐line treatment | 7 | 4 | 1 | 2 | 57.1% | 71.4% | 4.5 ± 2.9 |
| Second‐line treatment | 13 | 7 | 3 | 3 | 53.8% | 76.9% | 7.4 ± 4.8 |
| Smoker | 7 | 2 | 2 | 3 | 28,6% | 57.6% | 5.6 ± 3.2 |
| Non‐smoker | 13 | 9 | 3 | 1 | 69.2% | 92.3% | 6.8 ± 3.6 |
| IHC +/ FISH + | 11 | 8 | 2 | 1 | 72.3% | 90.1% | 8.8 ± 4.4 |
| IHC or FISH + | 4 | 0 | 1 | 3 | 0 | 25.0% | 2.8 ± 1.2 |
Abbreviations: DCR, disease control rate; N, member; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Seven patients who smoked accepted ALK treatment (two received alectinib, five received crizotinib). A total of 13 non‐smokers accepted ALK treatment (four received alectinib and nine received crizotinib). The objective response rate (ORR) was 28.6% (2/7) and 69.2% (9/13), respectively (p = 0.16). The disease control rate (DOC) was 57.6% and 69.2% (9/13), respectively (p = 0.28). The duration of treatment was almost 1.2 months longer than in smokers (smokers 5.6 ± 2.2 vs. 6.8 ± 3.6 months; p = 0.752). Therefore, smokers with ALK SCC who responded to crizotinib/alectinib showed a shorter duration time of treatment and worse response than non‐smokers.
In 20 cases confirmed to be ALK‐positive by IHC and FISH, 15 cases received an AIK‐inhibitor. There was one SD case in four patients assayed by IHC (+)/FISH (−) or IHC (−)/FISH (+). There were eight PR cases, two SD cases, and one PD case, out of a total of 11 patients, both IHC (+)/FISH (+). The DCR was 90.1% (10/11) and 25.0% (1/4), respectively (p = 0.01). Four patients in which ALK rearrangement was assayed by IHC (−)/FISH (+) or IHC (+)/FISH (−) did not respond to crizotinib, and the duration time of treatment decreased sharply from one to four months (p = 0.001). 26 , 27
DISCUSSION
The EML4‐ALK fusion gene is one of the most important molecular alterations in NSCLC, but is extremely rarely expressed in SCC. 1 , 2 In our center, IHC analysis includes p40, P63, cytokeratin (CK) 5/6 and CK7, thyroid transcription factor‐1 (TTF‐1) and napsin‐A. To confirm the diagnosis of SCC requires TTF‐1 and napsin‐A completely negative and other indicators positive. In our report IHC findings were strongly suggestive that the tumors were SCC.
Caliò et al. reported that ALK rearrangements may be found in pure lung squamous cell carcinomas and the frequency was 2.5% (one of 40 cases) in biopsy samples. The ALK‐positive rate depends on tissue size and has been found to be higher in surgical specimens in comparison to smaller biopsy specimens. 28 , 29 It has been confirmed that the whole specimen obtained surgically showed typical morphohistology of SCC, and contained no other histological type. Furthermore, heterogeneous expression of ALK‐protein was seen throughout the entire area of the tumor. There was a possibility that the tumor was not pure SCC. Occasionally, the histological type of the small amount of specimen obtained via TBB was difficult to determine in subsequent IHC and molecular analyses.
In our report, five patients were surgically diagnosed with eight diagnosed via small biopsy specimens. The majority of the 31 cases in our study were diagnosed using small biopsy specimens. Fusion genes are often seen in a younger age group (mean age: 55.6 years old) and female group (15/31), especially non‐smokers.
The recommended methods for detection of ALK rearrangement include Ventana IHC (D5F3), in‐situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT‐PCR). 8 , 9 Previous studies have found that ALK‐positive with SCC is 1%, as detected by IHC/FISH or PCR. 27 , 30 Because of the low frequency in squamous lung cancer, ALK fusion gene testing is not routinely recommended in the National Comprehensive Cancer Network guideline for the treatment of NSCLC. The China Food and Drug Administration (CFDA) granted full approval for crizotinib in the treatment of patients with ALK‐positive NSCLC on January 22, 2013 and Ventana IHC (D5F3) has been approved by the CFDA as an aid in identifying patients who are eligible for treatment with crizotinib. 31 Until now, the updated CAP/IASLC/AMP molecular testing guideline for lung cancer recommended IHC as an equivalent alternative to FISH for ALK testing. Since Ventana IHC (D5F3) is the most convenient and inexpensive method for ALK rearrangement, therapeutic decisions based on IHC results are clearly reasonable. 32
ALK‐inhibitor (crizotinib/alectinib) is much more effective than chemotherapy for ADC with harbored the ALK rearrangement. In advanced NSCLC, the disease control rate is 60% and median progression‐free survival of response is 4–10 months for first‐line combination chemotherapy. The disease control rate (DCR) decreases 20%–40% for second‐line chemotherapy and median duration of response is poor. 3 , 30 Morodomi et al. reported that NSCLC patients with EML4‐ALK fusion genes are relatively insensitive to cytotoxic chemotherapy for postoperative recurrent disease or advanced disease. 33
Our article retrospectively analyzed the clinical features of 31 patients with ALK‐rearranged SCC. A total of 20 cases received ALK‐TKI as first‐ or second‐line treatment. The response of ALK‐TKI treatment was much better than chemotherapy. The superiority of second‐line treatment ALK‐inhibitor compared to standard chemotherapy has previously been demonstrated. The median duration time of therapy was 6.4 ± 4.4 months, much longer than patients with chemotherapy.
The therapeutic effect of ALK‐inhibitor in patients with SCC remains controversial. Target therapy with ALK‐rearranged ADC was more effective than SCC. The duration time of treatment (our study: 6.4 ± 4.4 months) ALK‐rearranged SCC was shorter than those in previous clinical trials of ALK‐rearranged ADC studies (PROFILE1007:7.7 months, PROFILE1014:10.9 months; ALESIANCT02838420:14.6 months), 34 , 35 suggesting that outcomes are worse for ACC as compared to the ADC patient when ALK inhibitors are used. Therefore, ALK‐rearranged SCC patients obtained clinical benefit from ALK‐inhibitor drug treatment, but it was not as beneficial to those patients who had ALK‐rearranged ADC.
Smoking status was a negative indicator of the efficacy of ALK‐inhibitor targeted therapy for ALK‐rearranged SCC. In this study, 13 non‐smokers accepted ALK treatment and only one suffered PD, and the ORR and DCR was much higher than in those patients who were smokers. The treatment duration time was almost two months longer than those in smokers, suggesting that outcomes are worse for smokers. From these cases the implication is that non‐smoking status may be a predictive factor for treatment in ALK‐positive patients with SCC.
ALK‐positive patients with SCC detected by IHC (+)/FISH (+) tended to be positively correlated with duration of treatment and associated with initial ALK inhibitor response. Response rate and disease control rate were extremely good. The treatment duration time was almost six months longer than that of patients detected by IHC or FISH positive with ALK‐rearrangement (9.0 ± 4.2 vs. 2.8 ± 1.2 months, respectively; p = 0.001), suggesting better outcomes for the former as compared to the latter patient group when ALK inhibitors are used. Shi et al. also reported FISH (+)/IHC (−) of lung adenocarcinoma was resistant to crizotinib. 36 It is difficult to further interpret the molecular mechanism of the lower response. The rate of discordance between ALK rearrangement detection by FISH and IHC ranges from 0.3% to 2%. So far, false‐positive explanation of IHC and FISH may be the main reason, especially with results close to the cutoff value. 37 , 38 Therefore, in order to devise a treatment strategy, it is reasonable to choose various methods to identify ALK‐positive patients.
Immune checkpoint blockade therapy is one of the most successful in patients with advanced NSCLC without driver oncogenes. Encouragingly, the therapeutic efficacy of immune checkpoint blockade has significantly improved for patients with advanced ACC. However, there is less data on the immune treatment of patients with ACC ALK‐rearrangement. We found only one previous case reported in the literature. 27 With IHC, this case showed focal positivity, FISH confirmed that it was rearrangement‐negative, and next‐generation sequencing (NGS) confirmed an ALK point mutation (K1525E). The patient showed no response to crizotinib and remained in SD for three months following previous treatment, then subsequently received therapy with PD‐L1 immune checkpoint inhibitors. The patient's treatment continued to the last recorded visit. We seldom treat a patient in this situation, but a personalized medicine or treatment strategy is sometimes necessary. However, further case reports are required to confirm such an association.
In conclusion, because of the increasing number of reported cases of ALK SCC, this presents an opportunity to allow doctors to define specific guidelines. Molecular characteristics of lung cancer have underlined the pathogenic and therapeutic importance of specific genes involved in tumor growth. This report highlights the importance of searching for driver mutations in patients with SCC. ALK‐targeted therapy could be an effective treatment option. Non‐smoker status and IHC (+)/FISH (+) may be predictive factors in those patients. It is therefore strongly advised that ALK inhibitor drug treatment could be one of the preferred approaches in cases of non‐smoker patients with ALK SCC.
Meng Q, Dong Y, Tao H, et al. ALK‐rearranged squamous cell carcinoma of the lung. Thoracic Cancer. 2021;12:1106–1114. 10.1111/1759-7714.13818
Contributor Information
Shucai Zhang, Email: sczhang6304@163.com.
Zhe Liu, Email: lza@vip163.com.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Didkowska J, Wojciechowska U, Mańczuk M, Łobaszewski J. Lung cancer epidemiology:contemporary and future challenges worldwide. Ann Transl Med. 2016;4(8):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reck M, Popat S, Reinmuth N, de Ruysscher D, Kerr KM, Peters S, et al. Metastatic non‐small‐ cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2014;25(suppl 3):27–39. [DOI] [PubMed] [Google Scholar]
- 4. Shaw AT, Yeap BY, Mino‐Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harborEML4 ‐ALK. J Clin Oncol. 2009;27(26):4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J, Shen Q, Shi Q, Yu B, Wang X, Cheng K, et al. Detection of ALK protein expression in lung squamous cell carcinomas by immunohistochemistry. J Exp Clin Cancer Res. 2014;33(1):109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. [DOI] [PubMed] [Google Scholar]
- 7. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumor: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 8. Wynes MW, Sholl LM, Dietel M, Schuuring E, Tsao MS, Yatabe Y, et al. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. Thorac Oncol. 2014;9(5):631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsao MS, Hirsch FR, Yatabe Y. IASLC. ATLAS of ALK and ROS1 testing in lung cancer. 2nd ed. Aurora, CO, USA: International Association for the Study of Lung Cancer; 2016. [Google Scholar]
- 10. Kim H, Park E, Kim YJ, Chung JH. ALK rearrangement in a pure squamous cell carcinoma:the challenge of detection of ALK rearrangement. J Vir Arch. 2013;462(5):597–9. [DOI] [PubMed] [Google Scholar]
- 11. Ochi N, Yamane H, Yamagishi T, Takigawa N, Monobe Y. Can we eliminate squamous cell carcinoma of the lung from testing of EML4‐ALK fusion gene? Lung Cancer. 2013;79(1):94–5. [DOI] [PubMed] [Google Scholar]
- 12. Alrifai D, Popat S, Ahmed M, Gonzalez D, Nicholson AG, Parcq JD, et al. A rare case of squamous cell carcinoma of the lung harboring ALK and BRAF activating mutations. Lung Cancer. 2013;80(3):339–40. [DOI] [PubMed] [Google Scholar]
- 13. Wang Q, He Y, Yang X, Wang Y, Xiao H. Extraordinary response to crizotinib in a woman with squamous cell lung cancer after two courses of failed chemotherapy. BMC Pulmon Med. 2014;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mikes RE, Jordan F, Hutarew G, Studnicka M. First line crizotinib in anaplastic lymphoma kinase (ALK) rearranged squamous cell lung cancer. Lung Cancer. 2015;90(3):610–3. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Q, Wang J, Zhang S. ALK‐rearranged squamous cell lung cancer: a case reports. Int J Clin Exp Pathol. 2015;8(2):2195–8. [PMC free article] [PubMed] [Google Scholar]
- 16. Takanashi Y, Tajima S, Matsuura S, Koyama S, Takahashi T, Neyatani H. ALK‐rearranged squamous cell carcinoma of the lung. Respirol Case Rep. 2015;3(3):105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vergne F, Quere G, Andrieu‐Key S, Descourt R, Quintin‐Roué I, Talagas M, et al. ALK‐rearranged squamous cell lung carcinoma responding to crizotinib: a missing link in the field of non‐small cell lung cancer? Lung Cancer. 2016;91:67–9. [DOI] [PubMed] [Google Scholar]
- 18. Tamiya A, Shimizu S, Atagi S. A case of squamous cell carcinoma harboring an EML4‐ALK rearrangement that was unsuccessfully treated with the ALK inhibitor alectinib. J Thorac Oncol. 2015;10(8):e74. [DOI] [PubMed] [Google Scholar]
- 19. Wang W, Song Z, Zhang Y. Response to crizotinib in a squamous cell lung carcinoma patient harbouring echinoderm microtubule‐associated protein‐like 4‐anaplastic lymphoma translocation: a case report. Thorac Cancer. 2016;7(3):355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamoto Y, Kodama K, Maniwa T, Takeda M, Kishima H. Anaplastic lymphoma kinase‐positive squamous cell carcinoma of the lung: a case report. Mol Clin Oncol. 2016;5(1):61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mamesaya N, Nakashima K, Naito T, Nakajima T, Endo M, Takahashi T. ALK‐rearranged lung squamous cell carcinoma responding to alectinib: a case report and review of the literature. BMC Cancer. 2017;17(1):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bolzacchini E, Tuzi A, Pinotti G. ALK‐rearranged squamous cell carcinoma of the lung treated with two lines of ALK inhibitors. J Thorac Oncol. 2017;12(15):e55–7. [DOI] [PubMed] [Google Scholar]
- 23. Li Q, Wu J, Yan LX, Huang JW, Zhang Z, Zhang JE, et al. ALK and ROSE1 double‐rearranged lung squamous cell lung carcinoma responding to crizotinib treatment: a case report. J Thorac Oncol. 2017;12(12):e193–7. [DOI] [PubMed] [Google Scholar]
- 24. Sagawa R, Ohba T, Ito E, Isogai S. ALK‐positive squamous cell carcinoma dramatically responded to alectinib. Oncol Med. 2018;12:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang T, Engelmann BJ, Morgan RM, Absher KJ, Kolesar JM, Villano JL. EML4‐ALK rearrangement in squamous cell carcinoma shows significant response to anti‐ALK inhibitor drugs crizotinib and alectinib. Cancer Chemother Pharmacol. 2018;81(5):965–8. [DOI] [PubMed] [Google Scholar]
- 26. Watanabe J, Togo S, Sumiyoshi I, Namba Y, Suina K, Mizuno T, et al. Clinical features of squamous cell lung cancer with anaplastic lymphoma kinase (ALK)‐rearrangement: a retrospective analysis and review. Oncotarget. 2018;9(35):24000–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang H, Sun L, Sang Y, Yang X, Tian G, Wang Z, et al. A study of ALK‐positive pulmonary squamous‐cell carcinoma: from diagnostic methodologies to clinical efficacy. Lung Cancer. 2019;130:135–42. [DOI] [PubMed] [Google Scholar]
- 28. Caliò A, Nottegar A, Gilioli E, Bria E, Pilotto S, Peretti U, et al. ALK/EML4 fusion gene may be found in pure squamous carcinoma of the lung. J Thorac Oncol. 2014;9(5):729–32. [DOI] [PubMed] [Google Scholar]
- 29. Xiao D, Lu C, Zhu W, He Q, Li Y, Fu C, et al. Comparison of small biopsy specimens and surgical specimens for the detection of EGFR mutations and EML4‐ALK in non‐small cell lung cancer. Oncotarget. 2016;7(37):59049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371(23):2167–77. [DOI] [PubMed] [Google Scholar]
- 31. Chinese Association of Oncologists Chinese Society for Clinical Cancer Chemotherapy . The diagnosis and treatment guideline of Chinese patients with EGFR mutation and ALK fusion gene‐positive non‐small cell lung cancer (2013 version). Zhonghua Zhong Liu Za Zhi. 2013;35:478–80. [PubMed] [Google Scholar]
- 32. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13(3):323–58. [DOI] [PubMed] [Google Scholar]
- 33. Morodomi Y, Takenoyama M, Inamasu E, Toyozawa R, Kojo M, Toyokawa G, et al. Non‐small cell lung cancer patients with EML4‐ALK fusion gene are insensitive to cytotoxic chemotherapy. Anticancer Res. 2014;34(7):3825–30. [PubMed] [Google Scholar]
- 34. Solomon BJ, Cappuzzo F, Felip E, Blackhall FH, Costa DB, Kim DW, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK‐positive non‐small‐cell lung cancer: results from PROFILE1014. J Clin Oncol. 2016;34(24):2858–65. [DOI] [PubMed] [Google Scholar]
- 35. Elliott J, Bai Z, Hsieh S‐C, Kelly SE, Chen L, Skidmore B, et al. ALK inhibitor for non‐small cell lung cancer: a systematic review and network meta‐analysis. PLoS One. 2020;15(2):e0229179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi R, Varella‐Garcia M, Li M, Ludkovski O, Danesh A. An anaplastic lymphoma kinase immunohistochemistry negative but fluorescence in situ hybridization positive lung adenocarcinoma is resistant to crizotinib. J Thorac Oncol. 2016;11(12):2248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ilie MI, Bence C, Hofman V, Long‐Mira E, Butori C, Bouhlel L, et al. Discrepancies between FISH and immunohistochemistry for assessment of the ALK status are associated with ALK ‘borderline’‐positive rearrangements or a high copy number: a potential major issue for anti‐ALK therapeutic strategies. Ann Oncol. 2015;26(1):238–44. [DOI] [PubMed] [Google Scholar]
- 38. Van der Wekken AJ, Pelgrim R, Werner N, Mastik MF, Hendriks L, van der Heijden EH, et al. Dichotomous ALK IHC is a better predictor for ALK inhibition outcome than traditional ALK‐FISH in advanced non‐small cell lung cancer. Clin Cancer Res. 2017;23(15):4251–8. [DOI] [PubMed] [Google Scholar]


