Abstract
Background
The tumor microenvironment is associated with prognosis in advanced non‐small cell lung carcinoma (NSCLC). The aim of this study was to explore the relationship between blood T cell diversity and survival of patients treated with pemetrexed‐based chemotherapy for nonsquamous NSCLC.
Methods
This prospective clinical study enrolled 26 patients with advanced NSCLC treated with 4–6 cycles of first‐line pemetrexed combined with platinum‐based therapy. The complementarity‐determining region 3 (CDR3) located in the T cell receptor beta chain (TCR β chain) was captured and deeply sequenced using next‐generation sequencing (NGS) technology, and the correlation between TCR changes and efficacy after chemotherapy was analyzed.
Results
Patients with an inferior quarter diversity index showed a significantly shorter progression‐free survival (PFS) than the others (median, 5.0 months vs. 8.1 months, P = 0.014). After two cycles of chemotherapy, the TCR diversity was significantly higher than the baseline (P = 0.034). Just as with the baseline, patients with an inferior quarter diversity index at the endpoint of cycle 2 showed a shorter progression‐free survival (PFS) than the others (median, 5.0 months vs. 8.4 months, P = 0.024).
Conclusions
In advanced NSCLC patients treated with first‐line pemetrexed combined with platinum, the low level of blood TCR diversity at baseline with an endpoint of two cycles of chemotherapy was correlated with a poor prognosis.
Keywords: Advanced non‐small cell lung carcinoma, T cell diversity, tumor microenvironment

The aim of this study was to explore the relationship between blood T cell diversity and the survival of patients treated with pemetrexed‐based chemotherapy for nonsquamous non‐small cell lung cancer (NSCLC). In advanced NSCLC patients treated with first‐line pemetrexed combined with platinum, the low level of blood TCR diversity at baseline with an endpoint of two cycles of chemotherapy was correlated with a poor prognosis. The level of diversity after two cycles was the only independent prognostic factor among the multifactor analysis.
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of death from cancer worldwide. Among the various subtypes of lung cancer, treatment modes in adenocarcinoma are the most diverse. For advanced adenocarcinoma patients in which there is no opportunity for targeted treatment, or in whom targeted treatment has failed, pemetrexed is recommended as a first‐line therapy. In addition, the pemetrexed regimen also demonstrates strong immune synergy when combined with immunotherapy. According to previous studies, chemotherapy drugs can affect the immune system through a variety of mechanisms, 1 , 2 including pemetrexed. 3 However, the influence of pemetrexed on the human immune system is still unclear.
T cells are a major component of the immune system and there is evidence that a T cell receptor repertoire may be a potential biomarker for prognosis and a predictive marker for response to cancer treatment. 4 , 5 , 6 , 7 , 8 The characteristics of the peripheral blood T cell receptor repertoire may represent the imprint of the antigen spectrum present in various states of the disease, which can help distinguish patients with different diseases 9 and predict the efficacy and prognosis of immunotherapy in cancer patients. 5 , 10
Previous studies have reported a relationship between the TCR and lung cancer. 11 , 12 However, these studies either targeted a relatively broad population of non‐small cell lung cancer (NSCLC) and did not strictly distinguish pathological types or did not use uniform treatment options. Different pathological subtypes and treatment options might affect the TCR repertoire. Therefore, patients with lung adenocarcinoma who were enrolled in this study and whose disease had been confirmed by pathology all received chemotherapy with pemetrexed combined with platinum as a first‐line treatment. The value of the TCR repertoire in lung adenocarcinoma is explored on the premise of consistent pathology, consistent protocols, and similar staging.
Our study sought to explore the influence of immunological function on lung adenocarcinoma patients who received pemetrexed chemotherapy and further investigate the predictive value of the TCR repertoire in the prognosis and therapeutic efficacy among these patients.
Methods
Sample collection
Peripheral blood of 26 patients with lung cancer who received first‐line pemetrexed based chemotherapy at Peking University Third Hospital from 2017 to 2020 was collected. Patients with previous or present complications associated with autoimmune disease and acute infection were excluded from the study. Peripheral blood samples were collected from patients before treatment and after every two cycles of chemotherapy. The clinical information of patients was obtained from the hospital medical record system, and none of the patients had previously received first‐line chemotherapy or radiotherapy before sampling. All patient diseases were pathologically confirmed. Evaluation was performed according to the RECIST version 1.1. Follow‐up data came from medical records or telephone follow‐up. Clinical stage was based on the eighth edition of the AJCC Cancer Staging System and the study was conducted in compliance with the Helsinki Declaration of 1975 and approved by the Institutional Review Board of Peking University's Third Hospital. All subjects provided their written informed consent.
High‐throughput sequencing of the TCR β‐chain
Third complementarity‐determining region (CDR3) of the T cell receptor beta chain (TCR β‐chain) was amplified by a multiplex PCR platform, which was designed specifically for the study. A total of 600 ng peripheral blood lymphocyte (PBL) DNA was amplified by using two rounds of PCR for each sample. For the first round, 32 forward primers of V genes and 13 reverse primers of J genes were used to run 10 cycles of PCR to amplify the CDR3 fragments using a Multiplex PCR Kit (Qiagen, Germany). Primers were designed to maximize the coverage of the heterogeneous set of target sequences of V and J families for a minimal PCR bias. For the second round, PCR was performed using Illumina universal primers. Paired‐end sequencing of samples was performed with the read‐length of 151 bp using the MGIseq‐2000 sequencing system (BGI, Shenzhen, China). Raw data were processed and analyzed by the following steps. First, we removed the sequencing reads which did not contain the primers of multi‐PCR using Cutadapt (https://github.com/marcelm/cutadapt); second, we merged the rest of the high‐quality paired‐end reads to acquire contigs; and third, we identified the CDR3 region using MiXCR55 (https://github.com/milaboratory/mixcr/) with default parameters. T cell diversity was evaluated using the Shannon–Wiener index.
Statistical analysis
A Student's t‐test was used to evaluate the difference between the two groups. The difference between proportions of the two groups was assessed using Fisher's exact test. These tests, as well as descriptive statistics and Pearson correlation analysis, were used to assess correlations between experimental parameters and were performed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). Kaplan‐Meier survival analysis (SPSS 22.0, IBM) was used to compare PFS between subgroups. Results were considered statistically significant when the P‐value was less than 0.05.
Results
Baseline characteristics
We tested 26 patients and 19 healthy controls. The median age was 59 years (range: 40–72) for the patient cohort, among which 14 patients had a smoking history and eight patients had received targeted therapy and harbored established EGFR driver mutation with exon 19 deletion. Additionally, there were two cases of an exon 20 mutation, one case of a KRAS mutation, one case of a c‐met mutation, and one case of a CDKN2A mutation. Among the patients with mutations, only those with exon 19 deletion mutations had previously received targeted therapy. Three patients with high expression of PD‐L1 received chemotherapy combined with immunotherapy. Five patients received chemotherapy combined with antiangiogenic therapy. All patients in this cohort were clinical stage IIIB or beyond and pathologically determined as solely having adenocarcinoma. No statistically significant differences in TCR diversity were found in patients with a distinct smoking status, gender, or age. Additionally, after matching age, gender, and smoking factors, there were no significant differences in the TCR diversity between the healthy controls and patient cohort (Table 1).
Table 1.
Association between clinicopathological characteristics and TCR diversity
| Characteristics | N (%) | TCR diversity | |
|---|---|---|---|
| Total (n = 26) | Mean ± SEM | P‐value | |
| Age, years | |||
| ≤59 | 13 (50%) | 6.89 ± 0.43 | 0.2475 |
| >59 | 13 (50%) | 7.52 ± 0.32 | |
| Gender | |||
| Male | 14 (53.8%) | 7.17 ± 0.50 | 0.8727 |
| Female | 12 (46.2%) | 7.25 ± 0.27 | |
| Smoking | |||
| Yes | 13 (50%) | 7.33 ± 0.30 | 0.6738 |
| No | 13 (50%) | 7.10 ± 0.46 | |
| EGFR +/− | |||
| Positive | 8 (30.8%) | 7.44 ± 0.61 | 0.5666 |
| Negative | 18 (69.2%) | 7.11 ± 0.28 | |
Prognosis and diversity of different nodes during chemotherapy
Next, the patients were grouped using the quartile of baseline TCR diversity index. Notably, patients with an inferior quarter diversity index showed a significantly shorter PFS than the others (median, 5.0 months vs. 8.1 months, P = 0.014, Fig 1a). Likewise, patients with an inferior quarter diversity index at the endpoint of cycle 2 showed a significantly shorter PFS than the others (median, 5.0 months vs. 8.4 months, P = 0.024, Fig 1b). Similar tendencies were displayed for the TCR diversity estimated at the endpoint of cycle 4 (median, 5.3 months vs. 9.3 months, P = 0.311, Fig 1c) and cycle 6 (median, 8.6 months vs. 11.7 months, P = 0.352, Fig 1d), but without significant statistical differences. However, the PFS of several patients whose diversity kept in continuously high level was particularly long.
Figure 1.
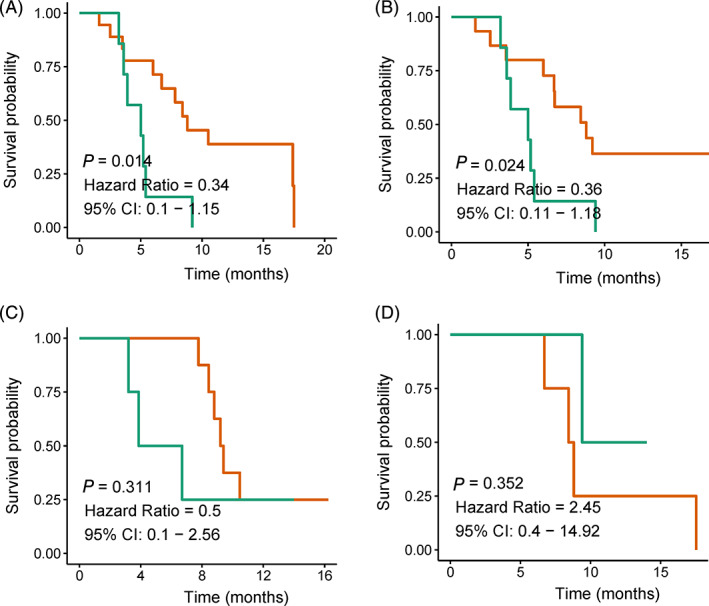
Diversity and prognosis. The relationship between diversity Shannon index and prognosis at (a) baseline ( ) >6.21(18) (
) >6.21(18) ( ) <6.21(7); (b) after two cycles (
) <6.21(7); (b) after two cycles ( ) >6.66(15) (
) >6.66(15) ( ) <6.66(7); (c) after four cycles (
) <6.66(7); (c) after four cycles ( ) > () 6.87(8) (
) > () 6.87(8) ( ) <6.87(4) and (d) after six cycles (
) <6.87(4) and (d) after six cycles ( ) >5.91(4)
) >5.91(4)  () <5.91(2).
() <5.91(2).
Changes of diversity during chemotherapy
After two cycles of chemotherapy, the TCR diversity of the patient cohort was significantly higher than the baseline (P = 0.034, Fig 2a), whereas after four cycles of chemotherapy, the TCR diversity was similar to the baseline (P = 0.259, Fig 2b). No difference was shown after two and four cycles (P = 0.630, Fig 2c).
Figure 2.
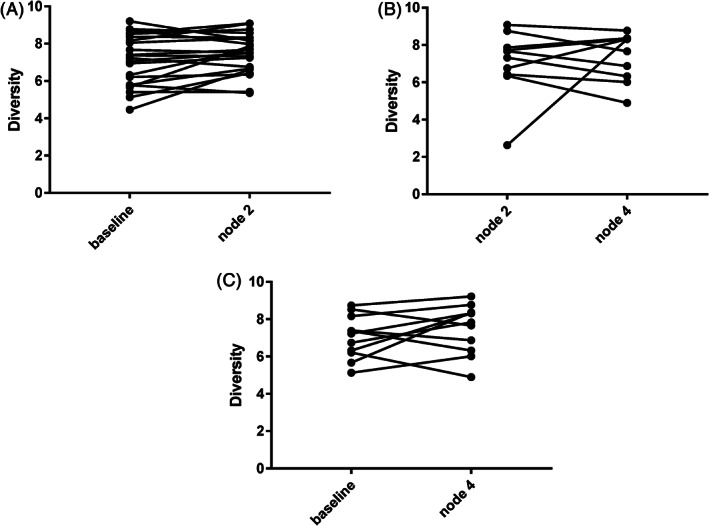
The diversity change between (a) baseline and after two cycles; (b) between after two and after four cycles; and (c) between baseline and after four cycles.
Difference of prognosis and diversity between driver gene‐positive and negative patients
Of the test subjects, eight patients with EGFR exon 19 deletion mutations progressed after receiving targeted therapy. The diversity changes after two cycles of chemotherapy of the eight patients are shown in Figure 3a. There were no significant differences between the baseline and after two cycles in these patients. The PFS of these eight EGFR‐mut patients were shorter than the EGFR‐wt patients (median, 5.7 months vs. 8.4 months, P = 0.328, Fig 3b). Moreover, the depth of response (DepOR) in the EGFR‐mut patients was slightly worse than that of the EGFR‐wt patients (−0.298 vs.−0.3647, P = 0.600, Fig 3c). Although there were no statistical differences between the two groups due to the limited sample size, the tendencies could be seen.
Figure 3.
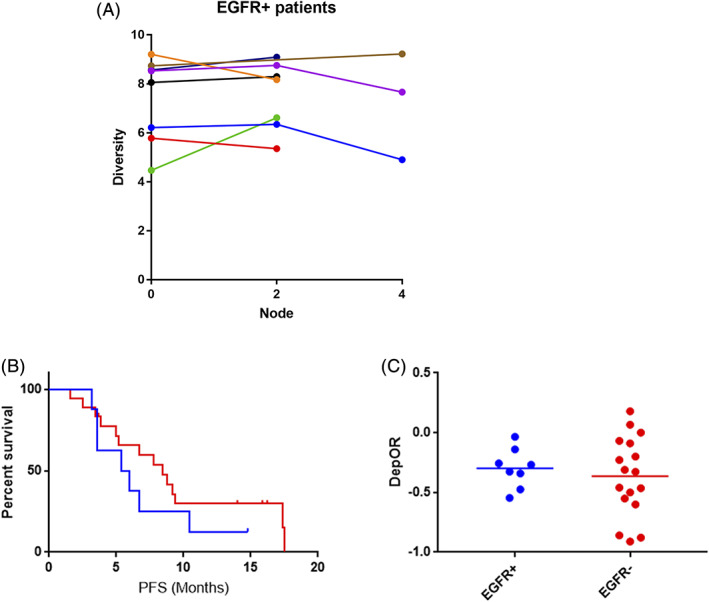
(a) The diversity changes of the eight EGFR exon 19 deletion mutation patients ( ) P001 (
) P001 ( ) P003 (
) P003 ( ) P011 (
) P011 ( ) P012 (
) P012 ( ) P020 (
) P020 ( ) P024 (
) P024 ( ) P026 (
) P026 ( ) P027. (b) Differences of PFS (5.7 months vs. 8.4 months, P = 0.328); and (c) DepOR (−0.298 vs.−0.3647, P = 0.600) between EGFR exon 19 deletion mutation patients and EGFR‐wt patients (
) P027. (b) Differences of PFS (5.7 months vs. 8.4 months, P = 0.328); and (c) DepOR (−0.298 vs.−0.3647, P = 0.600) between EGFR exon 19 deletion mutation patients and EGFR‐wt patients ( ) EGFR+ (
) EGFR+ ( ) EGFR‐.
) EGFR‐.
Chemotherapy combined with immunotherapy
Of the 26 patients enrolled, three received chemotherapy (pemetrexed, platinum) combined with PD‐L1 inhibitors. The patient characteristics are as follows: Patient No. 025 (marked in green in Fig 4f) (tumor cell PD‐L1 80%), who had a main tumor in the right lung and mediastinal lymph nodes (baseline) (Fig 4a), received chemotherapy alone because he had a fever during the first two cycles of treatments. The response evaluation result was stable disease (SD) (some lesions shrank and some lesions progressed significantly), and meanwhile the diversity decreased. After the first two cycles (Fig 4b), the temperature of the patient returned to normal, and a PD‐L1 inhibitor for the third and fourth cycles was added, and the response evaluation was that the patient obtained partial remission (PR) (Fig 4c); the TCR diversity increased significantly. The response evaluation was similar to the previous two cycles after the fifth and sixth cycles (Fig 4d). Local progress was noticeable after immunotherapy maintenance (Fig 4e), while the diversity began to decrease. Patient No. 023 (marked in red in Fig 4f) (tumor cell PD‐L1 90%) lacked a baseline, and was evaluated as SD after two cycles with low diversity. After four cycles, the diversity significantly increased with the evaluation as PR. Maintenance treatment was later commenced and the evaluation remained as PR after two cycles of maintenance; however, the treatment was discontinued because of immunologically associated pneumonia after three maintenance cycles. Patient No. 008 (marked in blue in Fig 4f) (tumor cell PD‐L1 50%) was evaluated as SD after the second and fourth cycles. After four cycles, pemetrexed combined with pembrolizumab was maintained for three cycles, and SD status was maintained throughout. The changes in diversity also increased and decreased slightly after two and four cycles, but with negligible fluctuation overall.
Figure 4.
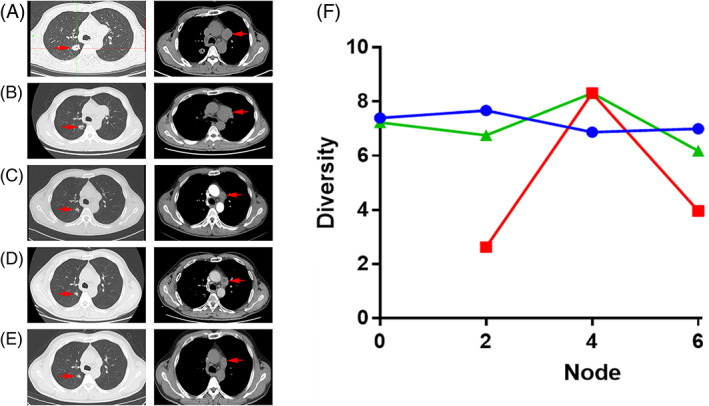
Diversity change of three patients treated by immunotherapy and CT performance of patient No. 025. (a) Chest image at baseline. (b) Chest image after two cycles. (c) Chest image after four cycles. (d) Chest image after six cycles. (e) Chest image after two cycles of monoimmunotherapy. (f) Diversity change of the three patients treated by immunotherapy ( ) P008 (
) P008 ( ) P023 (
) P023 ( ) P025.
) P025.
Discussion
In the era of immunotherapy, pemetrexed has also become one of the cornerstone chemotherapy drugs for combined immunotherapy. In combination with immunotherapy, even if pemetrexed requires pretreatment with dexamethasone, which will reduce the efficacy of the immunotherapy, it still results in a strong synergy in combination immunotherapy. The protocol involved in our study was pemetrexed combined with platinum. The clinical results of the KEYNOTE‐189 trial showed that pemetrexed/platinum chemotherapy combined with the anti‐PD‐1 antibody produces significant activity in patients with NSCLC. 13 These facts indicate that there may be a positive synergy between the two therapies.
Previous studies have suggested that chemotherapy has immunosuppressive or immune activating effects. 1 , 2 There have been only a few previous studies on the effects of pemetrexed on immune function, primarily on animals or in vitro studies. 14 , 15 It has been found that when pemetrexed is used in combination with anti‐PD‐L1 antibody, antitumor effects are increased and significant inflammation/immune activation is observed in animal experiments. 3 , 14 , 16 Meanwhile, the immunoregulatory effects of platinum have both negative regulatory effects 17 and positive regulatory effects. 18 , 19 , 20 , 21 , 22 Based on the uncertainty of the positive and negative directions of platinum drugs for immune regulation and the limited reports of pemetrexed on immune regulation, our study initially explored the effects of pemetrexed combined with platinum on immune function in humans.
In many tumor types, TCR repertoire diversity appears to be a prognostic factor for clinical outcomes in cancer patients. 23 , 24 , 25 , 26 , 27 , 28 The low TCR diversity of certain patients indicates that the immune status might be severely impaired which can be a potential negative predictor of immune checkpoint inhibitor response. 6 , 29 , 30 The above is consistent with our research which indicated that patients with increased diversity after treatment have a better prognosis. We also found that a longer remission period might also be obtained in patients with continuously increasing diversity. Meanwhile, the stagnant diversity level observed in some of our patients indicated a poor prognosis which may be because the immune system remains unchanged and the growing tumor cannot be controlled. 12 Therefore, TCR diversity is an indicator of immune function related to prognosis.
In our study, pemetrexed combined with platinum generally showed a positive immunomodulatory effect, especially after two cycles. With the extension of the number of chemotherapy cycles, the immune activation did not increase further but began to decline. The TCR diversity decreased after four instead of after two cycles. Yet, some patients in our study still maintained a high level of diversity after four cycles. When patients exhibit an increase in immune diversity for an extended duration of time, it suggests a very promising prognosis.
Not only a prognostic indicator, TCR diversity may also be a predictor of efficacy in anti‐PD‐1 immunotherapy. Three patients received PD‐L1 inhibitors in combination with pemetrexed platinum‐based chemotherapy. Superior efficacy was shown when diversity increased in all of these patients, and vice versa. For patient No.025, with a PD‐L1 of 80%, there was no change in the TCR diversity when treatment was carried out with chemotherapy or immunotherapy alone. However, after receiving chemotherapy combined with immunotherapy, the diversity increased, and was accompanied by significant efficacy. It is suggested that changes in TCR can indicate the effectiveness of treatment. The host's efficacy is related to TCR diversity. For this patient, neither chemotherapy nor immunotherapy was able to control the growth of this lesion. This may have been related to the synergistic antitumor effects of chemotherapy combined with immunotherapy. However, due to the limited number of patients who received immunotherapy, prognostic analysis was not easy to complete.
In terms of the synergistic effects, some believe that chemotherapy can induce mutations and produce new antigens, thereby enhancing the efficacy of immunotherapy. However, recent views suggest that, although chemotherapy induces neoantigens, chemotherapy is mostly a cytotoxic drug and rarely induces mutations. The amount of neoantigen mutations produced is, therefore, relatively small. 31 , 32 , 33 If cancer cells carrying neoantigens make up only a small portion of the tumor, there is little chance that they will be recognized by the immune system. 34 Even if it is identified, it will not trigger a significant therapeutic response.
Studies have also shown that chemotherapy can also change the tumor microenvironment by intensifying the infiltration of CD8+ T cells, decreasing regulatory T cells and myeloid suppressor cells which leads to an increased ratio of CD8+ T cells to regulatory T cells, promoting the maturation of antigen‐presenting cells. 35 , 36 , 37 Some chemotherapeutics have been reported to directly act on different immune cells, such as dendritic cells, macrophages, myeloid‐derived suppressor cells, T cells and natural killer cells. 38 All these effects can regulate the immune microenvironment. Nevertheless, the mechanism of the synergistic or additive effects for chemoimmunotherapy has not as yet been fully investigated.
Our study found that the diversity of EGFR‐mut patients after two cycles was similar to the baseline; meanwhile, the PFS and duration of response (DOR) of patients with EGFR‐mut was worse than in the patients with EGFR‐wt. One possible reason for this might be that for EGFR mutation‐positive patients, the PD‐L1 expression level may alter from low to high 39 and tumor mutation burden (TMB) has a tendency to be higher than before after EGFR‐TKI treatment. 40 The above changes could lead to insensitivity to chemotherapy. Another possibility is after EGFR‐TKI treatment, CD8+ and FOXP3+ TIL densities may be much lower, 40 which could cause tumor progression. Based on the results of the IMpower150 study, if patients with TKI resistance are given the option of combined immunotherapy with antiangiogenic therapy, they may achieve improved PFS/OS. 41 However, based on our medical insurance restrictions, fewer patients choose self‐funded antiangiogenic therapy.
Our study also has some limitations. First, it was not until we observed a preliminary trend in combined immunotherapy that we were able to determine that the patients receiving combined immunotherapy …
Second, when we enrolled patients into the study, bevacizumab was not included in the health care policy at the time, so almost all the patients enrolled in the study were patients who had not received combined antiangiogenic therapy. Finally, although enrollment into this study was strict, uniform and prospective, the overall enrollment population was small, and only a limited trend could be seen. These findings should be further investigated by expanding the sample size in the subsequent treatment population.
In conclusion, our data suggest that for NSCLC patients with advanced disease treated with first‐line pemetrexed combined with platinum, low level of blood TCR diversity at baseline with an endpoint of two cycles of chemotherapy correlated with a poor prognosis. Furthermore, the level of TCR diversity after two cycles of chemotherapy, which tend to be highest during chemotherapy, was also a prognostic factor. In addition, the longer duration of elevated TCR diversity suggests a better prognosis. Receiving combined immunotherapy with increased diversity also had better efficacy. In addition, chemotherapy appeared to be less effective in patients with an EGFR‐mut than those with an EGFR‐wt. However, due to the limited sample size, an expanded cohort is needed to further validate the findings in our study.
Disclosure
The authors confirm that there are no conflicts of interest.
Supporting information
Appendix S1 Supporting information
Contributor Information
Li Qian, Email: docliq@126.com.
Liang Li, Email: liang.dr@163.com.
References
- 1. Sakai H, Kokura S, Ishikawa T et al. Effects of anticancer agents on cell viability, proliferative activity and cytokine production of peripheral blood mononuclear. J Clin Biochem Nutr 2013; 52 (1): 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pol J, Vacchelli E, Aranda F et al. Trial watch: Immunogenic cell death inducers for anticancer. Chemotherapy 2015; 4 (4): e1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schaer DA, Geeganage S, Amaladas N et al. The folate pathway inhibitor pemetrexed pleiotropically enhances effects of cancer. Immunotherapy 2019; 25 (23): 7175–88. [DOI] [PubMed] [Google Scholar]
- 4. Durgeau A, Virk Y, Corgnac S, Mami‐Chouaib F. Recent advances in targeting CD8 T‐cell immunity for more effective cancer. Immunotherapy 2018; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Postow MA, Manuel M, Wong P et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer 2015; 3 (1): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Snyder A, Nathanson T, Funt SA et al. Contribution of systemic and somatic factors to clinical response and resistance to PD‐L1 blockade in urothelial cancer: An exploratory multi‐omic analysis. PLOS Med 2017; 14 (5):e1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robert L, Tsoi J, Wang X et al. CTLA4 blockade broadens the peripheral T‐Cell receptor repertoire. Clin Cancer Res 2014; 20 (9): 2424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tumeh PC, Harview CL, Yearley JH et al. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515 (7528): 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han Y, Liu X, Wang Y. Identification of characteristic TRB V Usage in HBV‐sssociated HCC by using differential expression profiling analysis. Oncoimmunology 2015; 4: e1021537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fang H, Yamaguchi R, Liu X et al. Quantitative T cell repertoire analysis by deep cDNA sequencing of T cell receptor α and β chains using next‐generation sequencing (NGS). Oncoimmunology 2014; 3 (12): e968467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reuben A, Gittelman RM, Gao J et al. TCR repertoire Intratumor heterogeneity in localized lung adenocarcinomas: An association with predicted neoantigen heterogeneity and postsurgical recurrence. Cancer Discov 2017; 7 (10): 1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Yang Q, Yang J et al. Characteristics and prognostic significance of profiling the peripheral blood T‐cell receptor repertoire in patients with advanced. Lung Cancer 2019; 145 (5): 1423–1431. [DOI] [PubMed] [Google Scholar]
- 13. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018; 378 (22): 2078–92. [DOI] [PubMed] [Google Scholar]
- 14. Schaer D, S/ G, Amaladas N et al. P1.04–07 Pemetrexed enhances anti‐tumor efficacy of PD‐L1 blockade by promoting intra‐tumor immune response via tumor and T cell‐intrinsic mechanisms. J Thorac Oncol 2018; 13 (10): S527. [Google Scholar]
- 15. Novosiadly R, Schaer D, Lu Z. P3.07–006 Pemetrexed exerts Intratumor immunomodulatory effects and enhances efficacy of immune checkpoint blockade in MC38 syngeneic mouse tumor model. J Thorac Oncol 2017; 12 (11): S2300. [Google Scholar]
- 16. Novosiadly R, Schaer D, Amaladas N et al. Pemetrexed Enhances Anti‐Tumor Efficacy of PD1 Pathway Blockade by Promoting Intra Tumor Immune Response Via Immunogenic Tumor Cell Death and T Cell Intrinsic Mechanisms. AACR 2018; 78 (13): 4549–4549. [Google Scholar]
- 17. Tr L, Allen CT, Xiao R et al. Cisplatin alters anti‐tumor immunity and synergizes with PD‐1/PD‐L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res 2017; 5: 1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ock CY, Kim S, Keam B et al. Changes in programmed death‐ligand 1 expression during cisplatin treatment in patients with head and neck squamous cell carcinoma. Oncotarget 2017; 8 (58): 97920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackaman C, Majewski D, Fox SA, Nowak AK, Nelson DJ. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother 2012; 61: 2343–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nio Y, Hirahara N, Minari Y, Iguchi C, Tamura KJ. Induction of tumor‐specific antitumor immunity after chemotherapy with cisplatin in mice bearing MOPC‐104E plasmacytoma by modulation of MHC expression on tumor surface. Anticancer Res 2002; 20 (5A): 3293–9. [PubMed] [Google Scholar]
- 21. Hu J, Kinn J, Zirakzadeh AA et al. The effects of chemotherapeutic drugs on human monocyte‐derived dendritic cell differentiation and antigen presentation. Clin Exp Immunol 2013; 172 (3): 490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang X, Cui S, Yongqian S. Cispla;tin selectively downregulated the frequency and mmunoinhibitory function of myeloid‐derived suppressor cells in a murine B16 melanoma model. Immunol Res 2015; 64: 160–70. [DOI] [PubMed] [Google Scholar]
- 23. Cui JH, Lin KR, Yuan SH et al. TCR repertoire as a novel indicator for immune monitoring and prognosis assessment of patients with cervical patients. Cancer 2018; 9: 2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin Y‐B, Luo W, Zhang G‐Y et al. TCR repertoire profiling of tumors, adjacent normal tissues, and peripheral blood predicts survival in nasopharyngeal carcinoma. Cancer Immunol Immunother 2018; 67: 1719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keane C, Gould C, Jones K et al. The T‐cell receptor repertoire influences the tumor microenvironment and is associated with survival in aggressive B‐cell lymphoma. Clin Cancer Res 2017; 23 (7): 1820–8. [DOI] [PubMed] [Google Scholar]
- 26. Lin KR, Deng FW, Jin YB et al. T cell receptor repertoire profiling predicts the prognosis of hbv‐associated hepatocellular carcinoma. Cancer Med 2018; 7 (8): 3755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manuel M, Trédan O, Bachelot T. Lymphopenia combined with low TCR diversity (Divpenia) predicts poor overall survival in metastatic breast cancer patients. Oncoimmunology 2012; 2 (4): 432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tamura K, Hazama S, Yamaguchi R et al. Characterization of the T cell repertoire by deep T cell receptor sequencing in tissues and blood from patients with advanced colorectal cancer. Oncol Lett 2016; 11: 3643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature 2017; 541 (7637): 321–30. [DOI] [PubMed] [Google Scholar]
- 30. Teng MWL, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T‐cell infiltration and PD‐L1. Cancer Res 2015; 75 (11): 2139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parris CN, Walker MC, Masters JW, Arlett C. Inherent sensitivity and induced resistance to chemotherapeutic drugs and irradiation in human cancer cell lines: relationship to mutation frequencies. Cancer Res 1991; 50 (23): 7513–8. [PubMed] [Google Scholar]
- 32. Helleday T, Arnaudeau C, Jenssen D. Effects of carcinogenic agents upon different mechanisms for intragenic recombination in mammalian cells. Carcinogenesis 1998; 19: 973–8. [DOI] [PubMed] [Google Scholar]
- 33. Arnaudeau C, Miranda ET, Jenssen D, Helleday T. Inhibition of DNA synthesis is a potent mechanism by which cytostatic drugs induce homologous recombination in mammalian cells. Mutat Res 2000; 461 (3): 221–8. [DOI] [PubMed] [Google Scholar]
- 34. Chang AY, Gejman RS, Jones H. Rejection of immunogenic tumor clones is limited by clonal fraction. eLife 2018; 7: e41090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre‐treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer 2010; 102 (1): 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tseng CW Hung CF, Alvarez RD et al. Pretreatment with cisplatin enhances E7‐specific CD8+ T‐cell‐mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res 2008; 14 (10): 3185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pfirschke C, Engblom C, Rickelt S et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016; 44 (2): 343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: Immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11 (3): 215–33. [DOI] [PubMed] [Google Scholar]
- 39. Haratani K, Hayashi] H, Tanaka T et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation‐positive non‐small‐cell lung cancer based on T790M status after disease progression during EGFR‐TKI treatment. Ann Oncol 2017; 28 (7): 1532–9. [DOI] [PubMed] [Google Scholar]
- 40. Isomoto K, Haratani K, Hayashi H et al. Impact of EGFR‐TKI treatment on the tumor immune microenvironment in EGFR mutation–positive non–small cell lung cancer. Clin Cancer Res 2020; 26 (8): 2037–46. [DOI] [PubMed] [Google Scholar]
- 41. Reck M, Mok TS, Nishio M et al. Atezolizumab plus bevacizumab and chemotherapy in non‐small‐cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open‐label phase 3 trial. Lancet Respir Med 2019; 7 (5): 387–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


