Abstract
Based on the development of new hybrid machines consisting of an MRI and a linear accelerator, magnetic resonance image guided radiotherapy (MRgRT) has revolutionized the field of adaptive treatment in recent years. Although an increasing number of studies have been published, investigating technical and clinical aspects of this technique for various indications, utilizations of MRgRT for adaptive treatment of head and neck cancer (HNC) remains in its infancy. Yet, the possible benefits of this novel technology for HNC patients, allowing for better soft-tissue delineation, intra- and interfractional treatment monitoring and more frequent plan adaptations appear more than obvious. At the same time, new technical, clinical, and logistic challenges emerge. The purpose of this article is to summarize and discuss the rationale, recent developments, and future perspectives of this promising radiotherapy modality for treating HNC.
Keywords: MRI, MR-guidance, IGRT (Image Guided Radiation Therapy), head and neck (H&N) cancer, adaptive radiotherapy, xerostoma, salivary gland
Introduction
In recent years magnetic resonance guidance (MRg) emerged as a new promising modality within the spectrum of image-guided radiotherapy (IGRT) (1), allowing for better tumor and soft tissue visualization, repetitive imaging without additional dose exposure, target volume gating, and online plan adaptation (2). Following the first platforms with these features, including low-field MR-imaging facilities and a cobalt source (3), soon the first hybrid platforms were developed combining this image modality with a linear accelerator (MR-Linacs) (4).
At present, MR-Linacs are widely used for treating various indications and tumor localizations, e.g., stereotactic body radiotherapy (SBRT) of the upper abdomen or the lung, prostate cancer, and other pelvic targets like the rectum (5). These applications are predominantly chosen due to the obvious benefits of daily plan adaptations when including target volumes and organs at risk (OAR) with distinct inter- and intrafractional motion or anatomical changes and due to the often used hypofractionated regimens limiting the efforts of repetitive adaptations (6, 7). On the other hand, implementation of this novel technology for treating head and neck cancer (HNC) remains at its infancy, and published data about its technical and clinical applications are scarce (8) and mainly limited to MR-cobalt platforms (9). However, despite the technical and clinical challenges of HNC-radiotherapy such as long-course regimens, enhanced acute toxicity, and patient immobilization using masks compromising treatment tolerance and more complex plans with a multitude of OAR, the first research groups have already started exploiting possible benefits of MR-Linacs for this indication (10–13).
The goal of this article is to present current developments in the field of MR-guided, adaptive radiotherapy for HNC and discuss clinical benefits and difficulties of the adoption of this promising technique. For this purpose, and because of the lack of a broad consensus regarding the MRg-definition, also data and knowledge gained from MR-planning guidance before x-ray IGRT were included.
Adaptive Treatment for Head and Neck Cancer and Potential Benefits
The concept of adaptive radiotherapy (ART) for HNC relies on accounting for potential anatomic changes during the treatment course, associated with, amongst others, tumor shrinkage, weight loss, or organ/structure migration and has been heavily exercised in the last two decades.
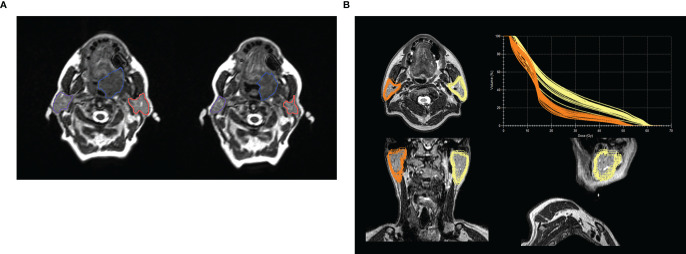
The original purpose of ART was to compensate for target position variability during radiotherapy in order to ensure correct dose accumulation, which led to the development of on-line 3D-imaging in the form of cone-beam-CT (CBCT) (14). Yet, most modern ART-approaches focus more on improving dose-sparing for specific OARs like the parotids (15–17). Although there is a lack of prospective clinical trials evaluating the objective benefit of ART for HNC, several dosimetric studies have been published so far, e.g., demonstrating an underestimation of the cumulative dose to the parotids when using the original non-adapted plan only, leading to increased probability for xerostomia (15, 18). Raghavan et al. were one of the first groups to demonstrate both a migration of the center of mass of the parotids, as well as a bilateral volume shrinkage in 6 HNC-patients, using an MRgRT-dedicated platform (19). An example of parotid migration and volume reduction demonstrated with the help of longitudinal imaging on the MR-Linac is shown on Figure 1A. An example of actual dose delivered to the parotid glands contoured offline after completion of treatment on a MR-Linac is shown in Figure 1B (20). Mohamad et al. showed that MRgART may be beneficial especially for swallowing related toxicities in HPV+ low risk HNC patients, especially at risk for long term toxicity due to the excellent outcome of these patients (21).
Figure 1.
(A) Example of volume changes and migration of parotid glands during the course of fractionated radiotherapy at an 0.35 T MR-Linac or a large base of tongue carcinoma between treatment start (left image) and beginning of the 7th treatment week - boost (right image). Left and right parotid glands are delineated in orange and violet respectively and the gross tumor volume in blue. The volume of the left and right parotid glands decreased by 8.2 cc and 10.0 cc, respectively. The inter-parotids distance changed from 11.0 cm to 10.3 cm. (B) Example of a post treatment analysis for a patient treated for a hypopharyngeal carcinoma with 70 Gy in 35 fractions. Parotid glands were contoured for each daily MRI during the course of fractionated radiotherapy at a 1.5 T MR-Linac and propagated to the T2w planning MRI, with the total plan DVH for each daily delivered plan in the upper right corner, showing the variance in actual delivered dose depending on volume of the parotid gland. Averaged Dmean of the anatomically corrected and daily adapted plans was 24.4 Gy and 16.5 Gy for the left and right parotid glands, respectively. The Dmean of the reference plan was 25.9 Gy for the left and 16.7 Gy for the right parotid gland. Baseline volume was 31.0 ccm for the right and 34.5 ccm for the left parotid gland. Mean volume (range) during treatment was 30.3 ccm (29.5–32.1) and 31.4 ccm (29.1–34.7). The example was presented as a poster at the congresses of DEGRO and AIRO 2019 by Monica lo Russo, MD (20).
In general, the use of MRI during the course of HNC treatment is beneficial because of the superior soft-tissue contrast, thereby allowing for more precise tumor delineation and margin reduction (22). Therefore, daily online adaptive MR-guided RT could potentially be beneficial for fast responding tumors, e.g., Epstein-Barr positive nasopharyngeal cancers or HPV-positive oropharyngeal cancers (OPC) (23). Also, patients with large respiration- or swallowing induced tumor motion, like in the case of laryngeal carcinoma could benefit from MRgRT (24). But, generally, anatomical changes in the head-and-neck region are slower, e.g., caused by weight loss or target volume changes. Several studies have investigated adaptive RT for head and neck treatments, but not many studies have considered this in the presence of a magnetic field. One study in 2018 has investigated plan quality after weight loss in the presence of a magnetic field (10), showing that the current approaches of offline planning once or twice per week might be sufficient for reducing the dosimetric impact of weight changes.
Besides improved soft-tissue contrast, another advantage of MRgRT is the potential for tumor response monitoring throughout treatment without additional imaging dose (25). One study from 2016 has studied the feasibility of treatment response assessment of head and neck cancer patients using diffusion-weighted (DW) MRI on a Cobalt-60 ViewRay system (26). This study showed variation in tumor apparent diffusion coefficient (ADC) values and consistent brainstem ADC values throughout treatment, potentially allowing for early treatment response assessment. Especially DWI is a promising candidate as a prognostic imaging biomarker in HNC (25, 27–31), but with still conflicting results depending on the parameters analyzed (32). Moreover, early changes in quantitative MR parameters in OAR such as parotid glands may help to predict late toxicity like xerostomia, enabling therapeutic interventions or plan adaptations (33, 34). Thus, MR-Linacs with their capability of longitudinal DWI, may facilitate a biologically adaptive treatment, depending on therapy response for tumors and/or OARs (35).
MR-Guidance in Head and Neck Radiotherapy: Current State of Research
Besides FDG-PET/CT, MRI has become an essential imaging modality in staging of HNC (36–39). Moreover MRI enables a better visualization of the macroscopic tumor for target volume definition and estimation/reduction of PTV margins during radical radiotherapy (40–42), as well as reduced interobserver variability (16, 18, 43–45), although prospective evaluation on primary outcome is lacking. Moreover, offline image registration remains a pitfall, if MRI is not performed in treatment position (46, 47). For treatment on the MR-Linac a simulation scan in RT position is readily available to overcome these difficulties and simultaneously offers one of the main benefits of these platforms.
Repetitive offline MR scans show, especially for HPV-associated OPC, a shrinkage already in the first weeks of therapy (48) up to a complete response in imaging in around 50% of the patients mid-treatment (49). Most of the existing data about MR-guidance in HNC treatment is in the setting of offline MRI, as online MRgRT is still a new development with only a handful of institutions treating patients with HNC on MR-Linacs and only limited data on feasibility of MRgRT in HNC published (8, 9). Tabular overview of published series or recruiting trials is provided in Table 1. With the above mentioned potential benefits for OAR sparing with ART (21) and the obvious advantage of daily MR-guided therapy at hand, a first prospective phase II trial for low risk HPV-associated OPC patients was initialized by the MD Anderson Cancer Center [NCT03224000 (50)]. In this trial, low risk HPV-associated OPC patients will be treated on the MR-Linac with a protocol based adaptation for the high dose volume depending on the shrinkage of the GTV. For adaptation to shrinkage of macroscopic disease an important issue may be the blurring of the tumor borders in MR-images, which is seen in studies of serial MRI during RT (48, 49). Because of this, there might be the necessity to include a GTV to CTV margin to account for these uncertainties, which need to be addressed in proper prospective clinical trials and post hoc analyses of the acquired imaging data with regimens not adapting the high dose target volume.
Table 1.
Overview of published and ongoing studies on MR-linac-based adaptive radiotherapy for head and neck cancer.
| First Author/PI | Year | Study design | Platform | Total patientsn | Timepoints of analysis/adaptation | Aim | Main finding/study endpoint | Relevance |
|---|---|---|---|---|---|---|---|---|
| Raghavan (19) | 2016 | Retrospective analysis | 0.35 T MRI-guided tri-cobalt 60 | 6 | Weekly | Quantify volume changes of parotid glands and GTV | Volume decrease of 31.3% (ipsilateral) and 21.8% (contralateral) and center of mass mitigation with increased dose compared to the reference plan; GTV shrinkage of 38.7% |
Possibility of underestimation of dose to the parotid glands without adaptation regarding in increased risk of xerostomia |
| Chen (8) | 2017 | Prospective institutional registry | 0.35 T MRI-guided tri-cobalt 60 | 12 | No pre-planned adaptation | Feasibility of MRg-SBRT in recurrent HNC | MRg-SBRT feasible, early toxicity within expected range | Due to MR-guidance potential to reduce margins |
| Chen (9) | 2018 | Prospective institutional registry | 0.35 T MRI-guided tri-cobalt 60 | 18 | No pre-planned adaptation | Feasibility of MRgRT in HNC | MRgRT feasible in primary treatment of HNC | Non-randomized data reporting feasibility of MRgRT in HNC with toxicity in expected range |
| Mohamed (21) | 2018 | Prospective planning study | Offline MRI | 5 | Weeks 2, 4, 6 | Adapative RT regarding GTV shrinkage in HPV+ OPC and impact on dose to OAR | GTV shrinkage of up to 100% in primary and 80% in LN; adaptive MRgRT lowers NTCP for Dysphagia and PEG dependency, no change in mean dose to parotid glands | Structured adaptive MRgRT for low risk HPV+ OPC may decrease risk for Dysphagia/PEG dependency |
| Bahig (50) | 2018 | Prospective two-stage Phase II trial | Offline MRI and Unity | 15 + 60 | Weekly adaptation | Adaptive RT for GTV shrinkage in HPV+ OPC with dose reduction | LRC at 6 month | Trial aiming to show safe dose reduction with adaptive MRgRT for shrinking GTV in low risk HPV+ OPC |
| Balermpas (13) | 2019 | Prospective phase II trial (NCT04242459) |
MRIdian | 44 | Weekly adaptation | Reduce incidence of Xerostomia | n.a. | Prospective study trying to show the potential benefit of adaptive MRgRT to reduce Xerostomia in HNC; finding new prognostic imaging biomarkers |
Several more prospective protocols are open for recruitment or will be opened soon to explore the role of MRgRT in HNC in various aspects: prospective basket trials, including various tumors and localizations, explore the feasibility of MRgRT depended on slots and patient burden, due to longer treatment time, noise, and claustrophobia (NCT04172753). Concerning clinical trials dedicated to HNC, the MARTHA-trial investigates potential benefits of weekly offline adaptation, narrow CTV to PTV margins and daily MRg-IGRT for reducing xerostomia in bilaterally irradiated patients over a conventionally fractionated, curative irradiation course of 7 weeks [NCT03972072 (13)]. Patient comfort and compliance will be also evaluated as secondary endpoints. Another trial will test the capability of SBRT in HNC for patients not fit for concomitant radiochemotherapy in combination with immune checkpoint inhibition (DEHART trial, NCT04477759). This is an intriguing approach for combined treatment, especially in HNC with a strong biological rationale, including the immunosensitizing effects of radiotherapy for this indication (51) or the interplay between hypoxia and immunotherapy (52). The number of running prospective trials for HNC cancer is limited so far and the existing studies do not implement identical approaches regarding the frequency and modality of adaptation, i.e., daily versus weekly, or online versus offline adaptive radiotherapy. Up to the present day, no results of prospective trials or registries have been published as a full paper but there were several presentations on congresses (1, 53–55).
Challenges Toward Online Adaptation
Although the number of patients with HNC treated in all of the commercially available MRgRT platforms is increasing worldwide, there still exist several open questions, both in terms of physics and logistics.
One technical challenge in treatments on the MR-Linac that is also relevant in HNC, is the electron return effect (ERE), caused by the influence of the magnetic field on secondary electrons, which results in dose enhancement and attenuation at interfaces between high/low density and low/high density tissue, respectively (56). The effect is more pronounced at higher magnetic field strength. Although this effect is taken into account during plan optimization, air-tissue interfaces, common in HNC-targets, might change during the course of treatment, resulting in variation in dose deposition and risk of hotspots where beams traverse from tissue to air. A recent study investigated the robustness of treatment plans with varying sinus filling (10), and showed that more robust plans can be generated by optimizing with an empty cavity, since the optimizer will then take into account the ERE. A recent planning study including ten patients with hypopharyngeal carcinoma studied the possible effect of a 1.5 T magnetic field on plan quality and dose to OAR. Overall there had been no significant differences in plan quality or doses to OAR, if the plan is optimized for the presence of the magnetic field (57). Nevertheless, the mean and maximal dose to the skin and maximal dose to larynx and trachea was significantly higher, which needs to be critically reviewed, when assessing clinical treatment plans. Moreover, differences in homogeneity and conformity can be observed, when compared to standard VMAT plans for conventional linacs, with unknown impact on outcome or QoL and future trials might need to address these differences, like when IMRT was introduced (58).
Another difficulty in head and neck treatments on the MR-Linac is the limited field of view (FOV), due to the design in which the MR gradient coil is physically split to enable a radiation window. The gap allows for maximum superior-inferior field sizes at isocenter of 22 cm for the Elekta Unity, and 28 cm for the Viewray MRIdian (59). Therefore, patients with extensive, multi-level, lymph node involvement, and/or tumors of the nasopharynx/sinonasal cavities might not be suitable for MR-Linac treatments with a single-isocenter. This, however, depends on the institutional delineation protocols and applied margins, as well on individual anatomic variations. A study from Chuter et al. (60) showed that 66.3% of the HNC-patients with a three dose-level treatment plan could be treated on the Elekta Unity, using a cranio-caudal margin of 1 cm. A reduction of this margin to 5 mm could increase the number of eligible patients by more than 15%. Another recent study showed that 6 out of 110 patients were not eligible for MR-Linac treatment with a single isocenter, including two nasopharynx patients, one oropharynx patient and three paranasal sinus patients (11). The authors stated that neutral neck position, as opposed to extended neck position, is favorable to maximise the number of patients treatable on the MR-Linac. Figure 2 depicts a real-life patient positioning for HNC treatment on both commercially available types of MR-Linacs, implementing neutral neck position and flexible receiver coils over a thermoplastic mask.
Figure 2.
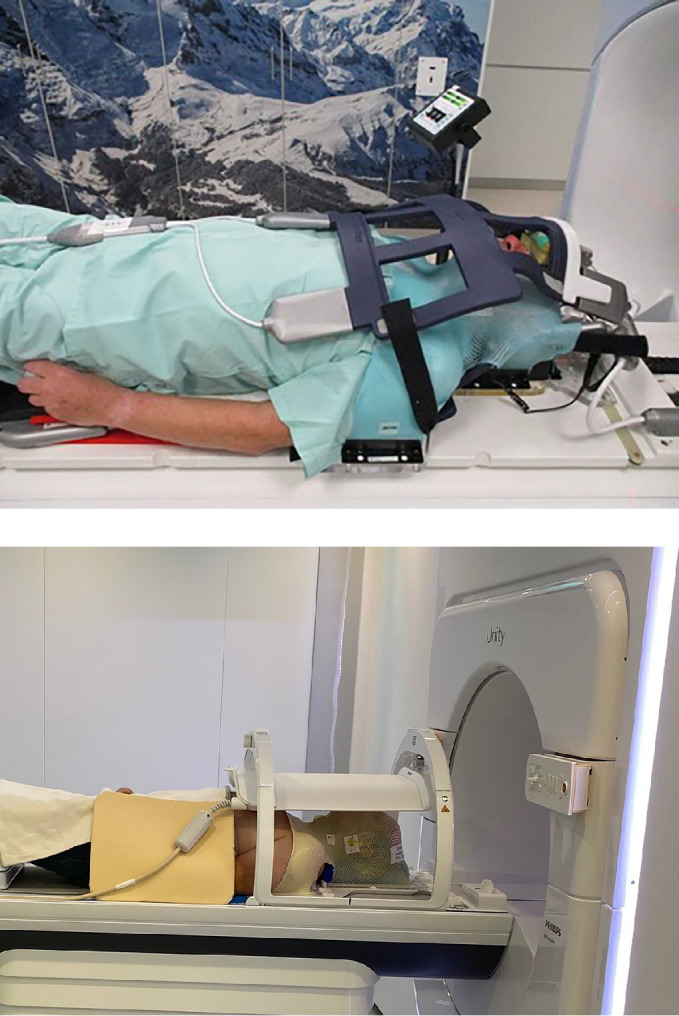
Patient positioning for MR-Linac based treatment for head and neck cancer in the two commercially available systems.
Up to this day, a planning CT is still used for routine treatments in most institutions. However, a straightforward solution for the problem of CT/MR mismatch mentioned above would be an MR-only workflow with the problem of the missing electron density information from the CT. In the adaptive online workflow of the Elekta Unity (Elekta AB, Sweden), a contour based bulk electron density override of structures such as soft tissue, bones and air contoured on the CT and propagated to the daily MR is provided for an online reoptimization. This delineation process is time consuming and error prone and could be overcome by the means of deep learning for bone structure delineation (61–63). When using bulk electron densities for dose calculation, the CT densities of patient positioning aids cannot be used. Therefore, all positioning devices, e.g., headrests, must be contoured with sufficient detail.
Furthermore, there is concern due to the noise for HNC patients on MR-Linacs, as headphones are not compatible with standard masks, so standard foam earplugs with the maximum noise reduction of up to 37 dB is recommended. Today, there is no prospective data published to assess the possible inner ear damage, but clinical experience for HNC patients treated in our institutions so far did not show any toxicity. To the author’s knowledge, the same problem is unsolved for MR-simulations, which are routinely used in daily routine.
Finally, at the present moment, there exist several aspects that make MRgRT for HNC time consuming with currently approximately 30 min needed for applying a single fraction, and 45–50 min if online-adaptation is performed (64, 65). One of the reasons is the limited dose rate due to the larger source isocenter distance on the Unity system (5), although this is more important for SBRT with large doses per fraction compared to conventionally fractionated HNC-treatment. The dose rate of the MRIdian system is 600 cGy/minute at 90 cm SAD and such comparable to that of a conventional linac. This prolonged treatment time leads to limitations regarding the number of patients treated daily and to compliance problems over a 6 or 7 week-course of radiotherapy as is usually performed for HNC treated with curative intent. Nevertheless, some of the reasons for this time- and resource-consuming procedures could be eliminated in the near future. Both commercially available platforms (MRIdian, ViewRay Inc, Oakwood, USA and Elekta Unity, Elekta AB, Stockholm, Sweden) are only capable of delivering step-and-shoot IMRT, but there do not seem to exist any insurmountable hardware limitations for introducing dynamic MLC or VMAT (66), which will lead to significantly faster radiotherapy applications. Moreover, recent research has demonstrated that a “full” online plan adaptation does not always show significant benefits (64) and that a simple plan re-optimization is often enough for providing plans of sufficient quality (7). Applying modern developments in artificial intelligence and machine learning, in order to improve image registration and automated segmentation, could further considerably reduce time for adaptation (67).
The above facts (noise, longer treatment-time etc.) demonstrate that current practice of MRgRT is most times associated with limitations, not only of technical nature (like VMAT versus IMRT), but also with a smaller or larger compromise in terms of patient comfort. This issue becomes even more significant as most of our current treatments are applied over 6–7 weeks.
For the intriguing concept of response or biologically adaptive radiotherapy, e.g., by the means of functional imaging, several important prerequisites, like accuracy and repeatability of the measured values as well as geometrical distortions need to be taken into account. First phantom studies showed that both platforms are capable of meeting these prerequisites (24). Nevertheless, as especially the head and neck area with movement of tissue due to breathing and swallowing as well as air-tissue interfaces and the missing dedicated head and neck coils, in-vivo data for serial DWI on MR-Linacs acquired with the recommended procedures (68, 69) is missing (70).
Discussion
Although MRgRT has advanced to an established modality for treating various tumor types, even for challenging tumor localizations like prostate cancer and moving targets like liver malignancies, implementation of this novel technique for irradiating HNC remains at its infancy. This article summarizes the most important rationales and obstacles behind this IGRT method so far and tries to present future directions of research in this quickly evolving field.
The possible benefits of adaptive MRgRT for HNC are obvious and have been exercised before with means of CT-scans, cone-beam-CT (CBCT) (16), or diagnostic MRI- (22, 45) and PET-imaging (71, 72) to serve as basis for adaptation during the 6-7 week treatment course. There are three main goals of adaptive RT cancer that can be more easily pursued with MRgRT as have been recently summarized by Corradini et al. (5): 1) adaptation to anatomical changes, 2) adaptation to tumor response, and 3) motion management. All of these issues are crucial for an effective and high-quality treatment of HNC and can be easily addressed with the new hybrid MR-Linac-platforms without additional dose exposure. The improved soft-tissue contrastation can provide -compared to CBCT- additional information not only about the external body contour and the tissue/air or tissue/bone interface, but also about relative interfractional changes of organs like the salivary glands or surgical flaps in the postoperative setting. Due to the better visualization and with more advanced adaptation algorithms and motion management strategies, classical irradiation masks may become obsolete potentially enhancing the patients comfort. First proof of principle for dedicated mask free radiotherapy planning for SRS in brain tumors showed good results for the mask free workflow (73). Furthermore, a daily monitoring of and quick reaction to tumor shrinkage, like in the case of viral-induced tumors will allow not only better sparing of OARs, but could pave the way for more elaborate dose-(des-)intensification and dose-painting trials (74), or even temporospatial fractionation approaches. Last but not least, the live, online, PTV-gating and cine-imaging allows for both 4D-planning and intrafractional motion monitoring to compensate for breathing or swallowing movement, an important feature in HNC, e.g., when treating glottic laryngeal cancer (75, 76). However, online motion management is not the only solution for such issues: Regarding motion-depended planning- and dosimetry uncertainties, offline-adaptation in different breathing/swallowing positions and calculation of the dosimetric impact might be an additional solution in these cases. Furthermore, exception gating could be applied in order to stop treatment in case of excessive motion (e.g., caused by coughing).
There still exist hurdles and handicaps in treatment planning and delivery, prohibiting a wider clinical use of MRgRT for HNC, with the most important ones being the lack of dynamic IMRT-approaches such as VMAT or dynamic MLC and the increased treatment delivery time with a potential higher treatment burden for the patient. Yet, technical advances are expected to solve these issues in the near future, making this innovative technique available for most HNC-patients. Until then, careful patient selection is of major importance. Patients with advanced tumors or nodal involvement, bilateral neck irradiation, target in proximity to sensible OARs and moving volumes are the most eager to benefit from MRgRT. Mathematical models to predict clinical benefit and guide slot allocation could facilitate patient selection, similar to the ones developed for proton treatment (77–79). Nevertheless, inter- and intra-fractional changes in head and neck tumors and anatomy do not usually take place as quick as, e.g., in the moving organs of the upper abdomen and as most of the times only conventional or slightly hypofractionated regimens are used, the potential additional benefits of online- over offline-adaptation should be always weighted against an extension of treatment time and compromise of the patient comfort. Until less time-consuming and more comfortable procedures are established, the decision regarding the time-point and frequency of plan adaptation has to be critically discussed, also considering the real clinical benefit. An, e.g., only weekly adaptation could be sufficient for many HN patients. In this case, the images could of course be directly acquired on the MR-Linac. Sufficient quality of these images and an MR-only planning procedure would simplify the process compared to an “external” MR-simulation with or without additional planning CT. Running and future trials should focus on possible toxicity reduction, but also on patient comfort, always involving patient reported outcomes (PROMs). Establishing novel, standardized patient positioning and immobilization devices or even treatment without masks based on the experience gathered by PROMs, as well as decision trees and standard operating procedures for the need of re-planning could facilitate a broader clinical implementation of MRgRT for head and neck cancer.
While there is still only a small number of prospective trials investigating applications of MRgRT for HNC, this is expected to increase in the next few years. Challenging fields of research could be not only the decrease of toxicity and the patient selection, but also the development of more advanced hardware, e.g., allowing for VMAT, or software, e.g., for monitor unit verification (66). Finally, the possibility to have access to daily, repetitive imaging during the whole course of radiotherapy could open completely new dimensions with respect to both functional imaging, like diffusion-weighted-MRI (80, 81), and radiomics (82) for predicting tumor response and normal tissue toxicity. This aspect becomes even more interesting through the possibility of comparison of high- (1.5 T) and low-field (0.35 T) magnetic resonance imaging provided by the different platforms.
This study has several limitations, most of all the non-systematic character of the review. Nevertheless, it is the first attempt to summarize the current stand of knowledge regarding MRgRT for the specific and challenging indication of head and neck cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
PB designed the manuscript structure and supervised the content. SB, DM, and JT devised additional ideas, provided images, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The MRgRT program in Tübingen received funding from the German Research Council (ZI736/2-1), University of Tuebingen.
Conflict of Interest
The authors declare that the Department of Radiation Oncology Tübingen receives within the frame of research agreements financial and technical support as well as sponsoring for travels and scientific symposia from Elekta AB (Stockholm, Sweden), TheraPanacea (Paris, France), Philips GmbH (Best, The Netherlands), Dr. Sennewald Medizintechnik GmbH (München, Germany), PTW Freiburg (Germany) and that the Department of Radiation Oncology of the Zurich University Hospital receives within the frame of research agreements funding for clinical trials from ViewRay Inc, Oakwood, USA. PB received research funding from ViewRay Inc, Oakwood, USA.
Acknowledgments
We would like to thank Dr. Monika lo Russo for providing Figure 1B.
References
- 1. Grégoire V, Guckenberger M, Haustermans K, Lagendijk JJW, Ménard C, Pötter R, et al. Image guidance in radiation therapy for better cure of cancer. Mol Oncol (2020) 14:1470–91. 10.1002/1878-0261.12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chin S, Eccles CL, McWilliam A, Chuter R, Walker E, Whitehurst P, et al. Magnetic resonance-guided radiation therapy: A review. J Med Imaging Radiat Oncol (2020) 64:163–77. 10.1111/1754-9485.12968 [DOI] [PubMed] [Google Scholar]
- 3. Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol (2014) 24:196–9. 10.1016/j.semradonc.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 4. Raaymakers BW, Jürgenliemk-Schulz IM, Bol GH, Glitzner M, Kotte ANTJ, van Asselen B, et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol (2017) 62:L41–50. 10.1088/1361-6560/aa9517 [DOI] [PubMed] [Google Scholar]
- 5. Corradini S, Alongi F, Andratschke N, Belka C, Boldrini L, Cellini F, et al. MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiat Oncol Lond Engl (2019) 14:92. 10.1186/s13014-019-1308-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wen N, Kim J, Doemer A, Glide-Hurst C, Chetty IJ, Liu C, et al. Evaluation of a magnetic resonance guided linear accelerator for stereotactic radiosurgery treatment. Radiother Oncol J Eur Soc Ther Radiol Oncol (2018) 127:460–6. 10.1016/j.radonc.2018.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Timmeren JE, Chamberlain M, Krayenbuehl J, Wilke L, Ehrbar S, Bogowicz M, et al. Treatment plan quality during online adaptive re-planning. Radiat Oncol Lond Engl (2020) 15:203. 10.1186/s13014-020-01641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen AM, Cao M, Hsu S, Lamb J, Mikaeilian A, Yang Y, et al. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Adv Radiat Oncol (2017) 2:167–75. 10.1016/j.adro.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen AM, Hsu S, Lamb J, Yang Y, Agazaryan N, Steinberg ML, et al. MRI-guided radiotherapy for head and neck cancer: initial clinical experience. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex (2018) 20:160–8. 10.1007/s12094-017-1704-4 [DOI] [PubMed] [Google Scholar]
- 10. Chuter RW, Pollitt A, Whitehurst P, MacKay RI, van Herk M, McWilliam A. Assessing MR-linac radiotherapy robustness for anatomical changes in head and neck cancer. Phys Med Biol (2018) 63:125020. 10.1088/1361-6560/aac749 [DOI] [PubMed] [Google Scholar]
- 11. Ng-Cheng-Hin B, Nutting C, Newbold K, Bhide S, McQuaid D, Dunlop A, et al. The impact of restricted length of treatment field and anthropometric factors on selection of head and neck cancer patients for treatment on the MR-Linac. Br J Radiol (2020) 93:20200023. 10.1259/bjr.20200023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinmann A, Alvarez P, Lee H, Court L, Stafford R, Sawakuchi G, et al. MRIgRT head and neck anthropomorphic QA phantom: Design, development, reproducibility, and feasibility study. Med Phys (2020) 47:604–13. 10.1002/mp.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balermpas P. MARTHA-trial: MRI - Guided Adaptive RadioTHerapy for Reducing XerostomiA in Head and Neck Cancer Including Longitudinal Evaluation of the Patient"s Immune Profile Under Radiotherapy. clinicaltrials.gov (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT03972072 (Accessed September 3, 2020).
- 14. Yan D, Lockman D, Martinez A, Wong J, Brabbins D, Vicini F, et al. Computed tomography guided management of interfractional patient variation. Semin Radiat Oncol (2005) 15:168–79. 10.1016/j.semradonc.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 15. Zhang P, Simon A, Rigaud B, Castelli J, Ospina Arango JD, Nassef M, et al. Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol (2016) 120:41–7. 10.1016/j.radonc.2016.05.028 [DOI] [PubMed] [Google Scholar]
- 16. Heukelom J, Fuller CD. Head and Neck Cancer Adaptive Radiation Therapy (ART): Conceptual Considerations for the Informed Clinician. Semin Radiat Oncol (2019) 29:258–73. 10.1016/j.semradonc.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castelli J, Simon A, Lafond C, Perichon N, Rigaud B, Chajon E, et al. Adaptive radiotherapy for head and neck cancer. Acta Oncol Stockh Swed (2018) 57:1284–92. 10.1080/0284186X.2018.1505053 [DOI] [PubMed] [Google Scholar]
- 18. Castelli J, Simon A, Louvel G, Henry O, Chajon E, Nassef M, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol Lond Engl (2015) 10:6. 10.1186/s13014-014-0318-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raghavan G, Kishan AU, Cao M, Chen AM. Anatomic and dosimetric changes in patients with head and neck cancer treated with an integrated MRI-tri-(60)Co teletherapy device. Br J Radiol (2016) 89:20160624. 10.1259/bjr.20160624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abstracts DEGRO 2019 . Strahlenther Onkol, Vol. 195. (2019). pp. 1–218. 10.1007/s00066-019-01465-2. [DOI] [PubMed] [Google Scholar]
- 21. Mohamed ASR, Bahig H, Aristophanous M, Blanchard P, Kamal M, Ding Y, et al. Prospective in silico study of the feasibility and dosimetric advantages of MRI-guided dose adaptation for human papillomavirus positive oropharyngeal cancer patients compared with standard IMRT. Clin Transl Radiat Oncol (2018) 11:11–8. 10.1016/j.ctro.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jager EA, Ligtenberg H, Caldas-Magalhaes J, Schakel T, Philippens ME, Pameijer FA, et al. Validated guidelines for tumor delineation on magnetic resonance imaging for laryngeal and hypopharyngeal cancer. Acta Oncol Stockh Swed (2016) 55:1305–12. 10.1080/0284186X.2016.1219048 [DOI] [PubMed] [Google Scholar]
- 23. Brown E, Porceddu S, Owen R, Harden F. Developing an Adaptive Radiotherapy Technique for Virally Mediated Head and Neck Cancer. J Med Imaging Radiat Sci (2013) 44:134–40. 10.1016/j.jmir.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 24. Bruijnen T, Stemkens B, Terhaard CHJ, Lagendijk JJW, Raaijmakers CPJ, Tijssen RHN. Intrafraction motion quantification and planning target volume margin determination of head-and-neck tumors using cine magnetic resonance imaging. Radiother Oncol (2019) 130:82–8. 10.1016/j.radonc.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 25. King AD, Thoeny HC. Functional MRI for the prediction of treatment response in head and neck squamous cell carcinoma: potential and limitations. Cancer Imaging (2016) 16:23. 10.1186/s40644-016-0080-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Y, Cao M, Sheng K, Gao Y, Chen A, Kamrava M, et al. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med Phys (2016) 43:1369–73. 10.1118/1.4942381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lambrecht M, Van Calster B, Vandecaveye V, De Keyzer F, Roebben I, Hermans R, et al. Integrating pretreatment diffusion weighted MRI into a multivariable prognostic model for head and neck squamous cell carcinoma. Radiother Oncol (2014) 110:429–34. 10.1016/j.radonc.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 28. Noij DP, Pouwels PJW, Ljumanovic R, Knol DL, Doornaert P, de Bree R, et al. Predictive value of diffusion-weighted imaging without and with including contrast-enhanced magnetic resonance imaging in image analysis of head and neck squamous cell carcinoma. Eur J Radiol (2015) 84:108–16. 10.1016/j.ejrad.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 29. Hauser T, Essig M, Jensen A, Gerigk L, Laun FB, Münter M, et al. Characterization and therapy monitoring of head and neck carcinomas using diffusion-imaging-based intravoxel incoherent motion parameters—preliminary results. Neuroradiology (2013) 55:527–36. 10.1007/s00234-013-1154-9 [DOI] [PubMed] [Google Scholar]
- 30. Vandecaveye V, Dirix P, De Keyzer F, Op de Beeck K, Vander Poorten V, Roebben I, et al. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur Radiol (2010) 20:1703–14. 10.1007/s00330-010-1734-6 [DOI] [PubMed] [Google Scholar]
- 31. Leibfarth S, Winter RM, Lyng H, Zips D, Thorwarth D. Potentials and challenges of diffusion-weighted magnetic resonance imaging in radiotherapy. Clin Transl Radiat Oncol (2018) 13:29–37. 10.1016/j.ctro.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peltenburg B, Driessen JP, Vasmel JE, Pameijer FA, Janssen LM, Terhaard CHJ, et al. Pretreatment ADC is not a prognostic factor for local recurrences in head and neck squamous cell carcinoma when clinical T-stage is known. Eur Radiol (2020) 30:1228–31. 10.1007/s00330-019-06426-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marzi S, Farneti A, Vidiri A, Di Giuliano F, Marucci L, Spasiano F, et al. Radiation-induced parotid changes in oropharyngeal cancer patients: the role of early functional imaging and patient–/treatment-related factors. Radiat Oncol (2018) 13:189. 10.1186/s13014-018-1137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stieb S, Elgohari B, Fuller CD. Repetitive MRI of organs at risk in head and neck cancer patients undergoing radiotherapy. Clin Transl Radiat Oncol (2019) 18:131–9. 10.1016/j.ctro.2019.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gurney-Champion OJ, Mahmood F, van Schie M, Julian R, George B, Philippens MEP, et al. Quantitative imaging for radiotherapy purposes. Radiother Oncol (2020) 146:66–75. 10.1016/j.radonc.2020.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nooij RP, Hof JJ, van Laar PJ, van der Hoorn A. Functional MRI for Treatment Evaluation in Patients with Head and Neck Squamous Cell Carcinoma: A Review of the Literature from a Radiologist Perspective. Curr Radiol Rep (2018) 6:2. 10.1007/s40134-018-0262-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adams S, Baum RP, Stuckensen T, Bitter K, Hör G. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med (1998) 25:1255–60. 10.1007/s002590050293 [DOI] [PubMed] [Google Scholar]
- 38. Wippold FJ2. Head and neck imaging: the role of CT and MRI. J Magn Reson Imaging JMRI (2007) 25:453–65. 10.1002/jmri.20838 [DOI] [PubMed] [Google Scholar]
- 39. Wong KH, Panek R, Bhide SA, Nutting CM, Harrington KJ, Newbold KL. The emerging potential of magnetic resonance imaging in personalizing radiotherapy for head and neck cancer: an oncologist’s perspective. Br J Radiol (2017) 90:20160768. 10.1259/bjr.20160768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chung N-N, Ting L-L, Hsu W-C, Lui LT, Wang P-M. Impact of magnetic resonance imaging versus CT on nasopharyngeal carcinoma: primary tumor target delineation for radiotherapy. Head Neck (2004) 26:241–6. 10.1002/hed.10378 [DOI] [PubMed] [Google Scholar]
- 41. Grégoire V, Evans M, Le Q-T, Bourhis J, Budach V, Chen A, et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol (2018) 126:3–24. 10.1016/j.radonc.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 42. Daisne J-F, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology (2004) 233:93–100. 10.1148/radiol.2331030660 [DOI] [PubMed] [Google Scholar]
- 43. Ahmed M, Schmidt M, Sohaib A, Kong C, Burke K, Richardson C, et al. The value of magnetic resonance imaging in target volume delineation of base of tongue tumours–a study using flexible surface coils. Radiother Oncol (2010) 94:161–7. 10.1016/j.radonc.2009.12.021 [DOI] [PubMed] [Google Scholar]
- 44. Rasch CRN, Steenbakkers RJHM, Fitton I, Duppen JC, Nowak PJCM, Pameijer FA, et al. Decreased 3D observer variation with matched CT-MRI, for target delineation in Nasopharynx cancer. Radiat Oncol Lond Engl (2010) 5:21. 10.1186/1748-717X-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ligtenberg H, Jager EA, Caldas-Magalhaes J, Schakel T, Pameijer FA, Kasperts N, et al. Modality-specific target definition for laryngeal and hypopharyngeal cancer on FDG-PET, CT and MRI. Radiother Oncol (2017) 123:63–70. 10.1016/j.radonc.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 46. Paulson ES, Crijns SPM, Keller BM, Wang J, Schmidt MA, Coutts G, et al. Consensus opinion on MRI simulation for external beam radiation treatment planning. Radiother Oncol (2016) 121:187–92. 10.1016/j.radonc.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 47. Kiser K, Meheissen MAM, Mohamed ASR, Kamal M, Ng SP, Elhalawani H, et al. Prospective quantitative quality assurance and deformation estimation of MRI-CT image registration in simulation of head and neck radiotherapy patients. Clin Transl Radiat Oncol (2019) 18:120–7. 10.1016/j.ctro.2019.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Subesinghe M, Scarsbrook AF, Sourbron S, Wilson DJ, McDermott G, Speight R, et al. Alterations in anatomic and functional imaging parameters with repeated FDG PET-CT and MRI during radiotherapy for head and neck cancer: a pilot study. BMC Cancer (2015) 15:137. 10.1186/s12885-015-1154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding Y, Hazle JD, Mohamed ASR, Frank SJ, Hobbs BP, Colen RR, et al. Intravoxel incoherent motion imaging kinetics during chemoradiotherapy for human papillomavirus-associated squamous cell carcinoma of the oropharynx: preliminary results from a prospective pilot study. NMR BioMed (2015) 28:1645–54. 10.1002/nbm.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bahig H, Yuan Y, Mohamed ASR, Brock KK, Ng SP, Wang J, et al. Magnetic Resonance-based Response Assessment and Dose Adaptation in Human Papilloma Virus Positive Tumors of the Oropharynx treated with Radiotherapy (MR-ADAPTOR): An R-IDEAL stage 2a-2b/Bayesian phase II trial. Clin Transl Radiat Oncol (2018) 13:19–23. 10.1016/j.ctro.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. von der Grün J, Rödel F, Brandts C, Fokas E, Guckenberger M, Rödel C, et al. Targeted Therapies and Immune-Checkpoint Inhibition in Head and Neck Squamous Cell Carcinoma: Where Do We Stand Today and Where to Go? Cancers (2019) 11. 10.3390/cancers11040472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eckert F, Zwirner K, Boeke S, Thorwarth D, Zips D, Huber SM. Rationale for Combining Radiotherapy and Immune Checkpoint Inhibition for Patients With Hypoxic Tumors. Front Immunol (2019) 10:407. 10.3389/fimmu.2019.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blinde S, Mohamed ASR, Al-Mamgani A, Newbold K, Karam I, Robbins JR, et al. Interobserver Variation in the International MRI Linear Accelerator Oropharyngeal Carcinoma Delineation Study. Int J Radiat Oncol Biol Phys (2018) 100:1362. 10.1016/j.ijrobp.2017.12.143 [DOI] [Google Scholar]
- 54. Christiansen RL, Johansen J, Zukauskaite R, Hansen CR, Bertelsen AS, Hansen O, et al. Accuracy of Automatic Structure Propagation for Daily High-Field Magnetic Resonance Image-Guided Head and Neck Radiotherapy. Int J Radiat Oncol Biol Phys (2019) 105:E786. 10.1016/j.ijrobp.2019.06.751 [DOI] [PubMed] [Google Scholar]
- 55. McDonald BA, Vedam S, Yang J, Wang J, Castillo P, Lee B, et al. Initial Feasibility and Clinical Implementation of Daily MR-guided Adaptive Head and Neck Cancer Radiotherapy on a 1.5T MR-Linac System: Prospective R-IDEAL 2a/2b Systematic Clinical Evaluation of Technical Innovation. Int J Radiation Oncol Biol Phys (2020) 1e13. 10.1016/j.ijrobp.2020.12.015 [DOI] [PMC free article] [PubMed]
- 56. Raaijmakers AJE, Raaymakers BW, Lagendijk JJW. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: dose increase at tissue-air interfaces in a lateral magnetic field due to returning electrons. Phys Med Biol (2005) 50:1363–76. 10.1088/0031-9155/50/7/002 [DOI] [PubMed] [Google Scholar]
- 57. Xia W, Zhang K, Li M, Tian Y, Men K, Wang J, et al. Impact of Magnetic Field on Dose Distribution in MR-Guided Radiotherapy of Head and Neck Cancer. Front Oncol (2020) 10:1739. 10.3389/fonc.2020.01739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eisbruch A, Harris J, Garden AS, Chao CKS, Straube W, Harari PM, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys (2010) 76:1333–8. 10.1016/j.ijrobp.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl Radiat Oncol (2019) 18:98–101. 10.1016/j.ctro.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chuter RW, Whitehurst P, Choudhury A, van Herk M, McWilliam A. Technical Note: Investigating the impact of field size on patient selection for the 1.5T MR-Linac. Med Phys (2017) 44:5667–71. 10.1002/mp.12557 [DOI] [PubMed] [Google Scholar]
- 61. Klages P, Benslimane I, Riyahi S, Jiang J, Hunt M, Deasy JO, et al. Patch-based generative adversarial neural network models for head and neck MR-only planning. Med Phys (2020) 47:626–42. 10.1002/mp.13927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jonsson J, Nyholm T, Söderkvist K. The rationale for MR-only treatment planning for external radiotherapy. Clin Transl Radiat Oncol (2019) 18:60–5. 10.1016/j.ctro.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chuter R, Prestwich R, Bird D, Scarsbrook A, Sykes J, Wilson D, et al. The use of deformable image registration to integrate diagnostic MRI into the radiotherapy planning pathway for head and neck cancer. Radiother Oncol (2017) 122:229–35. 10.1016/j.radonc.2016.07.016 [DOI] [PubMed] [Google Scholar]
- 64. Tetar SU, Bruynzeel AME, Lagerwaard FJ, Slotman BJ, Bohoudi O, Palacios MA. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imaging Radiat Oncol (2019) 9:69–76. 10.1016/j.phro.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Finazzi T, Palacios MA, Spoelstra FOB, Haasbeek CJA, Bruynzeel AME, Slotman BJ, et al. Role of On-Table Plan Adaptation in MR-Guided Ablative Radiation Therapy for Central Lung Tumors. Int J Radiat Oncol Biol Phys (2019) 104:933–41. 10.1016/j.ijrobp.2019.03.035 [DOI] [PubMed] [Google Scholar]
- 66. 4Graves SA, Snyder JE, Boczkowski A, St-Aubin J, Wang D, Yaddanapudi S, et al. Commissioning and performance evaluation of RadCalc for the Elekta unity MRI-linac. J Appl Clin Med Phys (2019) 20:54–62. 10.1002/acm2.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Simon A, Nassef M, Rigaud B, Cazoulat G, Castelli J, Lafond C, et al. Roles of Deformable Image Registration in adaptive RT: From contour propagation to dose monitoring. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf (2015) 2015:5215–8. 10.1109/EMBC.2015.7319567 [DOI] [PubMed] [Google Scholar]
- 68. O’Connor JPB, Aboagye EO, Adams JE, Aerts HJWL, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol (2017) 14:169–86. 10.1038/nrclinonc.2016.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kooreman ES, van Houdt PJ, Keesman R, Pos FJ, van Pelt VWJ, Nowee ME, et al. ADC measurements on the unity MR-linac – a recommendation on behalf of the elekta unity MR-linac consortium. Radiother Oncol (2020) 153:106–13. 10.1016/j.radonc.2020.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thorwarth D, Ege M, Nachbar M, Mönnich D, Gani C, Zips D, et al. Quantitative Magnetic Resonance Imaging on Hybrid Magnetic Resonance Linear Accelerators: Perspective on technical and clinical validation. Phys Imaging Radiat Oncol (2020) 16:69–73. 10.1016/j.phro.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bussink J, van Herpen CML, Kaanders JHAM, Oyen WJG. PET-CT for response assessment and treatment adaptation in head and neck cancer. Lancet Oncol (2010) 11:661–9. 10.1016/S1470-2045(09)70353-5 [DOI] [PubMed] [Google Scholar]
- 72. Mowery YM, Vergalasova I, Rushing CN, Choudhury KR, Niedzwiecki D, Wu Q, et al. Early (18)F-FDG-PET Response During Radiation Therapy for HPV-Related Oropharyngeal Cancer May Predict Disease Recurrence. Int J Radiat Oncol Biol Phys (2020) 108:969–76. 10.1016/j.ijrobp.2020.08.029 [DOI] [PubMed] [Google Scholar]
- 73. Nagtegaal SHJ, van Lier ALHMW, den Boer AA, Kramer MCA, Fanetti G, Eppinga WSC, et al. Does an immobilization mask have added value during planning magnetic resonance imaging for stereotactic radiotherapy of brain tumours? Phys Imaging Radiat Oncol (2020) 13:7–13. 10.1016/j.phro.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen S, Yan D, Qin A, Maniawski P, Krauss DJ, Wilson GD. Effect of Uncertainties in Quantitative (18) F-FDG PET/CT Imaging Feedback for Intratumoral Dose Response Assessment and Dose Painting by Number. Med Phys (2020) 47:5681-92. 10.1002/mp.14482 [DOI] [PubMed] [Google Scholar]
- 75. Levendag PC, Teguh DN, Keskin-Cambay F, Al-Mamgani A, van Rooij P, Astreinidou E, et al. Single vocal cord irradiation: a competitive treatment strategy in early glottic cancer. Radiother Oncol (2011) 101:415–9. 10.1016/j.radonc.2011.05.026 [DOI] [PubMed] [Google Scholar]
- 76. Elicin O, Terribilini D, Shelan M, Volken W, Mathier E, Dal Pra A, et al. Primary tumor volume delineation in head and neck cancer: missing the tip of the iceberg? Radiat Oncol Lond Engl (2017) 12:102. 10.1186/s13014-017-0838-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Austin AM, Douglass MJJ, Nguyen GT, Penfold SN. Patient selection for proton therapy: a radiobiological fuzzy Markov model incorporating robust plan analysis. Phys Eng Sci Med (2020) 43:493–503. 10.1007/s13246-020-00849-4 [DOI] [PubMed] [Google Scholar]
- 78. Mee T, Kirkby NF, Kirkby KJ. Mathematical Modelling for Patient Selection in Proton Therapy. Clin Oncol (2018) 30:299–306. 10.1016/j.clon.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 79. Bijman RG, Breedveld S, Arts T, Astreinidou E, de Jong MA, Granton PV, et al. Impact of model and dose uncertainty on model-based selection of oropharyngeal cancer patients for proton therapy. Acta Oncol (2017) 56:1444–50. 10.1080/0284186X.2017.1355113 [DOI] [PubMed] [Google Scholar]
- 80. Wiedenmann N, Grosu A-L, Büchert M, Rischke HC, Ruf J, Bielak L, et al. The utility of multiparametric MRI to characterize hypoxic tumor subvolumes in comparison to FMISO PET/CT. Consequences for diagnosis and chemoradiation treatment planning in head and neck cancer. Radiother Oncol (2020) 150:128–35. 10.1016/j.radonc.2020.06.013 [DOI] [PubMed] [Google Scholar]
- 81. Qamar S, King AD, Ai Q-YH, So TY, Mo FKF, Chen W, et al. Pre-treatment intravoxel incoherent motion diffusion-weighted imaging predicts treatment outcome in nasopharyngeal carcinoma. Eur J Radiol (2020) 129:109127. 10.1016/j.ejrad.2020.109127 [DOI] [PubMed] [Google Scholar]
- 82. Tanadini-Lang S, Balermpas P, Guckenberger M, Pavic M, Riesterer O, Vuong D, et al. Radiomic biomarkers for head and neck squamous cell carcinoma. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al (2020) 196:868–78. 10.1007/s00066-020-01638-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.