This cross-sectional study evaluates the reproducibility of a recently reported association between sex and mild ABCA4 alleles in patients with Stargardt disease.
Key Points
Question
Are some ABCA4 alleles disproportionately associated with female sex among patients with autosomal recessive Stargardt disease (STGD1)?
Findings
In this cross-sectional study, analysis of 644 patients with genetically confirmed STGD1 did not support the recently reported female predilection among patients with mild ABCA4 alleles.
Meaning
This independent analysis did not support sex as a potential disease-modifying variable in STGD1.
Abstract
Importance
Probing differences in disease prevalence between sexes is challenging, especially in mendelian diseases. Independent replication of any association study is warranted.
Objective
To evaluate whether the recently reported association between sex and mild ABCA4 alleles among patients with autosomal recessive Stargardt disease (STGD1) is reproducible.
Design, Setting, and Participants
Sequencing and clinical data from 644 unrelated patients with genetically confirmed STGD1 were analyzed in a cross-sectional study at the Department of Ophthalmology, Columbia University, New York, New York. Data were collected from June 1999 to October 2020.
Main Outcomes and Measures
Sex, best-corrected visual acuity, and age at onset among patients with STGD1 with and without mild ABCA4 alleles.
Results
A total of 644 patients with STGD1 with at least 2 pathogenic variants were included in the study. The mean (SD) age was 38.6 (17.2) years, and 352 participants (54.7%) were female. The proportion of women was slightly higher in the entire cohort and in most allele categories, although none of the differences were statistically significant. The proportion of women carrying the c.5603A>T p.(Asn1868Ile) allele was 7% (95% CI, −9 to 23) higher than in the subgroup not carrying any mild alleles (P = .32). The proportion of women carrying the c.5882G>A p.(Gly1961Glu) allele was 2% (95% CI, −12 to 15) higher than in the subgroup not carrying any mild alleles (P = .77). The difference between the total mild allele subcohort and the no mild allele subcohort was 3% (95% CI, −8 to 14; P = .48). Compared with patients in the no mild allele category, patients with mild alleles exhibited significantly delayed disease onset (mean [SD] age, 23.1 [11.6] for those with the c.5882G>A allele and 31.7 [13.5] years for those with the c.5603A>T allele vs 18.6 [11.8] years for those with no mild alleles; P < .001) and preserved visual acuity (5882G>A subgroup: mean [SD] logMAR, 0.65 [0.66]; 95% CI, 0.63-0.68; c.5603A>T subgroup: 0.64 [0.39]; 95% CI, 0.58-0.70; those with no mild alleles: 1.00 [0.57]; 95% CI, 0.96-1.03; P < .001).
Conclusions and Relevance
This independent analysis of a larger cohort of individuals with Stargardt disease did not support the association between sex and certain mild ABCA4 alleles. While sex is undoubtedly an important variable in medicine, its putative association with clinical outcomes should be rigorously scrutinized.
Introduction
Autosomal recessive Stargardt disease (STGD1) is the most common form of inherited retinal degeneration caused by mutations in the ABCA4 (OMIM 601691) locus.1 While the disease exhibits exceptional clinical heterogeneity, to our knowledge, all cases of STGD1 can be explained by variation in ABCA4. However, an article by Runhart et al2 reported an association between certain mild ABCA4 alleles and female sex, designating sex as a potential contributing factor in the multifactorial etiology of STGD1. To our knowledge, no such association exists for any other mendelian eye disease. Sex differences in disease cohorts are not an uncommon observation in the expanding epidemiological literature.3 However, such differences are often because of many factors. A meta-analysis4 of 77 published studies associating sex differences with genetic diseases found that most were inappropriately documented, not methodologically robust, or simply irreproducible. Narrowing the perspective to retinal disease, one finds similar deficiencies. Several large-scale studies of age-related macular degeneration (AMD), a nonmendelian multifactorial disease, have reported female prevalence in their respective cohorts.5,6 Data from 3 major population-based studies—the Beaver Dam Eye Study, the Blue Mountain Eye Study, and the Rotterdam Study—yielded a combined odds ratio of 1.15 (95% CI, 1.10-1.21) with increased risk of AMD in women.7 Yet other, more recent, large-scale studies found no female predilection in AMD.8,9 Accordingly, neither the American Academy of Ophthalmology10 nor the World Health Organization (2019 report) consider female sex a significant risk factor for AMD. A priori knowledge of such patterns warrants caution against drawing conclusions in studies without rigorous replication and careful consideration of confounding factors.
Methods
All study participants provided written consent under Columbia University Institutional Review Board protocol AAAI9906 and were not compensated for their participation. All procedures adhered to tenets established in the Declaration of Helsinki. In this cross-sectional study, complete ophthalmic examinations for all patients included slitlamp and dilated fundus examinations, retinal imaging, and full-field electroretinogram testing. ABCA4 sequencing was performed and analyzed as previously described.11 Patient sex was self-reported, obtained from written questionnaires on demographic and clinical characteristics submitted by patients prior to enrolling. All detected possibly pathogenic variants and their familial segregation were analyzed and confirmed by Sanger sequencing. Data were collected from June 1999 to October 2020 and analyzed from September to October 2020. Statistical analyses were performed by 2-tailed Fisher exact test and Mann-Whitney U test using R version 4.0.3 (the R Foundation). Significance was set at P < .05.
Results
A total of 644 unrelated patients with genetically confirmed STGD1 at Columbia University, New York, New York, were included in this analysis. The mean (SD) age at examination was 38.6 (17.2) years, and age was similar between women and men within and across each allele category. A total of 352 participants (54.7%) were female. We analyzed the sex distribution and clinical attributes across specific categories of ABCA4 variants (Table). While there was a slightly higher proportion of women in all reported categories (3% for the total subcohort and 2% to 7% for the 2 variants), the difference was not statistically significant. Specifically, the proportion of women carrying the c.5603A>T, p.(Asn1868Ile) allele was 7% (95% CI, −9 to 23) higher than in the subgroup not carrying any mild alleles (P = .32). The proportion of women carrying the c. 5882G>A p.(Gly1961Glu) allele was 2% (95% CI, −12 to 15) higher than in the subgroup not carrying any mild alleles (2-tailed Fisher exact test, P = .77). The difference was 3% (95% CI, −8 to 14; P = .48) between the total mild allele and no mild allele categories (Table).
Table. Sex Distribution Across ABCA4 Allele Categories in 644 Patients With Biallelic Stargardt Disease at Columbia University.
| Mild ABCA4 allele | No. (%)a | |
|---|---|---|
| Women | Men | |
| c.5603A>T | 48 (60.0) | 32 (40.0) |
| c.5882G>A | 82 (54.7) | 68 (45.3) |
| c.5714+5G>A | 16 (59.3) | 11.0 (40.7) |
| c.4253+43G>A | 6 | 9 |
| c.3113C>T | 8 | 4 |
| c.6089G>A | 6 | 4 |
| c.769-784C>Tb | 0 | 0 |
| c.2588G>Cb | 0 | 0 |
| Total mild allele | 166 (56.5) | 128 (43.5) |
| No mild allele | 186 (53.1) | 164 (46.9) |
| Total ABCA4 biallelic | 352 (54.7) | 292 (45.3) |
Percentages are presented for 10 or more patients to avoid implying a greater effect and in keeping with Runhart et al.2
These variants have not been identified as causal alleles in noncomplex form.
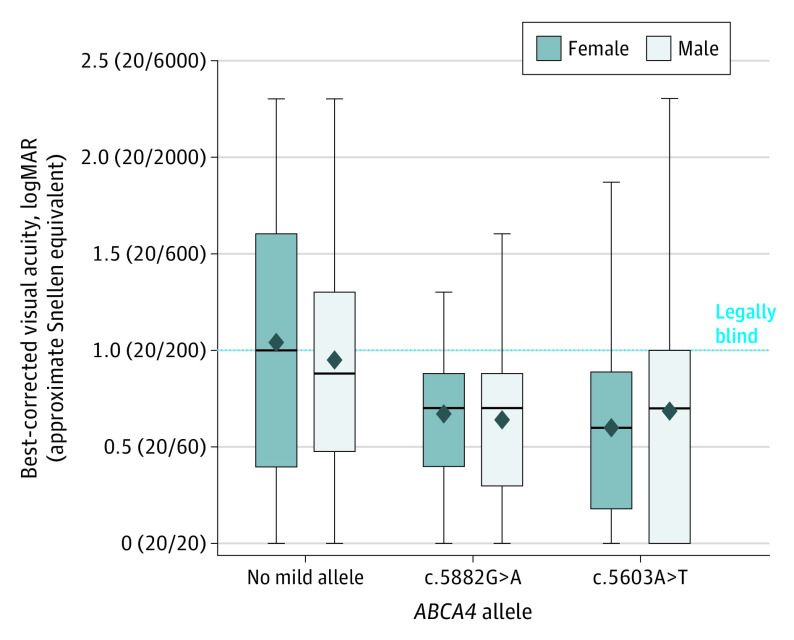
Age at onset was significantly younger in patients with no mild alleles (mean [SD] 18.6 [11.8] years; 95% CI, 17.6-19.5) compared with those with c.5882G>A alleles (mean [SD], 23.1 [11.6] years; 95% CI, 21.8-24.4; Mann Whitney U test, P = .007) and c.5603A>T alleles (mean [SD], 31.7 [13.5] years; 95% CI, 29.9-33.6; Mann-Whitney U test, P < .001), irrespective of sex. Mean (SD) best-corrected visual acuity in both eyes was also significantly poorer in the no mild allele subgroup (logMAR, 1.00 [0.57]; 95% CI, 0.96-1.03) compared with the 5882G>A subgroup (logMAR, 0.65 [0.66]; 95% CI, 0.63-0.68; P < .001) and the c.5603A>T subgroup (logMAR, 0.64 [0.39]; 95% CI, 0.58-0.70; P < .001), irrespective of sex (Figure).
Figure. Distribution of Best-Corrected Visual Acuity in Patients With No Mild Alleles, the c.5882G>A p.(Gly1961Glu) Allele, and the c.5603A>T p.(Asn1868Ile) Allele in ABCA4.
The box plots represent the distribution of best-corrected visual acuities of patients with autosomal recessive Stargardt disease. In each box plot, mean values are denoted by diamonds, and median values are denoted by midlines. Upper and lower limits of the box plots show the interquartile ranges. Whiskers indicate maximum and minimum values. Best-corrected visual acuity of each eye was measured by Snellen scale and converted to logMAR. The dotted line marks the lower limit of legal blindness, which is defined as 20/200 or worse (logMAR ≥ 1.0). A logMAR of 2.0 (20/2000) indicates counting fingers, and a logMAR of 2.5 (20/6000) indicates hand motions.
Discussion
While observed sex differences in disease cohorts may occur, conclusions about causal relationships should be made judiciously. Moreover, ascertaining a biological basis for this phenomenon is well beyond the scope of population-based cohort studies. We should therefore assess whether factors not directly linked to anatomical or genomic differences can yield such results. One consideration is selection bias. For various socioeconomic reasons, it has been extensively reported12,13 that women exhibit different health-seeking behavior compared with men in a way that may lead to overestimation of disease prevalence in women. Runhart et al2 acknowledged this phenomenon but disregarded its potential effect because the reference group of patients without mild alleles came from the same cohort; it would seem the authors assumed that the clinical manifestations of mild alleles compared with the clinical manifestation of severe alleles would have no bearing on the likelihood that certain patient groups would seek medical attention. To better understand this, it is necessary to look at the clinical attributes of each ABCA4 allele. As shown in their article,2 both groups of patients with mild alleles experienced a much later onset of disease (median age, approximately 20 years for c.5882G>A and approximately 41 years for c.5603A>T) compared with the reference group (median age, 13 years). Analysis of our data confirmed this observation. So was the health-seeking behavior the same in all groups? No, for a simple reason: patients with early-onset STGD1 (ie, those without mild alleles) experienced profound vision loss in childhood (Figure), which necessitated medical attention regardless of their desire. This choice was also parentally governed. For these 2 reasons, it is unlikely there was a selection bias from health-seeking behavior in this group. This cannot be said for individuals with much milder symptoms who become aware of their disease in adulthood, especially late adulthood for those with the c.5603A>T allele. In fact, the c.5603A>T allele phenotype would likely lend itself to sex-related disparities because of the attributes this phenotype shares with AMD, a factor that is often associated with the misdiagnosis of STGD1 as AMD.14 The assumption that socioeconomic and behavioral factors would have a singular effect across all ABCA4 genotypes is wrong, given that ABCA4 disease occupies a broad clinical spectrum and extends across age groups.14
Finally, it is important to consider why women should be more affected than men with a mendelian retinal disease caused by mutations in a transporter of vitamin A derivatives in the visual cycle. The authors2 propose a multifactorial etiology for ABCA4 disease, although no data support this proposition. The only complex traits with reported sex imbalance are AMD and central serous chorioretinopathy, in which hormonal imbalance is a proven cause.15 All data, along with our findings of no significant association, underscore the implausibility that STGD1 in approximately 25% of all patients “should be considered a polygenic or multifactorial disease rather than a disease caused by ABCA4 gene mutations alone.”2
Limitations
This study had limitations. Patients enrolled in our study were mainly recruited through 2 tertiary retinal disease clinics—Edward S. Harkness Eye Institute of Columbia University Medical Center, New York, New York, and The Chicago Lighthouse, Chicago, Illinois—and thus may not be representative of STGD1 across all populations. The proportion of observed features (eg, sex) are beholden to a number of factors, such as differences in health-seeking behavior between sexes.
Age at onset was self-reported by all patients as the time symptoms were first noticed. The variability and subjectivity associated with these data warrant careful consideration as they may not reflect the actual time of disease onset. Best-corrected visual acuity may vary according to factors such as ambient lighting and the efforts of the patient and test facilitator. While a visual acuity test provides a reasonable assessment of central macular health, it may not reflect disease changes in other parts of the retina.
Conclusions
In summary, our independent analysis of a larger cohort did not replicate the previously reported sex disparity in STGD1.2 Therefore, we cannot support the suggestion that “sex should be considered as a potential disease-modifying variable in both basic research and clinical trials on STGD1.”2 Unexplained sex-associated incongruities in disease are frequently observed across clinical and biomedical research, but it is difficult to establish their true underlying basis.
References
- 1.Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236-246. doi: 10.1038/ng0397-236 [DOI] [PubMed] [Google Scholar]
- 2.Runhart EH, Khan M, Cornelis SS, et al. ; ABCA4 Disease Consortium Study Group . Association of sex with frequent and mild ABCA4 alleles in Stargardt disease. JAMA Ophthalmol. 2020;138(10):1035-1042. doi: 10.1001/jamaophthalmol.2020.2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911-922. doi: 10.1038/nrg2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patsopoulos NA, Tatsioni A, Ioannidis JP. Claims of sex differences: an empirical assessment in genetic associations. JAMA. 2007;298(8):880-893. doi: 10.1001/jama.298.8.880 [DOI] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research Group . Risk factors associated with age-related macular degeneration. a case-control study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study report number 3. Ophthalmology. 2000;107(12):2224-2232. doi: 10.1016/s0161-6420(00)00409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119(3):571-580. doi: 10.1016/j.ophtha.2011.09.027 [DOI] [PubMed] [Google Scholar]
- 7.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108(4):697-704. doi: 10.1016/S0161-6420(00)00580-7 [DOI] [PubMed] [Google Scholar]
- 8.Buch H, Nielsen NV, Vinding T, Jensen GB, Prause JU, la Cour M. 14-Year incidence, progression, and visual morbidity of age-related maculopathy: the Copenhagen City Eye Study. Ophthalmology. 2005;112(5):787-798. doi: 10.1016/j.ophtha.2004.11.040 [DOI] [PubMed] [Google Scholar]
- 9.Laitinen A, Laatikainen L, Härkänen T, Koskinen S, Reunanen A, Aromaa A. Prevalence of major eye diseases and causes of visual impairment in the adult Finnish population: a nationwide population-based survey. Acta Ophthalmol. 2010;88(4):463-471. doi: 10.1111/j.1755-3768.2009.01566.x [DOI] [PubMed] [Google Scholar]
- 10.Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration preferred practice pattern. Ophthalmology. 2020;127(1):1-P65. doi: 10.1016/j.ophtha.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 11.Zernant J, Schubert C, Im KM, et al. Analysis of the ABCA4 gene by next-generation sequencing. Invest Ophthalmol Vis Sci. 2011;52(11):8479-8487. doi: 10.1167/iovs.11-8182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deveugele M, Derese A, van den Brink-Muinen A, Bensing J, De Maeseneer J. Consultation length in general practice: cross sectional study in six European countries. BMJ. 2002;325(7362):472. doi: 10.1136/bmj.325.7362.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract. 2016;17:38. doi: 10.1186/s12875-016-0440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cremers FPM, Lee W, Collin RWJ, Allikmets R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog Retin Eye Res. 2020;79:100861. doi: 10.1016/j.preteyeres.2020.100861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82-118. doi: 10.1016/j.preteyeres.2015.05.003 [DOI] [PubMed] [Google Scholar]