Key Points
Question
Can tislelizumab in combination with chemotherapy offer superior clinical benefit compared with chemotherapy alone as first-line treatment for patients with advanced squamous non–small-cell lung cancer?
Findings
In this phase 3 randomized clinical trial, tislelizumab plus chemotherapy resulted in significant improvement of progression-free survival and objective response rates compared with chemotherapy alone and demonstrated a manageable safety/tolerability profile. In exploratory analyses, neither progression-free survival nor objective response rates were significantly associated with programmed cell death 1 ligand 1 expression.
Meaning
The results of this trial suggest that tislelizumab in combination with chemotherapy is an appropriate first-line treatment option in patients with advanced squamous non–small-cell lung cancer.
Abstract
Importance
This study demonstrates that tislelizumab in combination with chemotherapy is associated with improved progression-free survival (PFS) in patients with advanced squamous non–small-cell lung cancer (sq-NSCLC).
Objective
To assess the efficacy and safety/tolerability of tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for patients with advanced sq-NSCLC.
Design, Setting, and Participants
This open-label, randomized phase 3 clinical trial was conducted at 46 sites in China between July 2018 and June 2019 and included patients with treatment-naive, histologically confirmed stage IIIB/IV sq-NSCLC. The data cutoff for these analyses was December 6, 2019; data extraction occurred on January 7, 2020.
Interventions
Patients were randomized (1:1:1) to receive 1 of the following regimens intravenously on a 21-day cycle: tislelizumab (200 mg, day 1) plus paclitaxel (175 mg/m2, day 1) and carboplatin (area under the concentration of 5, day 1) (arm A); tislelizumab plus nab-paclitaxel (100 mg/m2, days 1, 8, and 15) and carboplatin (arm B); and paclitaxel and carboplatin (arm C). Patients were stratified by disease stage and tumor programmed cell death 1 ligand 1 (PD-L1) expression (<1% vs 1%-49% vs ≥50%).
Main Outcomes and Measures
The primary end point was progression-free survival (PFS) assessed by an independent review committee (IRC). Secondary end points included overall survival, investigator-assessed (INV) PFS, IRC-assessed objective response rate (ORR), and IRC-assessed duration of response, as well as the incidence and severity of adverse events (AEs).
Results
Overall, 355 patients (median [range] age, 62 [34-74] years; 330 men [91.7%]) with sq-NSCLC received treatment. After a median study follow-up of 8.6 months (95% CI, 8.1-9.0 months), IRC-assessed PFS was significantly improved with tislelizumab plus chemotherapy (arm A, 7.6 months; arm B, 7.6 months) vs chemotherapy alone (arm C, 5.5 months; hazard ratios were 0.524 (95% CI, 0.370-0.742; P < .001 [A vs C]) and 0.478 (95% CI, 0.336-0.679; P < .001 [B vs C]). Higher IRC-assessed ORR and longer IRC-assessed duration of response were observed in arms A (72.5%; 8.2 months) and B (74.8%; 8.6 months) vs C (49.6%; 4.2 months). No association was observed between PD-L1 expression and IRC-assessed PFS or ORR. Discontinuation of any treatment because of AEs was reported in 15 (12.5%; arm A), 35 (29.7%; arm B), and 18 (15.4%; arm C) patients. In each arm, the most common grade of 3 or greater AE was decreased neutrophil levels, which aligned with known chemotherapy toxic effects. Six treatment-related AEs leading to death occurred; however, no deaths were solely attributed to tislelizumab.
Conclusions and Relevance
In this phase 3 randomized clinical trial, adding tislelizumab to chemotherapy was associated with significantly prolonged IRC-assessed PFS, higher IRC-assessed ORRs, and a manageable safety/tolerability profile in patients with advanced sq-NSCLC, regardless of PD-L1 expression.
Trial Registration
ClinicalTrials.gov Identifier: NCT03594747
This randomized clinical trial assesses the efficacy and safety of tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for patients with advanced squamous non–small-cell lung cancer.
Introduction
Lung cancer is a leading cause of cancer incidence and death.1 As most squamous non–small-cell lung cancers (sq-NSCLCs) are diagnosed at an advanced stage,2 disease management can be challenging.3 Prognosis has been poor for patients treated with standard platinum-based chemotherapy, highlighting a high unmet medical need.3,4,5,6 Combining standard first-line regimens with monoclonal antibodies that block programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) has led to improvements in the treatment of NSCLC.2,7
Three large phase 3 studies have previously compared the efficacy and safety/tolerability profile of PD-1/L1 inhibitors in combination with chemotherapy vs chemotherapy in patients with advanced sq-NSCLC who were unselected for PD-L1 expression.8,9,10 In KEYNOTE-407, patients with metastatic sq-NSCLC who received pembrolizumab (anti–PD-1 antibody) plus carboplatin and paclitaxel or nab-paclitaxel reported significantly prolonged overall survival (OS) and progression-free survival (PFS) (dual primary end points) compared with chemotherapy.8 IMpower131, which randomized patients with metastatic sq-NSCLC to receive atezolizumab (anti–PD-L1 antibody) plus chemotherapy or chemotherapy alone, met only 1 of its coprimary end points (PFS but not OS).10 In part 2 of CheckMate 227, patients with metastatic NSCLC (nonsquamous NSCLC, n = 543; sq-NSCLC, n = 212) received the anti–PD-1 antibody, nivolumab, plus histology-based chemotherapy or chemotherapy alone. While this trial did not meet its primary end point of OS, patients with sq-NSCLC who were treated with combination therapy had improved PFS and a higher objective response rate (ORR) compared with chemotherapy in an exploratory analysis.9 As, to our knowledge, pembrolizumab plus standard chemotherapy is the only approved treatment for advanced sq-NSCLC, further investigation into the combination of a PD-1/L1 inhibitor plus chemotherapy is warranted.
Tislelizumab, a monoclonal antibody with high binding affinity to the PD-1 receptor, was specifically engineered to minimize Fcγ receptor binding on macrophages, thereby abrogating antibody-dependent phagocytosis, a mechanism of T cell clearance and potential resistance to anti–PD-1 therapy.11,12 In 2 early-phase studies (NCT02407990; NCT04068519), tislelizumab monotherapy at the recommended dose of 200 mg administered intravenously every 3 weeks was generally well tolerated and demonstrated antitumor activity in Asian and non-Asian populations with advanced lung cancers, regardless of PD-L1 expression.13,14 In a phase 2 trial (NCT03432598), patients with advanced lung cancer had robust responses to first-line tislelizumab plus platinum-based chemotherapy.15 Objective response rates were 44% (non–sq-NSCLC), 77% (SCLC), and either 80% or 67% (sq-NSCLC) depending on the chemotherapy backbone administered. In this article, we present the efficacy and safety results from the pivotal phase 3 trial of tislelizumab plus chemotherapy (RATIONALE 307) in patients with treatment-naive advanced sq-NSCLC.
Methods
Patients
RATIONALE 307 is an open-label, randomized, multicenter phase 3 trial (eFigure 1 in Supplement 1) conducted in China according to the ethical principles of the Declaration of Helsinki, Good Clinical Practice guidelines, principles of informed consent, and requirements of publicly registered clinical trials (Supplement 2). Patients gave written informed consent before enrollment, and the participating institutions provided institutional review board approval.
Patients (aged 18-75 years) with treatment-naive, histologically confirmed locally advanced (stage IIIB) or metastatic (stage IV) sq-NSCLC as classified by the American Joint Committee Cancer, Cancer Staging Manual, 7th Edition were eligible. Patients were eligible if they were not amenable to curative surgery or radiotherapy, had measurable disease (Response Evaluation Criteria in Solid Tumors, version 1.1), and an Eastern Cooperative Oncology Group performance score of 1 or less. Patients with mixed histology were eligible when squamous histology was the major histological component. Newly extracted or archival tumor tissue samples were required for PD-L1 expression assessment. Patients with known EGFR-sensitizing sequence variants or ALK fusions, a history of interstitial lung disease, or noninfectious pneumonitis were ineligible. Additional inclusion/exclusion criteria are listed in the eMethods in Supplement 1.
Study Design and Treatment
Patients were randomized (1:1:1) to treatment by using an interactive response technology system. Randomization was stratified by disease stage (stage IIIB vs IV) and level of tumor cell (TC) PD-L1 expression (<1% vs 1%-49% vs ≥50%). Patients with tumors unevaluable for PD-L1 expression were included in the <1% TC PD-L1 expression group. Patients received 1 of the following regimens intravenously every 3 weeks: tislelizumab (200 mg, day 1) plus paclitaxel (175 mg/m2, day 1) and carboplatin (area under the concentration [AUC] of 5, day 1) (arm A); tislelizumab (200 mg, day 1) plus nab-paclitaxel (100 mg/m2, days 1, 8, and 15) and carboplatin (AUC of 5, day 1) (arm B); or paclitaxel (175 mg/m2, day 1) and carboplatin (AUC of 5, day 1) (arm C).
Tislelizumab was administered for 1 hour on day 1 of cycles 1 and 2 and for 30 minutes in subsequent infusions. Tislelizumab treatment continued every 3 weeks until lack of clinical benefit or intolerable toxicity. Doublet chemotherapy was given until completion of 4 to 6 cycles (at the investigator’s discretion), occurrence of disease progression (Response Evaluation Criteria in Solid Tumors, version 1.1), or intolerable toxicity, whichever occurred first. Patients in arm C could cross over to receive tislelizumab monotherapy if it was determined that they had independent review committee (IRC)–confirmed disease progression. The administered doses of paclitaxel and carboplatin represented approved treatment doses for advanced lung cancer in China16; the administered nab-paclitaxel dose is the approved dose for locally advanced or metastatic NSCLC in the US.17
End Points
The primary end point was the comparison of PFS assessed by IRC between tislelizumab combined with paclitaxel plus carboplatin (arm A) or nab-paclitaxel plus carboplatin (arm B) and paclitaxel plus carboplatin alone (arm C). Secondary end points included OS, investigator-assessed (INV) PFS, IRC-assessed ORR, and IRC-assessed duration of response (DoR), as well as the incidence and severity of adverse events (AEs). Other end points included disease control rate (DCR) by IRC and examination of TC PD-L1 expression as a potential predictive biomarker of response and survival. Radiologic images were assessed by independent central review (Bioclinica, Inc). Definitions of efficacy end points are included in the eMethods in Supplement 1.
Safety and tolerability were assessed throughout the trial by monitoring AEs/serious AEs (graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0). Potential immune-mediated AEs were selected from a group of preferred terms regardless of whether the investigator attributed the event to a treatment or considered the event to be immune related. Tumor assessments and screening for PD-L1 expression are described in the eMethods in Supplement 1; investigators, patients, and the sponsor were masked to PD-L1 results.
Statistical Analysis
The sample size was determined by the number of PFS events required to demonstrate superiority of tislelizumab-containing arms to chemotherapy. A sample size of 342 patients was estimated to allow an 80% power to detect a hazard ratio (HR) of 0.65 comparing arms A or B with arm C at a 1-sided α level of .025. The type I error was controlled at the α level of .025 by using sequential testing of arm A vs C followed by arm B vs C until the first nonrejection. Prespecified efficacy boundaries were 1-sided (P = .01). Efficacy analyses were assessed in the intent-to-treat (ITT) analysis set, which was defined as all randomized patients; safety/tolerability data were summarized from the safety analysis set, which included patients who received any dose of tislelizumab and/or chemotherapy. Statistical analyses were conducted using SAS (SAS Institute).
Categorical variables were summarized by the number (percentage) of patients; continuous variables were reported using descriptive statistics. Time-to-event end points were estimated using Kaplan-Meier analysis; the Brookmeyer and Crowley method was used to construct 95% CIs for the median PFS, OS, and DoR of each treatment arm. Hazard ratios for comparisons between arms A or B with arm C were estimated using the stratified Cox proportional hazards model; a stratified 1-sided log-rank test calculated the significance between treatment arms. The stratified Cochran-Mantel-Haenszel χ2 test assessed differences in ORR. The stratification factors of PD-L1 expression (<1% vs 1%-49% vs ≥50%) and disease stage (IIIB vs IV) were applied to all stratified analyses. To assess the predictive value of PD-L1 expression, a post hoc interaction analysis with PFS and response was performed. Cox and logistics models were used to estimate HRs and odds ratios, respectively. The covariates in the 2 models included treatment, PD-L1 expression (≥1% vs <1%), and interaction of treatment and PD-L1 expression, with disease stage as a stratification factor. An independent data monitoring committee reviewed an interim efficacy analysis for PFS after 75% of targeted events (approximately 130 PFS events) occurred in the ITT analysis set. In this article, we present the results of the interim analysis and post hoc PD-L1 interaction analysis.
Results
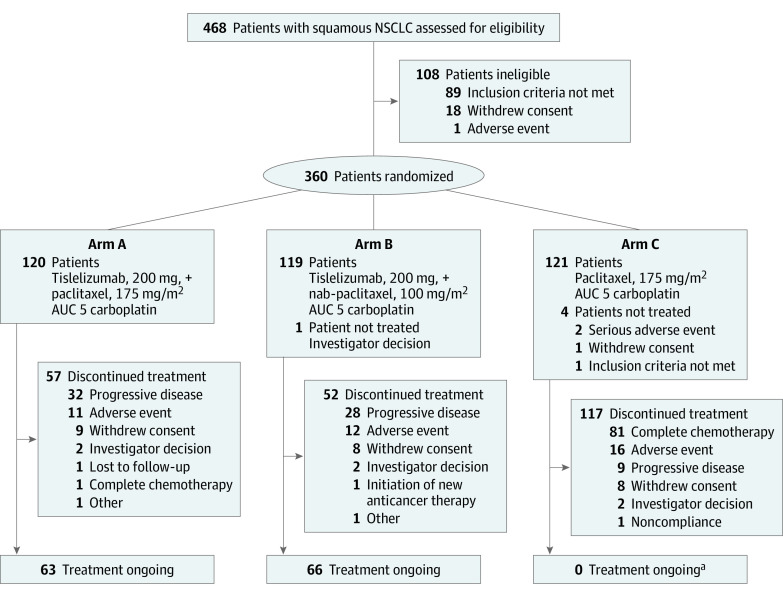
Between July 30, 2018, and June 13, 2019, 360 patients who met eligibility criteria were randomized into arm A (120 [33.3%]), arm B (119 [33.1%]), or arm C (121 [33.6%]) (Figure 1). Five patients (B, 1; C, 4) were randomized but did not receive study treatment; these patients were included in the ITT population but not the safety analysis set. The median study follow-up time was 8.6 months (95% CI, 8.1-9.0 months). At the time of data cutoff (December 6, 2019), 129 patients in arms A (63 [52.2%]) and B (66 [55.5%]) continued to receive treatment. Of the 121 patients randomized to arm C, 89 (73.6%) were still in follow-up, including 30 of the 54 who crossed over to tislelizumab monotherapy. Demographic and disease baseline characteristics were representative of the target patient population and generally well balanced between treatment arms, including disease stage and PD-L1 expression, which was consistent with randomization based on these stratification factors (Table 1).
Figure 1. Study Profile.
Patients who discontinued treatment for any reason were included in follow-up. AUC indicates area under the concentration; NSCLC, non–small-cell lung cancer.
aA total of 54 patients crossed over to receive tislelizumab; 2 patients who crossed over to receive tislelizumab discontinued because of an adverse event.
Table 1. Demographic and Baseline Disease Characteristics of Patients With Advanced Squamous Non–Small-Cell Lung Cancer.
| ITT population | No. (%) | |||
|---|---|---|---|---|
| Arm A: tislelizumab + PC (n = 120) | Arm B: tislelizumab + nab-PC (n = 119) | Arm C: PC (n = 121) | Total (N = 360) | |
| Age, median (range), y | 60 (41-74) | 63 (38-74) | 62 (34-74) | 62 (34-74) |
| Age group, y | ||||
| <65 | 81 (67.5) | 67 (56.3) | 85 (70.2) | 233 (64.7) |
| ≥65 | 39 (32.5) | 52 (43.7) | 36 (29.8) | 127 (35.3) |
| Sex | ||||
| Men | 107 (89.2) | 112 (94.1) | 111 (91.7) | 330 (91.7) |
| Women | 13 (10.8) | 7 (5.9) | 10 (8.3) | 30 (8.3) |
| Tobacco use | ||||
| Current/former | 96 (80.0) | 107 (89.9) | 98 (81.0) | 301 (83.6) |
| Never | 24 (20.0) | 12 (10.1) | 23 (19.0) | 59 (16.4) |
| ECOG status | ||||
| 0 | 31 (25.8) | 22 (18.5) | 32 (26.4) | 85 (23.6) |
| 1 | 89 (74.2) | 97 (81.5) | 89 (73.6) | 275 (76.4) |
| Solid tumor stage | ||||
| Stage IIIB | 38 (31.7) | 40 (33.6) | 44 (36.4) | 122 (33.9) |
| Stage IV | 82 (68.3) | 79 (66.4) | 77 (63.6) | 238 (66.1) |
| PD-L1 expression on tumor cells, % | ||||
| <1a | 48 (40.0) | 47 (39.5) | 49 (40.5) | 144 (40.0) |
| 1-49 | 30 (25.0) | 30 (25.2) | 31 (25.6) | 91 (25.3) |
| ≥50 | 42 (35.0) | 42 (35.3) | 41 (33.9) | 125 (34.7) |
| Confirmed distant metastatic site(s)b | ||||
| Bone | 24 (20.0) | 16 (13.4) | 21 (17.4) | 61 (16.9) |
| Liver | 15 (12.5) | 15 (12.6) | 14 (11.6) | 44 (12.2) |
| Brain | 2 (1.7) | 3 (2.5) | 1 (0.8) | 6 (1.7) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ITT, intent-to-treat; nab, nanoparticle albumin-bound; PC, paclitaxel and carboplatin; PD-L1, programmed cell death 1 ligand 1.
Patients with nonevaluable tumor samples were included in the less than 1% PD-L1 expression tumor cell subgroup.
A patient was counted only once within each category but may be counted in multiple categories.
The median tislelizumab treatment duration was 32.3 weeks (range, 3.0-64.0 weeks) for arm A and 30.9 weeks (range, 3.0-62.3 weeks) for arm B. The median number of tislelizumab treatment cycles was 10 (range, 1-20) for arm A and 10 (range, 1-19) for arm B. In total, 49 (40.8%) and 44 patients (37.3%) received 9 to 12 cycles of tislelizumab in arms A and B, respectively; 32 (26.7% [A]) and 28 patients (23.7% [B]) received more than 12 cycles of tislelizumab. The median relative dose intensity of tislelizumab was 97.7% (range, 63.6%-107.7%) in arm A and 91.3% (range, 54.5%-100.0%) in arm B (eTable 1 in Supplement 1).
The median (range) number of chemotherapy cycles was 4.5 (1-6), 4.0 (1-6), and 4.0 (1-6) cycles in arms A, B, and C, respectively. Chemotherapy exposure was similar between arms A and C regarding exposure duration, dose intensity, and the numbers and cycles of administration. The median relative dose intensities of nab-paclitaxel and carboplatin were lower in arm B vs paclitaxel and carboplatin in arms A or C (eTable 2 in Supplement 1).
Tislelizumab plus chemotherapy (arms A and B) vs chemotherapy (arm C) significantly prolonged IRC-assessed PFS (Figure 2, A and B). The median IRC-assessed PFS for arms A, B, and C was 7.6 months (95% CI, 6.0-9.8), 7.6 months (95% CI, 5.8–11.0), and 5.5 months (95% CI, 4.2-5.7), respectively. Stratified HRs were 0.524 (95% CI, 0.370-0.742; P < .001) between arms A and C and 0.478 (95% CI, 0.336-0.679; P < .001) between arms B and C. The 9-month PFS rate was 41.7% (95% CI, 30.9-52.1) and 47.2% (95% CI, 36.5-57.2) for arms A and B vs 17.5% (95% CI, 9.8-26.9) for chemotherapy. The INV PFS results were similar (eFigure 2 in Supplement 1).
Figure 2. Progression-Free Survival by Independent Review Committee.
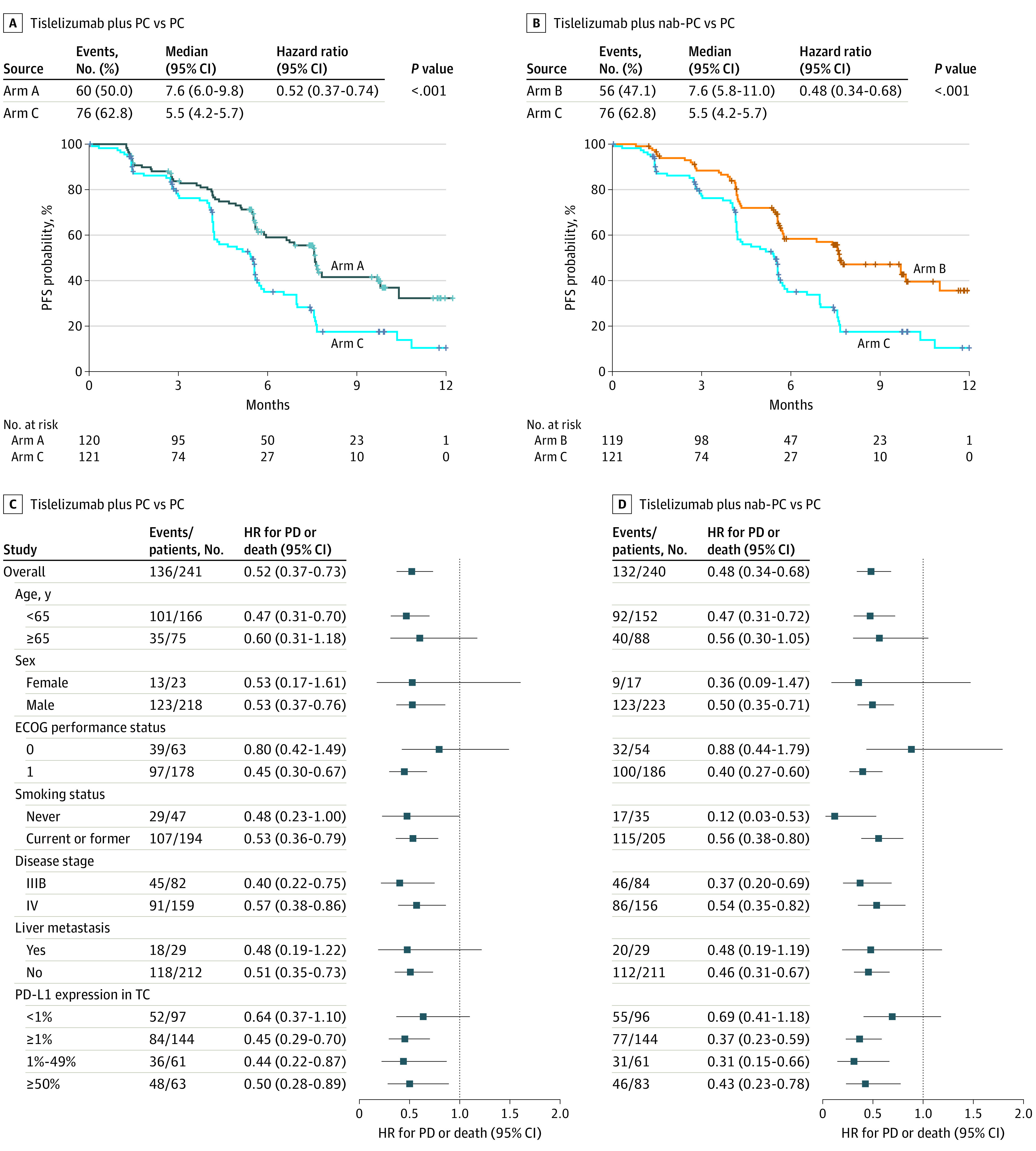
Kaplan-Meier curves for progression-free survival (PFS) of patients treated with tislelizumab plus paclitaxel and carboplatin (PC) vs PC (A) and tislelizumab plus nab-PC vs PC (B). Subgroup analysis of tislelizumab plus PC vs PC (C) and tislelizumab plus nab-PC vs PC (D). ECOG indicates Eastern Cooperative Oncology Group; HR, hazard ratio; nab, nanoparticle albumin-bound; PD, progressive disease; PD-L1, programmed cell death 1 ligand 1; TC, tumor cell.
Adding tislelizumab to chemotherapy prolonged IRC-assessed PFS vs chemotherapy alone across most subgroups (Figure 2, C and D). In patients with stage IIIB disease, the median PFS estimates were 9.8 (arm A), 11.0 (arm B), and 5.6 months (arm C); the risk of disease progression and death was reduced in both arms that contained tislelizumab compared with chemotherapy (arm A vs C: HR, 0.402; 95% CI, 0.215-0.750; arm B vs C: HR, 0.372; 95% CI, 0.202-0.686) (eTable 3 in Supplement 1). A similar trend was identified in patients with stage IV disease (arm A vs C: 7.6 vs 5.2 months; HR, 0.570; 95% CI, 0.376-0.862; arm B vs C: 7.4 vs 5.2 months; HR, 0.537; 95% CI, 0.350-0.824) (eTable 3 in Supplement 1). Improvements in PFS were observed across all PD-L1 subgroups (eFigures 3-5 and eTable 4 in Supplement 1). Despite a trend of greater PFS benefit observed in the PD-L1–positive subgroup at a 1% cutoff, interaction analyses did not detect predictive effects of PD-L1 for a PFS benefit of combination treatments (eTable 5 in Supplement 1).
Higher response rates were observed in arms A (73%; 95% CI, 63.6%-80.3%) and B (75%; 95% CI, 66.0%-82.3%) vs C (50%; 95% CI, 40.4%-58.8%) (Table 2). The median IRC-assessed DoR was longer with tislelizumab combination therapy (arm A: 8.2 months; 95% CI, 5.0-not estimable; arm B: 8.6 months; 95% CI, 6.3-not estimable) vs chemotherapy (arm C: 4.2 months; 95% CI, 2.8-4.9). Regardless of PD-L1 expression, tislelizumab plus chemotherapy trended toward increased ORR, and no predictive effect of PD-L1 at a 1% cutoff was observed (eFigure 6 and eTable 5 in Supplement 1). With a median follow-up of 8.6 months (95% CI, 8.1-9.0 months), OS data were not mature.
Table 2. Best Confirmed IRC-Assessed Overall Response in Patients With Advanced Squamous Non–Small-Cell Lung Cancer Treated With Tislelizumab Plus Doublet Chemotherapy or Chemotherapy Alone.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Arm A: tislelizumab + PC (n = 120) | Arm B: tislelizumab + nab-PC (n = 119) | Arm C: PC (n = 121) | |
| BOR | |||
| CR | 5 (4) | 3 (3) | 1 (<1) |
| PR | 82 (68) | 86 (72) | 59 (49) |
| SD | 18 (15) | 19 (16) | 36 (30) |
| Non-CR/non-PD | 0 | 0 | 1 (<1) |
| PD | 12 (10) | 5 (4) | 11 (9) |
| NE/missing | 3 (3) | 6 (5) | 13 (11) |
| ORR, % (95% CI) | 73 (63.6-80.3) | 75 (66.0-82.3) | 50 (40.4-58.8) |
| Odds ratio (95% CI) | 2.9 (1.65-4.95) | 3.1 (1.78-5.41) | NA |
| DCR, % (95% CI) | 88 (80.2-92.8) | 91 (84.1-95.3) | 80 (71.9-86.9) |
| CBR, % (95% CI)a | 81 (72.6-87.4) | 80 (71.5-86.6) | 56 (46.9-65.2) |
Abbreviations: BOR, best overall response; CBR, clinical benefit rate; CR, complete response; DCR, disease control rate; IRC, independent review committee; NA, not applicable; nab, nanoparticle albumin-bound; NE, not evaluable; ORR, objective response rate; PC, paclitaxel and carboplatin; PD, progressive disease; PR, partial response; SD, stable disease.
Includes patients with BOR in CR or PR or 24 weeks or greater SD.
Treatment-emergent AEs (TEAEs) occurred in nearly all patients (eTable 6 in Supplement 2). The TEAEs that occurred in 20% or more of patients in any arm are shown in Table 3; across all arms, the most common grade 3 or more TEAE was decreased neutrophil levels. Serious TEAEs were reported in 118 patients (44 [36.7%; A]; 45 [38.1%; B]; 29 [24.8%; C]). Discontinuation of any treatment component because of TEAEs was reported in 15 (12.5%; A), 35 (29.7%; B), and 18 patients (15.4%; C); TEAEs leading to permanent discontinuation of tislelizumab were similar between arms A (12 [10.0%]) and B (12 [10.2%]).
Table 3. Incidence of Treatment-Emergent Adverse Events Occurring in 20% or More of Patients.
| Preferred term | No. (%) | |||||
|---|---|---|---|---|---|---|
| Tislelizumab + paclitaxel + carboplatin (n = 120) | Tislelizumab + nab-paclitaxel + carboplatin (n = 118) | Paclitaxel + carboplatin (n = 117) | ||||
| All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | |
| Anemia | 106 (88.3) | 9 (7.5) | 110 (93.2) | 27 (22.9) | 94 (80.3) | 14 (12.0) |
| Alopecia | 77 (64.2) | 0 | 82 (69.5) | 0 | 72 (61.5) | 0 |
| Decreased neutrophil levels | 76 (63.3) | 62 (51.7) | 72 (61.0) | 54 (45.8) | 68 (58.1) | 53 (45.3) |
| Decreased white blood cell count | 64 (53.3) | 27 (22.5) | 68 (57.6) | 32 (27.1) | 62 (53.0) | 28 (23.9) |
| Leukopenia | 57 (47.5) | 19 (15.8) | 66 (55.9) | 30 (25.4) | 56 (47.9) | 21 (17.9) |
| Decreased appetite | 52 (43.3) | 1 (0.8) | 52 (44.1) | 1 (0.8) | 36 (30.8) | 1 (0.9) |
| Neutropenia | 51 (42.5) | 40 (33.3) | 50 (42.4) | 32 (27.1) | 55 (47.0) | 47 (40.2) |
| Increased ALT levels | 50 (41.7) | 2 (1.7) | 41 (34.7) | 2 (1.7) | 27 (23.1) | 0 |
| Increased AST | 43 (35.8) | 0 | 40 (33.9) | 1 (0.8) | 14 (12.0) | 0 |
| Decreased platelet cell count | 41 (34.2) | 5 (4.2) | 52 (44.1) | 16 (13.6) | 28 (23.9) | 2 (1.7) |
| Pain in extremity | 40 (33.3) | 3 (2.5) | 17 (14.4) | 0 | 27 (23.1) | 0 |
| Nausea | 36 (30.0) | 0 | 51 (43.2) | 0 | 35 (29.9) | 1 (0.9) |
| Constipation | 36 (30.0) | 0 | 33 (28.0) | 0 | 27 (23.1) | 0 |
| Thrombocytopenia | 33 (27.5) | 7 (5.8) | 47 (39.8) | 15 (12.7) | 32 (27.4) | 7 (6.0) |
| Asthenia | 29 (24.2) | 0 | 21 (17.8) | 0 | 24 (20.5) | 1 (0.9) |
| Vomiting | 28 (23.3) | 1 (0.8) | 27 (22.9) | 0 | 20 (17.1) | 2 (1.7) |
| Increased blood bilirubin levels | 27 (22.5) | 0 | 15 (12.7) | 0 | 15 (12.8) | 0 |
| Hypoesthesia | 27 (22.5) | 0 | 12 (10.2) | 0 | 19 (16.2) | 0 |
| Hypoalbuminemia | 27 (22.5) | 1 (0.8) | 21 (17.8) | 0 | 19 (16.2) | 0 |
| Rash | 25 (20.8) | 4 (3.3) | 26 (22.0) | 2 (1.7) | 4 (3.4) | 0 |
| Arthralgia | 25 (20.8) | 0 | 21 (17.8) | 0 | 19 (16.2) | 0 |
| Pyrexia | 24 (20.0) | 0 | 24 (20.3) | 1 (0.8) | 18 (15.4) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; nab, nanoparticle albumin-bound.
Treatment-related AEs (TRAEs) occurred in 353 patients (99.4%), most commonly anemia, alopecia, and decreased neutrophil levels (eTable 7 in Supplement 1). Grade 3 or higher TRAEs occurred in 296 patients (103 [85.8%; A]; 99 [83.9%; B]; 94 [80.3%; C]); grade 3 or higher TRAEs were mostly hematologic in nature and consistent with known chemotherapy AEs.18 Serious TRAEs occurring in 2% or more of patients included decreased neutrophil levels (4 [3.3%; A]; 4 [3.4%; B]; 2 [1.7%; C]), febrile neutropenia (2 [1.7%; A]; 3 [2.5%; B]; 1 [0.9%; C]), thrombocytopenia (1 [0.8%; A]; 1 [0.8%; B]; 3 [2.6%; C]), and pneumonitis (3 [2.5%; A]; 2 [1.7%; B]; 0 [0%; C]). Treatment-emergent AEs leading to death were similar across all 3 arms (4 [3.3%; A]; 5 [4.2%; B]; 5 [4.3%; C]). Six patients experienced TRAEs leading to death (arm A, 1; arm B, 2; arm C, 3), none of which were solely attributed to tislelizumab. These TRAEs were hydrocephalus (n = 1 [A]), hepatic failure (n = 1 [B]), death (n = 1 [B], n = 1 [C]), and septic shock (n = 2 [C]). Hyperglycemia, hypothyroidism, and pneumonia were the most common potential immune-mediated AEs in patients who received tislelizumab-containing therapy (eFigure 7 in Supplement 1). Most potential immune-mediated AEs were low grade (grade 1-2) and did not lead to treatment discontinuation.
Discussion
In this phase 3 trial, tislelizumab in combination with chemotherapy improved PFS in patients with locally advanced or metastatic sq-NSCLC. After a median study follow-up of 8.6 months, IRC-assessed PFS was significantly prolonged in both tislelizumab-containing arms compared with chemotherapy alone. These benefits were observed regardless of PD-L1 expression and in most prespecified subgroup analyses. In both tislelizumab-containing arms, ORR was greater than 70%. Observed responses were clinically meaningful and durable, with a doubling of DoR by more than 4 months compared with chemotherapy alone. The RATIONALE 307 results are consistent with similar studies in patients with advanced sq-NSCLC. KEYNOTE-407 reported prolonged OS and PFS, as well as improved response and median DoR after a median follow-up of 7.8 months.8 While IMpower131 demonstrated a significant benefit in median PFS, it did not show a significant OS benefit.10 An exploratory analysis in part 2 of CheckMate 227 reported improved median PFS and DoR.9
To our knowledge, RATIONALE 307 is one of the first phase 3 trials of a PD-1 inhibitor in combination with chemotherapy in sq-NSCLC to include patients with stage IIIB disease who were not amenable to curative surgery or chemoradiotherapy. Subgroup analyses of PFS demonstrated that tislelizumab plus chemotherapy provided benefits compared with chemotherapy alone, regardless of disease stage. As the median PFS was comparable for patients with stage IIIB and stage IV disease who were randomized to receive chemotherapy alone, our post hoc analysis suggests that patients with stage IIIB or stage IV disease have similar clinical prognosis. Additionally, the positive PFS benefits observed in patients with stage IIIB disease suggest a potential new treatment option for this patient population. Furthermore, the addition of tislelizumab to chemotherapy also prolonged PFS in patients with stage IV disease; these results are consistent with median PFS estimates in KEYNOTE-407 (6.4 months) and IMpower131 (6.3 months).8,10
Compared with nsq-NSCLC, the relationship between clinical outcomes and TC PD-L1 expression is less understood in sq-NSCLC, which may be because of the higher mutational burden in tumors of smokers with squamous disease.19,20,21,22 In RATIONALE 307, improvement in PFS estimates were observed in both tislelizumab-containing arms compared with chemotherapy alone regardless of PD-L1 status, as confirmed by a post hoc interaction analysis. The findings are similar to those reported in KEYNOTE-407,8 but not in IMpower131,10 in which PFS benefit was observed only in PD-L1–positive subgroups, with greater PFS benefit shown in patients with higher tumor PD-L1 expression. However, this variation may be because of differences in immunohistochemistry assays and scoring methods for PD-L1 expression.8,10,23
Although OS has been the standard measure of clinical benefit in recent oncology trials, it can be confounded by mortality that is unrelated to disease and crossover within the trial.24 With the development of novel agents or therapies in a second-line or later-line setting, the availability of treatment options after progression can also add to the confounding factors. RATIONALE 307 was designed with PFS as the primary end point, allowing patients who experienced radiologic disease progression while receiving chemotherapy alone to cross over and receive tislelizumab monotherapy, thus preventing delays in subsequent standard treatment. Based on the significant reduction in the risk of disease progression or death with tislelizumab in combination with chemotherapy, RATIONALE 307 demonstrated clinical benefit as a first-line treatment of sq-NSCLC.
First-line treatment with tislelizumab plus chemotherapy was generally well tolerated. Adverse events reported across both tislelizumab-containing arms were consistent with established safety profiles of PD-1/L1 inhibitors and chemotherapy.8,10,25 Most TEAEs were mild to moderate in severity. Most AEs were hematologic in nature and aligned with known toxicities of chemotherapy backbones. Overall, the rate of treatment discontinuations because of a TEAE was low. While the proportion of patients who discontinued participation because of AEs was comparable between the arms administered with paclitaxel, a higher incidence of AEs leading to discontinuation was observed in patients who were randomized to nab-paclitaxel. This increased rate may be because of more frequent administration and safety assessments with nab-paclitaxel (once weekly) compared with paclitaxel (every 3 weeks). Similar findings were observed for AEs leading to dose modification/delay and are aligned with observations in the IMpower131 and KEYNOTE-407 studies.8,10
Strengths and Limitations
As this study was an open-label design, one potential limitation is investigator bias; however, numerous measures were taken to ensure the validity of data. RATIONALE 307 used the same established response criteria and performed tumor assessments at the same frequency across the arms, as well as adhering to protocol-defined schedules. Additionally, evaluation of the primary end point was strengthened by the use of an IRC, which were consistent with that of the investigators, suggesting that the PFS end point was not confounded by the knowledge of treatment assignment.
Conclusions
The addition of tislelizumab to standard chemotherapy demonstrated a significant reduction to the risk of progression or death for patients with advanced sq-NSCLC. This represents an additional treatment option as first-line treatment for patients with sq-NSCLC.
eMethods. Criteria and end point defintions
eFigure 1. Study design
eFigure 2. Progression-free survival by investigator
eFigure 3. Progression-free survival by independent review committee in patients with ≥50% tumor cell PD-L1 expression
eFigure 4. Progression-free survival by independent review committee in patients with 1-49% tumor cell PD-L1 expression
eFigure 5. Progression-free survival by independent review committee in patients with <1% tumor cell PD-L1 expression
eFigure 6. Objective response rate by PD-L1 expression as assessed by independent review committee
eFigure 7. Potential immune-mediated AEs by preferred term occurring in >2 patients across treatment arms
eTable 1. Tislelizumab treatment exposure
eTable 2. Chemotherapy treatment exposure
eTable 3. Progression-free survival by disease stage per RECIST version 1.1 by independent review committee in ITT analysis set
eTable 4. Progression-free survival by PD-L1 per RECIST version 1.1 by independent review committee in ITT analysis set
eTable 5. Biomarker interaction analysis for PD-L1 at cutoff of 1% TC per RECIST version 1.1 by independent review committee in ITT analysis set
eTable 6. Overall summary of treatment emergent adverse events
eTable 7. Incidence of treatment-related adverse events occurring in ≥15% of patients
Trial protocol
Data sharing statement
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Carlisle JW, Ramalingam SS. A banner year for immunotherapy and targeted therapy. Nat Rev Clin Oncol. 2019;16(2):79-80. doi: 10.1038/s41571-018-0138-4 [DOI] [PubMed] [Google Scholar]
- 3.Socinski MA, Obasaju C, Gandara D, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13(2):165-183. doi: 10.1016/j.jtho.2017.11.111 [DOI] [PubMed] [Google Scholar]
- 4.Azzoli CG, Baker S Jr, Temin S, et al. ; American Society of Clinical Oncology . American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27(36):6251-6266. doi: 10.1200/JCO.2009.23.5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Non-small Cell Lung Cancer Collaborative Group . Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311(7010):899-909. doi: 10.1136/bmj.311.7010.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543-3551. doi: 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 7.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937. doi: 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L, Luft A, Vicente D, et al. ; KEYNOTE-407 Investigators . Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. doi: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Ciuleanu TE, Yu X, et al. Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as first-line (1L) treatment (tx) for advanced non-small cell lung cancer (aNSCLC): CheckMate 227—part 2 final analysis. Abstract presented at: European Society for Medical Oncology Immuno-Oncology Congress; December 12, 2019; Geneva, Switzerland. [Google Scholar]
- 10.Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351-1360. doi: 10.1016/j.jtho.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 11.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcyRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. 2015;28(3):285-295. doi: 10.1016/j.ccell.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079-1090. doi: 10.1007/s00262-018-2160-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai J, Deva S, Lee JS, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer. 2020;8(1):e000453. doi: 10.1136/jitc-2019-000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen L, Guo J, Zhang Q, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. 2020;8(1):e000437. doi: 10.1136/jitc-2019-000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Zhao J, Ma Z, et al. A phase 2 study of tislelizumab in combination with platinum-based chemotherapy as first-line treatment for advanced lung cancer in Chinese patients. Lung Cancer. 2020;147:259-268. doi: 10.1016/j.lungcan.2020.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Zhi XY, Wu YL, Bu H, et al. ; Lung Cancer Diagnosis and Treatment Expert Panel of the Chinese Ministry of Health . Chinese guidelines on the diagnosis and treatment of primary lung cancer (2011). J Thorac Dis. 2012;4(1):88-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraxane. Package insert. Celgene Corporation; 2013.
- 18.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055-2062. doi: 10.1200/JCO.2011.39.5848 [DOI] [PubMed] [Google Scholar]
- 19.Pirker R. Biomarkers for immune checkpoint inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol. 2019;31(1):24-28. doi: 10.1097/CCO.0000000000000496 [DOI] [PubMed] [Google Scholar]
- 20.Melosky B, Juergens R, Hirsh V, et al. Amplifying outcomes: checkpoint inhibitor combinations in first-line non-small cell lung cancer. Oncologist. 2020;25(1):64-77. doi: 10.1634/theoncologist.2019-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33(5):853-861.e4, e854. doi: 10.1016/j.ccell.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of Blueprint phase 2 project. J Thorac Oncol. 2018;13(9):1302-1311. doi: 10.1016/j.jtho.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill S, Berry S, Biagi J, et al. Progression-free survival as a primary endpoint in clinical trials of metastatic colorectal cancer. Curr Oncol. 2011;18(suppl 2):S5-S10. doi: 10.3747/co.v18iS2.958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Criteria and end point defintions
eFigure 1. Study design
eFigure 2. Progression-free survival by investigator
eFigure 3. Progression-free survival by independent review committee in patients with ≥50% tumor cell PD-L1 expression
eFigure 4. Progression-free survival by independent review committee in patients with 1-49% tumor cell PD-L1 expression
eFigure 5. Progression-free survival by independent review committee in patients with <1% tumor cell PD-L1 expression
eFigure 6. Objective response rate by PD-L1 expression as assessed by independent review committee
eFigure 7. Potential immune-mediated AEs by preferred term occurring in >2 patients across treatment arms
eTable 1. Tislelizumab treatment exposure
eTable 2. Chemotherapy treatment exposure
eTable 3. Progression-free survival by disease stage per RECIST version 1.1 by independent review committee in ITT analysis set
eTable 4. Progression-free survival by PD-L1 per RECIST version 1.1 by independent review committee in ITT analysis set
eTable 5. Biomarker interaction analysis for PD-L1 at cutoff of 1% TC per RECIST version 1.1 by independent review committee in ITT analysis set
eTable 6. Overall summary of treatment emergent adverse events
eTable 7. Incidence of treatment-related adverse events occurring in ≥15% of patients
Trial protocol
Data sharing statement