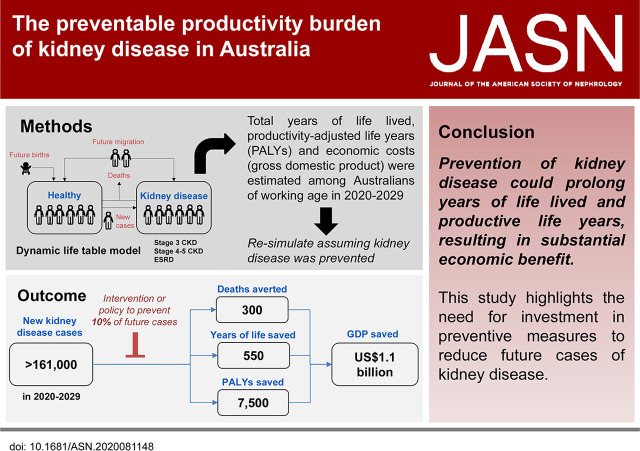
Significance Statement
Kidney disease is associated with reduced work productivity. The authors describe the preventable productivity burden of kidney disease in Australia over 10 years (2020–2029), using the novel metric “productivity-adjusted life year” (PALY). The PALY resembles the quality-adjusted life year, but it adjusts years of life lived for productivity loss resulting from ill health instead of quality-of-life impairment. They demonstrate that, if 10% of future cases of kidney disease can be prevented over this period, >7500 PALYs could be saved, equivalent to a gain of US$1.1 billion in gross domestic product. These results have potential to inform policy makers regarding the need and the substantial financial incentive for the prevention of kidney disease.
Keywords: chronic kidney disease, end stage kidney disease, productivity, incidence, dynamic life table model, burden
Visual Abstract
Abstract
Background
Kidney disease is associated with impaired work productivity. However, the collective effect of missed work days, reduced output at work, and early withdrawal from the workforce is rarely considered in health-economic evaluations.
Methods
To determine the effect on work productivity of preventing incident cases of kidney disease, using the novel measure “productivity-adjusted life year” (PALY), we constructed a dynamic life table model for the Australian working-age population (aged 15–69 years) over 10 years (2020–2029), stratified by kidney-disease status. Input data, including productivity estimates, were sourced from the literature. We ascribed a financial value to the PALY metric in terms of gross domestic product (GDP) per equivalent full-time worker and assessed the total number of years lived, total PALYs, and broader economic costs (GDP per PALY). We repeated the model simulation, assuming a reduced kidney-disease incidence; the differences reflected the effects of preventing new kidney-disease cases. Outcomes were discounted by 5% annually.
Results
Our projections indicate that, from 2020 to 2029, the estimated number of new kidney-disease cases will exceed 161,000. Preventing 10% of new cases of kidney disease during this period would result in >300 premature deaths averted and approximately 550 years of life and 7600 PALYs saved—equivalent to a savings of US$1.1 billion in GDP or US$67,000 per new case avoided.
Conclusions
Pursuing a relatively modest target for preventing kidney disease in Australia may prolong years of life lived and increase productive life years, resulting in substantial economic benefit. Our findings highlight the need for investment in preventive measures to reduce future cases of kidney disease.
In 2017, CKD affected 700 million people globally, resulting in 28.5 million years of life lost and 1.2 million deaths.1,2 CKD cost the Australian government A$5 billion in 2017, with A$1 billion attributable to RRT alone.3 Furthermore, the demand for RRT in Australia for patients with ESKD is projected to double by 2030.4,5 Self-reported data from the Australian Health Survey in 2012 indicated only 1% of the population had CKD.6 However, results from biomedical tests from the same study revealed at least 10% of the Australian population have positive indicators for CKD, highlighting significant unawareness and underdiagnosis.6
Kidney disease is associated with decreased physical and mental well-being,7 and, therefore, affects work productivity. In Australia, the total societal costs and government subsidies for kidney-disease management amount to A$4.3 billion per annum.8 Patients with CKD and ESKD miss more days from work ("absenteeism") and have reduced output while at work (“presenteeism”) compared with the general population.9 Renal insufficiency is also associated with early withdrawal from the labor force.10 However, the collective effect of absenteeism, presenteeism, and early withdrawal from the labor force associated with kidney disease is rarely considered in health-economic evaluations, and many studies assessing these parameters are outdated. Therefore, the aim of this study was to assess the effect of preventing new (incident) cases of kidney disease on work productivity in Australians of working age over the next 10 years, estimated using years of life lived and “productivity-adjusted life years” (PALYs).11–14 PALYs are akin to quality-adjusted life years (QALYs), but adjust years of life lived for productivity loss due to ill health rather than impairment in quality of life.
Methods
Dynamic Life Table Model
A dynamic, multistate, Markov model (Figure 1) was constructed to assess the effect of incident kidney disease on mortality, years of life lived, and work productivity among the Australian population of working age, over the next 10 years (from 2020 to 2029).11–14 Working age was defined as 15–69 years to reflect the commonly accepted employment age in Australia.15,16 The model comprised five health states: “alive without CKD,” “alive with stage 3 CKD,” “alive with stage 4–5 CKD (but not ESKD),” “alive with ESKD,” and “dead” (Figure 1). Stage 3 CKD was defined as an eGFR of 59–30 ml/min, whereas stage 4–5 CKD was defined as an eGFR of <30 ml/min, but not undergoing RRT. ESKD was defined as an eGFR <15 ml/min (or stage 5 CKD) and undergoing RRT (including dialysis or transplantation). Therefore, the “alive with ESKD” health state only included people with ESKD who were undergoing treatment. Subjects within the “alive without kidney disease” health state could develop CKD or die; whereas those in the “alive with stage 3 CKD” group could remain in that health state, move into the “alive with stage 4–5 CKD” health state, or die. Those within the “alive with stage 4–5 CKD (but not ESKD)” group could remain in that health state, develop ESKD requiring RRT (i.e., move into “alive with ESKD” health state), or die. Those within the “alive with ESKD” health state could remain in that health state or die. Thus, there was no recovery from CKD or ESKD.
Figure 1.
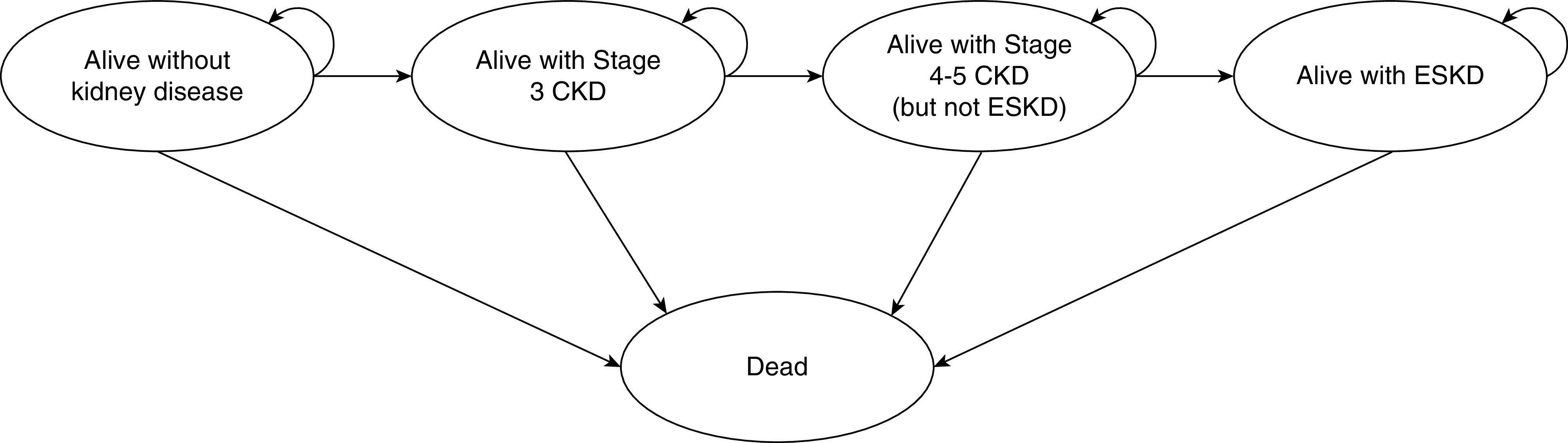
Markov model of the possible transitions and health states. The Australian population of working age was separated into those with and without kidney disease. People with kidney disease were further separated into those with stage 3 and 4–5 CKD and ESKD. Stage 3 CKD was defined as an eGFR of <60–30 ml/min. Stage 4–5 CKD was defined as an eGFR of <30 ml/min, but not undergoing RRT. ESKD was defined as those with an eGFR of <15 ml/min (stage 5 CKD) and undergoing RRT. With each annual cycle, those in the “alive without kidney disease” health state were at risk of developing CKD or dying, whereas those in the “alive with Stage 3 CKD” health state could remain alive, develop stage 4–5 CKD, or die. Those in the “alive with stage 4–5 CKD (but not ESKD)” health state could remain alive, develop ESKD, or die. Those in the “alive with ESKD” health state could remain alive or die.
The dynamic model accounted for predicted population changes over time, including births, deaths, migration, movement into and out of working age, progression of CKD and movement into CKD and ESKD. The base-case analysis estimated the total years of life lived, PALYs, and associated economic costs over the next 10 years for the Australian working-age population, stratified by disease status. The entire cohort was resimulated, hypothetically assuming all future cases of CKD and ESKD could be prevented. The difference in years of life lived, PALYs, and associated costs represented the potential gains from prevention of CKD and ESKD. All outcomes beyond the first year were discounted at a rate of 5% per annum as per Australian guidelines.17 All model inputs are summarized in Table 1.
Table 1.
Summary of model inputs
| Details and Unit | Point Estimate | Lower Bound | Upper Bound | Reference Range | Source |
|---|---|---|---|---|---|
| Abstenteeism, days per person per year | |||||
| Stage 3 CKD | 14.4 | 12.3 | 16.4 | 95% CI | van Haalen et al.9 |
| Stage 4–5 CKD | 20.3 | 17.8 | 22.9 | 95% CI | |
| ESKD | 29.3 | 26.6 | 32.0 | 95% CI | |
| Presenteeism, days per person per year | |||||
| Stage 3 CKD | 46.2 | 43.9 | 48.5 | 95% CI | van Haalen et al.9 |
| Stage 4–5 CKD | 66.3 | 63.5 | 69.2 | 95% CI | |
| ESKD | 83.3 | 80.5 | 86.0 | 95% CI | |
| Risk of early labor-force withdrawal, HR | |||||
| Stage 4–5 CKD | 5.4 | 3.8 | 7.0 | ±30% | Klarenbach et al.10 |
| ESKD | 7.9 | 1.6 | 39.4 | 95% CI | |
| Risk of all-cause mortality | |||||
| Stage 3 CKD, standardized incidence ratio | 2.2 | 2.1 | 2.4 | 95% CI | Eriksen et al.18 |
| Stage 4–5 CKD, HR | 3.6 | 3.3 | 4.0 | 95% CI | Neovius et al.19 |
| ESKD, HR | 5.3 | 4.2 | 6.6 | 95% CI | |
| Prevalence, % | |||||
| Stage 3 CKD | Age and sex specific | N/A | N/A | N/A | AIHW and NHS6,20 |
| Stage 4–5 CKD | |||||
| ESKD | Age and sex specific | N/A | N/A | N/A | AIHW6 |
| Transition probabilities (incidence), probability | |||||
| From healthy to stage 3 CKD | Age, sex, and year specific | N/A | N/A | N/A | Formulas adapted from Grams et al.21 |
| From stage 3 CKD to stage 4–5 CKD | |||||
| From stage 4–5 CKD to ESKD | |||||
| Other Australian population data | |||||
| General population mortality rates, % | Age, sex, and year specific | N/A | N/A | N/A | ABS22 |
| Net migration, no. of persons | Age, sex, and year specific | N/A | N/A | N/A | ABS22 |
| GDP per EFT worker, A$ | Year specific | N/A | N/A | N/A | ABS23 |
| Mean no. of hours worked | Age and sex specific | N/A | N/A | N/A | ABS24 |
NHS, National Health Survey; N/A, not applicable.
Population Estimates
All supporting data are included in Supplemental Tables 1–6. More detailed explanation of the methods can be found in Supplemental Table 1. Australian population data for the latest available year (2019) was obtained from the Australian Bureau of Statistics (ABS).25 Projected Australian mortality, migration, and birth data were also acquired from the ABS.22 The prevalence of CKD (pre-RRT) was extrapolated from the Australian Institute of Health and Welfare (AIHW) 2013 report,26 and adjusted to include only people with stage 3 and stage 4–5 CKD using sex- and eGFR-specific data reported in the 2011–2012 Australian National Health Survey (NHS) (Supplemental Table 1).20 The model sought to capture those with stage 3–5 CKD, because these stages represent a clinically significant loss of kidney function, which often translate into symptoms that interfere with patients’ daily lives.27 The NHS data represented biomedical measurement of eGFR and creatinine in approximately 25,000 households across Australia, which represented 31,837 unique individuals in the final sample. Prevalence of ESKD was adapted from an AIHW analysis of Australian and New Zealand Dialysis and Transplant Registry data in 2013.6 The incidence of stage 3 and stage 4–5 CKD and ESKD was calculated on the basis of the prevalence rates in 2013 and projected condition-specific mortality rates using the formulas reported by Grams et al.21 (details in Supplemental Table 1).
To determine condition-specific mortality, the increased risk of mortality associated with CKD and ESKD was used to adjust the projected all-cause mortality rates in the Australian population. The hazard ratio (HR) for all-cause mortality in those with stage 3 CKD was adapted from a Norwegian study by Eriksen et al.18 Compared with the general population, those with stage 3 CKD had a 2.2-fold increased risk (95% CI, 2.1 to 2.4) of all-cause mortality. The HR of all-cause mortality in those with stage 4–5 CKD and ESKD compared with the general population was derived from the Swedish study by Neovius et al.19 The HR of all-cause mortality was 3.6 (95% CI, 3.3 to 4.0) for stage 4–5 CKD, and 5.3 (95% CI, 4.2 to 6.6) for those with ESKD and undergoing RRT.19 Details underpinning mortality estimates are included in Supplemental Table 1.
Net overall migration was estimated using projected ABS overseas departure and arrival data.22 Net overall migration estimates were assumed to be apportioned into the four living health states of alive without kidney disease, alive with stage 3 CKD, alive with stage 4–5 CKD (but not ESKD), and alive with ESKD, as per the estimated prevalence of stage 3 and 4–5 CKD and ESKD.
Productivity Estimates
“Productivity indices” were generated to account for the proportional loss in productivity attributable to absenteeism and presenteeism due to CKD and ESKD.12–14 Productivity indices are represented by cardinal values ranging from zero (completely unproductive) to one (completely productive). These indices were determined on the basis of the average number of working days per year (240 days, assuming full-time workers have 4 weeks annual leave and, therefore, work 48 weeks per year, 5 days per week) and absenteeism and presenteeism data (Supplemental Table 1). For those with kidney disease, productivity effects were derived from data published by van Haalen et al.9 The study estimated productivity losses among patients with kidney disease (stages 3a, 3b, 4, and 5 CKD and ESKD) based on the Adelphi Real World Disease Specific Programmes conducted in France, Italy, the United Kingdom, Germany, Spain, the United States, and China. For stage 3 CKD, the weighted average number of working days lost to absenteeism and presenteeism were 14.4 (95% CI, 12.3 to 16.4) and 46.2 (95% CI, 43.9 to 48.5), respectively. For stage 4–5 CKD (non-RRT dependent), there was an estimated 17.0 (95% CI, 15.8 to 18.3) days of missed work and 53.5 (95% CI, 52.1 to 55.0) days of unproductive time at work per person per year. For those with RRT-dependent ESKD, the number of days lost to absenteeism and presenteeism were 29.3 (95% CI, 27.6 to 30.9) and 83.3 (95% CI, 81.6 to 85), respectively. Productivity effects for those without kidney disease were drawn from Australian general population data. Absenteeism estimates were adapted from the Australian Human Research Institute survey,28 and presenteeism from a report by Medibank Australia,29 with 8.8 days and 6.5 days lost per person per year, respectively (Supplemental Table 1). The estimated productivity index in those with stage 3 CKD was 0.748, whereas those with stage 4–5 CKD and ESKD had productivity indices of 0.639 and 0.531, respectively. The cohort without kidney disease had a productivity index of 0.936.
To estimate the number of PALYs, the total years of life lived for each year was multiplied by the productivity indices and the condition-specific proportion of equivalent full-time (EFT) workers. This is akin to the multiplication of years of life lived by utilities to generate QALYs.30 To obtain the condition-specific proportion of EFT workers, age- and sex-specific labor-force participation rates in those with and without kidney disease were calculated by applying the HR of withdrawal from the labor force in those with CKD and ESKD10 to the national labor-force participation rates for 201924 (Supplemental Table 1). The HR used was from a study by Klarenbach et al.10 (stage 4–5 CKD, HR, 5.4; 95% CI, not available; ESKD, HR, 7.9; 95% CI, 1.6 to 39.4). Due to a lack of data to inform the estimates, the proportion of EFT in those with stage 3 CKD was conservatively assumed to be the same as those without kidney disease. The estimated disease-specific labor-force participation rates were adjusted to the reported mean number of hours worked (based on 2016 employment data from ABS31) and the maximum total working hours for full-time employees (40 hours per week) to obtain the proportion of EFT workers for each age group and sex. For example, the adjusted labor-force participation rate in males aged 35 with ESKD was 24.8%, and males aged 35 work 42 hours per week on average. Therefore, the estimated proportion of EFT workers was 26% (24.8%×[42/40]). Details pertaining to the estimation of EFT workers are included in Supplemental Table 1. Age- and sex-specific proportions of EFT workers in the general population, and by condition, are presented in Supplemental Table 2.
The cohort of patients included in the studies from which productivity effects of kidney disease were drawn (premature deaths, absenteeism, presenteeism, and early withdrawal from the labor force) also reported common kidney-disease comorbidities, such as diabetes, hypertension, and cardiovascular disease. Thus, these inputs were suitable because the studies presented a broad representation of the typical profile of patients with kidney disease.9,10,19 It should be noted that our model only assessed the overall productivity effect of kidney-disease prevention and did not directly account for changes in productivity related to comorbid diseases.
To estimate the cost of lost productivity, each PALY was ascribed the value of Australian gross domestic product (GDP) per EFT worker. GDP is an appropriate measure because it broadly represents a country’s outputs (encompassing all goods and services produced and sold on the market) in monetary terms. Trend data on Australian GDP per hour worked (from 1975 to 2018) from the ABS was used to project the economic value of a PALY (details in Supplemental Table 1).23 The value of a PALY ranged from A$197,259 (US$133,787) in 2020 to A$217,983 (US$151,377) in 2029 (discounted). All costs were determined in 2020 Australian dollars and converted into US dollars using the latest available (2019) purchasing power parity rate of 1.440 (as of October 15, 2020).32
Sensitivity and Scenario Analyses
To determine the effect of uncertainty related to key input data on the model outputs, sensitivity analyses were undertaken. The key input parameters assessed included the increased risk of mortality and early withdrawal from the labor force due to kidney disease,10 and absenteeism and presenteeism estimates (Supplemental Table 3).33,34 To further test our model assumptions, we compared the base case to the following scenarios: (1) reducing the incidence of CKD and ESKD using a sliding scale (in 10% decrements) to assess more realistic and achievable targets, including a 10% reduction in kidney-disease incidence; (2) extending the time horizon to 20 years (projection until the year 2039); (3) assuming no temporal trend in GDP and, therefore, maintaining the economic value of a PALY using the latest available data (2018); (4) using the proportion of EFT for the total Australian population rather than the estimated condition-specific EFT (Supplemental Table 3); (5) using the HR of early labor-force withdrawal in those with stage 4–5 CKD into the stage 3 CKD cohort; (6) assuming those with ESKD had the same proportion of EFT as those without kidney disease; and (7) reducing the discount rate to 3% (World Health Organization standard)35 and (8) 0% (assuming undiscounted).
Results
In 2020, the estimated number of people with kidney disease in the Australian population of working age was estimated to be 456,576 (2.5%) (Table 2). This comprised 403,809 (88.4%) people with stage 3 CKD, 36,478 (8.0%) with stage 4–5 CKD and 16,289 (3.6%) with ESKD. Supplemental Table 4 present sex-specific projection estimates. Over the next 10 years, it was estimated there will be 161,470 new cases of kidney disease (Table 3). This comprised 85,556 new cases in males and 75,914 in females. In terms of stage-specific estimates, 151,138 new cases of stage 3 CKD were predicted, whereas the numbers of new cases of stage 4–5 CKD and ESKD were 9843 and 489, respectively. If we could prevent 10% of new cases of kidney disease (including CKD and ESKD) over this period, a total of 304 premature deaths would be averted (Table 3). This comprised 199 deaths averted among males and 105 among females (Supplemental Table 6).
Table 2.
The estimated number of years of life lived, PALYs, and the broader economic cost (in terms of GDP) for the total Australian population of working age (15–69 yr) over 10 years (2020–2029), with and without kidney disease
| Year | Total Number of People | Total Years of Life Lived | Total PALYs | Total Cost (US$) | ||||
|---|---|---|---|---|---|---|---|---|
| Without Kidney Disease | With Kidney Disease | Without Kidney Disease | With Kidney Disease | Without Kidney Disease | With Kidney Disease | Without Kidney Disease | With Kidney Disease | |
| 2020 | 17,599,628 | 456,576 | 16,646,273 | 433,166 | 10,309,192 | 185,679 | 1,412,207,817,948 | 25,435,292,555 |
| 2021 | 17,847,423 | 460,249 | 16,075,760 | 415,794 | 9,954,095 | 178,458 | 1,379,482,494,374 | 24,731,505,363 |
| 2022 | 18,104,021 | 464,311 | 15,528,105 | 399,335 | 9,608,890 | 171,573 | 1,347,008,202,560 | 24,051,725,470 |
| 2023 | 18,354,275 | 468,270 | 14,997,165 | 383,618 | 9,273,072 | 164,989 | 1,314,760,790,720 | 23,392,643,753 |
| 2024 | 18,599,258 | 472,345 | 14,477,030 | 368,498 | 8,945,994 | 158,672 | 1,282,692,412,421 | 22,750,688,112 |
| 2025 | 18,839,390 | 476,359 | 13,968,648 | 353,969 | 8,628,127 | 152,616 | 1,250,913,467,984 | 22,126,406,978 |
| 2026 | 19,066,116 | 479,990 | 13,469,368 | 339,830 | 8,318,138 | 146,752 | 1,219,272,676,710 | 21,510,920,823 |
| 2027 | 19,286,821 | 483,642 | 12,979,389 | 326,112 | 8,016,039 | 141,087 | 1,187,809,638,987 | 20,906,158,928 |
| 2028 | 19,505,990 | 487,194 | 12,503,096 | 312,905 | 7,723,433 | 135,648 | 1,156,802,227,248 | 20,317,186,776 |
| 2029 | 19,718,518 | 490,702 | 12,040,223 | 300,172 | 7,440,149 | 130,419 | 1,126,270,014,747 | 19,742,429,703 |
| Total | 186,921,439 | 4,739,638 | 142,685,055 | 3,633,398 | 88,217,129 | 1,565,894 | 12,677,219,743,699 | 224,964,958,461 |
| Combined totala | 191,661,077 | 146,318,452 | 89,783,023 | 12,902,184,702,161 | ||||
Years of life lived, PALYs, and costs were subjected to an annual discount rate of 5% applied beyond the first year, and, therefore, represent discounted values. All analyses were estimated in 2020 Australian dollars and converted into US dollars using the purchasing power parity rate of 1.440 (2019 data).32
With and without kidney disease.
Table 3.
The effect of preventing new cases of kidney disease in terms of deaths averted, years of life saved, PALYs saved, and costs saved among the total Australian population of working age (15–69 yr) over 10 years (2020–2029)
| Kidney Disease Incidence (%)a | Total New Kidney Disease Cases Avoided | Total Deaths Averted | Total Years of Life Saved | Total PALYs Saved | Total Cost Saved (US$) |
|---|---|---|---|---|---|
| 90 | 16,264 | 304 | 554 | 7590 | 1,109,660,926 |
| 80 | 32,502 | 606 | 1107 | 15,151 | 2,215,138,296 |
| 70 | 48,714 | 909 | 1660 | 22,684 | 3,316,414,987 |
| 60 | 64,900 | 1211 | 2211 | 30,189 | 4,413,473,818 |
| 50 | 81,061 | 1512 | 2762 | 37,664 | 5,506,297,549 |
| 40 | 97,195 | 1813 | 3313 | 45,111 | 6,594,868,886 |
| 30 | 113,303 | 2113 | 3862 | 52,529 | 7,679,170,471 |
| 20 | 129,385 | 2412 | 4411 | 59,918 | 8,759,184,892 |
| 10 | 145,441 | 2711 | 4959 | 67,278 | 9,834,894,676 |
| 0 | 161,470 | 3010 | 5506 | 74,609 | 10,906,282,293 |
Years of life lived, PALYs, and costs were subjected to an annual discount rate of 5% applied beyond the first year, and, therefore, represent discounted values. All analyses were estimated in 2020 Australian dollars and converted into US dollars using the purchasing power parity rate of 1.440 (2019 data).32
Assumes the incidence of kidney disease in all cohorts, including stage 3 and 4–5 CKD and ESKD, were concurrently reduced.
Years of Life Lived Saved
The total predicted number of years of life lived for the Australian population of working age over 10 years (2020–2029) was 146,318,452 (3,633,398 in those with kidney disease and 142,685,055 without kidney disease) (discounted). During this period, both prevalent and incident cases of kidney disease are estimated to incur a loss of 56,049 in years of life. If an intervention that could prevent 10% of future cases of kidney disease was implemented, a total of 554 years of life would be saved, equivalent to 0.03 years of life saved per person with kidney disease over 10 years (Table 3). In terms of sex differences, there were an estimated 364 years of life saved among males and 190 among females.
PALYs Saved
The total number of PALYs estimated over the next 10 years (2020–2029) for the Australian working-age population was 89,783,023 (1,565,894 in those with kidney disease and 88,217,29 without kidney disease), accruing >US$12.9 trillion in GDP (discounted) (Table 2). During this period, both prevalent and incident cases of kidney disease were estimated to incur a loss of 635,326 PALYs. Prevention of 10% of new cases of kidney disease over this time period would result in 7590 PALYs gained (or 0.4 PALYs per new case avoided over 10 years). The projected number of PALYs gained among males and females were 4827 and 2763, respectively (Supplemental Table 6). In terms of GDP, this amounted to a potential saving of US$1.1 billion, or US$67,544 per new case avoided (Table 3).
Sensitivity and Scenario Analyses
Figure 2 displays the effect of premature mortality, labor-force withdrawal, absenteeism, and presenteeism on productivity loss due to prevalent and incident cases of CKD and ESKD over 10 years. A total loss of US$91.2 billion was estimated to occur due to kidney disease–related impairment in work productivity. Of the estimated productivity loss, presenteeism in stage 3 CKD (US$43.8 billion or 48.0%) contributed nearly half, followed by early labor-force withdrawal in stage 4–5 CKD (US$18.5 billion or 20.3%). Males contributed slightly more (54.6%) to the total economic loss compared with females (45.4%) (Figure 2).
Figure 2.
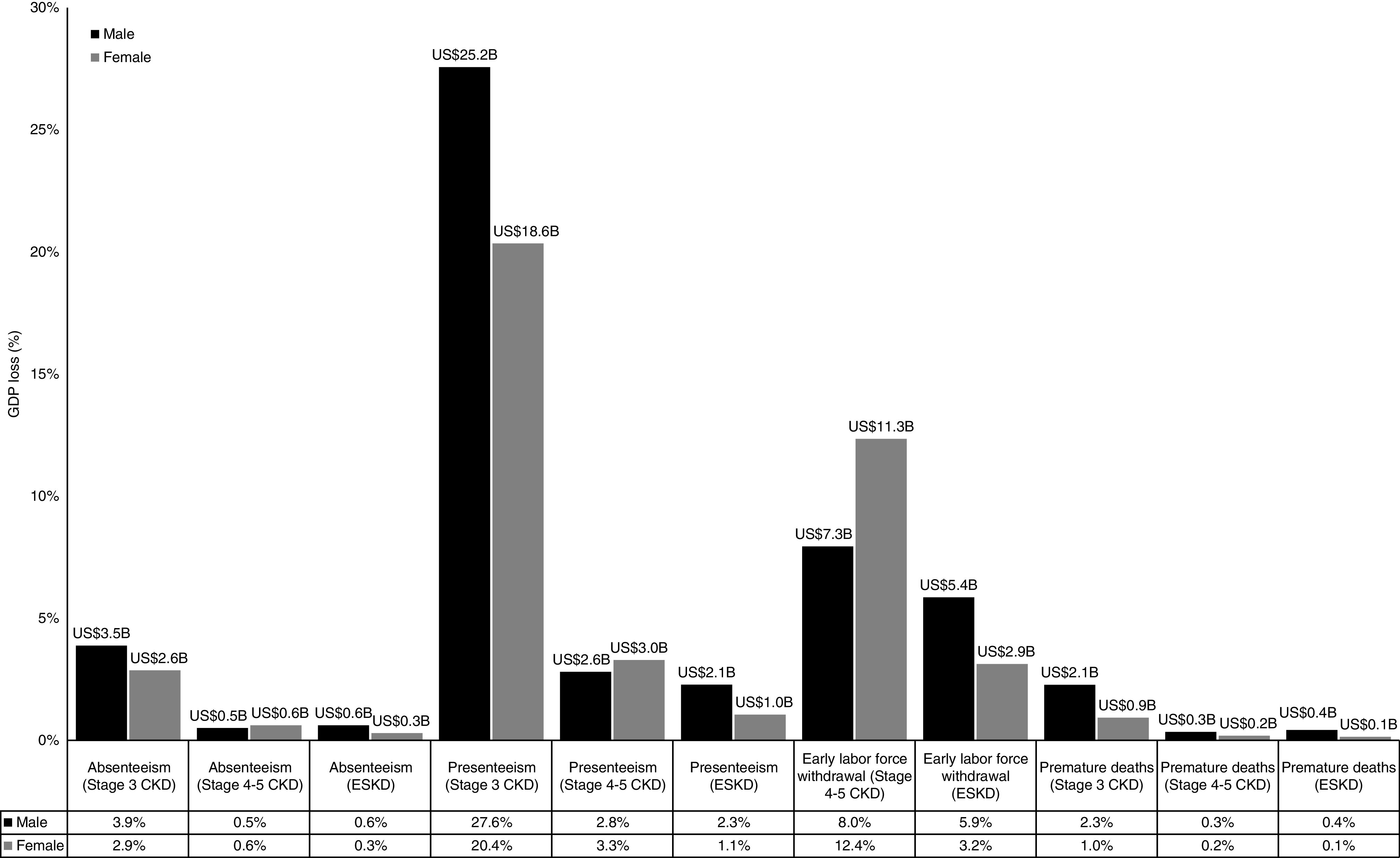
The impact of kidney disease on productivity in the Australian population of working age over the next 10 years (2020-2029), which amounts to US$91.2 billion in GDP. The economic burden is driven by kidney disease-related presenteeism, followed by early labor-force withdrawal. To assess each component (premature mortality, early labor-force withdrawal, absenteeism, and presenteeism), one factor at a time were inputted into the model while the others were “switched off.” The results were compared with the analysis that assumed kidney disease did not exist. Technical details are provided in Supplemental Table 3. All analyses were estimated in 2020 Australian dollars and converted into US dollars using the purchasing power parity rate of 1.440 (2019 data).32
The model was most sensitive to the uncertainty surrounding the increased risk of early withdrawal from the labor force (Figure 3). Applying the upper bound of the HR of early labor-force withdrawal due to ESKD increased PALYs by 732,755 (0.8%), whereas applying the lower bound decreased PALYs by 130,709 (0.1%). Due to a lack of data, the hazard of early labor-force withdrawal attributable to CKD was assumed to vary by 30%. Using the upper and lower bound of the estimate increased and reduced PALYs by 81,753 (0.09%) and 71,556 (0.08%), respectively. Variations in absenteeism and presenteeism inputs and HR for all-cause mortality affected the estimated number of PALYs the least, ranging from 0.00002% to 0.02% (Figure 3) compared with the base case.
Figure 3.
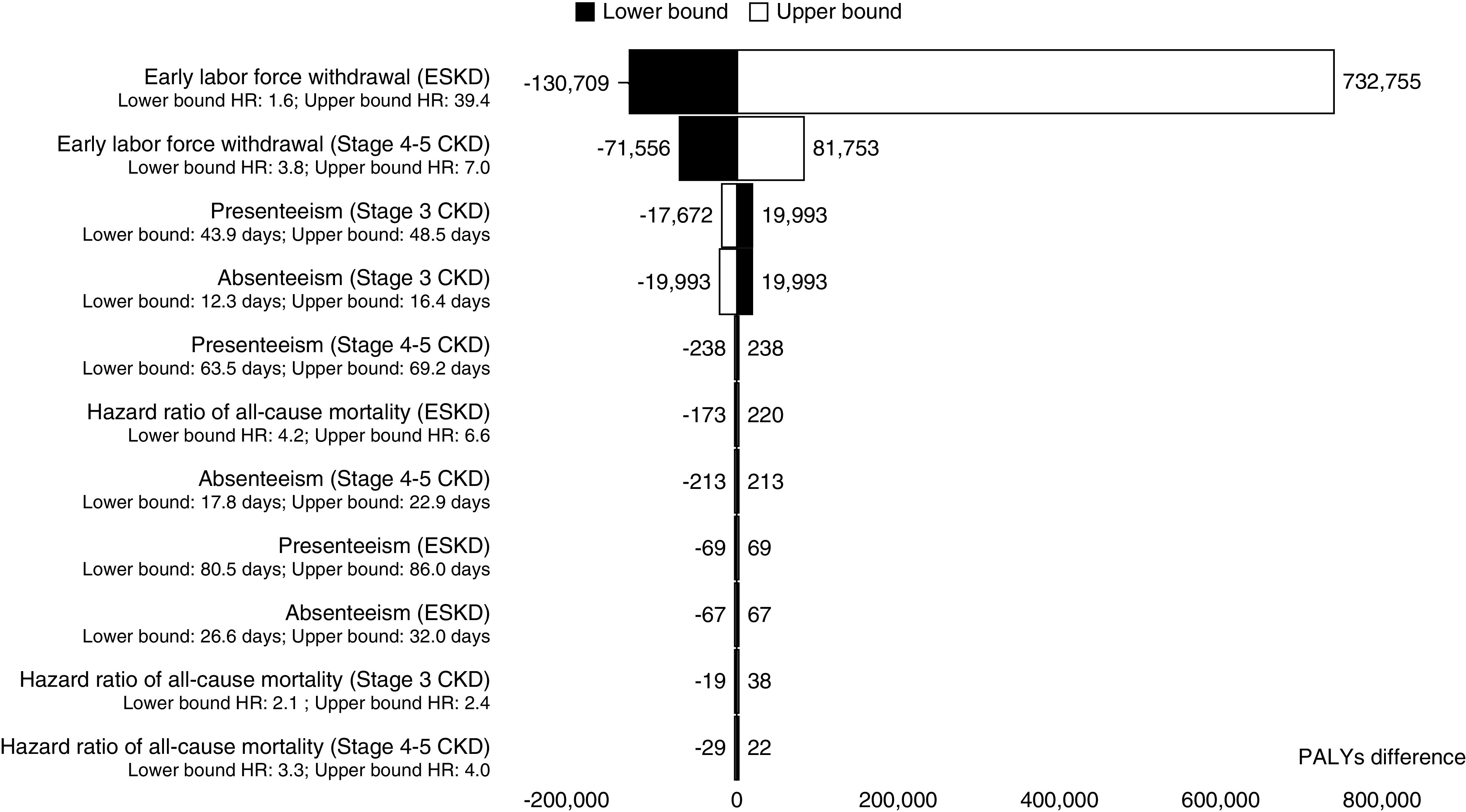
Sensitivity analyses assessing the effect of variation in key model parameters on the number of PALYs, compared with the base-case analysis. The tornado diagram demonstrates the model is most sensitive to variations in the increased risk of early labor force withdrawal associated with kidney disease. Black bars denote the lower-bound estimate has been used, whereas the white bars indicate use of the upper-bound estimate of the parameter. Bars represent the difference in the number of PALYs compared with the base case. In the base-case analysis, the total PALYs among working age Australians in 2020–2029 was 89,783,023 (Table 2). Note that due to a lack of available data for the intervals, the HR of early labor-force withdrawal due to CKD (as reported by Klarenbach et al.10) was assumed to vary by 30%.
Extending the time horizon to 20 years (2020–2039) resulted in 61.8 million additional PALYs (68.9% more than the base case) or US$9.9 trillion in GDP (Table 4). If 10% of future kidney-disease cases could be prevented over this 20-year time horizon, >US$3.1 billion in GDP could be gained. Assuming the discounting rate of 3%, there was an additional 9.6 million PALYs (10.7% increase to the base case). When outcomes were undiscounted, an extra 27.0 million PALYs were accrued (30.2% increase). Abolishing the risk of early labor-force withdrawal due to CKD and ESKD to estimate the proportion of EFT workers (refer to Supplemental Table 3 for details) reduced the total number of PALYs by 0.2% compared with the base case (Table 3). Employing the HR of early labor-force withdrawal in those with stage 4–5 CKD to the stage 3 CKD cohort resulted in a loss of 1.3 million PALYs (1.4% reduction compared with the base case). If we assume that those with ESKD had the same proportion of EFT workers as those without kidney disease, there would be an additional 35,000 PALYs (0.04%) compared to the base case.
Table 4.
Scenario analyses assessing the effect of different model assumptions on the estimated number of PALYs and associated economic costs among the total Australian population of working age (15–69 yr) over 10 years (2020–2029), compared with the base-case analysis
| Analysis | Details | PALYs Lost/Saved Compared with the Base Case (%) | Total GDP Lost/Gained (US$ billion) |
|---|---|---|---|
| Extending time horizon to 20 years (2020–2039) | Kidney-disease incidence assuming current trajectories | +68.9 | +9878 |
| Kidney-disease incidence reduced by 10% | +68.9 | +9881 | |
| Kidney-disease incidence reduced by 100% (no new cases) | +69.1 | +9908 | |
| GDP per EFT worker | No growth in GDP over time (2018 value) | N/A | +890 |
| Proportion of EFT | Abolishing disease-specific risk of labor-force withdrawal to estimate proportion of EFT (i.e., applying the general population EFT estimate to the entire cohort, with and without kidney disease) | −0.3 | −41 |
| Assuming those with stage 3 CKD have the same HR of early labor-force withdrawal as those with stage 4–5 CKD | −1.4 | −187 | |
| Assuming those with ESKD have the same proportion of EFT as those without kidney disease | +0.04 | +5 | |
| Discount rate | 3% | +10.7 | +1404 |
| 0% | +30.2 | +3966 |
The base-case analysis estimated the total PALYs and associated economic costs in the total Australian population of working age over the next 10 years, assuming the current trajectory of kidney disease in Australia. The total PALYs in the base case was 91,830,845 and the associated economic costs was US$13,196,459,568,023 (Table 2). All analyses were estimated in 2020 Australian dollars and converted into US dollars using the purchasing power parity rate of 1.440 (2019 data).32 N/A, not applicable.
Discussion
Our study highlights that, over the next 10 years, kidney disease will continue to impose a substantial burden on work productivity and the economy. Conservative assumptions of kidney-disease prevention during this period suggest significant economic benefit. For example, 7600 PALYs could be saved if incident cases of kidney disease were reduced by 10%. This is akin to preventing 1503 new cases of stage 3 CKD, 117 new cases of stage 4–5 CKD, and six new cases of ESKD per year over 10 years, and could result in US$1.1 billion in GDP gained. An analysis of Medicare data in the United States demonstrated that preventing a 10% reduction in eGFR over 10 years in patients with stage 3 CKD or higher could save US$27.6 billion in costs of care.36 This highlights that investment in preventive strategies addressing kidney disease would result in substantial savings due to improved work productivity, and reduced costs of care, the latter of which were not considered in our current model.
Absenteeism among patients with CKD who are non-RRT dependent (stage 4 and 5) in Italy was estimated to cost €553 per person per year,37 which is lower than our findings in the stage 4–5 CKD cohort (US$2889 per person over 10 years). Presenteeism was not reported in the Italian study; however, in our study, presenteeism contributed 6.5 times more to the overall GDP loss compared with absenteeism, which reflected the greater loss of productive hours worked in those with kidney disease. In terms of labor-force participation, approximately 14% and 7% of those with CKD and ESKD, respectively, were EFT workers. Our estimates are substantially lower than reports from other developed countries (CKD, 51%; ESKD, 9%–40%).38–41 These differences can be attributed to a broad definition of employment (e.g., inclusion of students as being employed38), small sample sizes,41 variation in the definition of working age, and—crucially—a lack of differentiation between part-time or full-time workers in these studies.38–41 By comparison, our estimates accounted for part- and full-time workers to determine the number of EFT workers,24 and the increased risk of early labor-force dropout.10 Indeed, the substantial reduction in workforce participation among those with stage 4–5 CKD was the second main driver of productivity loss in our model (20.3%).
Moderate CKD (stage 3) is often considered “clinically significant” because it represents an approximately 50% loss of normal renal function, and is the stage where patients would usually start exhibiting symptoms.27 These patients have higher risks of hospitalization and mortality compared with those with stage 1 and 2 CKD,42 and have increased disability and reduced productivity.9 Notably, due to a lack of data, we have conservatively assumed those with stage 3 CKD have the same proportion of EFT as those without kidney disease. This assumption is on the basis that CKD is generally asymptomatic until kidney impairment becomes severe (stage 4–5 CKD). However, given that those with stage 3 CKD are more likely to die earlier,18 and have higher absenteeism and presenteeism compared with the general population9 (albeit lower estimates than with stage 4–5 CKD and ESKD), it is very likely that labor-force participation in those with stage 3 CKD would still be affected to some extent. Productivity loss due to absenteeism, presenteeism, and premature deaths were the highest in stage 3 CKD cohort compared with stage 4–5 and ESKD (Figure 2), which is driven by a higher prevalence and incidence of stage 3 CKD. Given that 64% of the Australian population with CKD are in stages 1 and 2,6 more efforts are needed for the secondary prevention of CKD in these patients to curb the progression into stage 3 CKD and beyond. Our analysis demonstrated absolute prevention of future CKD cases (stage 3–5) could save >US$6770 per person per year due to increased productivity. This is double the yearly direct nonhealthcare cost and government subsidies (A$3661 or US$2542 per person) in an Australian cohort with stage 3–5 CKD, as reported by Wyld et al.8 In terms of ESKD, labor-force data analysis suggests a 1-year delay could result in 0.3 years in productivity gained per person.43 Our model also shows a gain of 0.1 PALYs per person for every new case of ESKD avoided over 10 years. Importantly, the burden of kidney disease has continued to increase over time. The number of Australians with stage 3–5 CKD has doubled between 2000 and 2012,4 and RRT prevalence increased by 19% in 2012–2020.3 Clearly, there is a pressing need to address the increasing burden of kidney disease, which exerts substantial negative effects on individual health, the society, and the economy.
One major barrier to early diagnosis and prevention of CKD is low awareness among the general public and primary care physicians.44 The AusHEART study found only 18% of patients with CKD were correctly diagnosed by primary care physicians.45 Furthermore, 25% of patients with ESKD requiring RRT within 90 days present late to nephrologists, therefore missing the opportunity for timely intervention.44 In recognition of these issues, preventive measures are now focused on (1) the long-term surveillance of high-risk predialysis cohorts, and (2) raising public awareness of CKD, particularly the importance of its early recognition.46 As a part of the Healthy People 2030 plan, the US Office of Disease Prevention and Health Promotion has aimed to reduce the future burden of kidney disease in the United States by 10%.47 Applying these goals to the Australian population, US$1.1 billion could potentially be spent on prevention strategies for kidney disease over the next 10 years as a break-even investment. Preventive strategies, particularly surrounding primary prevention (lifestyle, diet, environmental factors), could simply build on already existing campaigns. For context, it was estimated that implementing a 30% tax on alcohol or tobacco and a 10% tax on unhealthy foods would cost approximately US$14 million each, whereas imposing mandatory salt limits on processed food would cost US$49 million.48 Mass media campaigns to improve physical activity would cost US$9 million.48 In fact, all of these interventions have been shown to be cost saving if implemented in the Australian setting.48 Additionally, prevention, detection, and better management of comorbidities associated with CKD, such as diabetes and hypertension,2 have been shown to be cost-effective.49 For example, implementing angiotensin-converting enzyme inhibitor use in those with >15% risk of cardiovascular disease would cost US$49 million per annum, but the cost offset amounts to US$250 million.48 Therefore, it is likely that the economic gains achieved from prevention far outweigh the cost of implementation, which presents a potential for economic return.
The key strength of our study is the comprehensive inclusion of absenteeism, presenteeism, early labor-force withdrawal, and premature mortality data to reflect the productivity burden of CKD and ESKD. Unlike a closed-cohort model, which follows the same group of people over time,11–13 the dynamic nature of our model accounted for natural population movements over time, providing a more realistic estimation of the outcomes of interest. In addition, the PALY metric allowed productivity to be measured at a population level across different age groups and sex.11–13 This metric is simple, easy to use, and can be applied in the context of various other diseases in Australia11–13 and other countries.50,51 By measuring the effect of ill health on productivity, we consider the broader economic costs of disease, which often exceed the healthcare effects. To compare, other established metrics such as the QALY and disability-adjusted life year only measure the effect of ill health on quality-of-life impairment and disability, respectively. The PALY, therefore, offers a convenient method to measure broader economic effects of disease and would complement the QALY and disability-adjusted life year for healthcare planning and decision making.
Limitations
There are several limitations to our study. First, comorbidities that could affect kidney disease incidence and mortality, and also negatively affect productivity, were not directly captured in our model. However, the productivity inputs used in our model were derived from studies in patients with kidney disease, many of whom presented with comorbidities, including hypertension, diabetes, and cardiovascular disease.9,10,19 Public health measures to improve overall health (diet, lifestyle, and environmental factors) in patients at high risk of CKD or those with early CKD would likely help to synergistically reduce the incidence of various comorbid diseases. However, interventions such as increasing use of angiotensin-converting enzyme inhibitors would have little direct effect on some comorbid conditions (e.g., diabetes and depression), thus, the productivity effects of these comorbidities are likely to remain. Therefore, depending on the anticipated effects of the targeted intervention to be implemented, further evaluations beyond the scope of this model may be needed. Second, productivity effects were only considered for those with the illness who were employed, whereas productivity loss due to unpaid work or caregiving responsibilities for patients with kidney disease were not taken into account due to a lack of available data. Therefore, our results likely underestimated the true productivity effect of kidney disease. In addition, our analysis was limited to the working-age population, and, therefore, we have not fully captured the effects of kidney disease in older age groups, where the incidence of kidney disease increases. However, this is beyond the scope of the study. The HR associated with early labor-force withdrawal in those with ESKD that we used in our model was drawn from Klarenbach et al.,10 which also included patients with non-ESKD CKD, and was, therefore, underestimated. We were also unable to find Australian-specific inputs for the productivity effects of kidney disease (absenteeism, presenteeism, premature deaths, and early labor-force withdrawal); therefore, the best available evidence from other countries, with similar demographics to Australia, was used. We acknowledge these estimates may not be directly applicable to the Australian population and, thus, our results may not have accurately captured the productivity loss due to kidney disease in the Australian context. Nevertheless, employing the upper- and lower-bound estimates of these productivity inputs (Figure 3) would not have changed our conclusions regarding the substantial effect of kidney disease on work productivity. Last, the ESKD cohort in our model did not consider the type of RRT modalities. Several studies indicate patients with ESKD on transplantation (32%–40%) had higher rates of employment than those on dialysis (9%–31%).39,52 Disability payouts, as reported in a Canadian study by Manns et al.,53 would be reduced by CA$1.7 million if transplant rates were increased by 10%, assuming 20% of transplanted individuals would return to work post-transplantation. However, there are also reports suggesting transplantation does not result in higher rates of employment, but helps maintain employment given the patients were already employed before the procedure.54,55 In our model, if those with ESKD (and, therefore, those on RRT) were generously assumed to have the labor-force participation rates of those without kidney disease, there would be an additional 35,058 (0.04%) PALYs compared with the base case (Table 4), and preventing 10% of future kidney-disease cases in this scenario would add US$320 million to the US$1.1 billion of GDP savings estimated in our incidence-reduction analysis (Table 3). Thus, our conclusions remain unchanged. Nonetheless, robust data on employment among Australians with different modalities of RRT could further inform this parameter.
In conclusion, prevention of incident cases of kidney disease results in prolonged life, increased productivity, and substantial economic benefit. Policy makers and employers are encouraged to engage in preventive measures to limit the incidence and progression of kidney disease.
Disclosures
D. Liew reports receiving honoraria or study grants from Abbvie, Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Pfizer, Sanofi, and Shire. A.J. Owen is an editorial board member for Nutrients. F. Savira is supported by a Monash International Postgraduate Research Scholarship, Monash Graduate Scholarship, and Monash Postgraduate Publication Award. E. Zomer reports receiving study grants from Amgen, AstraZeneca, Pfizer, and Shire. This research funding is unrelated to this study and the funding organizations have no role in the design or interpretation of this study. D. Liew reports Consultancy Agreements with Abbvie, Astellas, AstraZeneca, Bristol-Myers Squibb, Edwards Lifesciences, Novartis, Pfizer, Sanofi, Shire; Research Funding from Abbvie, Astellas, AstraZeneca, Bristol-Myers Squibb, Novartis, Pfizer, Sanofi, Shire; Honoraria from Abbvie, Astellas, AstraZeneca, Bristol-Myers Squibb, Edwards Lifesciences, Novartis, Pfizer, Sanofi, and Shire. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
Dr. Ella Zomer, Dr. Feby Savira, and A/Prof. Zanfina Ademi designed the study; Dr. Feby Savira collected the model inputs; Dr. Ella Zomer, Dr. Feby Savira, and A/Prof. Zanfina Ademi performed input parameterization; Dr. Feby Savira, Dr. Ella Zomer, and A/Prof. Zanfina Ademi analyzed and interpreted the results; Dr. Feby Savira drafted the manuscript; A/Prof. Bing H. Wang, Dr. Andrew R. Kompa, A/Prof. Zanfina Ademi, Dr. Alice J. Owen, Dr. Ella Zomer, and Prof. Danny Liew validated the results and critically revised the manuscript for intellectual content. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated or resolved.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “QALYs, DALYs and Now PALYs: Strengthening the Argument for Prevention of CKD,” on pages 771–773.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020081148/-/DCSupplemental.
Supplemental Table 1. Description of the model inputs and data sources.
Supplemental Table 2. The estimated proportion of equivalent full time workers (%) in the general population, and separated by kidney disease status, among Australians of working age.
Supplemental Table 3. Description of the scenario analyses.
Supplemental Table 4. The estimated number of people with and without kidney disease, years of life lived, PALYs and the broader economic cost (in terms of GDP) among Australian males of working age (15–69 years) from 2020 to 2029.
Supplemental Table 5. The estimated number of people with and without kidney disease, years of life lived, PALYs and the broader economic cost (in terms of GDP) among Australian females of working age (15–69 years) from 2020 to 2029.
Supplemental Table 6. The impact of preventing new cases of kidney disease in terms of deaths averted, years of life saved, PALYs saved, and costs saved among Australian males and females of working age (15–69 years) over 10 years (2020 to 2029).
References
- 1.Cockwell P, Fisher L-A: The global burden of chronic kidney disease. Lancet 395: 662–664, 2020. 10.1016/S0140-6736(19)32977-0 [DOI] [PubMed] [Google Scholar]
- 2.GBD Chronic Kidney Disease Collaboration: Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395: 709–733, 2020. 10.1016/s0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Health Australia: National Strategic Action Plan for Kidney Disease. Canberra, Australia, Kidney Health Australia and Australian Government Department of Health, 2019 [Google Scholar]
- 4.Australian Institute of Health and Welfare: Chronic kidney disease. Available at: https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/cardiovascular-health-compendium/contents/impact. Accessed May 25, 2020
- 5.Tucker PS, Kingsley MI, Morton RH, Scanlan AT, Dalbo VJ: The increasing financial impact of chronic kidney disease in Australia. Int J Nephrol 2014: 120537, 2014. 10.1155/2014/120537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Australian Institute of Health and Welfare: Cardiovascular Disease, Diabetes and Chronic Kidney Disease: Australian Facts: Prevalence and Incidence, Canberra, Australia, Australian Institute of Health and Welfare, 2014 [Google Scholar]
- 7.Plantinga LC, Johansen K, Crews DC, Shahinian VB, Robinson BM, Saran R, et al.; CDC CKD Surveillance Team: Association of CKD with disability in the United States. Am J Kidney Dis 57: 212–227, 2011. 10.1053/j.ajkd.2010.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyld ML, Lee CM, Zhuo X, White S, Shaw JE, Morton RL, et al.: Cost to government and society of chronic kidney disease stage 1-5: A national cohort study. Intern Med J 45: 741–747, 2015. 10.1111/imj.12797 [DOI] [PubMed] [Google Scholar]
- 9.van Haalen H, Jackson J, Spinowitz B, Milligan G, Moon R: Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: Analysis of multinational real-world data. BMC Nephrol 21: 88, 2020. 10.1186/s12882-020-01746-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klarenbach S, Stafinski T, Longobardi T, Jacobs P: The effect of renal insufficiency on workforce participation in the United States: An analysis using National Health and Nutrition Examination Survey III data. Am J Kidney Dis 40: 1132–1137, 2002. 10.1053/ajkd.2002.36854 [DOI] [PubMed] [Google Scholar]
- 11.Hird TR, Zomer E, Owen AJ, Magliano DJ, Liew D, Ademi Z: Productivity burden of hypertension in Australia. Hypertension 73: 777–784, 2019. 10.1161/hypertensionaha.118.12606 [DOI] [PubMed] [Google Scholar]
- 12.Magliano DJ, Martin VJ, Owen AJ, Zomer E, Liew D: The productivity burden of diabetes at a population level. Diabetes Care 41: 979–984, 2018. 10.2337/dc17-2138 [DOI] [PubMed] [Google Scholar]
- 13.Owen AJ, Maulida SB, Zomer E, Liew D: Productivity burden of smoking in Australia: A life table modelling study. Tob Control 28: 297–304, 2019. 10.1136/tobaccocontrol-2018-054263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan QY, Zomer E, Owen AJ, Chin KL, Liew D: Impact of tobacco use on health and work productivity in Malaysia. Tob Control 29: 111–117, 2020. 10.1136/tobaccocontrol-2018-054677 [DOI] [PubMed] [Google Scholar]
- 15.Youth Law Australia: When can I start working? Available at: https://yla.org.au/vic/topics/employment/when-can-i-start-working/. Accessed March 12
- 16.Australian Bureau of Statistics: Retirement and retirement intentions, Australia, Canberra, Australia, Australian Bureau of Statistics, 2020. Available at: https://www.abs.gov.au/ausstats/abs@.nsf/mf/6238.0. Accessed December 27, 2020 [Google Scholar]
- 17.National Health and Medical Research Council: How to Compare the Costs and Benefits: Evaluation of the Economic Evidence, Canberra, Australia, National Health and Medical Research Council, 2001, p 43 [Google Scholar]
- 18.Eriksen BO, Ingebretsen OC: The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int 69: 375–382, 2006. 10.1038/sj.ki.5000058 [DOI] [PubMed] [Google Scholar]
- 19.Neovius M, Jacobson SH, Eriksson JK, Elinder CG, Hylander B: Mortality in chronic kidney disease and renal replacement therapy: A population-based cohort study. BMJ Open 4: e004251, 2014. 10.1136/bmjopen-2013-004251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Australian Bureau of Statistics: Table 6: Kidney Disease Biomarkers. In: Australian Health Survey: Biomedical results for chronic diseases, Canberra, Australia, Australian Bureau of Statistics, 2013 [Google Scholar]
- 21.Grams ME, Chow EK, Segev DL, Coresh J: Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis 62: 245–252, 2013. 10.1053/j.ajkd.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Australian Bureau of Statistics: Population projections, Australia, Canberra, Australia, Australian Bureau of Statistics, 2018. Available at: https://www.abs.gov.au/AUSSTATS/abs@.nsf/mf/3222.0. Accessed July 8, 2020 [Google Scholar]
- 23.Australian Bureau of Statistics: 5204.0 - Australian system of national accounts, 2017–2018, Canberra, Australia, Australian Bureau of Statistics, 2018. Available at: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/5204.02017-18?OpenDocument. Accessed July 8, 2020 [Google Scholar]
- 24.Australian Bureau of Statistics: Labour force, Australia, detailed - electronic delivery, Canberra, Australia, Australian Bureau of Statistics, 2019 [Google Scholar]
- 25.Australian Bureau of Statistics : National state and territory populations, Canberra, Australia, Australian Bureau of Statistics, 2019. Available at: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Jun%202019?OpenDocument. Accessed May 14, 2020
- 26.Lee-Ko CHE, Matur S: Cardiovascular Disease, Diabetes and Chronic Kidney Disease (Australian Fact): Prevalence and Incidence, Canberra, Australia, Australian Institute of Health and Welfare, 2014, p 102 [Google Scholar]
- 27.Couser WG, Remuzzi G, Mendis S, Tonelli M: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80: 1258–1270, 2011. 10.1038/ki.2011.368 [DOI] [PubMed] [Google Scholar]
- 28.Australian Human Resources Institute: HR Pulse Survey Report: Absence Management, Melbourne, Australia, Australian Human Resources Institute, 2016, p 19 [Google Scholar]
- 29.Medibank, KPMG Econtech: Sick at Work: The cost of presenteeism to your business and the economy. Melbourne, Australia, Medibank, 2011 [Google Scholar]
- 30.Torrance GW, Feeny D: Utilities and quality-adjusted life years. Int J Technol Assess Health Care 5: 559–575, 1989. 10.1017/s0266462300008461 [DOI] [PubMed] [Google Scholar]
- 31.Australian Bureau of Statistics , Census TableBuilder 2016 Census: Employment, Income and Education Australian Bureau of Statistics Canberra, Australia 2019. Available at https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/6202.0Aug%202019?OpenDocument
- 32.Organisation for Economic Co-operation and Development: Purchasing power parities (PPP). Available at: https://data.oecd.org/conversion/exchange-rates.htm#indicator-chart. Accessed February 22, 2020
- 33.Goetzel RZ, Hawkins K, Ozminkowski RJ, Wang S: The health and productivity cost burden of the “top 10” physical and mental health conditions affecting six large U.S. employers in 1999. J Occup Environ Med 45: 5–14, 2003. 10.1097/00043764-200301000-00007 [DOI] [PubMed] [Google Scholar]
- 34.Stewart WF, Ricci JA, Chee E, Morganstein D: Lost productive work time costs from health conditions in the United States: Results from the American productivity audit. J Occup Environ Med 45: 1234–1246, 2003. 10.1097/01.jom.0000099999.27348.78 [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization: Making Choices in Health: WHO Guide to Cost- Effectiveness Analysis, edited by Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL, Geneva, Switzerland, World Health Organization, 2003 [Google Scholar]
- 36.Trivedi H: Cost implications of caring for chronic kidney disease: Are interventions cost-effective? Adv Chronic Kidney Dis 17: 265–270, 2010. 10.1053/j.ackd.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 37.Turchetti G, Bellelli S, Amato M, Bianchi S, Conti P, Cupisti A, et al.; On Behalf of the Tuscany CKD Study Group: The social cost of chronic kidney disease in Italy. Eur J Health Econ 18: 847–858, 2017. 10.1007/s10198-016-0830-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erickson KF, Zhao B, Ho V, Winkelmayer WC: Employment among patients starting dialysis in the United States. Clin J Am Soc Nephrol 13: 265–273, 2018. 10.2215/cjn.06470617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helanterä I, Haapio M, Koskinen P, Grönhagen-Riska C, Finne P: Employment of patients receiving maintenance dialysis and after kidney transplant: A cross-sectional study from Finland. Am J Kidney Dis 59: 700–706, 2012. 10.1053/j.ajkd.2011.08.025 [DOI] [PubMed] [Google Scholar]
- 40.van Manen JG, Korevaar JC, Dekker FW, Reuselaars MC, Boeschoten EW, Krediet RT; NECOSAD Study Group. Netherlands Cooperative Study on Adequacy of Dialysis: Changes in employment status in end-stage renal disease patients during their first year of dialysis. Perit Dial Int 21: 595–601, 2001 [PubMed] [Google Scholar]
- 41.Jansen DL, Rijken M, Heijmans MJWM, Kaptein AA, Groenewegen P: Psychological and social aspects of living with chronic kidney disease. In: Chronic Kidney Disease and Renal Transplantation, edited by Sahay M, The Netherlands, InTech Open, 2012, 10.5772/25992 [Google Scholar]
- 42.Yang WS, Chang YC, Hsieh ML, Wang JL, Wu LC, Chang CH: Stratified risks of infection-related hospitalization in patients with chronic kidney disease - a prospective cohort study. Sci Rep 10: 4475, 2020. 10.1038/s41598-020-61144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Vries EF, Rabelink TJ, van den Hout WB: Modelling the cost-effectiveness of delaying end-stage renal disease. Nephron 133: 89–97, 2016. 10.1159/000446548 [DOI] [PubMed] [Google Scholar]
- 44.Chen T, Harris DC: Challenges of chronic kidney disease prevention. Med J Aust 203: 209–210, 2015. 10.5694/mja15.00241 [DOI] [PubMed] [Google Scholar]
- 45.Razavian M, Heeley EL, Perkovic V, Zoungas S, Weekes A, Patel AA, et al.: Cardiovascular risk management in chronic kidney disease in general practice (the AusHEART study). Nephrol Dial Transplant 27: 1396–1402, 2012. 10.1093/ndt/gfr599 [DOI] [PubMed] [Google Scholar]
- 46.Venuthurupalli SK, Hoy WE, Healy HG, Cameron A, Fassett RG: CKD screening and surveillance in Australia: Past, present, and future. Kidney Int Rep 3: 36–46, 2017. 10.1016/j.ekir.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Office of Disease Prevention and Health Promotion : Healthy People 2030: Chronic kidney disease. Available at: https://health.gov/healthypeople/objectives-and-data/browse-objectives/chronic-kidney-disease#:~:text=Healthy%20People%202030%20focuses%20on,help%20prevent%20or%20delay%20CKD. Accessed July 16, 2020
- 48.Vos T, Carter R, Barendregt J, Mihalopoulos C, Veerman L, Magnus A, et al.; ACE-Prevention Team: Assessing cost-effectiveness in prevention (ACE-Prevention): Final report, Brisbane, Australia, University of Queensland, Brisbane and Deakin University, 2010 [Google Scholar]
- 49.Howard K, Salkeld G, White S, Chadban S, Craig J, McDonald S, et al.: The Cost-Effectiveness of Early Detection and Intervention to Prevent the Progression of Chronic Kidney Disease in Australia, Melboune, Australia, Kidney Health Australia, 2006 [Google Scholar]
- 50.Afroz A, Hird TR, Zomer E, Owen A, Chen L, Ademi Z, et al.: The impact of diabetes on the productivity and economy of Bangladesh. BMJ Glob Health 5: e002420, 2020. 10.1136/bmjgh-2020-002420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hird TR, Zomer E, Owen A, Chen L, Ademi Z, Magliano DJ, et al.: The impact of diabetes on productivity in China. Diabetologia 62: 1195–1203, 2019. 10.1007/s00125-019-4875-4 [DOI] [PubMed] [Google Scholar]
- 52.Julián-Mauro JC, Cuervo J, Rebollo P, Callejo D: Employment status and indirect costs in patients with renal failure: Differences between different modalities of renal replacement therapy. Nefrologia 33: 333–341, 2013. 10.3265/Nefrologia.pre2012.Dec.11767 [DOI] [PubMed] [Google Scholar]
- 53.Manns B, McKenzie SQ, Au F, Gignac PM, Geller LI; Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD) Network: The financial impact of advanced kidney disease on Canada pension plan and private disability insurance costs. Can J Kidney Health Dis 4: 2054358117703986, 2017. 10.1177/2054358117703986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danuser B, Simcox A, Studer R, Koller M, Wild P; Psychosocial Interest Group, Swiss Transplant Cohort Study: Employment 12 months after kidney transplantation: An in-depth bio-psycho-social analysis of the Swiss Transplant Cohort. PLoS One 12: e0175161, 2017. 10.1371/journal.pone.0175161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parajuli S, Singh J, Sandal S, Liebman SE, Demme RA: Self-reported employment status and social participation after successful kidney transplantation. Prog Transplant 26: 92–98, 2016. 10.1177/1526924816633956 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.