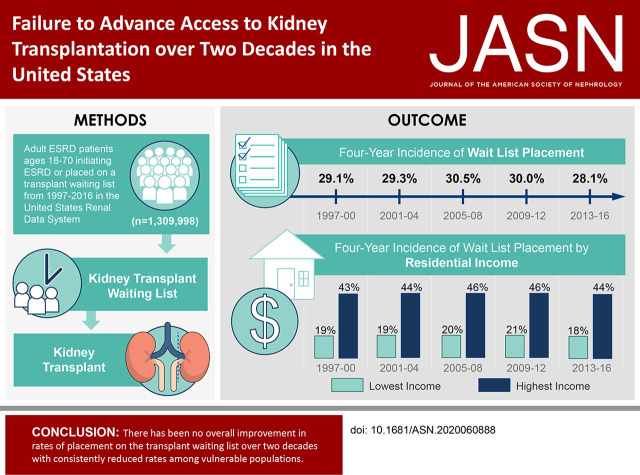
Significance Statement
There have been numerous research studies and policies developed to improve access to kidney transplantation among patients with ESKD over past decades. In this retrospective cohort study, the authors evaluated the longitudinal pattern of rates of placement on the transplant waiting list and transplantation among the ESKD population, as well as whether patterns were consistent in the population or varied among patient groups with historically low rates of access to transplantation. The study’s primary findings indicated no improvement in the incidence of placement on the transplant waiting list over a two-decade period from 1997 to 2016. In addition, rates of wait list placement and transplantation were consistently reduced among vulnerable populations. These results indicate that more effective interventions are needed to improve access to transplantation in the United States.
Keywords: kidney transplantation, end-stage renal disease, risk factors
Visual Abstract
Abstract
Background
Extensive research and policies have been developed to improve access to kidney transplantation among patients with ESKD. Despite this, wide variation in transplant referral rates exists between dialysis facilities.
Methods
To evaluate the longitudinal pattern of access to kidney transplantation over the past two decades, we conducted a retrospective cohort study of adult patients with ESKD initiating ESKD or placed on a transplant waiting list from 1997 to 2016 in the United States Renal Data System. We used cumulative incidence models accounting for competing risks and multivariable Cox models to evaluate time to waiting list placement or transplantation (WLT) from ESKD onset.
Results
Among the study population of 1,309,998 adult patients, cumulative 4-year WLT was 29.7%, which was unchanged over five eras. Preemptive WLT (prior to dialysis) increased by era (5.2% in 1997–2000 to 9.8% in 2013–2016), as did 4-year WLT incidence among patients aged 60–70 (13.4% in 1997–2000 to 19.8% in 2013–2016). Four-year WLT incidence diminished among patients aged 18–39 (55.8%–48.8%). Incidence of WLT was substantially lower among patients in lower-income communities, with no improvement over time. Likelihood of WLT after dialysis significantly declined over time (adjusted hazard ratio, 0.80; 95% confidence interval, 0.79 to 0.82) in 2013–2016 relative to 1997–2000.
Conclusions
Despite wide recognition, policy reforms, and extensive research, rates of WLT following ESKD onset did not seem to improve in more than two decades and were consistently reduced among vulnerable populations. Improving access to transplantation may require more substantial interventions.
Access to kidney transplantation is a critical lifesaving therapeutic option, roughly doubling life expectancy for patients with ESKD.1,2 Kidney transplantation is associated with longer survival and higher quality of life, and it is cost effective relative to the alternative treatment modality of maintenance dialysis.3–6 Kidney transplantation is available to eligible patients on the basis of access to a living donor or placement on a waiting list to receive a deceased donor transplant from a national allocation system.7 In 2019, there were 23,401 kidney transplants performed in the United States. As of November 2020, there were >91,000 patients on the national kidney transplant waiting list.8 The majority of patients with severe kidney disease initiate maintenance dialysis prior to receiving a transplant.
There is extensive literature documenting systemic barriers to kidney transplantation as well as individual characteristics associated with delayed access to transplantation for patients with ESKD. These include diminished access to transplant among patients from minority race/ethnic groups, lower socioeconomic status, older patients, and patients with comorbidities.9–17 Education about transplantation is a requirement for dialysis providers treating patients with ESKD.16,18 Despite this, there is wide variation in transplant referral rates between dialysis facilities, potentially exacerbated by poor understanding of the allocation system by dialysis providers and staff.15,19–24 There have been numerous studies identifying disparities, interventions, and policy reforms aimed at improving access to transplantation over the past 30 years.16,25–28 Recognition of the national imperative to promote transplantation was highlighted by an executive order by the federal government in 2019 aimed to markedly increase organ donation and transplantation rates in the United States.29
The primary aim of this study was to assess secular trends in access to transplantation for patients with ESKD over the past two decades in the United States. We sought to evaluate longitudinal changes in time to placement on the waiting list for deceased donor transplantation or receipt of a living donor transplant among adult patients from initial ESKD onset. In addition, we sought to evaluate changes in access to transplantation on the basis of demographic, clinical, and socioeconomic characteristics of the ESKD population.
Methods
Data Source and Study Population
The study population derived from the United States Renal Data System (USRDS). USRDS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases and is a national data registry that collects information, including treatment and outcomes, on the ESKD population in the United States.30 This study population included adult patients aged 18–70 with initial ESKD onset, wait list placement, or transplant between 1997 and 2016.
Exposure Variable
The primary exposure variable was year of initial ESKD onset or preemptive placement on the transplant waiting list. This date represented the initial dialysis or transplant date or date of placement on the waiting list for patients in the USRDS registry. For patients with repeated listing or transplant dates during the study period, only the initial event was considered for this study. In order to depict results more clearly, we also categorized year of initial ESKD onset or wait list placement into five eras: 1997–2000, 2001–2004, 2005–2008, 2009–2012, and 2013–2016.
Outcomes
We used a composite end point of time to the earliest date of either placement on the kidney transplant waiting list or receipt of living donor transplantation (waiting list placement or transplantation [WLT]) if this occurred prior to placement on the waiting list. Policy changed over the study period such that in earlier years, patients could receive a living donor transplant without being placed on the waiting list, whereas in more recent years (since September 1, 2014), all patients are placed on the waiting list even if the intent is to receive a living donor transplant. We examined the proportion and adjusted likelihood of WLT preemptively, prior to the initiation of dialysis, as well as separate analyses for time to WLT from patients’ initial date of dialysis initiation. We also depicted the cumulative incidence of WLT incorporating both preemptive WLT and following the initiation of dialysis. We also examined time to death prior to WLT and time to deceased and living donor transplantation from dialysis onset. Cumulative incidence models were administratively censored at 4 years following the inception date for each patient as well as the end of the study period (December 31, 2016). Given that the most recent era (2013–2016) had few patients with full 4-year follow-up, as a sensitivity analysis, we also generated cumulative incidence and multivariable Cox models censored at the last date of each respective era.
Covariates
The covariates included demographic information collected at initial ESKD onset and were selected a priori on the basis of prior research findings that have evaluated access to transplantation. Race was combined with Hispanic ethnicity using six categories of non-Hispanic White, non-Hispanic Black, Hispanic White, Hispanic Black, Asian, and other race/ethnicity. Primary diagnoses were maintained in the registry as diabetes, hypertension, cystic disease, GN, and other. The 18 ESKD regional networks were incorporated in the analysis on the basis of initial assignment. We merged data from the Centers for Medicare & Medicaid Services (CMS)-ESKD Medical Evidence 2728 form and used the USRDS crosswalk file to reclassify comorbid conditions that changed definition over time. The final study population only included data with a 2728 form submitted within 24 months of initial ESKD onset date (n=1,309,998). There were no marked differences in patients who had missing data for these for this reason of the initial population of n=1,347,099. Missing levels were categorized using a missing level in all analyses. Excluding patients with missing levels did not alter any of the primary results depicted in this study. We combined data from American Community Surveys, including median household income of patients, on the basis of residential zip code. We used three time periods of income data (2000, 2008, and 2016) and assigned the median income on the basis of the most proximate year of ESKD onset. We adjusted income levels using the Consumer Price Index to account for inflation and adjusted to 2016 United States dollars.
Statistical Methods
We used cumulative incidence plots accounting for competing risks of mortality to evaluate rates of WLT. For these models, we incorporated the minimum of ESKD onset or preemptive WLT as the inception point. For preemptively listed or transplanted patients, the events were coded as occurring on day 1, and all other patients’ time at risk initiated at the time of ESKD. On the basis of the five eras used to evaluate secular trends, we administratively censored models at 4 years post-ESKD initiation along with last follow-up for the study period of December 31, 2016. We used a multivariable logistic model for the outcome of preemptive WLT. We used multivariable Cox proportional hazard models to assess time to WLT (censored at death) as well as time to death (censored at WLT). The inception points for the Cox models were at the time of ESKD initiation, and as such, preemptively listed patients were not included in these models. We also used multivariable Cox proportional hazard models to evaluate time to deceased and living donor transplantation from the time of dialysis initiation. These models were censored for the alternative transplant type (e.g., deceased donor models were censored at the time of living donor transplantation). Analyses were conducted using SAS software (v. 9.4; Cary, NC). The study was approved by the Cleveland Clinic Institutional Review Board.
Results
Study Population Characteristics
The study population included 1,309,998 patients with mean age of 54.4 years (SD=11.6 years). Fifty-seven percent were men, and the most common race/ethnicities were non-Hispanic White (46%), non-Hispanic Black (32%), and Hispanic White (15%) (Table 1). Diabetes was the most common primary diagnosis (49%) followed by hypertension (23%). The most frequent body mass index (BMI) group was 25–29 kg/m2 (28%), and the most common household median income was $36,001–$47,000 (27%). There were some variations in patient characteristics over time, including increases in older patients, Hispanics, men, hypertension, increasing BMI, and region. The number of patients increased over time such that the most recent era (2013–2016) had 22% of patients compared with 17% in 1997–2000 (Table 1).
Table 1.
Patient characteristics by era (n=1,309,998)
| Patient Characteristics, n | Year of Initial ESKD Onset or Wait List Placement, % | ||||
|---|---|---|---|---|---|
| 1997–2000, n=217,989 | 2001–2004, n=244,601 | 2005–2008, n=270,258 | 2009–2012, n=284,772 | 2013–2016, n=292,378 | |
| Age at ESKD onset | |||||
| 18–39, n=154,749 | 14.4 | 12.6 | 11.8 | 10.9 | 10.3 |
| 40–49, n=214,610 | 17.9 | 17.4 | 16.7 | 15.6 | 14.9 |
| 50–59, n=383,385 | 27.2 | 29.4 | 30.5 | 29.8 | 29.0 |
| 60–70, n=557,254 | 40.5 | 40.6 | 41.0 | 43.8 | 45.8 |
| Race/ethnicity | |||||
| Non-Hispanic White, n=603,369 | 46.8 | 45.8 | 45.7 | 46.0 | 46.1 |
| Non-Hispanic Black, n=421,801 | 33.5 | 33.4 | 30.0 | 31.2 | 30.4 |
| Asian, n=40,855 | 2.7 | 2.9 | 3.1 | 3.3 | 3.6 |
| Hispanic White, n=191,078 | 11.5 | 13.1 | 14.6 | 16.2 | 16.6 |
| Hispanic Black, n=6361 | 0.6 | 0.5 | 0.5 | 0.5 | 0.4 |
| Other, n=46,534 | 4.9 | 4.3 | 3.1 | 2.9 | 2.9 |
| Sex | |||||
| Women, n=562,886 | 45.7 | 44.4 | 42.9 | 41.7 | 41.0 |
| Men, n=746,977 | 54.3 | 55.6 | 57.1 | 58.3 | 59.0 |
| Unknown, n=135 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Primary diagnosis | |||||
| Diabetes, n=642,778 | 48.9 | 49.0 | 48.2 | 48.5 | 50.6 |
| Hypertension, n=298,072 | 20.6 | 21.2 | 21.8 | 23.5 | 25.8 |
| GN, n=145,895 | 13.9 | 12.5 | 11.2 | 10.1 | 8.8 |
| Cystic disease, n=40,470 | 3.2 | 3.1 | 3.3 | 3.1 | 2.8 |
| Other/missing, n=182,783 | 13.4 | 14.1 | 15.5 | 14.8 | 12.0 |
| BMI, kg/m2 | |||||
| 13–18, n=38,482 | 5.5 | 3.1 | 2.6 | 2.2 | 2.0 |
| 19–24, n=276,845 | 25.5 | 24.3 | 20.8 | 18.5 | 18.1 |
| 25–29, n=365,309 | 27.6 | 29.8 | 28.2 | 27.3 | 26.8 |
| 30–34, n=265,657 | 16.3 | 19.6 | 20.7 | 21.6 | 22.1 |
| 35–39, n=154,884 | 7.9 | 10.6 | 12.1 | 13.5 | 13.9 |
| 40+, n=158,432 | 6.7 | 9.9 | 12.7 | 14.6 | 15.0 |
| Missing, n=50,389 | 10.5 | 2.7 | 2.9 | 2.5 | 2.1 |
| ESKD network | |||||
| (S-CA) Southern California Network, n=97,068 | 7.0 | 7.5 | 7.2 | 7.5 | 7.7 |
| (N-CA) Trans-Pacific ESRD Network, n=62,034 | 4.7 | 4.6 | 4.7 | 4.8 | 4.9 |
| (WA) Northwest Renal Network, n=36,239 | 2.6 | 2.7 | 2.7 | 3.0 | 2.9 |
| (CO) Inter-Mountain ESRD Network, n=61,848 | 4.4 | 4.6 | 4.7 | 4.9 | 4.9 |
| (TX) Network of Texas, n=119,147 | 8.0 | 8.4 | 8.9 | 9.4 | 10.3 |
| (OK) ESRD Network 13, n=58,976 | 4.6 | 4.6 | 4.4 | 4.4 | 4.5 |
| (MO) ESRD Network 12, n=47,957 | 3.8 | 3.6 | 3.7 | 3.6 | 3.7 |
| (MN) Renal Network of Upper Midwest, n=81,884 | 6.4 | 6.5 | 6.5 | 6.1 | 5.9 |
| (IL) Renal Network of Illinois, n=55,605 | 4.4 | 4.3 | 4.3 | 4.2 | 4.0 |
| (IN) Tri-State R. N., n=96,347 | 7.3 | 7.3 | 7.4 | 7.5 | 7.3 |
| (MS) Network 8, n=76,491 | 5.7 | 5.6 | 5.9 | 5.9 | 6.0 |
| (FL) ESRD Network of Florida, n=76,617 | 5.5 | 5.6 | 5.9 | 6.1 | 6.1 |
| (NC) Southeastern Kidney Council, n=122,919 | 9.2 | 9.3 | 9.5 | 9.3 | 9.6 |
| (VA) Mid-Atlantic R. C., n=79,256 | 6.4 | 6.4 | 6.2 | 5.9 | 5.6 |
| (PA) ESRD Network Organization, n=56,443 | 4.7 | 4.4 | 4.4 | 4.2 | 3.9 |
| (NJ) Trans-Atlantic R. C., n=58,218 | 4.9 | 4.7 | 4.4 | 4.3 | 4.1 |
| (NY) Network of New York, n=80,726 | 6.9 | 6.5 | 6.1 | 5.8 | 5.7 |
| (CT) Network of New England, n=41,527 | 3.5 | 3.3 | 3.2 | 3.0 | 2.9 |
| Missing, n=696 | 0.1 | 0.1 | <0.1 | <0.1 | <0.1 |
| Comorbid conditions | |||||
| Congestive heart failure, n=335,151 | 26.3 | 25.7 | 26.6 | 25.4 | 24.2 |
| Cancer, n=60,005 | 3.6 | 4.1 | 5.0 | 5.2 | 4.8 |
| Atherosclerotic heart disease, n=212,996 | 20.6 | 20.8 | 16.2 | 14.6 | 10.9 |
| Tobacco use, n=98,508 | 6.6 | 6.5 | 8.0 | 8.2 | 8.0 |
| Hypertension, n=1,087,041 | 75.3 | 80.0 | 83.8 | 86.2 | 87.4 |
| Alcohol abuse, n=27,311 | 2.0 | 1.8 | 2.2 | 2.3 | 2.1 |
| Peripheral vascular disease, n=144,161 | 12.2 | 11.6 | 11.8 | 10.8 | 9.2 |
| Median household income level, $ | |||||
| <29,000, n=108,464 | 6.7 | 6.3 | 9.1 | 9.6 | 9.1 |
| 29,001–36,000, n=154,661 | 10.0 | 9.9 | 12.1 | 12.8 | 13.5 |
| 36,001–47,000, n=352,327 | 26.0 | 25.8 | 27.2 | 27.9 | 27.2 |
| 47,001–61,000, n=335,675 | 27.7 | 28.1 | 24.9 | 24.0 | 24.3 |
| 61,001–80,000, n=209,576 | 17.0 | 17.5 | 15.6 | 15.2 | 15.1 |
| >80,000, n=127,359 | 10.4 | 10.9 | 9.4 | 8.9 | 9.4 |
| Missing, n=21,936 | 2.2 | 1.7 | 1.7 | 1.5 | 1.4 |
| All patients | 16.6 | 18.7 | 20.6 | 21.7 | 22.3 |
S-CA, Southern California; N-CA, Northern California; R. N., renal network; R. C., renal coalition.
Cumulative Incidence of Wait Listing or Transplant by Era
The overall incidence of WLT within 1 year of ESKD among the study population was 19%. This proportion included 7.4% of patients with preemptive WLT in addition to patients with WLT within 1 year of dialysis onset. The proportion of 1-year WLT remained relatively consistent over the eras: 18% in 1997–2000, 20% in 2005–2008, and 19% in 2013–2016. As expected, the proportion varied by age, with higher WLT among younger patients. The proportion declined over time for younger patients, including a 16% decline among patients 18–29 years old and a 22% decline among patients 30–39 years old (Figure 1). In contrast, the proportion increased for patients aged 60–64 years old (23% increase) and 65–70 years old (126% increase) (Figure 1). The cumulative incidence of WLT by era is depicted in Figure 2. Preemptive WLT significantly increased over time (5.2%–9.8%). However, within 1 year of ESKD, the cumulative incidences were similar by era (ranging between 18.1% and 19.9%). Similarly, 4-year incidences were similar by era, ranging between 28.1% in the 2013–2016 era and 30.5% in the 2005–2008 era.
Figure 1.
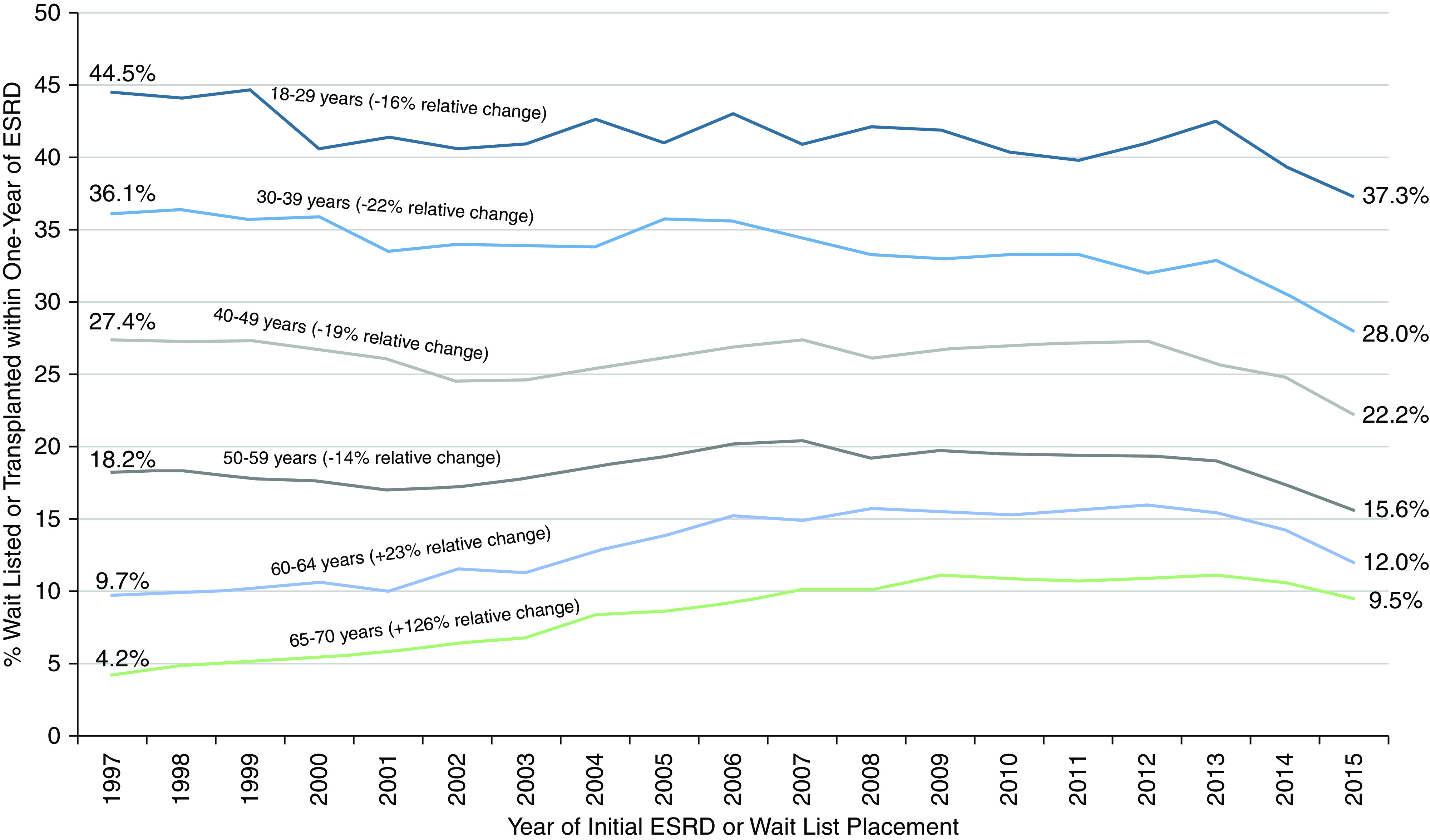
Proportion of adult patients 18–70 years placed on the waiting list or transplanted within 1 year of ESKD. Patients placed on the waiting list either prior to or within 1 year of ESKD onset.
Figure 2.
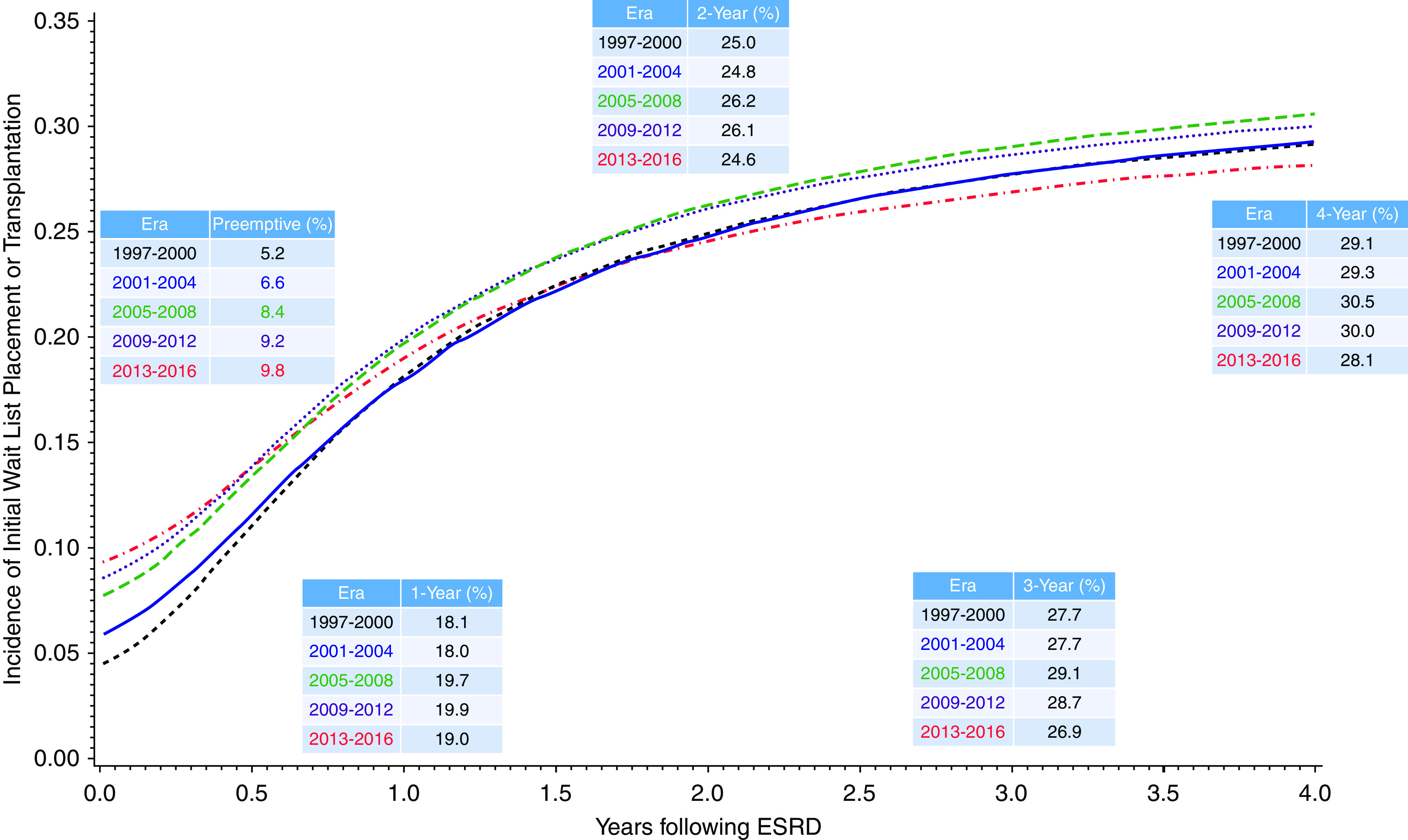
Cumulative incidence of wait list placement or transplant by era onset. Competing risk model with inception point minimum of ESKD onset date or placement on the transplant waiting list, with death a competing risk (censored at last follow-up).
Cumulative Incidence of Mortality prior to WLT by Era
The cumulative incidence rates of mortality within 1, 2, 3, and 4 years of ESKD onset were 14%, 23%, 31%, and 37%, respectively. The cumulative incidence of mortality declined over the study period, including 4-year incidence of mortality of 41.0% in the 1997–2000 era versus 33.6% in the 2013–2016 era (Figure 3).
Figure 3.
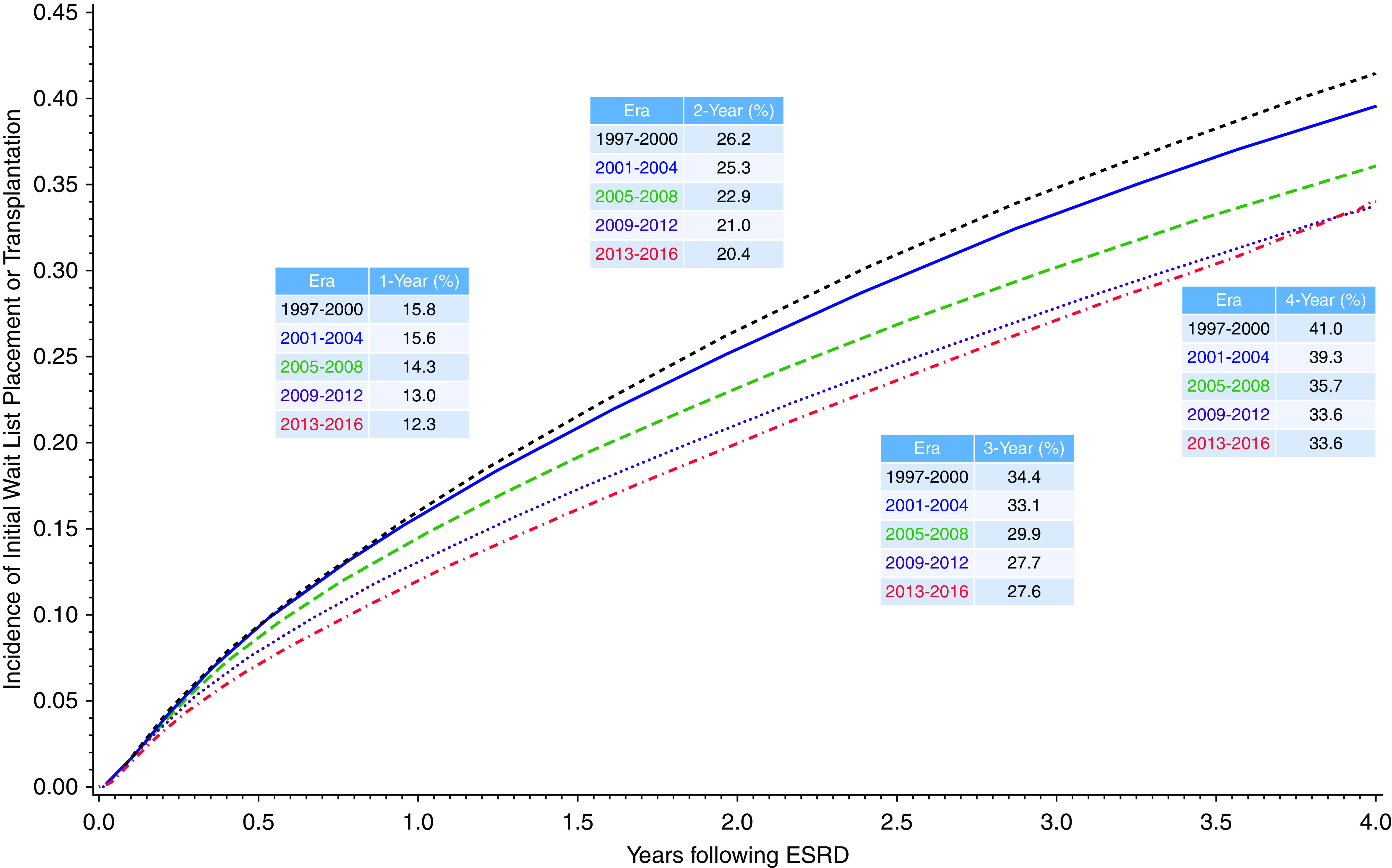
Cumulative incidence of mortality prior to wait list placement or transplantation by era from time of ESKD. Competing risk model with inception point at ESKD onset date and WLT a competing risk (censored at last follow-up).
Cumulative Incidence of WLT by Patient Characteristics
The 4-year incidence of WLT among patients 18–39 years old declined over time (55.8%–48.8%) (Table 2). Older patients (ages 60–70) had the lowest overall WLT incidence but a significant increase by era (13.4%–19.8%). Asian patients had the highest rate of WLT across eras, whereas Black patients consistently had the lowest WLT rates. Men had higher incidence of WLT compared with women. There was lower incidence among patients with diabetes and morbidly obese patients and significant variation by ESKD network. Incidence of WLT was also progressively higher by residential median income level, and the significant differences by median income level were consistent over the study period (Figure 4). The 4-year incidence of deceased donor transplantation declined from 9.8% in 1997–2000 to 6.8% in 2013–2016 (Supplemental Figure 1). Similarly, living donor transplant rates were 6.9% in 1997–2000 and 5.3% in 2013–2016 (Supplemental Figure 2).
Table 2.
Cumulative incidence of 4-year WLT by patient characteristics and ESKD network and era (n=1,309,998)
| Patient Characteristics | Year of ESKD Onseta | ||||
|---|---|---|---|---|---|
| 1997–2000 | 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 | |
| Age at ESKD onset | |||||
| 18–39 (11.8%) | 55.8 | 54.4 | 53.4 | 52.0 | 48.8 |
| 40–49 (16.4%) | 41.4 | 39.8 | 40.3 | 40.9 | 38.4 |
| 50–59 (29.3%) | 30.7 | 30.2 | 31.0 | 30.4 | 28.6 |
| 60–70 (42.5%) | 13.4 | 16.3 | 19.7 | 20.5 | 19.8 |
| Race/ethnicity | |||||
| Non-Hispanic White (46.1%) | 32.7 | 31.9 | 32.1 | 30.4 | 28.3 |
| Non-Hispanic Black (32.2%) | 22.9 | 23.2 | 25.3 | 25.9 | 24.4 |
| Asian (3.1%) | 45.3 | 49.3 | 48.9 | 49.4 | 47.4 |
| Hispanic White (14.6%) | 31.7 | 32.5 | 34.3 | 33.8 | 30.8 |
| Hispanic Black (0.5%) | 27.7 | 29.2 | 31.6 | 26.7 | 29.2 |
| Other (3.6%) | 23.7 | 25.4 | 28.3 | 27.0 | 24.7 |
| Sexb | |||||
| Women (43.0%) | 25.9 | 26.5 | 28.0 | 27.5 | 25.5 |
| Men (57.0%) | 31.8 | 31.5 | 32.5 | 31.9 | 30.0 |
| Primary diagnosis | |||||
| Diabetes (49.1%) | 22.4 | 23.0 | 25.0 | 24.8 | 22.1 |
| Hypertension (22.8%) | 26.7 | 26.8 | 28.0 | 28.1 | 27.1 |
| GN (11.1%) | 51.8 | 52.5 | 54.6 | 54.5 | 52.0 |
| Cystic disease (3.1%) | 67.4 | 70.9 | 72.9 | 71.8 | 69.8 |
| Other (14.0%) | 25.5 | 25.5 | 25.5 | 25.0 | 26.5 |
| BMI, kg/m2b | |||||
| 13–18 (2.9%) | 23.2 | 21.1 | 21.8 | 23.7 | 20.1 |
| 19–24 (21.1%) | 29.6 | 30.1 | 31.3 | 30.8 | 29.7 |
| 25–29 (27.9%) | 32.1 | 32.9 | 34.6 | 34.6 | 32.4 |
| 30–34 (20.3%) | 30.4 | 31.7 | 34.7 | 34.3 | 32.7 |
| 35–39 (11.8%) | 26.1 | 27.4 | 30.1 | 30.2 | 28.1 |
| 40+ (12.1%) | 19.3 | 17.4 | 17.0 | 16.0 | 13.1 |
| ESKD networkb | |||||
| (S-CA) Southern California network (7.4%) | 31.8 | 33.4 | 31.7 | 29.2 | 29.2 |
| (N-CA) Trans-Pacific ESRD Network (4.7%) | 40.3 | 44.7 | 47.4 | 42.4 | 37.7 |
| (WA) Northwest Renal Network (2.8%) | 35.7 | 33.5 | 31.0 | 29.5 | 27.2 |
| (CO) Inter-Mountain ESRD Network (4.7%) | 32.6 | 30.6 | 32.9 | 33.0 | 30.8 |
| (TX) Network of Texas era (9.1%) | 26.0 | 25.9 | 28.9 | 29.7 | 26.1 |
| (OK) ESRD Network 13 (4.5%) | 22.4 | 19.7 | 21.1 | 20.2 | 19.4 |
| (MO) ESRD Network 12 (3.7%) | 28.6 | 27.4 | 28.8 | 28.0 | 27.6 |
| (MN) Renal Network of Upper Midwest (6.3%) | 37.4 | 38.0 | 36.0 | 34.9 | 34.2 |
| (IL) Renal Network of Illinois era (4.2%) | 31.1 | 30.8 | 32.0 | 33.1 | 29.6 |
| (IN) Tri-State R. N. era (7.4%) | 26.2 | 25.0 | 25.1 | 23.5 | 22.0 |
| (MS) Network 8 era (5.8%) | 24.5 | 25.7 | 26.0 | 26.0 | 21.6 |
| (FL) ESRD Network of Florida (5.9%) | 24.2 | 23.7 | 24.8 | 24.9 | 23.4 |
| (NC) Southeastern Kidney Council (9.4%) | 21.6 | 21.5 | 23.6 | 24.7 | 25.0 |
| (VA) Mid-Atlantic R. C. era (6.1%) | 31.6 | 29.6 | 29.4 | 31.1 | 29.3 |
| (PA) ESRD Network Organization (4.3%) | 32.8 | 33.7 | 36.3 | 34.4 | 34.1 |
| (NJ) Trans-Atlantic R. C. era (4.4%) | 26.3 | 29.4 | 30.9 | 30.7 | 28.7 |
| (NY) Network of New York era (6.2%) | 28.8 | 30.6 | 37.2 | 37.0 | 33.4 |
| (CT) Network of New England era (3.2%) | 35.8 | 37.0 | 40.0 | 40.9 | 38.8 |
| Median household income level, $b | |||||
| <29,000 (8.3%) | 19.3 | 19.1 | 20.2 | 20.8 | 18.4 |
| 29,001–$36,000 (11.8%) | 21.2 | 21.1 | 22.9 | 23.1 | 21.2 |
| 36,001–47,000 (26.9%) | 24.8 | 24.1 | 26.8 | 26.4 | 24.0 |
| 47,001–61,000 (25.6%) | 30.0 | 29.6 | 32.3 | 31.9 | 29.7 |
| 61,001–80,000 (16.0%) | 35.6 | 36.1 | 38.1 | 37.0 | 35.7 |
| >80,000 (9.7%) | 42.7 | 44.1 | 45.5 | 46.0 | 44.0 |
| All patients | 29.1 | 29.3 | 30.6 | 30.1 | 28.1 |
S-CA, Southern California; N-CA, Northern California; R. N., renal network; R. C., renal coalition.
Cumulative incidence on the basis of competing risk models with death included as a competing risk; includes patients listed or transplanted preemptively as well as following dialysis initiation.
Missing levels are not displayed.
Figure 4.
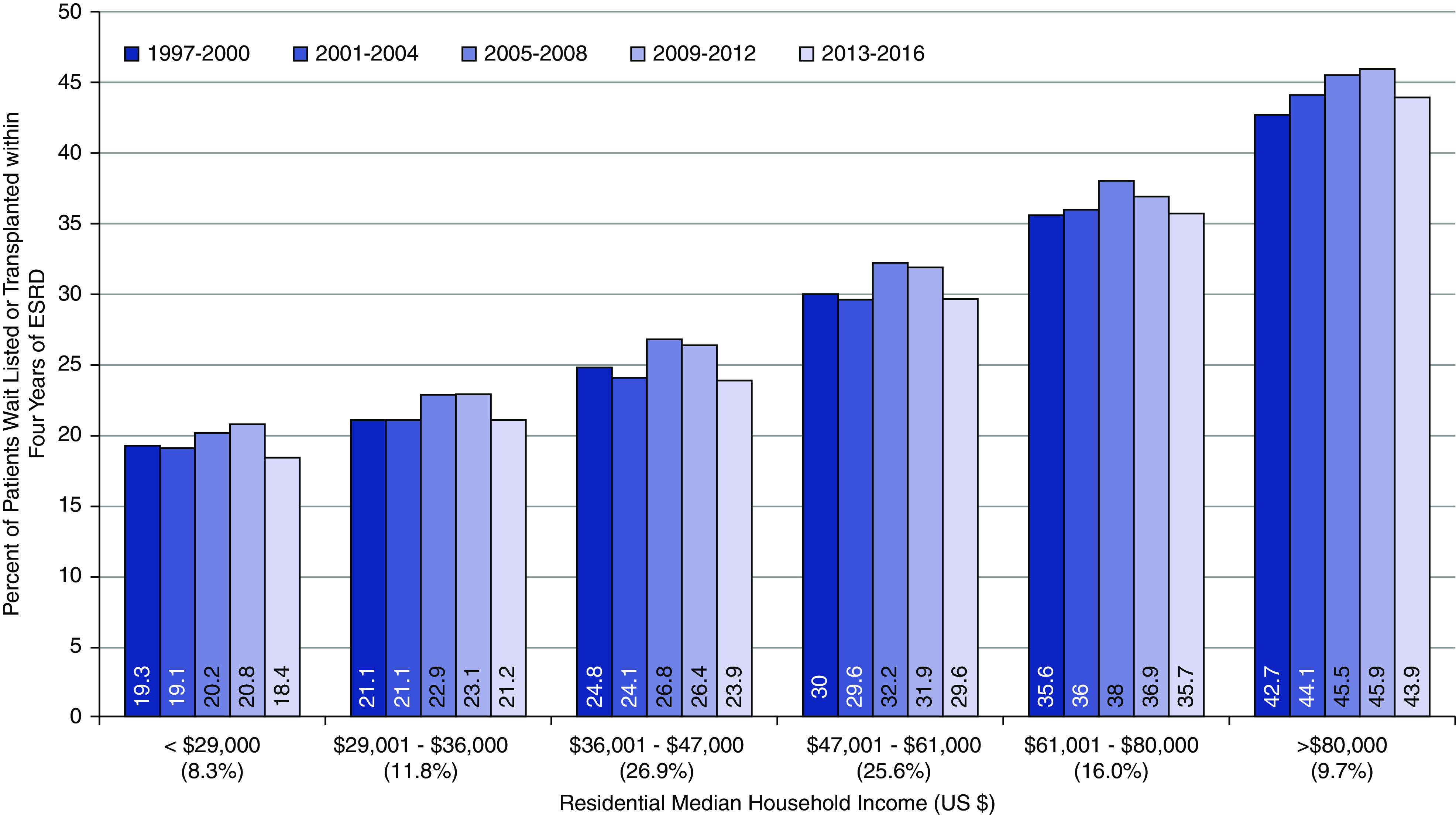
Cumulative incidence of 4-year wait list placement or transplantation by median zip code level household income and era. Competing risk model with inception point minimum of ESKD onset date or placement on the transplant waiting list with death a competing risk (censored at last follow-up). Median household income estimated from patients’ primary residential zip code and the American Community Surveys from 2000, 2008, and 2016.
Adjusted Risks of Wait List Placement, Transplantation, and Mortality
The adjusted likelihood of preemptive WLT increased by era (adjusted odds ratio [AHR], 3.09; 95% confidence interval [95% CI], 3.01 to 3.18 for 2013–2016 relative to 1997–2000). Other significant independent factors associated with higher likelihood of preemptive WLT included higher household median income, absence of comorbid conditions, employed working status, cystic disease, women, non-Hispanic Whites, and younger age (Supplemental Figure 3). Factors associated with increased adjusted hazard of WLT following dialysis initiation included higher median income, absence of comorbid conditions, employed working status, midlevel BMI, cystic disease and GN, men, non-Black race, and younger age (Supplemental Figure 4). The most recent era was associated with reduced likelihood of WLT (AHR, 0.80; 95% CI, 0.79 to 0.82) relative to the 1997–2000 era (Table 3). ESKD region was also significantly associated with differences in the cumulative incidence of WLT and adjusted time to WLT. Figure 5 depicts the 4-year cumulative incidence and AHRs of time to WLT by the 18 ESKD regions for the years 2013–2016.
Table 3.
Adjusted wait list and transplant outcomes by era
| Eraa | Adjusted Odds of Preemptive WLTb | Adjusted Hazards of Time to WLT following Maintenance Dialysis Initiationc | Adjusted Hazards of Time to Death following Maintenance Dialysis Initiation prior to WLTc | Adjusted Hazards of Time to Deceased Donor Transplantation following Maintenance Dialysis Initiationd | Adjusted Hazards of Time to Living Donor Transplantation following Maintenance Dialysis Initiationd |
|---|---|---|---|---|---|
| 1997–2000 | Reference | Reference | Reference | Reference | Reference |
| 2001–2004 | 1.46 (1.42 to 1.50) | 0.96 (0.94 to 0.97) | 0.94 (0.94 to 0.95) | 0.87 (0.86 to 0.89) | 1.00 (0.98 to 1.03) |
| 2005–2008 | 2.26 (2.20 to 2.32) | 0.99 (0.98 to 1.00) | 0.85 (0.84 to 0.86) | 0.82 (0.80 to 0.83) | 0.85 (0.83 to 0.88) |
| 2009–2012 | 2.81 (2.73 to 2.88) | 0.95 (0.94 to 0.96) | 0.79 (0.78 to 0.79) | 0.66 (0.65 to 0.67) | 0.69 (0.67 to 0.71) |
| 2013–2016 | 3.09 (3.01 to 3.18) | 0.80 (0.79 to 0.82) | 0.75 (0.74 to 0.76) | 0.53 (0.51 to 0.55) | 0.54 (0.52 to 0.56) |
Year of initial ESKD or wait list placement.
Likelihood of preemptive WLT among all patients in the study population (n=1,310,396); model was adjusted for age, race/ethnicity, BMI, primary diagnosis, sex, employment status, chronic heart failure, atherosclerotic heart disease, history of cancer, tobacco use, alcohol abuse, peripheral vascular disease, median household income, ESKD network, and Medicaid insurance coverage.
Hazards of time to wait list placement or death among patients initiated on maintenance dialysis prior to WLT (n=1,214,461); models were censored at death. Models were adjusted for age, race/ethnicity, BMI, primary diagnosis, sex, employment status, chronic heart failure, atherosclerotic heart disease, history of cancer, tobacco use, alcohol abuse, peripheral vascular disease, median household income, ESKD network, and Medicaid insurance coverage.
Hazards of time to deceased or living donor transplantation among patients initiated on maintenance dialysis prior to WLT (n=1,214,461); models were censored at death and time of alternative donor type. The model was adjusted for age, race/ethnicity, BMI, primary diagnosis, sex, employment status, chronic heart failure, atherosclerotic heart disease, history of cancer, tobacco use, alcohol abuse, peripheral vascular disease, median household income, ESKD network, and Medicaid insurance coverage.
Figure 5.
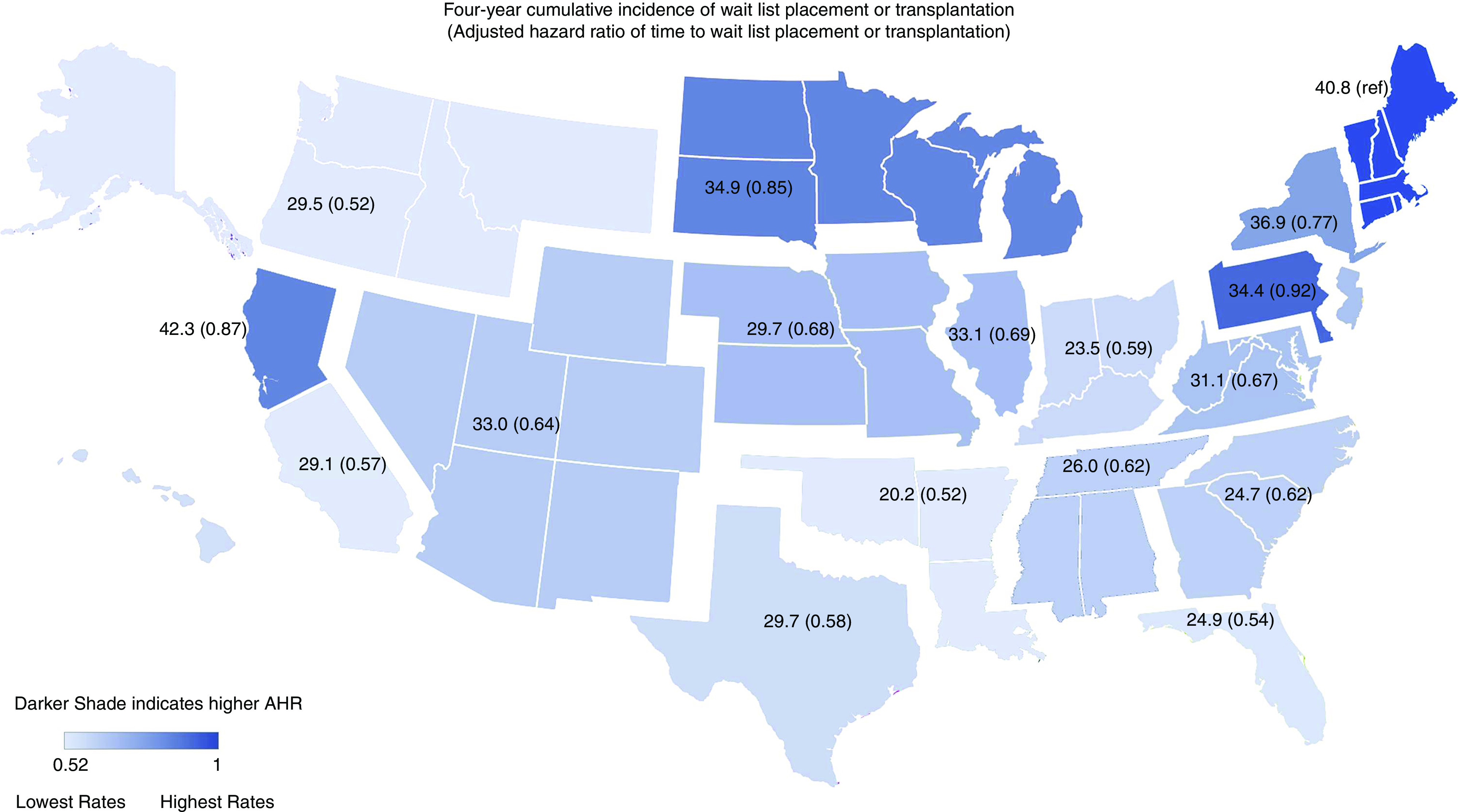
Cumulative incidence of 4-year wait list placement or transplantation and adjusted hazards for time to wait list placement or transplantation among patients in 2013–2016 by ESKD network. Darker shade indicates higher adjusted hazard ratio for wait list placement of transplantation. Ref, reference ESKD network.
By more narrowly categorizing era 5 to the period prior to 2013 to December 3, 2014 and following the implementation of the revised Kidney Allocation System (KAS), the adjusted rates were lower in each period but particularly reduced after KAS implementation. The adjusted WLT from 2013 to December 3rd, 2014 was 0.87 (95% CI, 0.86 to 0.88), and post-KAS (December 4, 2014–2016), AHR was 0.76 (95% CI, 0.75 to 0.77). Adjusted mortality was progressively reduced over time (AHR, 0.75; 95% CI, 0.74 to 0.76 for 2013–2016 relative to 1997–2000) and lower among patients with higher median income. Mortality was higher among patients with comorbid conditions and nonemployed patients and lower among patients who were obese, nondiabetic, non-Hispanic White, and of younger age. The adjusted relative hazard of deceased and living donor transplantation declined over time (Table 3). Rates of both deceased and living donor transplantation were also consistently lower throughout the study period for patients residing in lower-income residential households.
As sensitivity analyses, we administratively censored cumulative incidence and multivariable models as the last date of each respective era (e.g., December 31, 1999 for era 1997–2000). Overall, the qualitative results for each model were very consistent with modest changes in point estimates. The 4-year incidences of wait list placement or transplant by era were 29.2%, 29.1%, 31.0%, 30.2%, and 28.1% for eras 1–5, respectively. The AHRs for time to wait list placement or transplant following ESKD (with era 1 as reference) were 0.92 (95% CI, 0.91 to 0.94) for era 2, 1.00 (95% CI, 0.99 to 1.01) for era 3, 0.97 (95% CI, 0.96 to 0.98) for era 4, and 0.81 (95% CI, 0.80 to 0.82) for era 5. Results using the revised censoring approach were also consistent with the primary analyses for the outcomes of time to deceased and living donor transplantation.
Discussion
The primary findings of the study demonstrate that there has been no measurable progress in improving access to kidney transplantation over the past two decades. The 4-year incidence of placement on the waiting list or transplantation following initial ESKD onset has been stagnant at approximately 30% for the entire 20-year study period. These disappointing results exist despite vast research literature depicting barriers to transplantation, numerous interventional studies demonstrating effective modalities for improving access to care, significant advances in clinical transplant medicine and surgery, and robust empirical studies validating the benefits of transplantation, including in relatively high-risk populations. Although results of the study indicate increased rates of preemptive access to transplantation, the majority of patients do not gain access to transplant until after dialysis initiation, and access to transplantation declined over time following dialysis initiation. In addition, results indicated prominent socioeconomic barriers to transplantation that did not change over the study period. Overall, results suggest that the culmination of efforts over the study period have had no marked effect on improving access to transplantation.
The proportion of patients with ESKD who would qualify as eligible for transplantation is not known, which complicates the ability to define the accurate level of unmet care. However, regardless of the specific proportion, there has been no measurable improvement over the duration of the study. Survival on dialysis prior to wait list placement has modestly improved, but it is important to recognize that there remains a substantial survival advantage of transplantation.31–34 The incidence of mortality decreased to approximately 33% at 4 years post-ESKD onset in the most recent era; however, 4-year mortality among kidney transplant recipients is <10%.8 On the basis of available demographic and clinical data, there are substantial numbers of patients with ESKD who would qualify for transplantation but are never placed on the waiting list.35 Survival on the kidney transplant wait list has markedly improved over time, which may suggest that transplant centers are more selective for transplant eligibility.36 There are clear clinical factors associated with transplant eligibility and wait list placement. As evidenced in this study, rates of wait list placement are significantly higher among patients with cystic disease as a primary etiology of ESKD, younger patients, and patients with absence of documented comorbid conditions. However, there is also evidence that nonclinical, systemic, or other underlying factors, including poor understanding of the advantages of transplantation by dialysis staff, explain a large proportion of patients not being placed on the waiting list. These factors are exacerbated by the fact that transplant education is not consistently disseminated across health care providers and varies by patient population despite requirements at the time of completion of the medical evidence form for Medicare eligibility.37–40 Thus, the failure to improve access to transplantation is likely not due to limitations of medically qualified patients but rather, is due to the ability to promote access to transplantation more broadly to all patient groups.
There were numerous patient characteristics with differential rates and secular changes of WLT. Patients older than 60 had increases in WLT rates, which may reflect increased acceptance of the survival benefit in this cohort.41,42 In contrast, the declining rates of WLT among younger patients are difficult to explain and discouraging given their potential benefit with transplantation.1,43 Lower rates among younger patients may reflect the significant association and greater prevalence of lower socioeconomic status or lack of access to care independent of clinical risk factors. Further understanding of if these barriers to care are increasing among younger patients is important to evaluate. In contrast, older patients have significantly increased rates of wait list placement, suggesting that rates of wait list placement over time are not simply reflections of stagnant secular changes overall but vary within the population. Interestingly, women had higher rates of preemptive wait list placement or transplant, whereas men had higher rates after initiating maintenance dialysis. Although crude incidence rates were similar by sex, women had lower residential income and employment rates, which may have explained higher risk-adjusted incidence rates for preemptive listing.
In 2014, the KAS was significantly revised. One change was to prioritize access to transplantation with higher-quality deceased donor organs to patients with higher expected post-transplant survival (the Estimated Post-Transplant Survival [EPTS] score).7,26,44 On the basis of the EPTS scoring algorithm, many younger patients with ESKD would qualify with a low EPTS score affording these patients accelarated priority to ideal donor kidney offers.45 There is potential that lower rates of WLT since implementation of KAS may be related to changes in allocation backdating priority to initial dialysis onset. However, the stagnant rates of access to transplant in the overall population preceded KAS, and the implementation of this policy did not seem to significantly alter the secular trends. In addition, it is important to acknowledge that many patients receive offers early after placement on the waiting list, despite lengthening waiting times, and as such, delayed access to the waiting list may still have prominent detrimental effects of prospective transplant candidates.46,47
Among many other significant factors associated with diminished rates of WLT, the effects of socioeconomic status were glaring and unchanged over time. Certainly, the social environment and available support are important considerations for candidate transplant viability and may be a greater concern among patients who have financial concerns.48–51 Health literacy and ability to navigate the steps necessary to obtain a transplant may also be more challenging among these patient groups.28,51–53 This study was limited on the basis of lack of information about whether patients were never referred for transplantation, failed to complete their evaluation, or were denied eligibility after referral. However, referral rates have been shown to be lower among minority patients and patients with lower socioeconomic status.15,16,23 Patients with lower socioeconomic status may also have more underlying health conditions that may reduce viability for the procedure.21,54–57 However, there is no evidence that social factors or comorbid conditions were more prevalent among lower socioeconomic groups over time, and they do not seem to explain the stagnant rates in this study. Rates of both deceased and living donor transplantation were also lower among patients residing in lower-income communities. These differences were consistent over the study period and parallel the differences in wait list placement. The marked geographic heterogeneity in rates of wait list placement may explain some of the differences in results by race/ethnicity and socioeconomic status. There is also no relationship between dialysis quality measures and transplant referral rates, suggesting that regional dialysis facility practices may affect transplant rates.58 Further examination of the interaction of geography with consistently lower rates of wait list placement and transplantation among racial minorities and patients residing in lower-income communities is warranted.
An important result of the study is that preemptive wait list rates have increased, which has been associated in part with improved access to care with the Affordable Care Act.59,60 However, disparities remain among patients who are preemptively listed.61,62 Furthermore, the actual number of patients who may be appropriate for preemptive listing (i.e., patients with a low GFR of <20 ml/min who do not have contraindications to transplant) is not known. Therefore, the improved preemptive proportion may reflect the growing burden of stage 4 CKD and thus, more patients qualifying for referral to transplant as opposed to an indication of improved access to care in the overall population. In addition, despite improvements in preemptive listing, within 1 year of ESKD onset WLT rates remained stagnant and attenuated the longer patients remained on dialysis. Region and profit status of dialysis facilities are important sources of variation in WLT rates, and these results suggest that novel interventions are particularly needed after dialysis initiation.25,63,64
There may be concerns about efforts to promote access to transplantation given limited available donor organs. In fact, the number of kidney transplants has significantly increased in the United States, roughly in proportion to the increase in the dialysis population. However, there are several factors to consider for continuing to prioritize access to transplantation. First, failure to promote transplantation has disproportionate effects on certain patient groups and exacerbates health disparities. Second, there are opportunities to receive donor kidney offers in a relatively short period of time in certain regions of the country, particularly for harder to place organs that still convey a significant survival benefit.1,65,66 Third, early promotion of transplantation can lead to identification of living donors, which is associated with excellent long-term outcomes. Fourth, providing opportunities for all potential candidates may stimulate further innovation and research to identify additional organ donors and develop more efficient allocation algorithms.46,67–70 Finally, there is a notably high deceased donor kidney discard rate in the United States relative to other countries, and improving placement of patients on transplant waiting lists may identify further uses for organs that otherwise are not allocated in a timely manner to patients who may benefit.67 Ultimately, optimizing care for all potential candidates is a priority, and further efforts to identify transplant opportunities will also be crucial.
In recognition of the benefits of transplantation, the federal administration enacted the Advancing Kidney Health Executive Order, which included aims to markedly increase donor availability and kidney transplantation in the United States.29 One of the intents of this executive order will be to improve access to transplantation on the basis of incentives to care providers and organ procurement organizations. In addition, recent changes to CMS policy to reduce rigorous quality monitoring may improve access to transplantation for patients with ESKD who may have been excluded due to concerns about the detrimental effects on measured performance.71–75
There are several prominent limitations of this study that should be acknowledged. The data are from a retrospective observational study, and thus, statistically significant associations do not necessarily imply causation. There is significant potential for residual confounding on the basis of unobserved factors associated with access to transplantation that are not available for analysis or risk adjustment. In this case, the degree to which these factors may have changed over time may also imply that secular changes could have been influenced by these noncodified factors. The actual denominator of patients who are medically and socially appropriate candidates is not known, including those patients with CKD who have not yet initiated dialysis. This analysis was also restricted to patients’ initial ESKD onset, and as such, patients with primary transplant graft failure and potential relisting or retransplantation were not incorporated into the analyses. This limitation may have affected rates of wait list placement and transplantation over time as the retransplant population has increased over the study period. The results do not also address specific interventions that may improve access to care but rather, suggest that prior efforts may not have accomplished intended goals of improving access and attenuating disparities.
In summary, results of the study highlight failure to advance access to kidney transplantation for the ESKD population over the prior two decades. These concerning results likely represent systematic barriers to care that have not been fully addressed with prior interventions and policy reforms. The effects of newer policies on improving access to transplantation will be critical to monitor. Equitable and optimal care to patients with ESKD includes promoting access to transplantation, and these results highlight that progress is urgently needed.
Disclosures
A. Huml reports being a scientific advisor or member with Cleveland Minority Organ and Tissue Transplant Education Program and IPRO ESRD Network of the Ohio River Valley Medical Review Board and other interests/relationships with Cleveland Kidney Precision Medicine Project Community Advisory Board. S. Mohan reports consultancy agreements with Angion Biomedica; being a scientific advisor or member of the American Society of Nephrology Quality Committee, member of Angion Pharma scientific advisory board, Deputy Editor of Kidney International Reports (International Society of Nephrology), member of Scientific Registry of Transplant Recipients Visiting Committee, and Vice Chair of United Network for Organ Sharing Data Advisory Committee; and other interests/relationships from research funding from the National Institutes of Health (the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Minority Health and Health Disparities, and National Institute of Biomedical Imaging and Bioengineering). E.D. Poggio reports consultancy agreements with Renalytix; and honoraria from CareDx, Novartis, and Reata. J.D. Schold reports consultancy agreements with Guidry and East, Novartis, Sanofi Corporation, and Transplant Management Group; honoraria from Novartis and Sanofi Inc.; and being a scientific advisor or member as Data Safety Monitoring Board Member for Bristol Myers Squibb. J.D. Schold reports receiving funding unrelated to this study from the Leonard C. Rosenberg Foundation, National Institute on Alcohol Abuse and Alcoholism, National Heart, Lung and Blood Institute, National Institute of Allergy and Infectious Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, National Science Foundation, and Patient-Centered Outcomes Research Institute. J.R. Sedor reports consultancy agreements with Goldfinch Bio, Maze, and Sanofi Genzyme; research funding from Calliditas and Novartis for clinical trials; honoraria from Chugai Pharmaceutical Co. (Next Generation Kidney Research Meeting, Tokyo), Drexel University, Maze, National Kidney Foundation Arizona, the University of Maryland, the University of Michigan, and the University of Minnesota; patents and inventions: APOL1 transgenic mice licensed to Sanofi Genzyme; invention disclosure for machine learning analysis of kidney biopsies; scientific advisor or membership with Kidney Foundation of Ohio (kidney patient organization for direct aid) and Board of Directors, NephCure Kidney International; and other interests/relationships on editorial boards for Seminars in Nephrology, JASN, and American Journal of Nephrology and member of International Society of Nephrology. All remaining authors have nothing to disclose.
Funding
None.
Data Sharing Statement
The data reported here have been supplied by the USRDS.
Supplementary Material
Acknowledgments
The interpretations and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.76
S. Mohan, E.D. Poggio, and J.D. Schold designed the study; J.D. Schold analyzed the data and made the figures; J.J. Augustine, L.D. Buccini, A. Huml, S. Mohan, E.D. Poggio, J.D. Schold, and J.R. Sedor drafted and revised the paper; and all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020060888/-/DCSupplemental.
Supplemental Figure 1. Cumulative incidence of time to deceased donor transplantation by era.
Supplemental Figure 2. Cumulative incidence of time to living donor transplantation by era.
Supplemental Figure 3. Forest plot of multivariable model for preemptive wait list placement or transplantation.
Supplemental Figure 4. Forest plot of multivariable model for time to wait list placement or transplantation after ESKD onset.
References
- 1.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, et al.: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al.: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang SH, et al.: An economic assessment of contemporary kidney transplant practice. Am J Transplant 18: 1168–1176, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Held PJ, McCormick F, Ojo A, Roberts JP: A cost-benefit analysis of government compensation of kidney donors. Am J Transplant 16: 877–885, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landreneau K, Lee K, Landreneau MD: Quality of life in patients undergoing hemodialysis and renal transplantation--a meta-analytic review. Nephrol Nurs J 37: 37–44, 2010 [PubMed] [Google Scholar]
- 6.Tucker EL, Smith AR, Daskin MS, Schapiro H, Cottrell SM, Gendron ES, et al.: Life and expectations post-kidney transplant: A qualitative analysis of patient responses. BMC Nephrol 20: 175, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedewald JJ, Samana CJ, Kasiske BL, Israni AK, Stewart D, Cherikh W, et al.: The kidney allocation system. Surg Clin North Am 93: 1395–1406, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, et al.: OPTN/SRTR 2018 annual data report: Kidney. Am J Transplant 20[Suppl s1]: 20–130, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Arce CM, Goldstein BA, Mitani AA, Lenihan CR, Winkelmayer WC: Differences in access to kidney transplantation between Hispanic and non-Hispanic whites by geographic location in the United States. Clin J Am Soc Nephrol 8: 2149–2157, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelrod DA, Guidinger MK, Finlayson S, Schaubel DE, Goodman DC, Chobanian M, et al.: Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA 299: 202–207, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM: The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med 341: 1661–1669, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, et al.: Racial disparities in access to renal transplantation–clinically appropriate or due to underuse or overuse? N Engl J Med 343: 1537–1544, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacLaughlin HL, Campbell KL: Obesity as a barrier to kidney transplantation: Time to eliminate the body weight bias? Semin Dial 32: 219–222, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Salter ML, McAdams-Demarco MA, Law A, Kamil RJ, Meoni LA, Jaar BG, et al.: Age and sex disparities in discussions about kidney transplantation in adults undergoing dialysis. J Am Geriatr Soc 62: 843–849, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU: Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol 6: 1760–1767, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Waterman AD, Peipert JD, Hyland SS, McCabe MS, Schenk EA, Liu J: Modifiable patient characteristics and racial disparities in evaluation completion and living donor transplant. Clin J Am Soc Nephrol 8: 995–1002, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan S, Mutell R, Patzer RE, Holt J, Cohen D, McClellan W: Kidney transplantation and the intensity of poverty in the contiguous United States. Transplantation 98: 640–645, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucirka LM, Purnell TS, Segev DL: Improving access to kidney transplantation: Referral is not enough. JAMA 314: 565–567, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander GC, Sehgal AR; Transplant Task Force of the Renal Network, Inc.: Variation in access to kidney transplantation across dialysis facilities: Using process of care measures for quality improvement. Am J Kidney Dis 40: 824–831, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Garg PP, Frick KD, Diener-West M, Powe NR: Effect of the ownership of dialysis facilities on patients’ survival and referral for transplantation. N Engl J Med 341: 1653–1660, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Mathur AK, Ashby VB, Sands RL, Wolfe RA: Geographic variation in end-stage renal disease incidence and access to deceased donor kidney transplantation. Am J Transplant 10: 1069–1080, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Patzer RE, Basu M, Smith KD, Plantinga L, Mohan S, Escoffery C, et al.: Awareness of the new kidney allocation system among United States dialysis providers with low waitlisting. Am J Nephrol 47: 115–119, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patzer RE, Plantinga LC, Paul S, Gander J, Krisher J, Sauls L, et al.: Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA 314: 582–594, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou S, Massie AB, Luo X, Ruck JM, Chow EKH, Bowring MG, et al.: Geographic disparity in kidney transplantation under KAS. Am J Transplant 18: 1415–1423, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB: Geographic variability in access to primary kidney transplantation in the United States, 1996-2005. Am J Transplant 7: 1412–1423, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Formica RN Jr.: Perspectives on the strengths and weaknesses of the national kidney allocation system. Clin J Am Soc Nephrol 12: 2056, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patzer RE, Paul S, Plantinga L, Gander J, Sauls L, Krisher J, et al.; Southeastern Kidney Transplant Coalition: A randomized trial to reduce disparities in referral for transplant evaluation. J Am Soc Nephrol 28: 935–942, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan C, Leon JB, Sayre SS, Marbury M, Ivers M, Pencak JA, et al.: Impact of navigators on completion of steps in the kidney transplant process: A randomized, controlled trial. Clin J Am Soc Nephrol 7: 1639–1645, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas E, Milton J, Cigarroa FG: The Advancing American Kidney Health Executive Order: An opportunity to enhance organ donation [published online ahead of print September 20, 2019]. JAMA 10.1001/jama.2019.14500 [DOI] [PubMed] [Google Scholar]
- 30.United States Renal Data Sytem : 2018. USRDS annual data report. Available at: https://www.usrds.org/annual-data-report/previous-adrs/. Accessed November 2, 2020
- 31.Clark S, Kadatz M, Gill J, Gill JS: Access to kidney transplantation after a failed first kidney transplant and associations with patient and allograft survival: An analysis of national data to inform allocation policy. Clin J Am Soc Nephrol 14: 1228–1237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill J, Dong J, Rose C, Gill JS: The risk of allograft failure and the survival benefit of kidney transplantation are complicated by delayed graft function. Kidney Int 89: 1331–1336, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Gill JS, Lan J, Dong J, Rose C, Hendren E, Johnston O, et al.: The survival benefit of kidney transplantation in obese patients. Am J Transplant 13: 2083–2090, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Perl J, Dong J, Rose C, Jassal SV, Gill JS: Is dialysis modality a factor in the survival of patients initiating dialysis after kidney transplant failure? Perit Dial Int 33: 618–628, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schold JD, Srinivas TR, Kayler LK, Meier-Kriesche HU: The overlapping risk profile between dialysis patients listed and not listed for renal transplantation. Am J Transplant 8: 58–68, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Schold JD, Arrigain S, Flechner SM, Augustine JJ, Sedor JR, Wee A, et al.: Dramatic secular changes in prognosis for kidney transplant candidates in the United States. Am J Transplant 19: 414–424, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Kucirka LM, Grams ME, Balhara KS, Jaar BG, Segev DL: Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant 12: 351–357, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Taylor DM, Bradley JA, Bradley C, Draper H, Dudley C, Fogarty D, et al.; ATTOM investigators: Limited health literacy is associated with reduced access to kidney transplantation. Kidney Int 95: 1244–1252, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Waterman AD, Peipert JD, Goalby CJ, Dinkel KM, Xiao H, Lentine KL: Assessing transplant education practices in dialysis centers: Comparing educator reported and Medicare data. Clin J Am Soc Nephrol 10: 1617–1625, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waterman AD, Peipert JD, McSorley AM, Goalby CJ, Beaumont JL, Peace L: Direct delivery of kidney transplant education to black and low-income patients receiving dialysis: A randomized controlled trial. Am J Kidney Dis 74: 640–649, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legeai C, Andrianasolo RM, Moranne O, Snanoudj R, Hourmant M, Bauwens M, et al.: Benefits of kidney transplantation for a national cohort of patients aged 70 years and older starting renal replacement therapy. Am J Transplant 18: 2695–2707, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Pérez-Sáez MJ, Arcos E, Comas J, Crespo M, Lloveras J, Pascual J; Catalan Renal Registry Committee: Survival benefit from kidney transplantation using kidneys from deceased donors aged ≥75 years: A time-dependent analysis. Am J Transplant 16: 2724–2733, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, et al.: Association of race and age with survival among patients undergoing dialysis. JAMA 306: 620–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Melanson TA, Plantinga LC, Basu M, Pastan SO, Mohan S, et al.: Racial/ethnic disparities in waitlisting for deceased donor kidney transplantation 1 year after implementation of the new national kidney allocation system. Am J Transplant 18: 1936–1946, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Husain SA, King KL, Dube GK, Tsapepas D, Cohen DJ, Ratner LE, et al.: Regional disparities in transplantation with deceased donor kidneys with kidney donor profile index less than 20% among candidates with top 20% estimated post transplant survival. Prog Transplant 29: 354–360, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Husain SA, King KL, Pastan S, Patzer RE, Cohen DJ, Radhakrishnan J, et al.: Association between declined offers of deceased donor kidney allograft and outcomes in kidney transplant candidates. JAMA Netw Open 2: e1910312, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King KL, Husain SA, Mohan S: Geographic variation in the availability of deceased donor kidneys per wait-listed candidate in the United States. Kidney Int Rep 4: 1630–1633, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraser SD, Roderick PJ, Casey M, Taal MW, Yuen HM, Nutbeam D: Prevalence and associations of limited health literacy in chronic kidney disease: A systematic review. Nephrol Dial Transplant 28: 129–137, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Garg J, Karim M, Tang H, Sandhu GS, DeSilva R, Rodrigue JR, et al.: Social adaptability index predicts kidney transplant outcome: A single-center retrospective analysis. Nephrol Dial Transplant 27: 1239–1245, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Mehrotra R, Marsh D, Vonesh E, Peters V, Nissenson A: Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 68: 378–390, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Schaeffner ES, Mehta J, Winkelmayer WC: Educational level as a determinant of access to and outcomes after kidney transplantation in the United States. Am J Kidney Dis 51: 811–818, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Ladin K, Rodrigue JR, Hanto DW: Framing disparities along the continuum of care from chronic kidney disease to transplantation: Barriers and interventions. Am J Transplant 9: 669–674, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sehgal AR: Impact of quality improvement efforts on race and sex disparities in hemodialysis. JAMA 289: 996–1000, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Galea S, Tracy M, Hoggatt KJ, Dimaggio C, Karpati A: Estimated deaths attributable to social factors in the United States. Am J Public Health 101: 1456–1465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM: Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 20: 1333–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schold JD, Flechner SM, Poggio ED, Augustine JJ, Goldfarb DA, Sedor JR, et al.: Residential area life expectancy: Association with outcomes and processes of care for patients with ESRD in the United States. Am J Kidney Dis 72: 19–29, 2018 [DOI] [PubMed] [Google Scholar]
- 57.Winkelmayer WC, Liu J, Brookhart MA: Altitude and all-cause mortality in incident dialysis patients. JAMA 301: 508–512, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Plantinga LC, Pastan SO, Wilk AS, Krisher J, Mulloy L, Gibney EM, et al.: Referral for kidney transplantation and indicators of quality of dialysis care: A cross-sectional study. Am J Kidney Dis 69: 257–265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harhay MN, McKenna RM, Boyle SM, Ranganna K, Mizrahi LL, Guy S, et al.: Association between medicaid expansion under the affordable care act and preemptive listings for kidney transplantation. Clin J Am Soc Nephrol 13: 1069–1078, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harhay MN, McKenna RM, Harhay MO: Association between Medicaid expansion under the Affordable Care Act and Medicaid-covered pre-emptive kidney transplantation. J Gen Intern Med 34: 2322–2325, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King KL, Husain SA, Jin Z, Brennan C, Mohan S: Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol 14: 1500–1511, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schold JD, Augustine JJ, Huml AM, O’Toole J, Sedor JR, Poggio ED: Modest rates and wide variation in timely access to repeat kidney transplantation in the United States. Am J Transplant 20: 769–778, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gander JC, Zhang X, Ross K, Wilk AS, McPherson L, Browne T, et al.: Association between dialysis facility ownership and access to kidney transplantation. JAMA 322: 957–973, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Lipford KJ, McPherson L, Hamoda R, Browne T, Gander JC, Pastan SO, et al.: Dialysis facility staff perceptions of racial, gender, and age disparities in access to renal transplantation. BMC Nephrol 19: 5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bowring MG, Holscher CM, Zhou S, Massie AB, Garonzik-Wang J, Kucirka LM, et al.: Turn down for what? Patient outcomes associated with declining increased infectious risk kidneys. Am J Transplant 18: 617–624, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kadatz M, Klarenbach S, Gill J, Gill JS: Cost-effectiveness of using kidneys from hepatitis C nucleic acid test-positive donors for transplantation in hepatitis C-negative recipients. Am J Transplant 18: 2457–2464, 2018 [DOI] [PubMed] [Google Scholar]
- 67.Cooper M, Formica R, Friedewald J, Hirose R, O’Connor K, Mohan S, et al.: Report of National Kidney Foundation consensus conference to decrease kidney discards. Clin Transplant 33: e13419, 2019 [DOI] [PubMed] [Google Scholar]
- 68.Mohan S, Chiles MC, Patzer RE, Pastan SO, Husain SA, Carpenter DJ, et al.: Factors leading to the discard of deceased donor kidneys in the United States. Kidney Int 94: 187–198, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohan S, Foley K, Chiles MC, Dube GK, Patzer RE, Pastan SO, et al.: The weekend effect alters the procurement and discard rates of deceased donor kidneys in the United States. Kidney Int 90: 157–163, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanriover B, Mohan S, Cohen DJ, Radhakrishnan J, Nickolas TL, Stone PW, et al.: Kidneys at higher risk of discard: Expanding the role of dual kidney transplantation. Am J Transplant 14: 404–415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowring MG, Massie AB, Craig-Schapiro R, Segev DL, Nicholas LH: Kidney offer acceptance at programs undergoing a Systems Improvement Agreement. Am J Transplant 18: 2182–2188, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandraker A, Andreoni KA, Gaston RS, Gill J, Locke JE, Mathur AK, et al.; AST/ASTS Transplant Metrics Taskforce: Time for reform in transplant program-specific reporting: AST/ASTS transplant metrics taskforce. Am J Transplant 19: 1888–1895, 2019 [DOI] [PubMed] [Google Scholar]
- 73.Schold JD, Buccini LD, Goldfarb DA, Flechner SM, Poggio ED, Sehgal AR: Association between kidney transplant center performance and the survival benefit of transplantation versus dialysis. Clin J Am Soc Nephrol 9: 1773–1780, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schold JD, Patzer RE, Pruett TL, Mohan S: Quality metrics in kidney transplantation: Current landscape, trials and tribulations, lessons learned, and a call for reform. Am J Kidney Dis 74: 382–389, 2019 [DOI] [PubMed] [Google Scholar]
- 75.White SL, Zinsser DM, Paul M, Levine GN, Shearon T, Ashby VB, et al.: Patient selection and volume in the era surrounding implementation of Medicare conditions of participation for transplant programs. Health Serv Res 50: 330–350, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.United States Renal Data Sytem : 2019. USRDS annual data report: Epimeiology of Kidney Disease in the United States. Available at: https://www.usrds.org/annual-data-report/previous-adrs/. Accessed November 3, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.